Design of Healthy Snack Based on Kiwifruit
Abstract
1. Introduction
2. Results
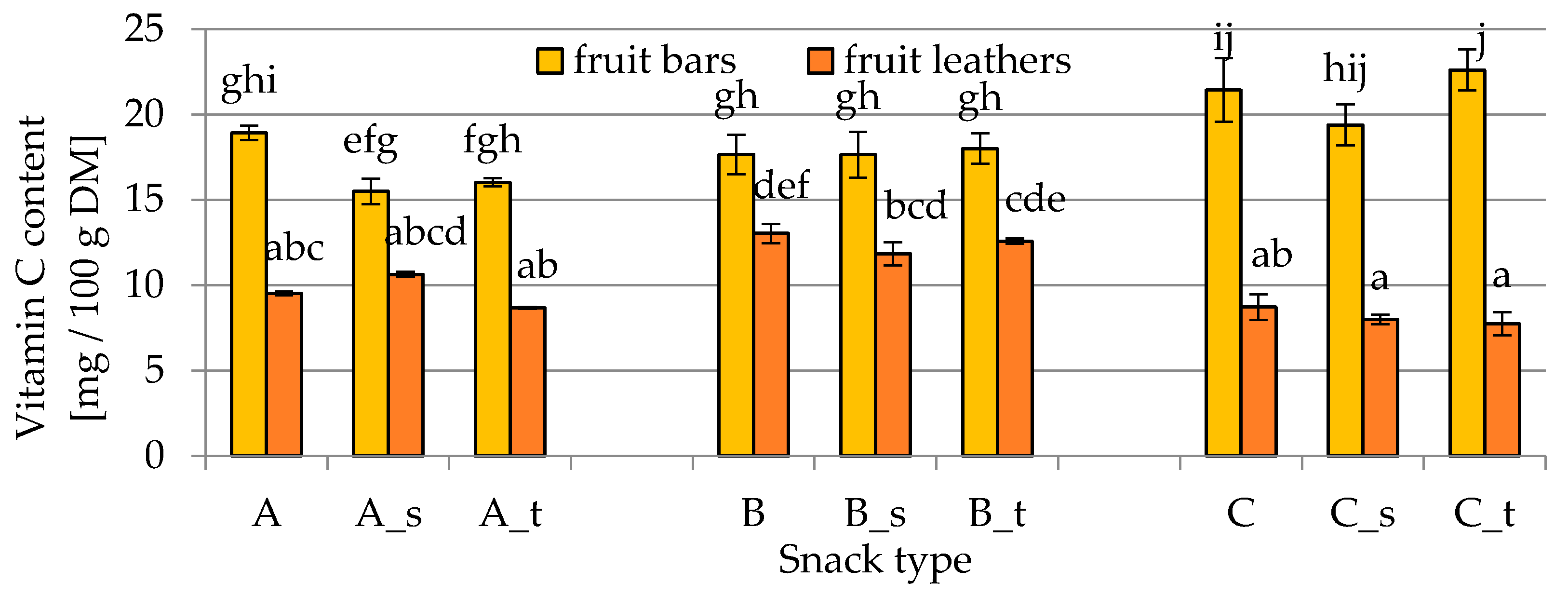
2.1. Vitamin C Content
2.2. Total Polyphenol Content (TPC)
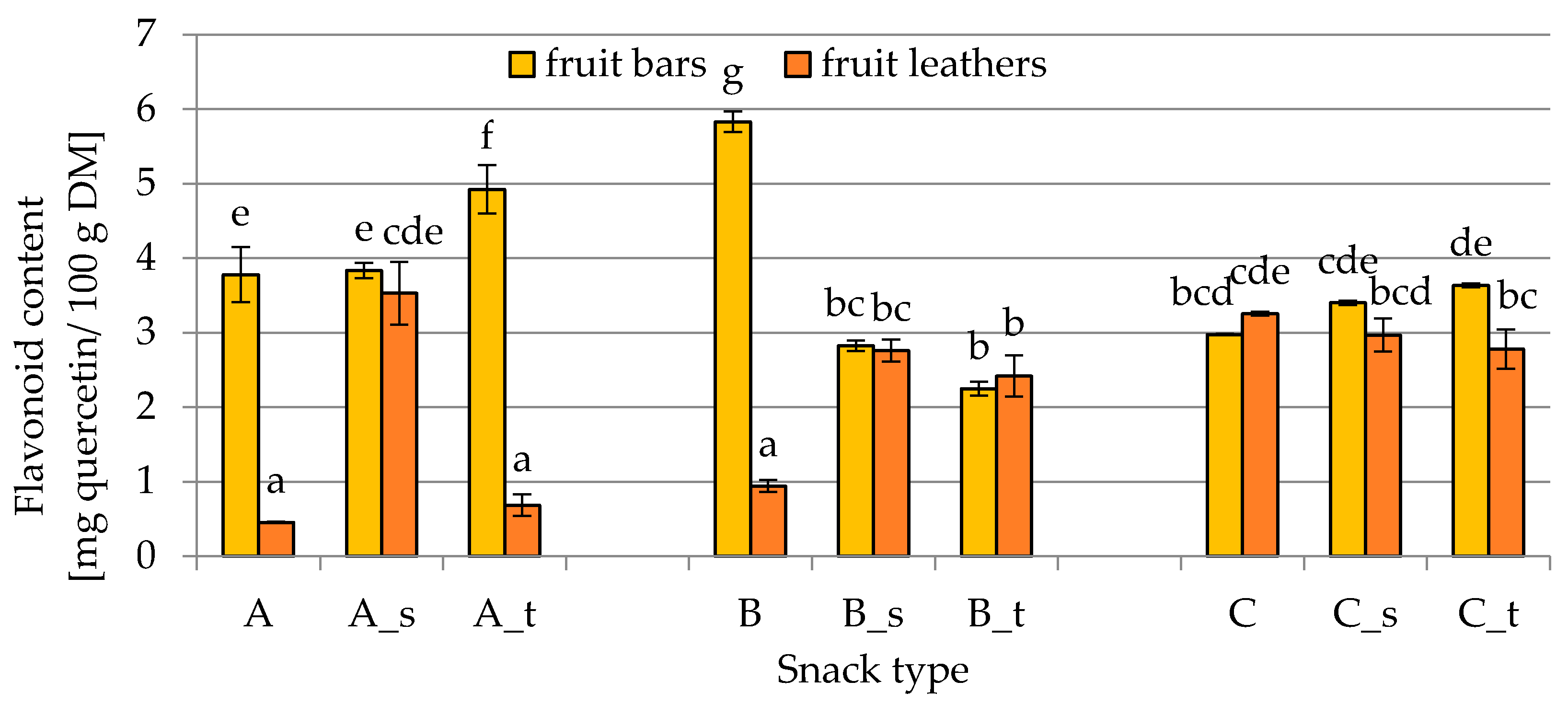
2.3. Flavonoid Content
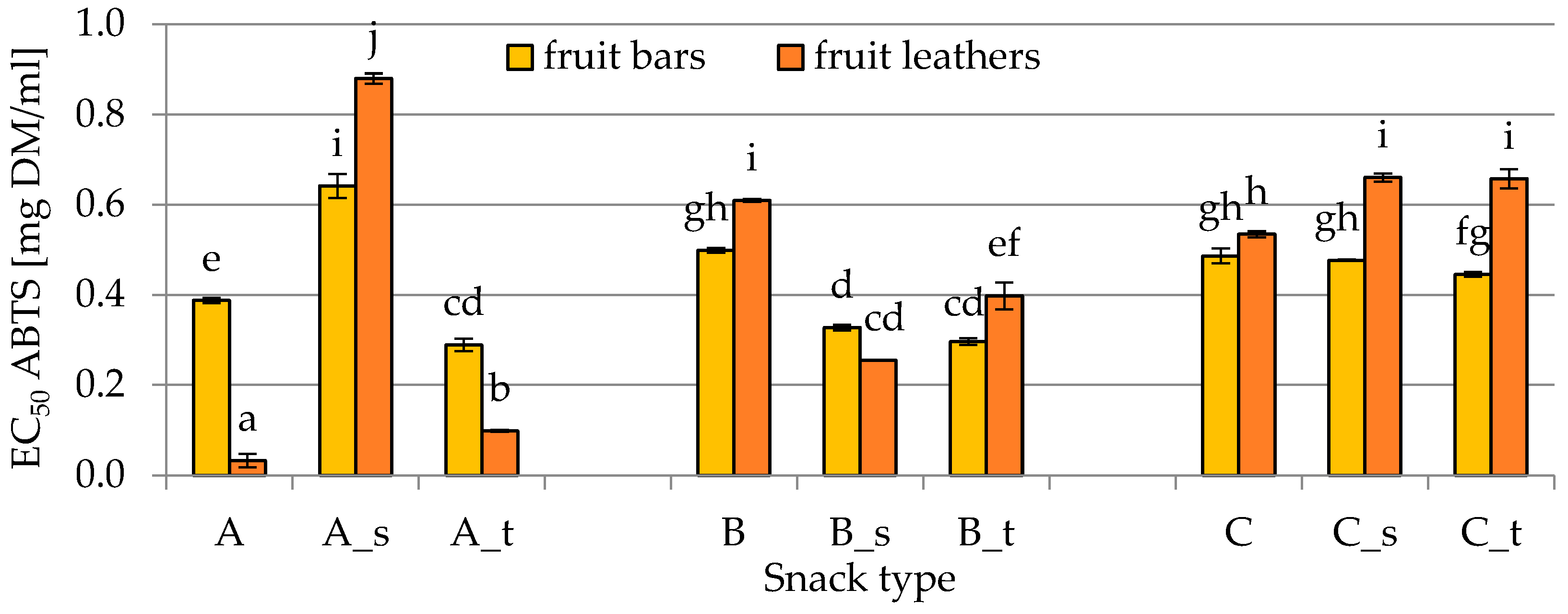
2.4. Antioxidant Activity
3. Discussion
4. Materials and Methods
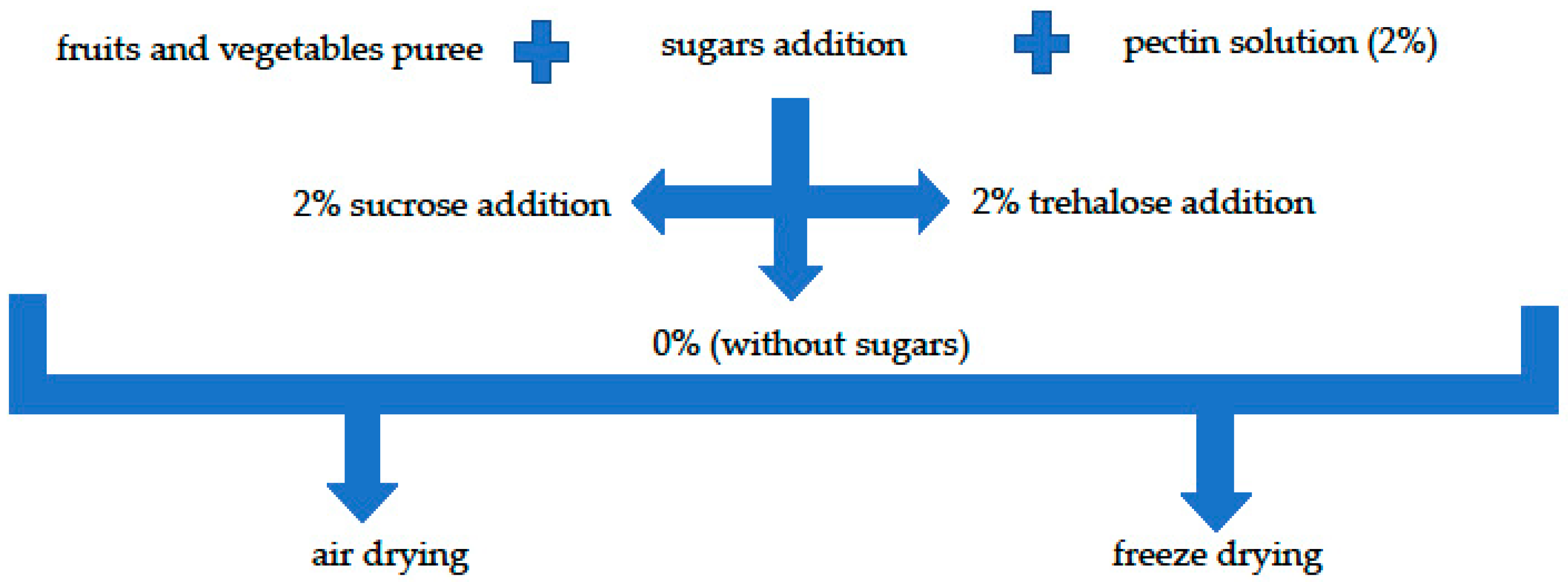
4.1. Snack Preparation
4.1.1. Materials and Puree Preparation
4.1.2. Fruit Leathers Preparation by Air Dying
4.1.3. Fruit Bars Preparation by Freeze-Drying
4.2. Chemical Analysis
4.2.1. Vitamin C Content
4.2.2. Total Polyphenol Content (TPC)
4.2.3. Flavonoid Content
4.2.4. Antioxidant Activity
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Cieśluk, P.; Barwińska, M.; Marczak, W.; Ordyniak, A.; Lenart, A.; Janowicz, M. Eating Habits and Sustainable Food Production in the Development of Innovative “Healthy” Snacks. Sustainability 2019, 11, 2800. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputod, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, H.R.; Beckley, J.H.; Resurreccion, A.V.A. So what are the practical considerations in actually running a test? What do I need to know? What does the rest of the company need to know? In Sensory and Consumer Research in Food Product Design and Development, 2nd ed.; Moskowitz, H.R., Beckley, J.H., Resurreccion, A.V.A., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 321–363. [Google Scholar]
- Hess, J.M.; Jonnalagadda, S.S.; Slavin, J.L. What Is a Snack, Why Do We Snack, and How Can We Choose Better Snacks? A Review of the Definitions of Snacking, Motivations to Snack, Contributions to Dietary Intake, and Recommendations for Improvement. Adv. Nutr. 2016, 7, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.M.S.C.; Morais, A.M.M.B.; Dammak, I.; Bonilla, J.; Sobral, P.J.A.; Laguerre, J.