Veronica austriaca L. Extract and Arbutin Expand Mature Double TNF-α/IFN-γ Neutrophils in Murine Bone Marrow Pool
Abstract
1. Introduction
2. Results
2.1. Identification of Metabolites in Veronica austriaca Extracts
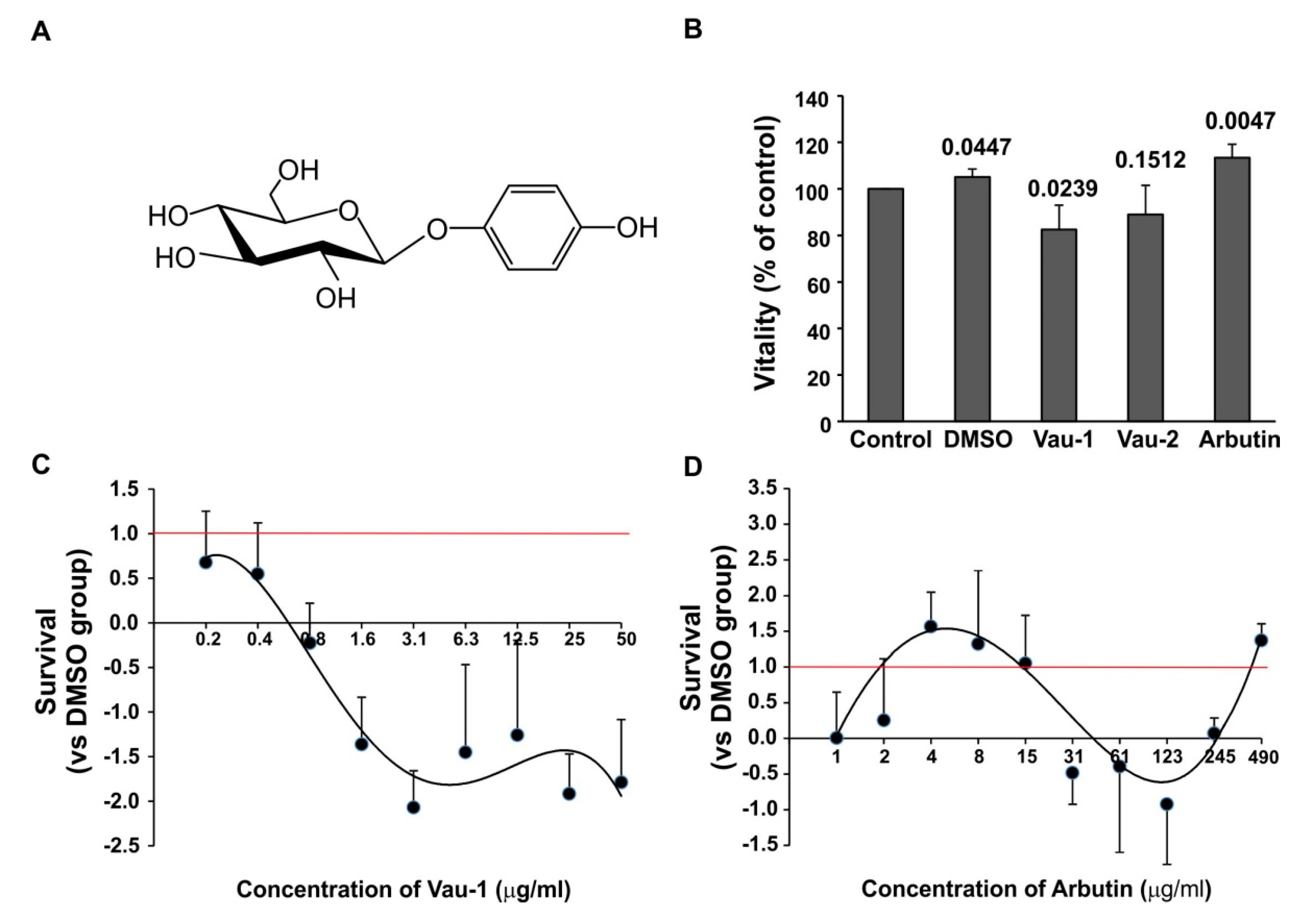
2.2. Effect of the Vau-1/Vau-2 Extracts or Arbutin on Vitality of BM-Derived Neutrophils
2.3. Effect of Vau-1/Vau-2 Extracts or Arbutin on TNF-α and IFN-γ Production in BM-Derived Neutrophils
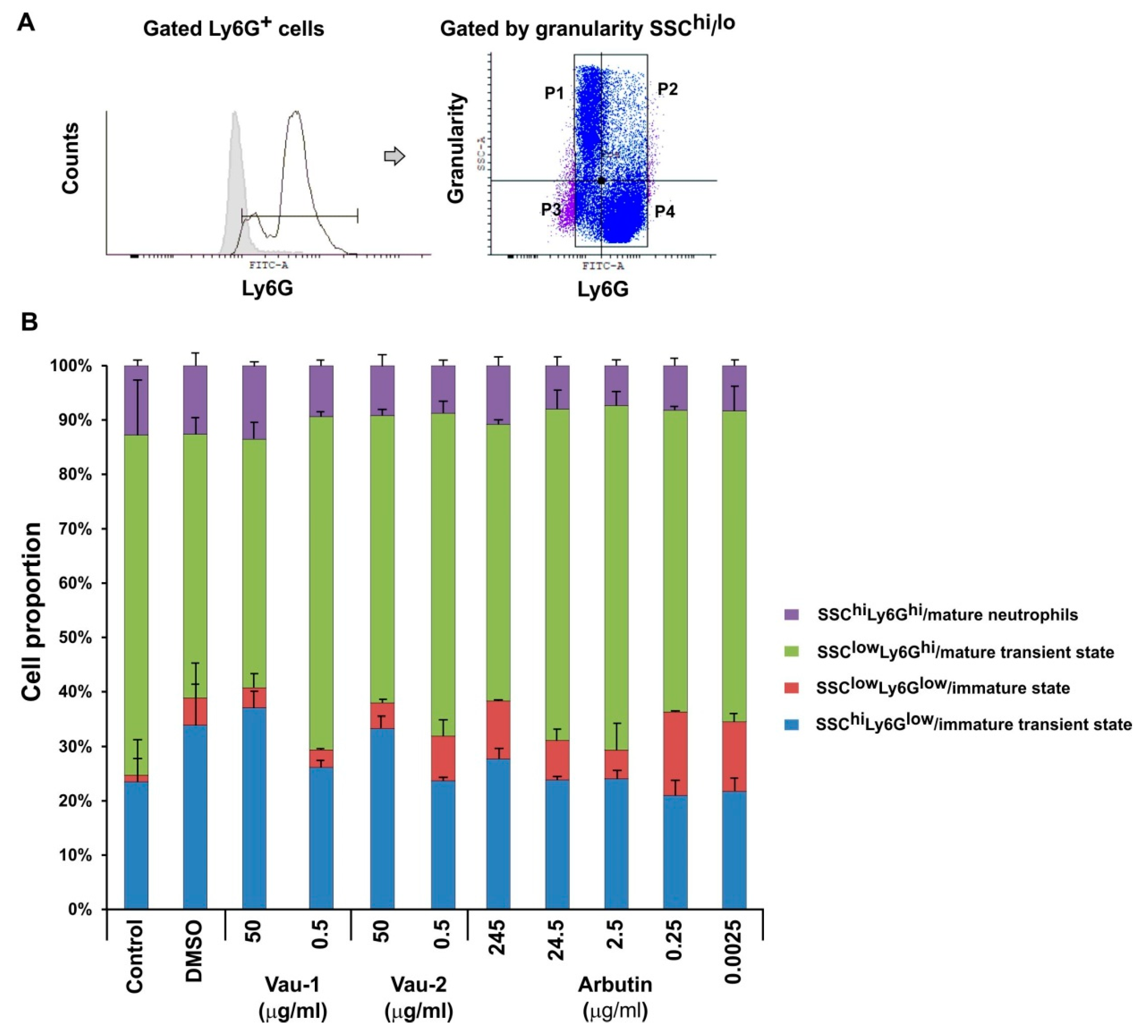
2.4. Effect of Vau-1/Vau-2 or Arbutin on the Pattern of Immature or Mature Neutrophils Expressing TNF-α and IFN-γ
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction Protocol and NMR Analysis
4.3. Extraction Protocol for HPLC Analysis and Biological Tests
4.4. HPLC Analysis of Arbutin Content in V. austriaca Extracts
4.5. Animal Studies
4.6. Isolation of Bone-Marrow-Derived Neutrophils
4.7. Cell Vitality
4.8. Intracellular Flowcytometry
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Peev, D. Genus Veronica L. In Flora of the Republic of Bulgaria; Kozuharov, S., Kuzmanov, B., Eds.; Prof. M. Drinov Publishin House: Sofia, Bulgaria, 1995; pp. 145–202. [Google Scholar]
- Salehi, B.; Shetty, M.S.; Kumar, N.V.A.; Živković, J.; Calina, D.; Docea, A.O.; Emamzadeh-Yazdi, S.; Kılıç, C.S.; Goloshvili, T.; Nicola, S.; et al. Veronica plants—Drifting from Pharm to Traditional Healing, Food Application, and Phytopharmacology. Molecules 2019, 24, 2454. [Google Scholar] [CrossRef]
- Raclariu, A.C.; Mocan, A.; Popa, M.O.; Vlase, L.; Ichim, M.C.; Crisan, G.; Brysting, A.K.; de Boer, H. Veronica officinalis Product authentification using DNA metabarcoding and HPLC-MS reveals widespread adulteration with Veronica chamaedrys. Front. Pharm. 2017, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Albach, D.; Grayer, R.J.; Kite, G.C.; Marker, S. Veronica: Acylated flavone glycosides as chemosystematic markers. Biochem. Syst. Ecol. 2005, 33, 1167–1177. [Google Scholar] [CrossRef]
