Lignan from Alnus japonica Inhibits Adipocyte Differentiation via Cell Cycle and FOXO1 Regulation
Abstract
1. Introduction
2. Results
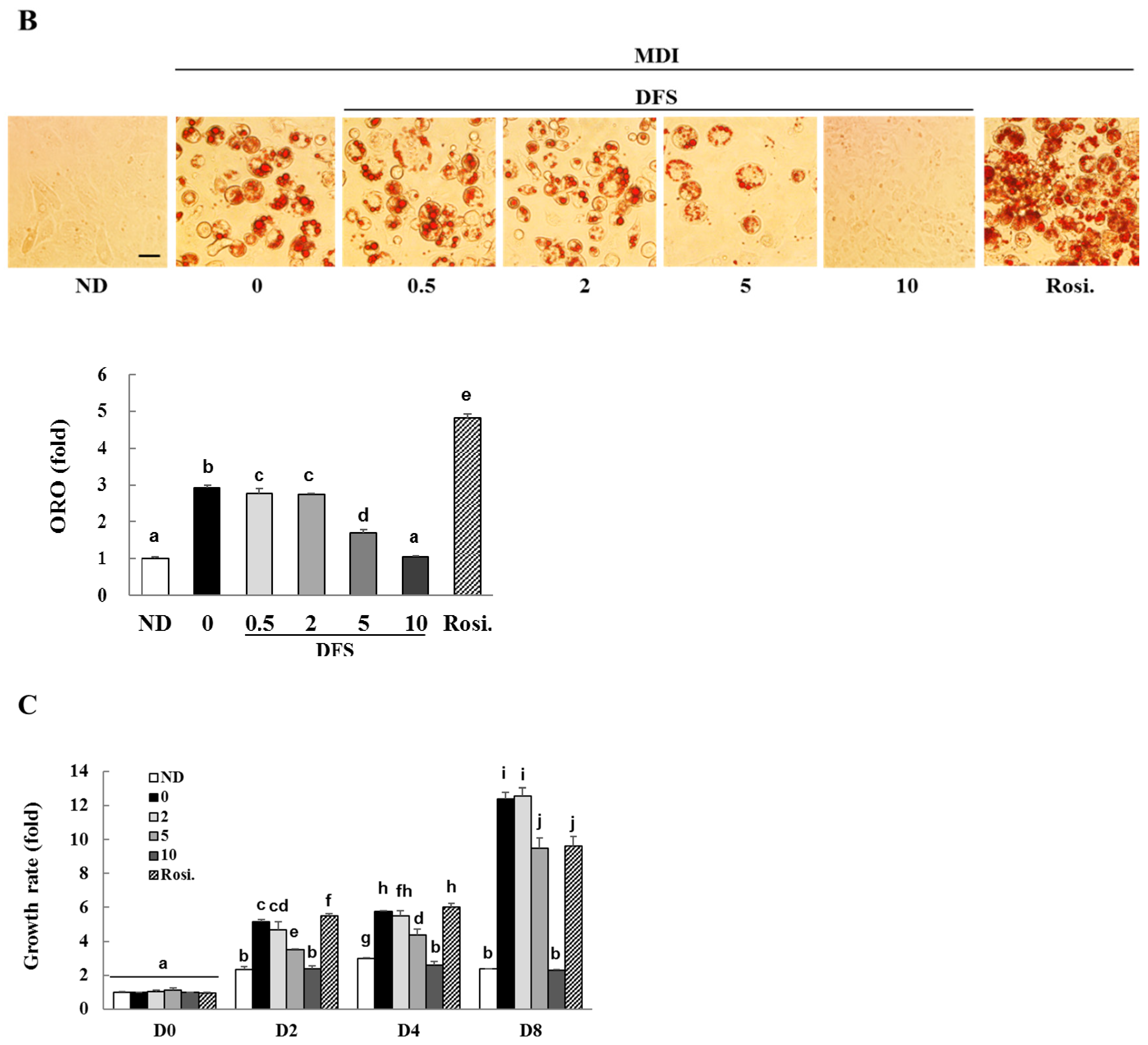
2.1. A Lignan Isolated from A. Japonica Inhibits Adipocyte Differentiation
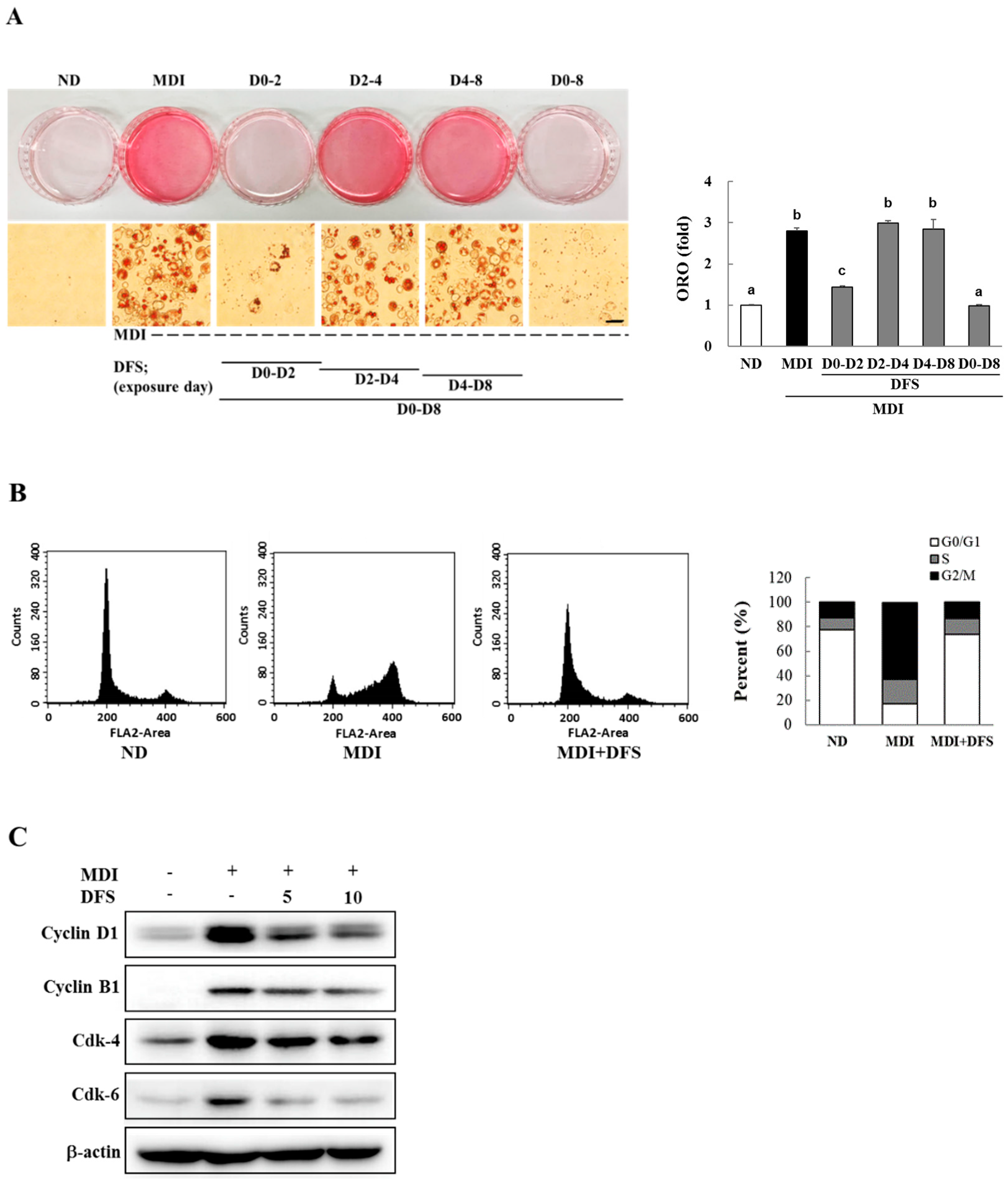
2.2. DFS Suppresses Adipocyte Differentiation via Cell Cycle Arrest during Mitotic Clonal Expansion
2.3. DFS Decreases Gene Expression of Adipogenic Factors during Adipocyte Differentiation
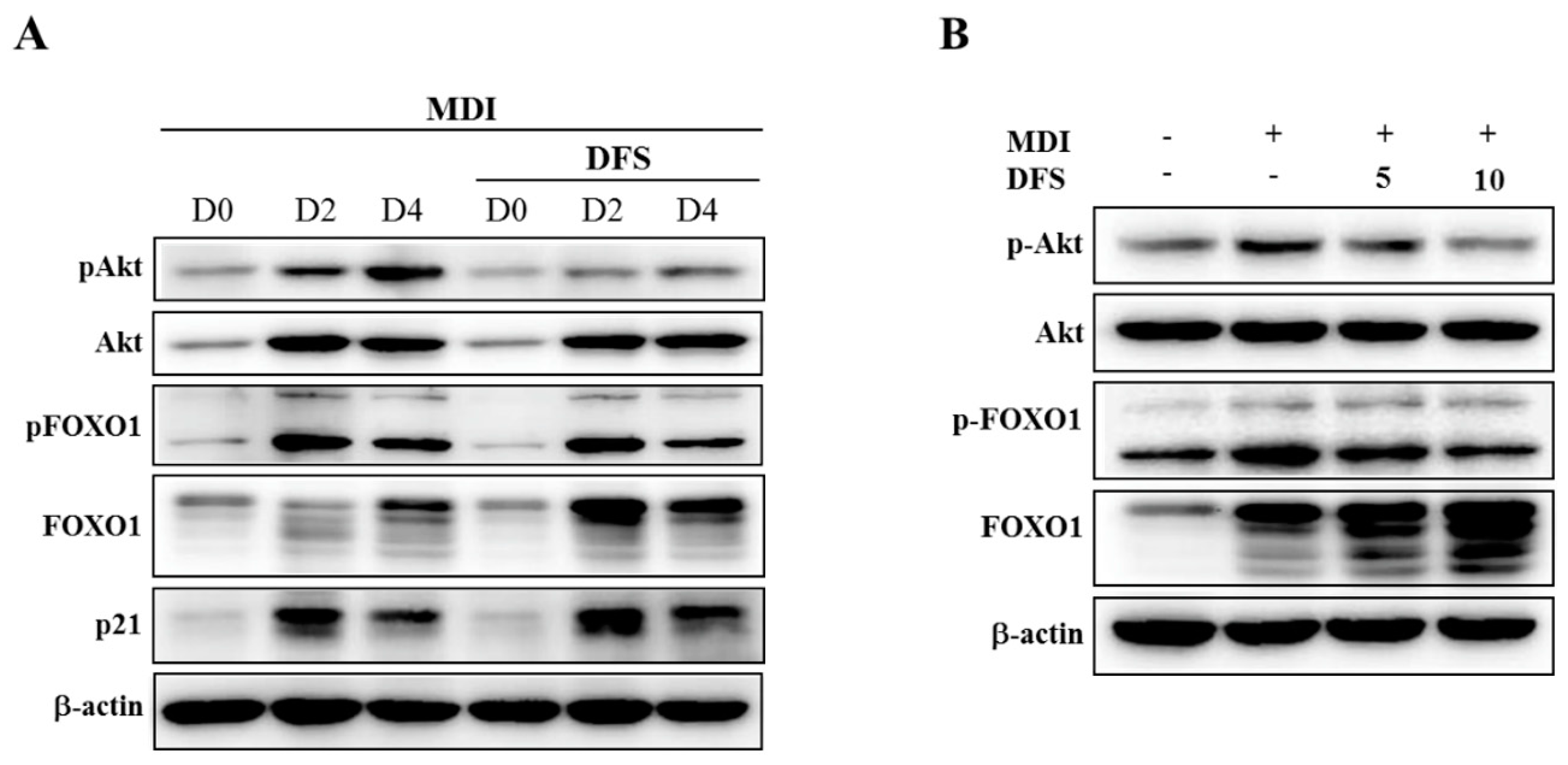
2.4. DFS Regulates Akt-FOXO1 Pathway during Adipocyte Differentiation
3. Discussion
4. Materials and Methods
4.1. Isolation of (−)-(2R,3R)-1,4-O-diferuloylsecoisolariciresinol (DFS) from A. Japonica
4.2. Culture and Pre-Adipocyte Differentiation
4.3. MTT Assay and Oil Red-O (ORO) Staining
4.4. Flow Cytometry
4.5. RNA Extraction and Quantitative Real-Time PCR (qRCR)
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Egger, G.; Dixon, J. Obesity and chronic disease: Always offender or often just accomplice? Br. J. Nutr. 2009, 102, 1238–1242. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Anti-Obesity Drugs: Long-Term Efficacy and Safety: An Updated Review. World J. Mens Health. 2020, 1, 5521–5534. [Google Scholar] [CrossRef]
