Hemicellulose Recovery from Spent-Sulfite-Liquor: Lignin Removal by Adsorption to Resins for Improvement of the Ultrafiltration Process
Abstract
:1. Introduction
2. Results and Discussion
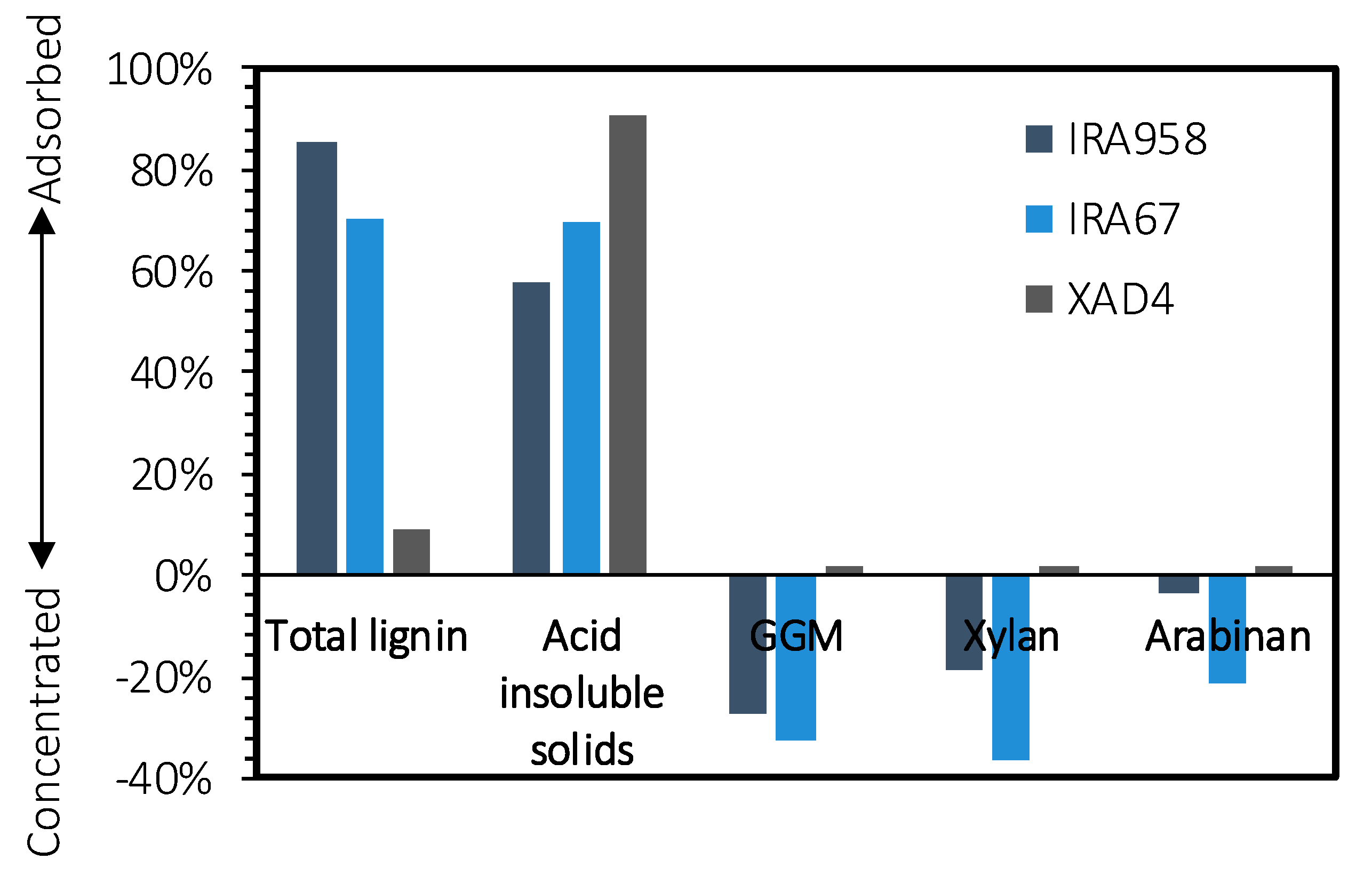
2.1. Screening of Adsorbents
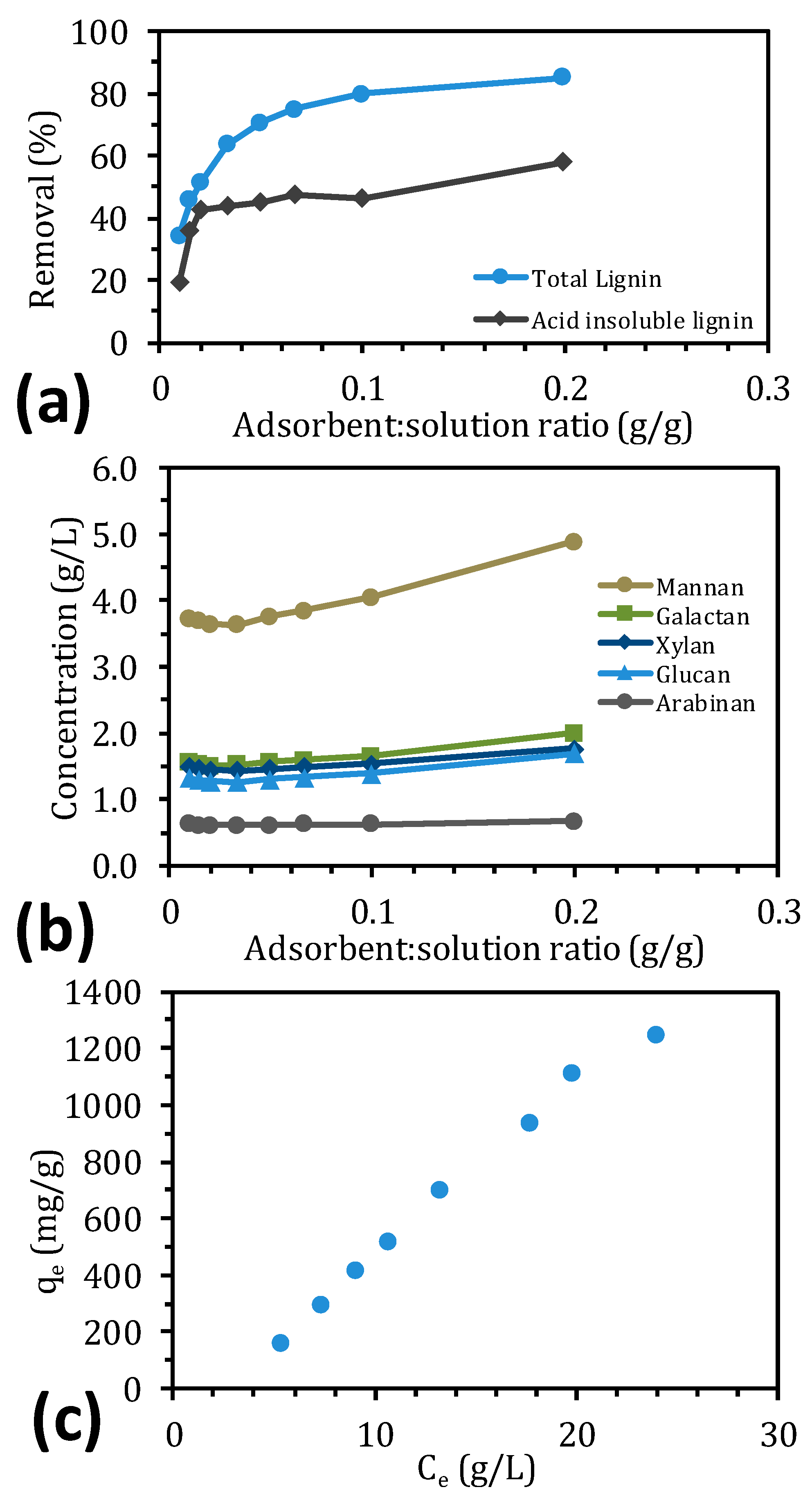
2.2. Equilibrium Adsorption for IRA958
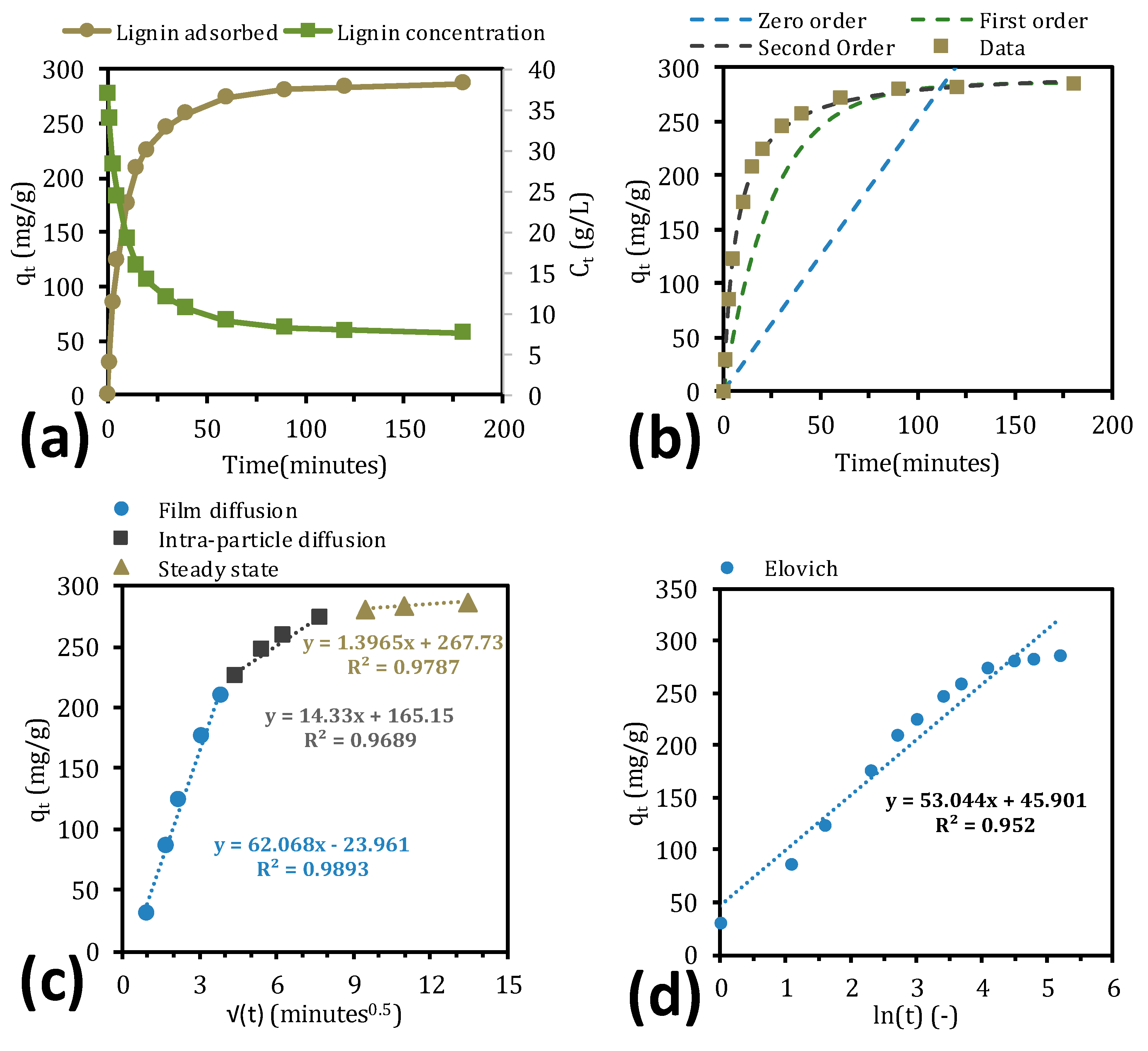
2.3. Adsorption Kinetics
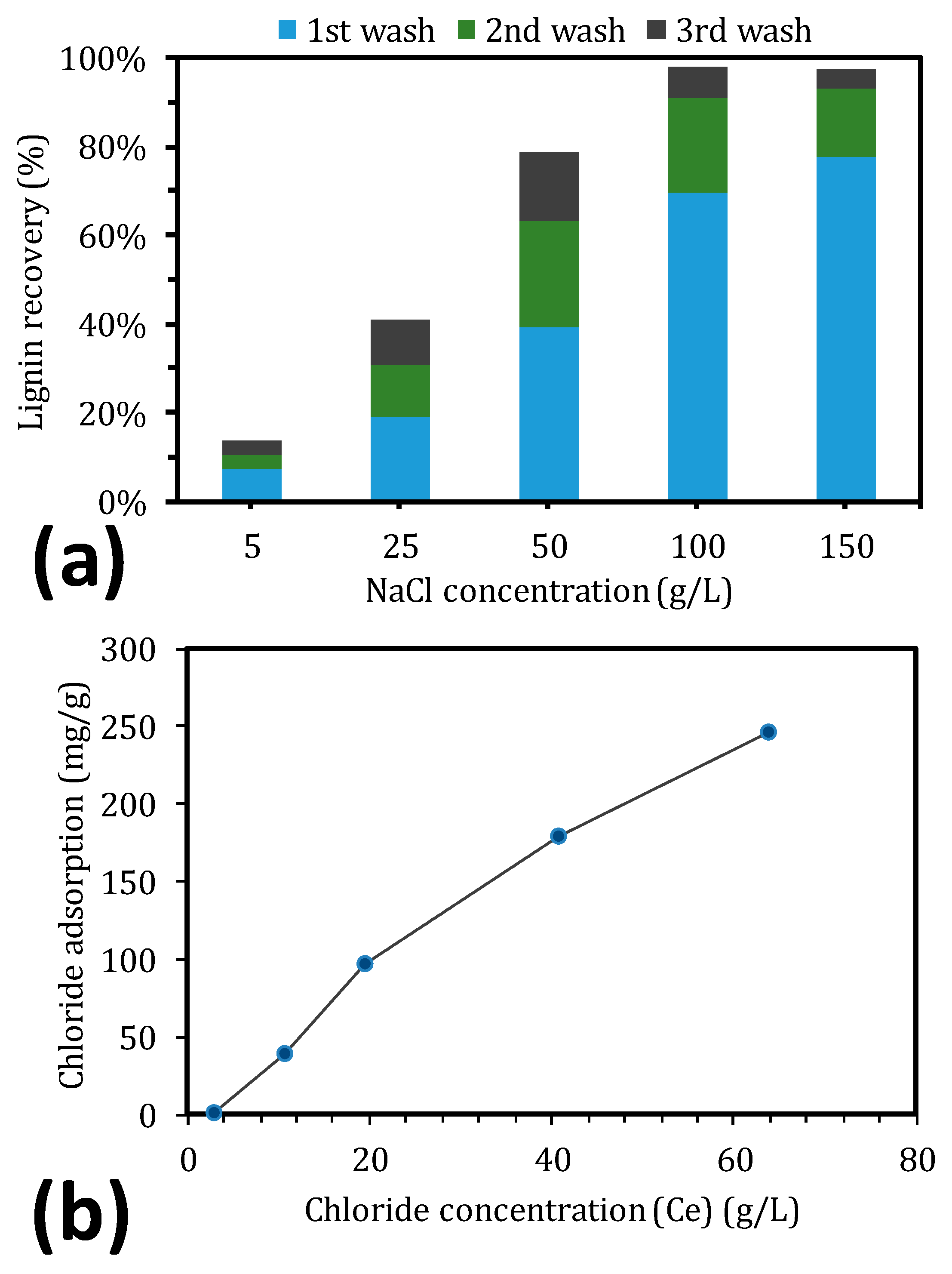
2.4. Lignin Desorption
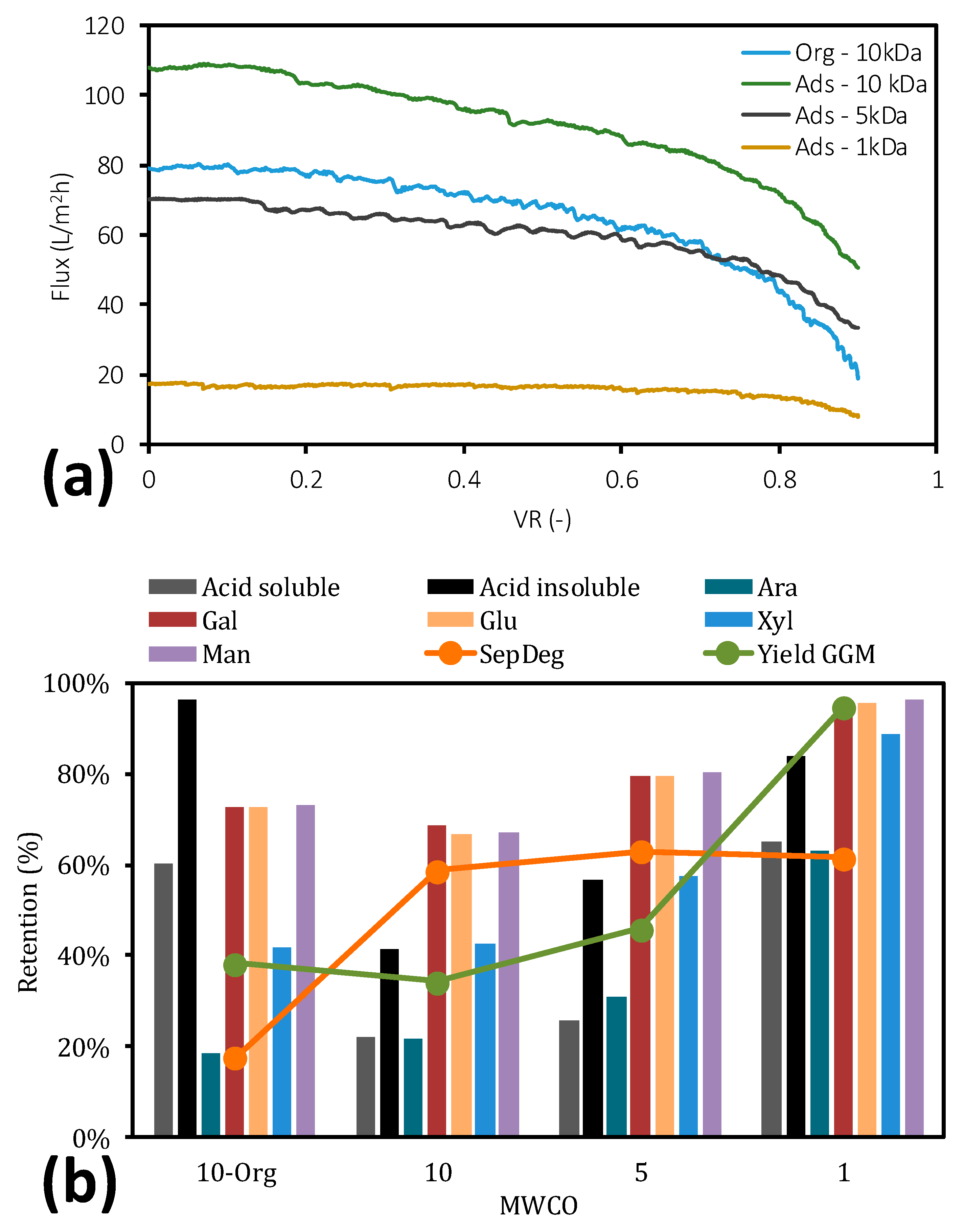
2.5. Ultrafiltration before and after Adsorption
3. Materials and Methods
3.1. Raw Material
3.2. Preparation of the Adsorbents
3.3. Adsorbent Screen and Equilibrium Adsorption Studies
3.4. Adsorption Kinetics
3.5. Lignin Desorption
3.6. Membrane Filtration
3.7. Analytical Measurements
3.7.1. Total Dry and Ash Content
3.7.2. Hemicellulose and Acid-Insoluble Solids
3.7.3. Lignin and Chloride Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro-and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Schneider, M.H.; Phillips, J.G. Furfuryl Alcohol and Lignin Adhesive Composition. U.S. Patent 6,747,076 B2, 8 June 2004. [Google Scholar]
- Kamoun, A.; Jelidi, A.; Chaabouni, M. Evaluation of the performance of sulfonated esparto grass lignin as a plasticizer–water reducer for cement. Cem. Concr. Res. 2003, 33, 995–1003. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Al-Rudainy, B.; Galbe, M.; Arcos Hernandez, M.; Jannasch, P.; Wallberg, O. Impact of Lignin Content on the Properties of Hemicellulose Hydrogels. Polymers 2018, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Rosengren, A.; Butler, S.J.; Arcos-Hernandez, M.; Bergquist, K.-E.; Jannasch, P.; Stålbrand, H. Enzymatic synthesis and polymerisation of β-mannosyl acrylates produced from renewable hemicellulosic glycans. Green Chem. 2019, 21, 2104–2118. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef]
