Hyperpolarization of Nitrile Compounds Using Signal Amplification by Reversible Exchange
Abstract
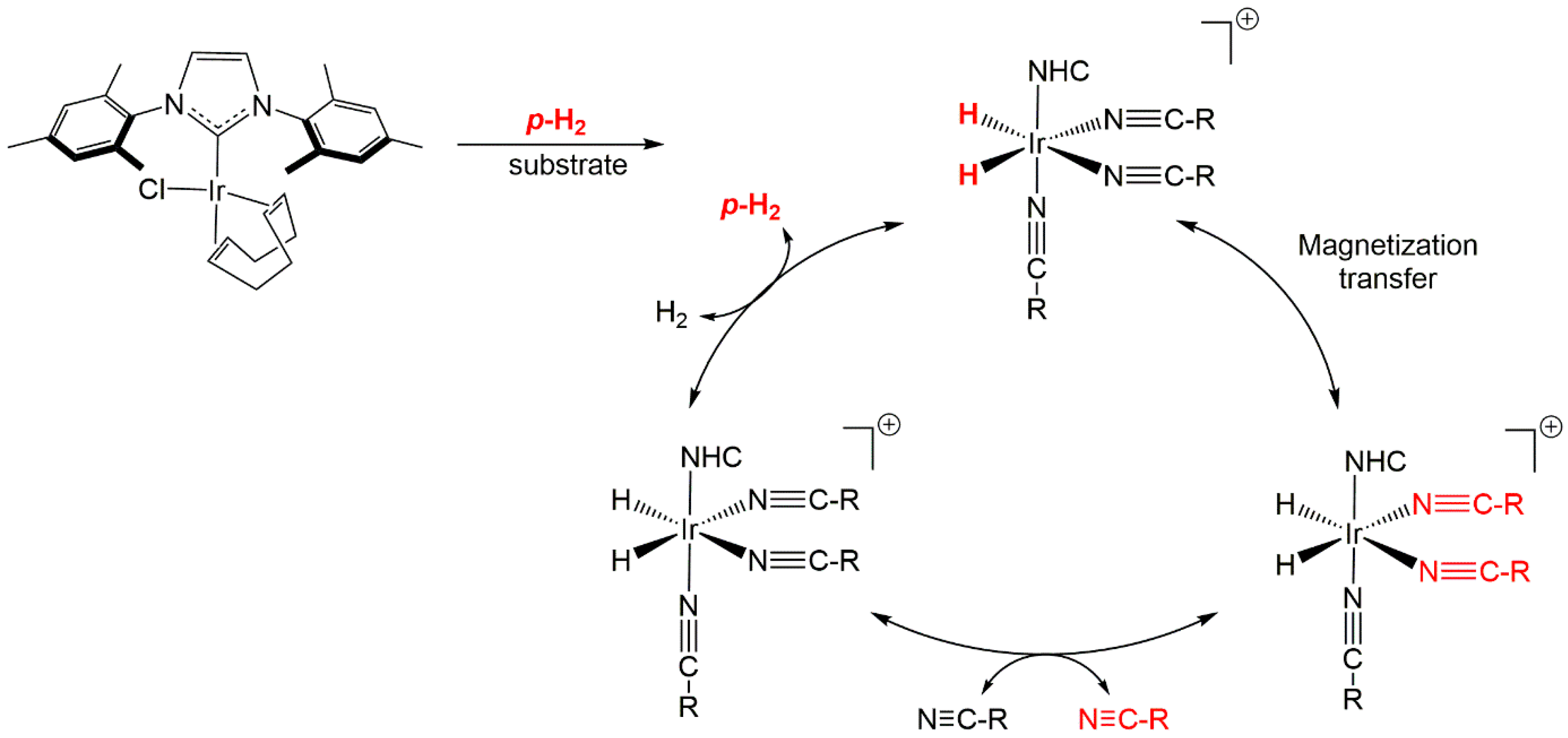
:1. Introduction
2. Results and Discussion
2.1. SABRE on Acetonitrile
2.2. SABRE on Propionitrile
2.3. SABRE on Butyronitrile and Isobutyronitrile
2.4. SABRE on Valeronitrile
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, M.; Jóhannesson, H.; Axelsson, O.; Karlsson, M. Design and implementation of 13C hyper polarization from para-hydrogen, for new MRI contrast agents. Comptes Rendus Chim. 2006, 9, 357–363. [Google Scholar] [CrossRef]
- Bowers, C.R.; Weitekamp, D.P. Transformation of symmetrization order to nuclear-spin magnetization by chemical reaction and nuclear magnetic resonance. Phys. Rev. Lett. 1986, 57, 2645–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maly, T.; Debelouchina, G.T.; Bajaj, V.S.; Hu, K.N.; Joo, C.G.; Mak-Jurkauskas, M.L.; Sirigiri, J.R.; Van Der Wel, P.C.A.; Herzfeld, J.; Temkin, R.J.; et al. Dynamic nuclear polarization at high magnetic fields. J. Chem. Phys. 2008, 128, 052211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, K.L.; Yurkovskaya, A.V.; Vieth, H.-M. Parahydrogen Induced Polarization in Scalar Coupled Systems: Analytical Solutions for Spectral Patterns and their Field Dependence. Zeitschrift für Phys. Chemie 2012, 226, 1315–1342. [Google Scholar] [CrossRef] [Green Version]
- Bowers, C.R.; Weitekamp, D.P. Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. [Google Scholar] [CrossRef] [Green Version]
- Matthes, J.; Gründemann, S.; Buntkowsky, G.; Chaudret, B.; Limbach, H.H. NMR Studies of the Reaction Path of the o-H2/p-H2 Spin Conversion Catalyzed by Vaska’s Complex in the Solid State. Appl. Magn. Reson. 2013, 44, 247–265. [Google Scholar] [CrossRef]
- Natterer, J.; Bargon, J. Para-Hydrogen Induced Polarization (PHIP). Prog. Nucl. Magn. Reson. Spectrosc. 1997, 31, 293–315. [Google Scholar] [CrossRef]
- Eisenschmid, T.C.; Kirss, R.U.; Deutsch, P.P.; Hommeltoft, S.I.; Eisenberg, R.; Bargon, J.; Lawler, R.G.; Balch, A.L. Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc. 1987, 109, 8089–8091. [Google Scholar] [CrossRef]
- Adams, R.W.; Aguilar, J.A.; Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.P.; Duckett, S.B.; Green, G.G.R.; Khazal, I.G.; Lopez-Serrano, J.; Williamson, D.C. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323, 1708–1711. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.W.; Duckett, S.B.; Green, R.A.; Williamson, D.C.; Green, G.G.R. A theoretical basis for spontaneous polarization transfer in non-hydrogenative parahydrogen-induced polarization. J. Chem. Phys. 2009, 131, 194505. [Google Scholar] [CrossRef] [PubMed]
