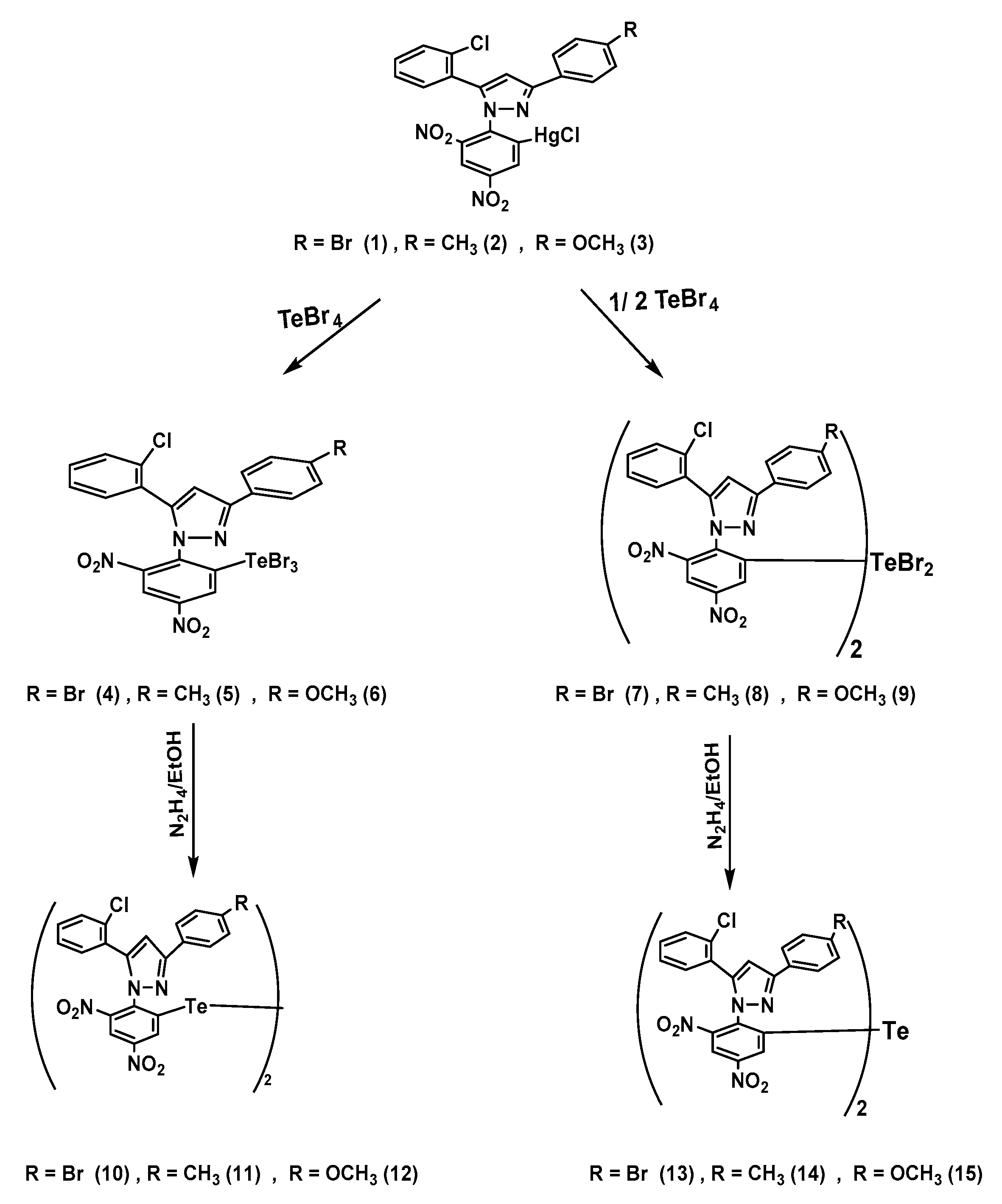
3.2. Synthesis
3.2.1. General Method for the Preparation of Aryl Mercury(II) Chlorides
2-(3-(4-Substitutedphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)mercury(II) chloride 1–3
A mixture of compound 2-hydrazinyl-3,5-dinitrophenylmercury chloride (3 mmol) in 25 mL of acetic acid and (4 mmol) of chalcones: 3-(2-chlorophenyl)-1-(4-bromophenyl)-prop-2-en-1-one, 3-(2-chlorophenyl)-1-(4-methylphenyl)prop-2-en-1-one and 3-(2-chlorophenyl)-1-(4-methoxy phenyl)prop-2-en-1-one), respectively, was refluxed for 5 h. Then, a catalytic amount of HCl (6–8 drops) was added and the mixture was refluxed for 1 h. After cooling, 50 mL of ice water was added to obtain a yellowish brown solid. The resulting precipitate was filtered, washed several times with water, and recrystallized (twice) from ethanol to obtain yellow solid in 65–77% yields.