- Hartman, J.; Albertsson, A.-C.; Sjöberg, J. Surface-and bulk-modified galactoglucomannan hemicellulose films and film laminates for versatile oxygen barriers. Biomacromolecules 2006, 7, 1983–1989. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Wallberg, O. From lab-scale to on-site pilot trials for the recovery of hemicellulose by ultrafiltration: Experimental and theoretical evaluations. Sep. Purif. Technol. 2020, 117187. [Google Scholar] [CrossRef]
- Liu, S. Woody biomass: Niche position as a source of sustainable renewable chemicals and energy and kinetics of hot-water extraction/hydrolysis. Biotechnol. Adv. 2010, 28, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, J.; Jacobs, A.; Palm, M.; Zacchi, G.; Dahlman, O.; Stålbrand, H. Characterization of galactoglucomannan extracted from spruce (Picea abies) by heat-fractionation at different conditions. Carbohydr. Polym. 2003, 51, 203–211. [Google Scholar] [CrossRef]
- Li, J.; Henriksson, G.; Gellerstedt, G. Carbohydrate reactions during high-temperature steam treatment of aspen wood. Appl. Biochem. Biotechnol. 2005, 125, 175–188. [Google Scholar] [CrossRef]
- Polari, L.; Ojansivu, P.; Mäkelä, S.; Eckerman, C.; Holmbom, B.; Salminen, S. Galactoglucomannan Extracted from Spruce (Picea abies) as a Carbohydrate Source for Probiotic Bacteria. J. Agric. Food. Chem. 2012, 60, 11037–11043. [Google Scholar] [CrossRef]