- Rayner, P.J.; Norcott, P.; Appleby, K.M.; Iali, W.; John, R.O.; Hart, S.J.; Whitwood, A.C.; Duckett, S.B. Fine-tuning the efficiency of para-hydrogen-induced hyperpolarization by rational N-heterocyclic carbene design. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Gordji-Nejad, A.; Blümich, B.; Appelt, S. Trace analysis by low-field NMR: Breaking the sensitivity limit. Anal. Chem. 2010, 82, 7078–7082. [Google Scholar] [CrossRef]
- Hövener, J.-B.B.; Schwaderlapp, N.; Borowiak, R.; Lickert, T.; Duckett, S.B.; Mewis, R.E.; Adams, R.W.; Burns, M.J.; Highton, L.A.R.R.; Green, G.G.R.R.; et al. Toward biocompatible nuclear hyperpolarization using signal amplification by reversible exchange: Quantitative in situ spectroscopy and high-field imaging. Anal. Chem. 2014, 86, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xu, J.; Gillen, J.; McMahon, M.T.; Artemov, D.; Tyburn, J.-M.; Lohman, J.A.B.; Mewis, R.E.; Atkinson, K.D.; Green, G.G.R.; et al. Optimization of SABRE for polarization of the tuberculosis drugs pyrazinamide and isoniazid. J. Magn. Reson. 2013, 237, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowley, M.J.; Adams, R.W.; Atkinson, K.D.; Cockett, M.C.R.; Duckett, S.B.; Green, G.G.R.; Lohman, J.A.B.; Kerssebaum, R.; Kilgour, D.; Mewis, R.E. Iridium N-heterocyclic carbene complexes as efficient catalysts for magnetization transfer from para-hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Serrano, L.D.; Owens, B.T.; Buriak, J.M. The search for new hydrogenation catalyst motifs based on N-heterocyclic carbene ligands. Inorganica Chim. Acta 2006, 359, 2786–2797. [Google Scholar] [CrossRef]
- Semenova, O.; Richardson, P.M.; Parrott, A.J.; Nordon, A.; Halse, M.E.; Duckett, S.B. Reaction Monitoring Using SABRE-Hyperpolarized Benchtop (1 T) NMR Spectroscopy. Anal. Chem. 2019, 91, 6695–6701. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Choi, J.W.; Kim, C.S.; Jeong, K. Parahydrogen-induced polarization in the hydrogenation of lignin-derived phenols using Wilkinson’s catalyst. Fuel 2019, 255, 115845. [Google Scholar] [CrossRef]
- HyeJin, J.; Sein, M.; Heelim, C.; Sara, K.; Gunwoo, L.; Sung Keon, N.; Keunhong, J. Signal Amplification by Reversible Exchange for COVID-19 Antiviral Drug Candidates. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Kovtunov, K.V.; Barskiy, D.A.; Shchepin, R.V.; Coffey, A.M.; Waddell, K.W.; Koptyug, I.V.; Chekmenev, E.Y. Demonstration of heterogeneous parahydrogen induced polarization using hyperpolarized agent migration from dissolved Rh(I) complex to gas phase. Anal. Chem. 2014, 86, 6192–6196. [Google Scholar] [CrossRef] [PubMed]
- Barskiy, D.A.; Knecht, S.; Yurkovskaya, A.V.; Ivanov, K.L. SABRE: Chemical kinetics and spin dynamics of the formation of hyperpolarization. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114–115, 33–70. [Google Scholar] [CrossRef] [PubMed]