-C.; Afonso, M.J.; Ramalhosa, E.C.D. Functional Dehydrated Foods for Health Preservation. J. Food Qual. 2018, 3, 1–29. [Google Scholar]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Stanisz, E.; Waśkiewicz, A. Potential health benefits and quality of dried fruits: Goji fruits, cranberries and raisins. Food Chem. 2017, 221, 228–236. [Google Scholar] [CrossRef]
- Vatthanakul, S.; Jangchud, A.; Jangchud, K.; Therdthai, N.; Wilkinson, B. Gold kiwifruit leather product development using Quality function deployment approach. Food Qual. Prefer. 2010, 21, 339–345. [Google Scholar] [CrossRef]
- Orrego, C.E.; Salgado, N.; Botero, C.A. Developments and Trends in Fruit Bar Production and characterization. Crit. Rev. Food Sci. Nutr. 2014, 54, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Ciurzyńska, A.; Marczak, W.; Lenart, A.; Janowicz, M. Production of innovative freeze-dried vegetable snack with hydrocolloids in terms of technological process and carbon footprint calculation. Food Hydrocoll. 2020, 108, 105993. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Teoh, A.; Massarotto, C.; Wibisono, R.; Wadhwa, S. Comparative analysis of fruit-based functional snack bars. Food Chem. 2010, 119, 1369–1379. [Google Scholar] [CrossRef]
- The Publications Office of the European Union. COMMISSION REGULATION (EC) No 1673/2004 of 24 September 2004 Laying Down the Marketing Standard Applicable to Kiwifruit; The Publications Office of the European Union: Luxembourg, 2004; pp. 5–10. [Google Scholar]