2-(3-(4-Bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl) mercury (II) chloride (1)
Light-yellow crystalline solid; Yield: 77%; M.p.: 193–195 °C; Rf = 0.55 (ethyl acetate-n-hexane); Molar conductance (Λm, ohm−1 cm−1 mol−1): 35; FTIR (KBr) cm−1: FTIR (KBr) cm−1: 3069 w, 1600 s, 1510 s, 1465 s, 1438 s, 1390 s, 1275 m, 1211 s, 1175 m, 1107 m, 1065 m, 1107 m, 1028, 976 m, 880 s, 825 m, 756 m; 1H NMR (500 MHz, DMSO-d6, δ /ppm): 5.96 (s, 1H, H4), 7.45–8.23 (m, 10H, Ar-H); UV-Vis (λmax, nm): 360; Anal. Calculated for C21H11BrCl2HgN4O4: C 34.33, H 1.51, N 7.63, Found C 34.40, H 1.51, N 7.69%.
2-(5-(2-Chlorophenyl)-3-(4-methyl phenyl)-1H-pyrazol-1-yl)-3,5-dinitro phenyl) mercury(II) chloride (2)
Bright-yellow crystalline solid; Yield: 65%; M.p.: 138–140 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 31; Rf = 0.68 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3063 w, 2974 w, 2913 w, 1600 s, 1465 s, 1438 s, 1392 s, 1323 m, 1273 s, 1107 s, 1064 s, 1030 m, 976, 821 m, 756 m; 1H NMR (500 MHz, DMSO-d6, δ /ppm): 1H NMR (500 MHz, DMSO-d6, δ/ppm): 2.20(s, 3H, CH3); 5.90 (s, 1H, H4), 7.50–8.20 (m, 10H, Ar–H); UV-Vis (λmax, nm): 350; Anal. Calculated for C22H14Cl2HgN4O4:C 32.98, H 1.76, N 6.99, Found: C 33.02, H 1.80, N 7.01%.
2-(5-(2-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)mercury(II) chloride (3)
Light-yellow crystalline solid; Yield: 72%; M.p.: 180–182 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 39; Rf = 0.51 (ethyl acetate-n-hexane); FT-IR (KBr) cm−1: 3063 w, 2974 w, 2931 w, 2839 w, 1600 s, 1604 s, 1570 s,1508 s, 1462 s, 1427 s, 1327 s, 1257 s, 1222 m, 1180 s, 1111 s, 1030 s, 976 m, 825 m, 752 m, 678 m; 1H NMR (500 MHz, DMSO-d6, δ /ppm): 3.83(s, 6H, OCH3), 5.81 (s, 2H, H4 and H4’), 7.43–8.22 (m, 10H, Ar–H); UV-Vis (λmax, nm): 348; Anal. Calculated for C22H14Cl2HgN4O5:C 38.41, H 2.34, N 8.14, Found: C 38.47, H 2.39, N 8.21%.
3.2.2. General Method for the Preparation of Aryl Tellurium Tribromides
(2-(3-(4-Substitutedphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium tribromide 4–6
A mixture of tellurium tetrabromide (1.78 g, 4.00 mmol) in 35 mL of dry dioxane and (4.00 mmol) aryl mercuric chlorides 1, 2, or 3, respectively, in 30 mL of dry dioxane was refluxed with stirring for 6 h under an argon atmosphere. The resulting solution was filtered hot and on cooling deposited in a 2:1 complex of dioxane and mercuric chloride as white plates, which was filtered off. The filtrate was reduced by a rotary evaporator to give a brown precipitate. Recrystallization of the crude product from a mixture of chloroform and hexane (1:4) gave a yellow crystalline solid in 60–68% yields.