- Wenzl, H. The Chemical Technology of Wood; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Macleod, J.M.; Benko, J.V. Sugars and Sugar Acids in Lignosulphonate Products. J. Wood Chem. Technol. 1982, 2, 207–219. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Schagerlöf, H.; Wallberg, O. Antisolvent precipitation of hemicelluloses, lignosulfonates and their complexes from ultrafiltrated spent sulfite liquor (SSL). Holzforschung 2018, 72, 839. [Google Scholar] [CrossRef]
- Berglund, J.; Azhar, S.; Lawoko, M.; Lindström, M.; Vilaplana, F.; Wohlert, J.; Henriksson, G. The structure of galactoglucomannan impacts the degradation under alkaline conditions. Cellulose 2019, 26, 2155–2175. [Google Scholar] [CrossRef]
- Persson, T.; Jönsson, A.-S. Fouling of ultrafiltration membranes during isolation of hemicelluloses in the forest industry. Sch. Res. Exch. 2009, 2009, 624012. [Google Scholar] [CrossRef]
- Steinmetz, V.; Villain-Gambier, M.; Klem, A.; Gambier, F.O.; Dumarcay, S.P.; Trebouet, D. Unveiling TMP Process Water Potential as an Industrial Sourcing of Valuable Lignin–Carbohydrate Complexes toward Zero-Waste Biorefineries. ACS Sustain. Chem. Eng. 2019, 7, 6390–6400. [Google Scholar] [CrossRef]
- Krawczyk, H.; Oinonen, P.; Jönsson, A.-S. Combined membrane filtration and enzymatic treatment for recovery of high molecular mass hemicelluloses from chemithermomechanical pulp process water. Chem. Eng. J. 2013, 225, 292–299. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Lipnizki, F.; Wallberg, O. Galactoglucomannan Recovery with Hydrophilic and Hydrophobic Membranes: Process Performance and Cost Estimations. Membranes 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuvander, J.; Lipnizki, F.; Jönsson, A.-S. On-Site Recovery of Hemicelluloses from Thermomechanical Pulp Mill Process Water by Microfiltration and Ultrafiltration. J. Wood Chem. Technol. 