- Vissers, M.C.M.; Carr, A.C.; Pullar, J.M.; Bozonet, S.M. The Bioavailability of Vitamin Cfrom Kiwifruit. In Advances in Food and Nutrition Research; Boland, M., Moughan, P.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 68, pp. 125–147. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Du, G.R.; Li, M.J.; Ma, F.W.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Landi, M.; Tardelli, F.; Remorini, D.; Massai, R.; Guidi, L. Do sun-versus shade-grown kiwifruits perform differently upon storage? An overview of fruit maturity and nutraceutical properties of whole and fresh-cut produce. J. Agric. Food Chem. 2014, 62, 4377–4383. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The Impact of Pulsed Electric Field Treatment on Selected Bioactive Compounds Content and Color of Plant Tissue. Innov. Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Cozzolino, R.; De Giulio, B.; Petriccione, M.; Martignetti, A.; Malorni, L.; Zampella, L.; Laurino, C.; Pellican, M.P. Comparative analysis of volatile metabolites, quality and sensory attributes of Actinidia chinensis fruit. Food Chem. 2020, 316, 126340. [Google Scholar] [CrossRef]
- Sapei, L.; Hwa, L. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef]
- Salama, Z.A.; El Baz, F.K.; Gaafar, A.A.; Fathy Zaki, M. Antioxidant activities of phenolics, flavonoids and vitamin C in two cultivars of fennel (Foeniculum vulgare Mill.) in responses to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2015, 14, 91–99. [Google Scholar] [CrossRef]
- Urbach, E.M.D. Introduction to nutritional tables. In Skin Diseases Nutrition and Metabolism; Butterworth-Heinemann: Oxford, UK, 2013; pp. 563–597. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Gamboa-Santosa, J.; Megías-Pérez, R.; Soria, A.C.; Olano, A.; Montilla, A.; Villamiel, M. Impact of processing conditions on the kinetic of vitamin C degradation and 2-furoylmethyl amino acid formation in dried strawberries. Food Chem. 2014, 153, 164–170. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Dadan, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D. Effect of ultrasound treatment during osmotic dehydration on bioactive compounds of cranberries. Ultrasonics 2018, 83, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hiwilepo-van Hal, P.; Bosschaart, C.; van Twisk, C.; Verkerk, R.; Dekker, M. Kinetics of thermal degradation of vitamin C in marula fruit (Sclerocarya birrea subsp. caffra) as compared to other selected tropical fruits. Food Sci. Technol. 2012, 49, 188–191. [Google Scholar] [CrossRef]
- Orikasa, T.; Koide, S.; Okamoto, S.; Imaizumi, T.; Muramatsu, Y.; Takeda, J.; Shiina, T.; Tagawa, A. Impacts of hot air and vacuum drying on the quality attributes of kiwifruit slices. J. Food Eng. 2014, 125, 51–58. [Google Scholar] [CrossRef]
- Martínez-Navarrete, N.; Salvador, A.; Oliva, C.; Camacho, M.M. Influence of biopolymers and freeze-drying shelf temperature on the quality of a mandarin snack. Food Sci. Technol. 2019, 99, 57–61. [Google Scholar] [CrossRef]
- Materska, M. Bioactive phenolics of fresh and freeze-dried sweet and semi-spicy pepper fruits (Capsicum annuum L.). J. Funct. Foods 2014, 7, 269–277. [Google Scholar] [CrossRef]
- Ozcelik, M.; Ambros, S.; Freitas Morais, S.I.; Kulozik, U. Storage stability of dried raspberry foam as a snack product: Effect of foam structure and microwave-assisted freeze drying on the stability of plant bioactives and ascorbic acid. J. Food Eng. 2019, 270, 109779. [Google Scholar] [CrossRef]
- Tylewicz, U.; Mannozzi, C.; Romani, S.; Castagnini, J.M.; Samborska, K.; Rocculi, P.; Dalla Rosa, M. Chemical and physicochemical properties of semi-dried organic strawberries enriched with bilberry juice-based solution. LWT 2019, 114, 108377. [Google Scholar] [CrossRef]