(2-(3-(4-Bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium tribromide (4)
Light-yellowish-brown crystalline solid; Yield: 68%; M.p.: 212–214 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 27; Rf = 0.45 ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3063 w, 1600 s, 1465 s, 1438 s, 1392 s, 1273 m, 1211 s, 1172 m, 1107 m, 1064 m, 1107 m, 1026, 976 m, 880 s, 821 m, 756 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 5.96 (s, 1H, H4), 7.46–8.20 (m, 10H, Ar–H); UV-Vis (λmax, nm): 395; Anal. Calcd for C21H11Br4ClN4O4Te:C 29.13, H 1.28,N 6.47, Found: C 29.18,H 1.31, N 6.50%.
(2-(5-(2-Chlorophenyl)-3-(4-methylphenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium tribromide (5)
Light-yellowish-brown crystalline solid; Yield: 60%; M.p.: 159–161 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 29; Rf = 0.75 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3075 m, 2925 w, 1592 m, 1463 m, 1439 m, 1396 m, 1325 s, 1277 m, 1212 m,1172 m, 1065 m, 1026 m, 978 s, 820 m, 756 s, 710 m, 685 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 2.20(s, 3H, CH3); 5.92 (s, 1H, H4), 7.45–8.25 (m, 10H, Ar–H); UV-Vis (λmax, nm): 382; Anal. Calculated for C22H14Br3ClN4O4Te: C 32.98, H 1.76, N 6.99, Found: C 33.02, H 1.80, N 7.01%.
(2-(5-(2-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-1-yl)-3,5dinitrophenyl)tellurium tribromide (6)
Light-yellowish brown solid; Yield: 67%; M.p.: 195–197 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 26; Rf = 0.61 (ethyl acetate- n-hexane); FTIR (KBr) cm−1: 3063 w, 2904 w, 2833 w, 1604 s, 1570 s, 1512 m, 1465 m, 1427 s, 1327 s, 1264 m, 1226 m, 1194 m, 1114 m, 1003 s, 914 m, 823 m, 754 m; 1H NMR (500 MHz, DMSO-d6, δ /ppm): 3.86(s, 3H, OCH3), 5.84 (s, 1H, H4), 7.45–8.20 (m, 10H, Ar-H); UV-Vis (λmax, nm): 379; Anal. Calculated for C22H14Br3ClN4O4Te: C 32.34, H 1.73, N 6.86, Found: C 32.30, H 1.78, N 6.99%.
3.2.3. General Method for the Preparation of Diaryl Tellurium Dibromides
Bis[(2-(3-(4-substitutedphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium dibromide 7–9
A mixture of tellurium tetrabromide (0.89 g, 2.00 mmol) and aryl mercuric chloride 1, 2, or 3 (4.00 mmol) in 35 mL of dry dioxane was refluxed with stirring for 6 h under an argon gas atmosphere. The resulting solution was filtered hot and cooled to room temperature. On cooling, a 2:1 complex of dioxane and mercuric halides was separated as white plates and was filtered off immediately. Recrystallization of the product from a mixture of dichloromethane and hexane (1:4) gave an orange-brown to yellowish brown solid in 70–75% yield.
Bis[(2-(3-(4-bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium dibromide (7)
Light-orange-brown crystalline solid; Yield: 75%; M.p.: 209–211 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 32; Rf = 0.50 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3059 w, 1604 s, 1570 s, 1516 s, 1465 m, 1438 m, 1338 s, 1311 m, 1273 m, 1211 s, 1180 m, 1157 m, 1041 s, 972 s, 860 s, 790 m, 752 m, 717 m, 690 m, 655 m, 578 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 5.95 (s, 2H, H4 and H4′), 7.46–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 380; Anal. Calculated for C42H22Br4Cl2N8O8Te: C 39.26, H 1.73, N 8.72, Found: C 39.30, H 1.75, N 8.74%.