- Wu, L.Y.; Chen, C.W.; Chen, L.K.; Chou, H.Y.; Chang, C.L.; Juan, C.C. Curcumin attenuates adipogenesis by inducing preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Nam, H.; Morrison, R.F. Curcumin inhibits 3T3-L1 preadipocyte proliferation by mechanisms involving post-transcriptional p27 regulation. Biochem. Biophys. Rep. 2016, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J.; Xian, T.; Zhou, Y.; Yuan, W.; Wang, M.; Gan, Y.; Wang, K.; Xiong, S.; Ma, C.; et al. Epigallocatechin-3-gallate suppresses differentiation of adipocytes via regulating the phosphorylation of FOXO1 mediated by PI3K-AKT signaling in 3T3-L1 cells. Oncotarget 2018, 9, 7411–7423. [Google Scholar] [CrossRef] [PubMed]
- Mitterberger, M.C.; Zwerschke, W. Mechanisms of resveratrol-induced inhibition of clonal expansion and terminal adipogenic differentiation in 3T3-L1 preadipocytes. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1356–1376. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Tian, M.; Huang, Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J. Mol. Endocrinol. 2019, 62, R239–R253. [Google Scholar] [CrossRef]
- Han, G.H.; Chay, D.B.; Nam, S.; Cho, H.; Chung, J.Y.; Kim, J.H. Prognostic implications of forkhead box protein O1 (FOXO1) and paired box 3 (PAX3) in epithelial ovarian cancer. BMC cancer 2019, 19, 1202–1211. [Google Scholar] [CrossRef]