2019, 39, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Karthikeyan, J. Removal of lignin and tannin colour from aqueous solution by adsorption onto activated charcoal. Environ. Pollut. 1997, 97, 183–187. [Google Scholar] [CrossRef]
- Song, T.; Pranovich, A.; Holmbom, B. Separation of polymeric galactoglucomannans from hot-water extract of spruce wood. Bioresour. Technol. 2013, 130, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Zasadowski, D.; Yang, J.; Edlund, H.; Norgren, M. Antisolvent precipitation of water-soluble hemicelluloses from TMP process water. Carbohydr. Polym. 2014, 113, 411–419. [Google Scholar] [CrossRef]
- Koivula, E.; Kallioinen, M.; Sainio, T.; Antón, E.; Luque, S.; Mänttäri, M. Enhanced membrane filtration of wood hydrolysates for hemicelluloses recovery by pretreatment with polymeric adsorbents. Bioresour. Technol. 2013, 143, 275–281. [Google Scholar] [CrossRef]
- Nitzsche, R.; Groengroeft, A.; Kraume, M. Separation of lignin from beech wood hydrolysate using polymeric resins and zeolites–Determination and application of adsorption isotherms. Sep. Purif. Technol. 2019, 209, 491–502. [Google Scholar] [CrossRef]
- Schwartz, T.J.; Lawoko, M. Removal of acid-soluble lignin from biomass extracts using Amberlite XAD-4 resin. BioResources 2010, 5, 2337–2347. [Google Scholar]
- Sumerskii, I.; Korntner, P.; Zinovyev, G.; Rosenau, T.; Potthast, A. Fast track for quantitative isolation of lignosulfonates from spent sulfite liquors. RSC Adv. 2015, 5, 92732–92742. [Google Scholar] [CrossRef]