- Johansen, M.; Larsen, T.S.; Mattebjerg, M.A.; Gotfredsen, C.H.; Marker, S. Chemical markers in Veronica sect. Hebe. Biochem. Syst. Ecol. 2007, 35, 614–620. [Google Scholar] [CrossRef]
- Maggi, A.; Taskova, R.; Gotfredsen, C.H.; Bianco, A.; Marker, S. Chemical markers in Veronica sect. Hebe. III. Biochem. Syst. Ecol. 2009, 37, 731–736. [Google Scholar] [CrossRef]
- Pedersen, P.; Gotfredsen, C.H.; Wagstaff, S.J.; Marker, S. Chemical markers in Veronica sect. Hebe. II. Biochem. Syst. Ecol. 2007, 35, 777–784. [Google Scholar] [CrossRef]
- Taskova, R.M.; Gotfredsen, C.H.; Marker, S. Chemotaxonomy of Veroniceae and its allies in the Plantaginaceae. Phytochemistry 2006, 67, 286–301. [Google Scholar] [CrossRef]
- Taskova, R.; Peev, D.; Handjieva, N. Iridoid glucosides of the genus Veronica s.l. and their systematic significance. Plant Syst. Evol. 2002, 231, 1–17. [Google Scholar] [CrossRef]
- Beara, I.; Živković, J.Č.; Lesjak, M.; Ristic, J.; Šavikin, K.; Maksimović, Z.; Janković, T. Phenolic profile and anti-inflammatory activity of three Veronica species. Ind. Crop. Prod. 2015, 63, 276–280. [Google Scholar] [CrossRef]
- Živković, J.Č.; Barreira, J.C.M.; Šavikin, K.P.; Alimpić, A.Z.; Stojković, D.; Dias, M.I.; Santos-Buelga, C.; Duletić-Laušević, S.; Ferreira, I.C.F.R. Chemical Profiling and Assessment of Antineurodegenerative and Antioxidant Properties of Veronica teucrium L. and Veronica jacquinii Baumg. Chem. Biodivers. 2017, 14, e1700167. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.; Yordanova, Z.P.; Alipieva, K.; Zahmanov, G.; Rusinova-Videva, S.; Kapchina-Toteva, V.; Simova, S.; Popova, M.; Georgiev, M.I. Genetic transformation of rare Verbascum eriophorum Godr. plants and metabolic alterations revealed by NMR-based metabolomics. Biotechnol. Lett. 2016, 38, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, P.; Alipieva, K.; Grozdanova, T.; Simova, S.; Bankova, V.; Georgiev, M.; Popova, M.P. New