Bis[(2-(5-(2-Chlorophenyl)-3-(4-methylphenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium dibromide (8)
Light-yellowish brown crystalline solid; Yield: 70%; M.p.: 150–152 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 25; Rf = 0.40 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3063 m, 2924 w, 1604 s, 1593 m, 1512 m, 1469 m, 1442 m, 1315 s, 1273 m, 1215 m, 1037 m, 1014 m, 976 m, 752 m, 578 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 2.06(s, 6H, 2CH3); 5.92 (s, 2H, H4 and H4′), 7.45–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 373; Anal. Calculated for C44H28Br2Cl2N8O8Te: C 45.75, H 2.44, N 9.70, Found: C 45.84, H 2.51, N 9.76%.
Bis[2-(5-(2-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-1-yl)-3,5dinitro phenyl)]tellurium dibromide (9)
Light-yellowish brown crystalline solid; Yield: 71%; M.p.: 191–193 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 30; Rf = 0.70 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3063 w, 2931 w, 2850 w, 1606 s, 1571 s, 1520 m, 1460 m, 1430 m, 1330 s, 1261 m, 1227 m, 1180 m, 1034 m, 1014 m, 978 s, 830 m, 759 m, 682 m, 578 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 3.86 (s, 6H, 2OCH3), 5.80 (s, 2H, 2H4), 7.45–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 371; Anal. Calculated for C44H28Br2Cl2N8O10Te: C 44.52, H 2.38, N 9.44, Found: C 44.60, H 2.41, N 9.45%.
3.2.4. General Method for the Preparation of Diaryl Ditellurides
Bis[(2-(3-(4-substituted phenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)] ditelluride 10–12
Aryl tellurium tribromide (3.00 mmol) was refluxed in ethanol (25 mL). An ethanolic solution of hydrazine hydrate was added drop by drop to the refluxing solution until the evolution of nitrogen ceased. The resulting solution was cooled to room temperature and poured into 100 mL of distilled water and extract with diethyl ether (4 × 30 mL). The etheric extracts were dried over an anhydrous calcium chloride. Evaporation of solvent afforded a dark red solid of compounds. The resulting precipitate was recrystallized by ethanol and gave a dark red solid in 61–68% yields.
Bis[(2-(3-(4-bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)] ditelluride (10)
Dark red crystalline solid; Yield: 68%; M.p.: 100–102 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 9; Rf = 0.62 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3072 w, 2928 w, 28431 w, 1600 s, 1570 s, 1520 m, 1465 m, 1431 m, 1331 s, 1266 m, 1226 m, 1181 m, 1033 m, 1010 m, 976 s, 829 m, 758 m, 684 m, 578 m; 1H NMR (500 MHz, DMSO-d6, δ /ppm): 5.96 (s, 2H, H4, and H4’), 7.47–8.23 (m, 20H, Ar–H); UV-Vis (λmax, nm): 500; Anal. Calculated for C42H22Br2Cl2N8O8Te2: C 40.27, H 1.77, N 8.95, Found: C 40.33, H 1.80, N 9.00%.
Bis[(2-(5-(2-Chlorophenyl)-3-(4-methylphenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)] ditelluride (11)
Dark red crystalline solid; Yield: 61%; M.p.: 91–93 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 11; Rf = 0.68 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3063 w, 2924 w, 1631 s, 1569 m, 1539 m, 1481 m, 1455 m, 1399 m, 1335 s, 1269 s, 1211 m, 1134 m, 1068 m, 1045 m, 852 m, 826 m, 768 m, 628 w; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 2.33(s, 6H, 2CH3); 5.91 (s, 2H, H4 and H4’), 7.42–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 491; Anal. Calculated for C44H28Cl2N8O8Te2: C 47.07, H 2.51, N 9.98, Found: C 47.10, H 2.50, N 10.10%.