- Song, H.M.; Song, J.L.; Li, D.F.; Hua, K.Y.; Zhao, B.K.; Fang, L. Inhibition of FOXO1 by small interfering RNA enhances proliferation and inhibits apoptosis of papillary thyroid carcinoma cells via Akt/FOXO1/Bim pathway. Onco Targets Ther. 2015, 8, 3565–3573. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Kitamura, T.; Kitamura, Y.; Biggs, W.H.; Arden, K.C.; Accili, D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 2003, 4, 119–129. [Google Scholar] [CrossRef]
- Ren, X.; He, T.; Chang, Y.; Zhao, Y.; Chen, X.; Bai, S.; Wang, L.; Shen, M.; She, G. The genus Alnus, A Comprehensive outline of its chemical constituents and biological activities. Molecules 2017, 22, 1383. [Google Scholar] [CrossRef] [PubMed]
- Sati, S.C.; Sati, N.; Sati, O.P. Bioactive constituents and medicinal importance of genus Alnus. Pharmacogn. Rev. 2011, 5, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Lee, M. Anti-adipogenic activity of a new cyclic diarylheptanoid isolated from Alnus japonica on 3T3-L1 cells via modulation of PPARgamma, C/EBPalpha and SREBP1c signaling. Bioorg. Med. Chem. Lett. 2015, 25, 4648–4651. [Google Scholar] [CrossRef]
- Dong, G.Z.; Jeong, J.H.; Lee, Y.I.; Han, Y.E.; Shin, J.S.; Kim, Y.J.; Jeon, R.; Kim, Y.H.; Park, T.J.; Kim, K.I.; et al. A lignan induces lysosomal dependent degradation of FoxM1 protein to suppress β-catenin nuclear translocation. Sci. Rep. 2017, 7, 45951–45960. [Google Scholar] [CrossRef]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell line models for differentiation: Preadipocytes and adipocytes. Ex. Biol. Med. (Maywood) 2010, 235, 1185–1193. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Lee, H.; Li, H.; Kweon, M.; Choi, Y.; Kim, M.J.; Ryu, J.H. Isobavachalcone from Angelica keiskei Inhibits Adipogenesis and Prevents Lipid Accumulation. Int. J. Mol. Sci. 2018, 19, 1693. [Google Scholar] [CrossRef]
- Chang, E.; Kim, C.Y. Natural products and obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Lee, Y.S.; Sin, D.M.; Lee, S.; Lee, M.K.; Lee, Y.M.; Hong, J.T.; Yun, Y.P.; Yoo, H.S. Sulforaphane inhibits mitotic clonal expansion during adipogenesis through cell cycle arrest. Obesity 2012, 20, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J.; Liu, X.S.; et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Armoni, M.; Harel, C.; Karni, S.; Chen, H.; Bar-Yoseph, F.; Ver, M.R.; Quon, M.J.; Karnieli, E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J. Biol. Chem. 2006, 281, 19881–19891. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tindall, D.J. CDK2 and FOXO1: A fork in the road for cell fate decisions. Cell Cycle 2007, 6, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.P.; Liao, Y.; Xia, W.; Spohn, B.; Lee, M.-H.; Hung, M.-C. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 2001, 3, 245–252. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural products with anti-obesity effects and different mechanisms of action. J. Agri. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Hu, Y.; Davies, G.E. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia 2010, 81, 358–366. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| PPARγ | AACTCTGGGAGATTCTCCTGTTGA | GAAGTGCTCATAGGCAGTGCAT |
| C/EBPα | TGCACCACCAACTGCTTAG | AAACCATCCTCTGGGTCTCC |
| Adiponectin | TGTAGGATTGTCAGTGGATCTG | GCTCTTCAGTTGTAGTAACGTCATC |
| LPL | AGGACCCCTGAAGACAC | GGCACCCAACTCTCATA |
| FAS | AGCGGCCATTTCCATTGCCC | CCATGCCCAGAGGGTGGTTG |
| GAPDH | TGCACCACCAACTGCTTAG | GGCATGGACTGTGGTCATGAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Jeong, J.H.; Ryu, J.-H. Lignan from Alnus japonica Inhibits Adipocyte Differentiation via Cell Cycle and FOXO1 Regulation. Molecules 2020, 25, 3346. https://doi.org/10.3390/molecules25153346
Lee H, Jeong JH, Ryu J-H. Lignan from Alnus japonica Inhibits Adipocyte Differentiation via Cell Cycle and FOXO1 Regulation. Molecules. 2020; 25(15):3346. https://doi.org/10.3390/molecules25153346
Chicago/Turabian StyleLee, Hyejin, Ji Hye Jeong, and Jae-Ha Ryu. 2020. "Lignan from Alnus japonica Inhibits Adipocyte Differentiation via Cell Cycle and FOXO1 Regulation" Molecules 25, no. 15: 3346. https://doi.org/10.3390/molecules25153346
APA StyleLee, H., Jeong, J. H., & Ryu, J.-H. (2020). Lignan from Alnus japonica Inhibits Adipocyte Differentiation via Cell Cycle and FOXO1 Regulation. Molecules, 25(15), 3346. https://doi.org/10.3390/molecules25153346