- Kovtunov, K.V.; Pokochueva, E.V.; Salnikov, O.G.; Cousin, S.F.; Kurzbach, D.; Vuichoud, B.; Jannin, S.; Chekmenev, E.Y.; Goodson, B.M.; Barskiy, D.A.; et al. Hyperpolarized NMR Spectroscopy: D-DNP, PHIP, and SABRE Techniques. Chem. Asian J. 2018, 13, 1857–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, K.; Min, S.; Chae, H.; Namgoong, S.K. Detecting low concentrations of unsaturated C—C bonds by parahydrogen-induced polarization using an efficient home-built parahydrogen generator. Magn. Reson. Chem. 2018, 56, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Min, S.; Chae, H.; Namgoong, S.K. Monitoring of hydrogenation by benchtop NMR with parahydrogen-induced polarization. Magn. Reson. Chem. 2019, 57, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Jeong, K.; Shim, J.H.; Lee, H.J.; Min, S.; Chae, H.; Namgoong, S.K.; Kim, K. SQUID-based ultralow-field MRI of a hyperpolarized material using signal amplification by reversible exchange. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.; Min, S.; Jeong, H.J.; Namgoong, S.K.; Oh, S.; Kim, K.; Jeong, K. Organic Reaction Monitoring of a Glycine Derivative Using Signal Amplification by Reversible Exchange-Hyperpolarized Benchtop Nuclear Magnetic Resonance Spectroscopy. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Mewis, R.E.; Green, R.A.; Cockett, M.C.R.; Cowley, M.J.; Duckett, S.B.; Green, G.G.R.; John, R.O.; Rayner, P.J.; Williamson, D.C. Strategies for the hyperpolarization of acetonitrile and related Ligands by SABRE. J. Phys. Chem. B 2015, 119, 1416–1424. [Google Scholar] [CrossRef]
- Fekete, M.; Bayfield, O.; Duckett, S.B.; Hart, S.; Mewis, R.E.; Pridmore, N.; Rayner, P.J.; Whitwood, A. Iridium(III) hydrido N-heterocyclic carbene-phosphine complexes as catalysts in magnetization transfer reactions. Inorg. Chem. 2013, 52, 13453–13461. [Google Scholar] [CrossRef]
- Colell, J.F.P.; Logan, A.W.J.; Zhou, Z.; Shchepin, R.V.; Barskiy, D.A.; Ortiz, G.X.; Wang, Q.; Malcolmson, S.J.; Chekmenev, E.Y.; Warren, W.S.; et al. Generalizing, Extending, and Maximizing Nitrogen-15 Hyperpolarization Induced by Parahydrogen in Reversible Exchange. J. Phys. Chem. C 2017, 121, 6626–6634. [Google Scholar] [CrossRef]
- Barskiy, D.A.; Shchepin, R.V.; Tanner, C.P.N.; Colell, J.F.P.; Goodson, B.M.; Theis, T.; Warren, W.S.; Chekmenev, E.Y. The Absence of Quadrupolar Nuclei Facilitates Efficient 13C Hyperpolarization via Reversible Exchange with Parahydrogen. ChemPhysChem 2017, 18, 1493–1498. [Google Scholar] [CrossRef]
- Fleming, F.F.; Yao, L.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, A.W.; Wood, W.J.L.; Hornsby, M.; Lesley, S.; Spraggon, G.; Ellman, J.A. Identification of selective, nonpeptidic nitrile inhibitors of cathepsin S using the substrate activity screening method. J. Med. Chem. 2006, 49, 6298–6307. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.J.; Crane, S.N.; Robichaud, J.; Scheigetz, J.; Black, W.C.; Chauret, N.; Wang, Q.; Massé, F.; Oballa, R.M. Investigation of ketone warheads as alternatives to the nitrile for preparation of potent and selective cathepsin K inhibitors. Bioorganic Med. Chem. Lett. 2009, 19, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, J.; Oballa, R.; Prasit, P.; Falgueyret, J.P.; Percival, M.D.; Wesolowski, G.; Rodan, S.B.; Kimmel, D.; Johnson, C.; Bryant, C.; et al. A novel class of nonpeptidic biaryl inhibitors of human cathepsin K. J. Med. Chem. 2003, 46, 3709–3727. [Google Scholar] [CrossRef] [PubMed]
- Appleby, K.M.; Mewis, R.E.; Olaru, A.M.; Green, G.G.R.; Fairlamb, I.J.S.; Duckett, S.B. Investigating pyridazine and phthalazine exchange in a series of iridium complexes in order to define their role in the catalytic transfer of magnetisation from para-hydrogen. Chem. Sci. 2015, 6, 3981–3993. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Min, S.; Chae, H.; Namgoong, S.K.; Jeong, K. Low Cost and Portable Parahydrogen Generator for the PHIP. J. Korean Magn. Reson. Soc. 2017, 21, 126–130. [Google Scholar]
- Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.P.; Duckett, S.B.; Green, G.G.R.; López-Serrano, J.; Whitwood, A.C. Spontaneous transfer of Parahydrogen derived spin order to pyridine at low magnetic field. J. Am. Chem. Soc. 2009, 131, 13362–13368. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, J.; McMahon, M.T.; Lohman, J.A.B.; Van Zijl, P.C.M. Achieving 1% NMR polarization in water in less than 1 min using SABRE. J. Magn. Reson. 2014, 246, 119–121. [Google Scholar] [CrossRef] [Green Version]
- Hövener, J.B.; Pravdivtsev, A.N.; Kidd, B.; Bowers, C.R.; Glöggler, S.; Kovtunov, K.V.; Plaumann, M.; Katz-Brull, R.; Buckenmaier, K.; Jerschow, A.; et al. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed. 2018, 57, 11140–11162. [Google Scholar] [CrossRef]
- Reile, I.; Eshuis, N.; Hermkens, N.K.J.; Van Weerdenburg, B.J.A.; Feiters, M.C.; Rutjes, F.P.J.T.; Tessari, M. NMR detection in biofluid extracts at sub-μM concentrations: Via para-H2 induced hyperpolarization. Analyst 2016, 141, 4001–4005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Min, S.; Chae, H.; Jeong, H.J.; Namgoong, S.K.; Oh, S.; Jeong, K. Hyperpolarization of Nitrile Compounds Using Signal Amplification by Reversible Exchange. Molecules 2020, 25, 3347. https://doi.org/10.3390/molecules25153347
Kim S, Min S, Chae H, Jeong HJ, Namgoong SK, Oh S, Jeong K. Hyperpolarization of Nitrile Compounds Using Signal Amplification by Reversible Exchange. Molecules. 2020; 25(15):3347. https://doi.org/10.3390/molecules25153347
Chicago/Turabian StyleKim, Sarah, Sein Min, Heelim Chae, Hye Jin Jeong, Sung Keon Namgoong, Sangwon Oh, and Keunhong Jeong. 2020. "Hyperpolarization of Nitrile Compounds Using Signal Amplification by Reversible Exchange" Molecules 25, no. 15: 3347. https://doi.org/10.3390/molecules25153347