- Westerberg, N.; Sunner, H.; Gunnar, H.; Mikaela, H.; Martin, L.; Rasmuson, A. Separation of galactoglucomannans, lignin and lignin-carbohydrate complexes from hot-water-extracted Norway spruce by cross-flow filtration and adsorption chromatography. BioResources 2012, 7, 4501–4516. [Google Scholar] [CrossRef] [Green Version]
- Narron, R.H.; Chang, H.-M.; Jameel, H.; Park, S. Soluble lignin recovered from biorefinery pretreatment hydrolyzate characterized by lignin–carbohydrate complexes. ACS Sustain. Chem. Eng. 2017, 5, 10763–10771. [Google Scholar] [CrossRef]
- Heinonen, J.; Sanlaville, Q.; Niskakoski, H.; Tamper, J.; Sainio, T. Separation and recovery of lignin from hydrolysates of lignocellulose with a polymeric adsorbent. Sep. Purif. Technol. 2017, 186, 125–134. [Google Scholar] [CrossRef]
- Van Blaricom, L.E.; Russell, G.K. Lignosulfonate Recovery from Waste Sulfite Liquor. International Patent Application No U.S. 29230652A, 6 July 1955. [Google Scholar]
- Takahashi, S.; Tanifuji, K.; Shiell, K.; Fatehi, P.; Jahan, M.S.; Ohi, H.; Ni, Y. Removal of acetic acid from spent sulfite liquor using anion exchange resin for effective xylose fermentation with Pichia stipitis. BioResources 2013, 8, 2417–2428. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Ren, J.; Zhang, Y.; Liu, X.; Ouyang, J. Simultaneously separation of xylo-oligosaccharide and lignosulfonate from wheat straw magnesium bisulfite pretreatment spent liquor using ion exchange resin. Bioresour. Technol. 2018, 249, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, B.; Wang, H.; Liu, C.; Mu, X. Preparation of concrete superplasticizer by oxidation-sulfomethylation of sodium lignosulfonate. BioResources 2013, 8, 1055–1063. [Google Scholar] [CrossRef]