iridoids from Verbasum nobile and their effect on lectin-induced T cell activation and proliferation. Food Chem. Toxicol 2018, 111, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, P.; Alipieva, K.; Stojanov, K.; Milanova, V.; Georgiev, M.I. Plant-derived verbascoside and isoverbascoside regulate Toll-like receptor 2 and 4-driven neutrophils priming and activation. Phytomedicine 2019, 55, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhao, C.; Wang, Y.; Peng, Y.; Cheng, J.; Li, Z.; Wu, L.; Jin, M.; Feng, H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell. Mol. Med. 2019, 23, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H. Inhibitory effects of catalpol coordinated with budesonide and their relationship with cytokines and Interleukin-13 expression. Am. J. Transl. Res. 2019, 11, 6413–6421. [Google Scholar]
- Kostadinova, E.P.; Alipieva, K.; Kokubun, T.; Taskova, R.; Handjieva, N.V. Phenylethanoids, iridoids and a spirostanol saponin from Veronica turrilliana. Phytochemistry 2007, 68, 1321–1326. [Google Scholar] [CrossRef]
- Migas, P.; Krauze-Baranowska, M. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Berná, J.; Rodriguez-Lopez, J.N.; Tudela, J.; Cánovas, F.G. Action of tyrosinase on alpha and beta-arbutin: A kinetic study. PLoS ONE 2017, 12, e0177330. [Google Scholar] [CrossRef]
- Cheng, S.-L.; Liu, R.H.; Sheu, J.-N.; Chen, S.-T.; Sinchaikul, S.; Tsay, G.J. Toxicogenomics of A375 human malignant melanoma cells treated with arbutin. J. Biomed. Sci. 2006, 14, 87–105. [Google Scholar] [CrossRef]
- Saracoglu, I.; Oztunca, F.H.; Nagatsu, A.; Harput, U.S. Iridoid content and biological activities of Veronica cuneifolia subsp. Cuneifolia and V. cymbalaria. Pharm. Biol. 2011, 49, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Escobar, J.A.; Bazaldúa, S.; Villarreal, M.L.; Bonilla-Barbosa, J.R.; Mendoza, S.; López, V.R. Cytotoxic and antioxidant activities of selected Lamiales species from Mexico. Pharm. Boil. 2011, 49, 1243–1248. [Google Scholar] [CrossRef]