- Ioannou, I.; Ghoul, M. Prevention of Enzymatic Browing in Fruit and Vegetables. Eur. Sci. 2013, 9, 1857–7431. [Google Scholar]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- Chin, S.K.; Siew, E.S.; Soon, W.L. Drying characteristics and quality evaluation of kiwi slices under hot air natural convective drying method. Int. Food Res. J. 2015, 22, 2188–2195. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- M’hiri, N.; Ghali, R.; Ben Nasr, I.; Boudhrioua, N. Effect of different drying processes on functional properties of industrial lemon byproduct. Process Saf. Environ. Prot. 2018, 116, 450–460. [Google Scholar] [CrossRef]
- Oliveira, G.; Tylewicz, U.; Dalla Rosa, M.; Andlid, T.; Alminger, M. Effects of Pulsed Electric Field-Assisted Osmotic Dehydration and Edible Coating on the Recovery of Anthocyanins from In Vitro Digested Berries. Foods 2019, 8, 505. [Google Scholar] [CrossRef] [PubMed]
- Olszowy, M.; Dawidowicz, L.A. Is it possible to use the DPPH and ABTS methods for reliable estimation of antioxidant power of colored compounds? Chem. Pap. 2018, 72, 393–400. [Google Scholar] [CrossRef]
- Fidrianny, I.; Octaviani, D.G.; Kusmardiyani, S. Study of Antioxidant Profile and Phytochemical Content of Different Organs Extracts of Morinda citrifolia L. J. Pharm. Sci. 2018, 10, 2102–2105. [Google Scholar]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; Di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 3–44. [Google Scholar]