- Qiu, X.; Kong, Q.; Zhou, M.; Yang, D. Aggregation behavior of sodium lignosulfonate in water solution. J. Phys. Chem. B 2010, 114, 15857–15861. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, J.; Teleman, A.; Junel, L.; Zacchi, G.; Dahlman, O.; Tjerneld, F.; Stålbrand, H. Isolation and characterization of galactoglucomannan from spruce (Picea abies). Carbohydr. Polym. 2002, 48, 29–39. [Google Scholar] [CrossRef]
- Al-Rudainy, B.; Galbe, M.; Wallberg, O. Influence of prefiltration on membrane performance during isolation of lignin-carbohydrate complexes from spent sulfite liquor. Sep. Purif. Technol. 2017, 187, 380–388. [Google Scholar] [CrossRef]
- Korntner, P.; Schedl, A.; Sumerskii, I.; Zweckmair, T.; Mahler, A.K.; Rosenau, T.; Potthast, A. Sulfonic acid group determination in lignosulfonates by headspace gas chromatography. ACS Sustain. Chem. Eng. 2018, 6, 6240–6246. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G.; Kazemian, H. Insights into the S-shaped sorption isotherms and their dimensionless forms. Microporous Mesoporous Mater. 2018, 272, 166–176. [Google Scholar] [CrossRef]
- Hinz, C. Description of sorption data with isotherm equations. Geoderma 2001, 99, 225–243. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Kumara, N.; Hamdan, N.; Petra, M.I.; Tennakoon, K.U.; Ekanayake, P. Equilibrium isotherm studies of adsorption of pigments extracted from kuduk-kuduk (Melastoma malabathricum L.) pulp onto TiO2 nanoparticles. J. Chem. 2014, 2014, 468975. [Google Scholar] [CrossRef] [Green Version]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 11. [Google Scholar] [CrossRef]