Bis[(2-(5-(2-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-1-yl)-3,5dinitro phenyl)] ditelluride (12)
Dark red crystalline solid; Yield: 62%; M.p.: 99–101 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 10; Rf = 0.55 (ethyl acetate- n-hexane); IR (KBr) cm−1: 3063 w, 2974 w, 2931 w, 2839 w, 1604 m, 1570 s, 1512 m, 1485 m,1427 m, 1327 m, 1261 s, 1226 m, 1184 m, 1033 s, 1014 m, 976 m, 825 m, 756 m, 682 m, 578 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm) 3.86 (s, 6H, 2OCH3), 5.80 (s, 2H, 2H4), 7.45–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 490; Anal. Calculated for C44H28Cl2N8O10Te2: C 45.70, H 2.35, N 9.68, Found: C 45.76, H 2.44, N 9.70%.
3.2.5. General Method for the Preparation of Diaryl Tellurides
Bis[(2-(3-(4-substituted phenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)] telluride 13–15
Diaryl tellurium dibromides (i.e., compounds 7, 8, or 9) (2.00 mmol) was dissolved in 25 mL of ethanol and refluxed. A solution of hydrazine hydrate in ethanol was added drop wisely to the refluxed solution until nitrogen evaluation ceased. The resulting solution was poured into 500 mL of distilled ice water to afford a yellow solid. The crude product was twice recrystallized from a mixture of ethanol and dichloromethane to obtain a yellow or yellowish brown solid in 58–67% yields.
Bis[(2-(3-(4-bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)] telluride (13)
Light-yellowish brown crystalline solid; Yield: 67%; M.p.: 88–90 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 13; Rf = 0.73 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3063 w, 1609 s, 1597, 1465 m, 1438 m, 1323 s, 1273 m, 1311 m, 1272 m, 1211 s, 1181 m, 1157 m, 1041 s, 986 s, 860 s, 790 m, 756 m, 719 m, 667 m, 655 m, 578 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 5.94 (s, 2H, H4 and H4′), 7.44–8.23 (m, 20H, Ar–H); UV-Vis (λmax, nm): 358; Anal. Calculated for C21H11BrCl3N4O4Te: C 34.43, H 1.51, N 7.65, Found: C 34.50, H 1.51, N7.71%.
Bis[(2-(5-(2-Chlorophenyl)-3-(4-methylphenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)] telluride (14)
Light-yellow crystalline solid; Yield: 58%; M.p.: 80–82 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 8; Rf = 0.65 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3059 w, 1600 s, 1567 s, 1513 s, 1460 m, 1440 m, 1332 s, 1310 m, 1270 m, 1210 s, 1181 m, 1154 m, 1040 s, 970 s, 861 s, 788 m, 751 m, 719 m, 691 m, 657 m, 573 m; 1H NMR (500 MHz, DMSO-d6, δ /ppm): 2.06 (s, 6H, 2CH3); 5.90 (s, 2H, H4 and H4′), 7.46-8.20 (m, 20H, Ar–H); UV-Vis (λ max, nm): 345; Anal. Calculated for C44H28Cl2N8O8Te: C 53.10, H 2.84, N 11.26, Found: C 53.13, H 2.90, N 11.33%.
Bis[(2-(5-(2-Chlorophenyl)-3-(4-methoxyphenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)] telluride (15)
Light-yellow crystalline; Yield: 65%; M.p.: 87–85 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 11; Rf = 0.60 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3063 w, 2974 w, 2931 w, 2839 w, 1604 m, 1570 s, 1512 s, 1465 m, 1427 m, 1327 s, 1261 m, 1226 m, 1180 m, 1111 m, 1033 m, 1014 m, 976 s, 825 m, 756 m, 682 m, 578 m, 505 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 3.83 (s, 6H, 2OCH3), 5.81 (s, 2H, H4, and H4′), 7.43–8.22 (m, 20H, Ar–H); UV-Vis (λmax, nm): 341; Anal. Calculated for C44H28Cl2N8O10Te: C 51.45, H 2.75, N 10.91, Found: C 51.19, H 2.82, N 11.01%.
3.2.6. General Method for the Preparation of Diaryl Tellurium Trichlorides
(2-(3-(4-substituedphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium trichloride 16–21
Thionyl chloride (0.12 g, 1.00 mmol) in 15 mL of ethanol was added drop wisely to an ethanolic solution of diaryl ditellurides compounds (i.e., compounds 10, 11 or 12) (1.00 mmol) with stirring at room temperature for 30 minutes. A yellow precipitate was formed immediately. Recrystallization by ethanol gave an yellow solid of compounds 16–18.