- Alhamdan, A.; Hassan, B.; Alkahtani, H.; Abdelkarim, D.; Younis, M. Cryogenic freezing of fresh date fruits for quality preservation during frozen storage. J. Saudi Soc. Agric. Sci. 2018, 17, 9–16. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of innovative technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Wiktor, A.; Dadan, M.; Tylewicz, U.; Dalla Rosa, M.; Witrowa-Rajchert, D. Influence of power ultrasound on the main quality properties and cell viability of osmotic dehydrated cranberries. Ultrasonics 2018, 83, 33–41. [Google Scholar] [CrossRef]

| Snack Symbol | Fruit and Vegetable Ingredients (%) | Sugar Addition | Addition of 2% Pectin Solution | Abbre-Viation |
|---|---|---|---|---|
| A | 44% kiwifruit | 0 | 20% | A |
| 21.6% strawberry | sucrose | A_s | ||
| 12% fennel | trehalose | A_t | ||
| 2.4% lemon juice | ||||
| B | 52% kiwifruit | 0 | 20% | B |
| 24% fennel | sucrose | B_s | ||
| 3.88% lemon juice | trehalose | B_t | ||
| 0.12% lemon peel | ||||
| C | 56% kiwifruit | 0 | 20% | C |
| 16% fennel | sucrose | C_s | ||
| 8% spinach | trehalose | C_t |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Dalla Rosa, M.; Mozzon, M. Design of Healthy Snack Based on Kiwifruit. Molecules 2020, 25, 3309. https://doi.org/10.3390/molecules25143309
Tylewicz U, Nowacka M, Rybak K, Drozdzal K, Dalla Rosa M, Mozzon M. Design of Healthy Snack Based on Kiwifruit. Molecules. 2020; 25(14):3309. https://doi.org/10.3390/molecules25143309
Chicago/Turabian StyleTylewicz, Urszula, Malgorzata Nowacka, Katarzyna Rybak, Kinga Drozdzal, Marco Dalla Rosa, and Massimo Mozzon. 2020. "Design of Healthy Snack Based on Kiwifruit" Molecules 25, no. 14: 3309. https://doi.org/10.3390/molecules25143309
APA StyleTylewicz, U., Nowacka, M., Rybak, K., Drozdzal, K., Dalla Rosa, M., & Mozzon, M. (2020). Design of Healthy Snack Based on Kiwifruit. Molecules, 25(14), 3309. https://doi.org/10.3390/molecules25143309