- Mezenner, N.Y.; Bensmaili, A. Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem. Eng. J. 2009, 147, 87–96. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Villarante, N.R.; Bautista, A.P.R.; Sumalapao, D.E.P. Batch adsorption study and kinetic profile of Cr (VI) using lumbang (Aleurites moluccana)-derived activated carbon-chitosan composite crosslinked with epichlorohydrin. Orient. J. Chem. 2017, 33, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Lin, X.; Xie, J.; Huang, X. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. RSC Adv. 2017, 7, 4492–4500. [Google Scholar] [CrossRef] [Green Version]
- Hilal, N.; Al-Zoubi, H.; Mohammad, A.; Darwish, N. Nanofiltration of highly concentrated salt solutions up to seawater salinity. Desalination 2005, 184, 315–326. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC press: Boka Raton, FL, USA, 2014. [Google Scholar]
- Luis, P. Fundamental Modeling of Membrane Systems: Membrane and Process Performance; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Stade, S.; Kallioinen, M.; Tuuva, T.; Mänttäri, M. Compaction and its effect on retention of ultrafiltration membranes at different temperatures. Sep. Purif. Technol. 2015, 151, 211–217. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds SSL and purified GGM are available from the authors. |

| Concentration (g/L) | |
|---|---|
| Total dry substance | 84.7 |
| Ash | 31.3 |
| Acid soluble lignin | 34.1 |
| Acid insoluble lignin | 2.0 |
| Arabinan | 0.6 |
| Galactan | 1.6 |
| Glucan | 1.4 |
| Xylan | 1.5 |
| Mannan | 3.9 |
| Model | Fitted Parameters | Unit | R2 | Equation | Ref |
|---|---|---|---|---|---|
| Adsorption isotherms | |||||
| Linear | Klin = 51.67 | (mL/g) | 0.9679 | [47] | |
| Langmuir | QL = −1621.8 KL = −0.0202 | (mg/g) (mL/mg) | 0.5445 | [48] | |
| Freundlich | KF = 31.06 nF = 1.1774 | (mLnFmg1−nF/g) (-) | 0.9855 | [48] | |