- Banerjee, A.; Gerondakis, S. Coordinating TLR-activated signaling pathways in cells of the immune system. Immunol. Cell Boil. 2007, 85, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, P.; Kostadinova, E.; Milanova, V.; Alipieva, K.; Georgiev, M.I.; Ivanovska, N. Antiinflammatory Properties of Extracts and Compounds Isolated from Verbascum xanthophoeniceum Griseb. Phytotherapy Res. 2012, 26, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-κB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef]
- Wen, Y.; Huo, S.; Zhang, W.; Xing, H.; Qi, L.; Zhao, D.; Li, N.; Xu, J.; Yan, M.; Chen, X. Pharmacokinetics, Biodistribution, Excretion and Plasma Protein Binding Studies of Acteoside in Rats. Drug Res. 2015, 66, 148–153. [Google Scholar] [CrossRef]
- Park, K.S. Aucubin, a naturally occurring iridoid glycoside inhibits TNF-α-induced inflammatory responses through suppression of NF-κB activation in 3T3-L1 adipocytes. Cytokine 2013, 62, 407–412. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of arbutin in lipopolysaccharide-stimulated BV2 microglial cells. Inflamm. Res. 2012, 61, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Harput, U.S.; Genc, Y.; Khan, N.; Saracoglu, I. Radical scavenging effects of different Veronica species. Rec. Nat. Prod. 2011, 5, 100–107. [Google Scholar]
- Živković, J.; Cebovic, T.; Maksimović, Z. In vivo and in vitro antioxidant effects of three Veronica species. Open Life Sci. 2012, 7, 559–568. [Google Scholar] [CrossRef]
- Tada, M.; Kohno, M.; Niwano, Y. Alleviation effect of arbutin on oxidative stress generated through tyrosinase reaction with l-tyrosine and l-DOPA. BMC Biochem. 2014, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Frontispiece: Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chem. Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M.I. Metabolic differentiations of dwarf elder by NMR-based metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Rathi, D.; Pareek, A.; Zhang, T.; Pang, Q.; Chen, S.; Chakraborty, S.; Chakraborty, N. Metabolite signatures of grasspea suspension-cultured cells illustrate the complexity of dehydration response. Planta 2019, 250, 857–871. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



| Metabolite | Vau-1 1 | Vau-2 1 | Selected Signals, Multiplicity and Coupling Constant 2 |
|---|---|---|---|
| Alanine | + | + | δ 3.71 (q)/δ 1.48 (d, J = 7.4) |
| α-Glucose | + | + | δ 5.18 (d, J = 3.8)/δ 3.49 m |
| β-Glucose | + | + | 4.57 (d, J = 7.9)/3.19 (dd, J = 7.9, 9.2 ) |
| Sucrose | + | + | δ 5.40 (d, J = 3.9) |
| Acetic acid | + | − | δ 1.93 (s) |
| Lactic acid | + | + | δ 4.06 m/δ 1.27 (d, J = 6.5) |
| Succinic acid | + | + | δ 2.47 (s) |
| Formic acid | + | + | δ 8.46 (s) |
| Choline | + | + | δ 3.21 (s) |
| Arbutin | ++++ | ++++ | δ 7.00 (d, J = 8.9)/δ 6.79 (d, J = 8.9)/δ 4.85 (d, J = 7.7)/δ 3.87 (dd, J = 12.3, 2.0)/δ 3.72 (dd, J = 12.3, 5.4) |
| Aucubin | ++ | + | δ 6.29 (dd, J = 6.2, 1.9)/δ 5.79 (t, J = 1.7)/δ 5.09 (dd, J = 6.3, 2.4)/δ 5.07 (d, J = 6.1)/δ 4.70 (d, J = 7.9) |
| Catalpol | + | ++ | δ 6.37 (dd, J = 6.0, 1.8)/δ 5.11 (dd, J = 6.0, 4.6)/δ 5.03 (d, J = 9.8)/δ 4.18 (d, J = 13.3)/δ 3.98 (dd, 8.3, 1.3)/δ 3.78 (d, 13.3)/δ 3.55 (brs)/δ 2.58 (dd, J = 9.8, 7.7)/δ 2.27 m |
| Verbascoside | + | ++ | δ 7.63 (d, J = 15.9)/δ 7.14 (d, J = 2.0)/7.05 (dd, J = 8.3, 2.0)/δ 6.67 (dd, J = 8.3, 2.0)/δ 6.34 (d, J = 15.9)/4.93 (t, J = 9.6)/4.47 (d, J = 7.9)/δ 2.81 (t, J = 7.2) 1.04 (d, J = 6.4) |
| Concentration of Extracts | Arbutin in Vau-1 | Arbutin in Vau-2 | ||
|---|---|---|---|---|
| µg/mL | µg | µM | µg | µM |
| 1000.0 | 308.2 | 1133.1 | 382.9 | 1407.7 |
| 795.0 | 245.0 | 900.8 | 304.4 | 1119.1 |
| 500.0 | 154.1 | 566.5 | 191.5 | 703.9 |
| 250.0 | 77.1 | 283.3 | 95.7 | 351.9 |
| 125.0 | 38.5 | 141.6 | 47.9 | 176.0 |