| Sips (Langmuir–Freundlich) | QS = 1947.2 KS = 0.0035 nS = 1.9616 | (mg/g) (mL/mg) (-) | 0.9978 | [43] | |
| Modified Brunauer–Emmett–Teller (BET) | QB = 24,728 KB = 0.3926 CS = 216.74 | (mg/g) (mL/mg) (mg/mL) | 0.9839 | [49] | |
| Adsorption kinetics | |||||
| Pseudo-zero-order | k0 = 2.5145 | (mg/(g min)) | −0.6910 | [50] | |
| Pseudo-first-order | k1 = 0.0389 qe = 286.44 | (1/min) (mg/g) | 0.9781 | [50] | |
| Pseudo-second-order | k2 = 4.869e−4 qe = 299.22 | (g/(mg·min)) (mg/g) | 0.9997 | [48] | |
| Elovich | α = 126.02 β = 0.0189 | (mg/(g·min)) (g/mg) | 0.9520 | [48] | |
| Intra-particle diffusion | kp = 14.33 m = 165.15 | (mg/(g·min0.5)) (mg/g) | 0.9689 | [48] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rudainy, B.; Galbe, M.; Wallberg, O. Hemicellulose Recovery from Spent-Sulfite-Liquor: Lignin Removal by Adsorption to Resins for Improvement of the Ultrafiltration Process. Molecules 2020, 25, 3435. https://doi.org/10.3390/molecules25153435
Al-Rudainy B, Galbe M, Wallberg O. Hemicellulose Recovery from Spent-Sulfite-Liquor: Lignin Removal by Adsorption to Resins for Improvement of the Ultrafiltration Process. Molecules. 2020; 25(15):3435. https://doi.org/10.3390/molecules25153435
Chicago/Turabian StyleAl-Rudainy, Basel, Mats Galbe, and Ola Wallberg. 2020. "Hemicellulose Recovery from Spent-Sulfite-Liquor: Lignin Removal by Adsorption to Resins for Improvement of the Ultrafiltration Process" Molecules 25, no. 15: 3435. https://doi.org/10.3390/molecules25153435
APA StyleAl-Rudainy, B., Galbe, M., & Wallberg, O. (2020). Hemicellulose Recovery from Spent-Sulfite-Liquor: Lignin Removal by Adsorption to Resins for Improvement of the Ultrafiltration Process. Molecules, 25(15), 3435. https://doi.org/10.3390/molecules25153435