| 62.5 | 19.3 | 70.8 | 23.9 | 88.0 |
| 50.0 | 15.4 | 56.7 | 19.1 | 70.4 |
| 31.3 | 9.6 | 35.5 | 12.0 | 44.1 |
| 25.0 | 7.7 | 28.3 | 9.6 | 35.2 |
| 15.6 | 4.8 | 17.7 | 6.0 | 22.0 |
| 12.5 | 3.9 | 14.2 | 4.8 | 17.6 |
| 7.81 | 2.4 | 8.8 | 3.0 | 11.0 |
| 3.91 | 1.2 | 4.4 | 1.5 | 5.5 |
| 3.13 | 1.0 | 3.5 | 1.2 | 4.4 |
| 1.95 | 0.6 | 2.2 | 0.7 | 2.7 |
| 0.98 | 0.3 | 1.1 | 0.4 | 1.4 |
| 0.78 | 0.2 | 0.9 | 0.3 | 1.1 |
| 0.50 | 0.2 | 0.6 | 0.2 | 0.7 |
| 0.25 | 0.1 | 0.28 | 0.1 | 0.35 |
| 0.025 | 0.01 | 0.028 | 0.01 | 0.035 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrova, P.A.; Alipieva, K.; Grozdanova, T.; Leseva, M.; Gerginova, D.; Simova, S.; Marchev, A.S.; Bankova, V.; Georgiev, M.I.; Popova, M.P. Veronica austriaca L. Extract and Arbutin Expand Mature Double TNF-α/IFN-γ Neutrophils in Murine Bone Marrow Pool. Molecules 2020, 25, 3410. https://doi.org/10.3390/molecules25153410
Dimitrova PA, Alipieva K, Grozdanova T, Leseva M, Gerginova D, Simova S, Marchev AS, Bankova V, Georgiev MI, Popova MP. Veronica austriaca L. Extract and Arbutin Expand Mature Double TNF-α/IFN-γ Neutrophils in Murine Bone Marrow Pool. Molecules. 2020; 25(15):3410. https://doi.org/10.3390/molecules25153410
Chicago/Turabian StyleDimitrova, Petya A., Kalina Alipieva, Tsvetinka Grozdanova, Milena Leseva, Dessislava Gerginova, Svetlana Simova, Andrey S. Marchev, Vassya Bankova, Milen I. Georgiev, and Milena P. Popova. 2020. "Veronica austriaca L. Extract and Arbutin Expand Mature Double TNF-α/IFN-γ Neutrophils in Murine Bone Marrow Pool" Molecules 25, no. 15: 3410. https://doi.org/10.3390/molecules25153410
APA StyleDimitrova, P. A., Alipieva, K., Grozdanova, T., Leseva, M., Gerginova, D., Simova, S., Marchev, A. S., Bankova, V., Georgiev, M. I., & Popova, M. P. (2020). Veronica austriaca L. Extract and Arbutin Expand Mature Double TNF-α/IFN-γ Neutrophils in Murine Bone Marrow Pool. Molecules, 25(15), 3410. https://doi.org/10.3390/molecules25153410