(2-(3-(4-Bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium trichloride (16)
Yellow brown crystalline solid; 82 Yield: %; M.p.: 168–170 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 33; Rf (ethyl acetate- n-hexane) = 0.81; FT-IR (KBr) cm−1: 3059 w, 1604 s, 1570 s, 1465 s, 1438 s, 1138 s, 1311 s, 1273 s, 1211 s, 1180 s, 1157 s, 1041 m, 972 s, 850 m, 790 m, 717 m, 748 s, 717 m, 690 m, 659 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 5.95 (s, 2H, H4 and H4′), 7.45–8.20 (m, 20H, Ar-H); UV-Vis (λmax, nm): 400; Anal. Calculated for C21H11BrCl4N4O4Te: C 51.45, H 2.75, N 10.91, Found: C 51.19, H 2.82, N 11.01%.
(2-(3-(4-Methylphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium trichloride (17)
Yellow crystalline solid; 76 Yield: %; M.p.: 160–162 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 30; Rf (ethyl acetate- n-hexane) = 0.75; FTIR (KBr) cm−1: FT-IR (KBr) cm−1: 3065 m, 2930 w, 1631 m, 1590 m, 1463 m, 1433 m, 1390 m, 1327 s, 1275 m, 1216 m,1170 m, 1066 m, 1022 m, 971 s, 821 m, 756 s, 710 m, 687 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 2.20 (s, 6H, 2CH3), 5.90 (s, 2H, H4 and H4′), 7.45–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 375; Anal. Calculated for C22H14Cl4N4O4Te: C 38.64, H 2.06, N 8.19, Found: C 38.70, H 2.00, N 8.24%.
(2-(3-(4-Methoxyphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium trichloride (18)
Yellow crystalline solid; 79 Yield: %; M.p.: 155–156 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 24; Rf (ethyl acetate- n-hexane) = 0.70; FTIR (KBr) cm−1: 3060 w, 2911 w, 2835 w, 1600 s, 1568 s, 1511 m, 1462 m, 1425 s, 1324 s, 1265 m, 1230 m, 1195m, 1112 m, 1005 s, 914 m, 825 m, 759 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 1H NMR (500 MHz, DMSO-d6, δ /ppm): 3.86 (s, 3H, OCH3), 5.82 (s, 1H, H4), 7.45–8.22 (m, 10H, Ar–H); UV-Vis (λmax, nm): 370; Anal. Calculated for C22H14Cl4N4O4Te: C 51.45, H 2.75, N 10.91, Found: C 51.19, H 2.82, N 11.01%.
3.2.7. General Method for the Preparation of Diaryl Tellurium Triiodides
(2-(3-(4-Substitutedphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium triiodide 19–21
A solution of iodine (0.10 g, 0.78 mmol) in 10 mL of ethanol added to a solution of diaryl ditelluride compounds 10, 11, or 12 (0.78 mmol) in 20 mL ethanol with stirring at room temperature for 30 min gave a brown solid of compounds 19, 20, and 21, respectively.
(2-(3-(4-Bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium triiodide (19)
Yellowish brown crystalline solid; 73 Yield: %; M.p.: 147–159 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 30; Rf (ethyl acetate-n-hexane) = 0.45; FTIR (KBr) cm−1: FTIR (KBr) cm−1: 3055 w, 1591 s, 1460 s, 1439 s, 1391 s, 1268 m, 1214 s, 1177 m, 1100 m, 1065 m, 1105 m, 1025, 971 m, 882 s, 820 m, 756 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 5.96 (s, 2H, H4, and H4′), 7.45–8.22 (m, 20H, Ar–H); UV-Vis (λmax, nm): 390; Anal. Calculated for C21H11I3BrClN4O4Te: C 25.05, H 1.10, N 5.56, Found: C 25.09, H 1.17, N 5.63.01%.
(2-(3-(4-Methylphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium triiodide (20)
Yellow crystalline solid; 76 Yield: %; M.p.: 122–120 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 29; Rf (ethyl acetate-n-hexane) = 0.61; FTIR (KBr) cm−1: 3063 m, 2924 w, 1597 m, 1465 m, 1438 m, 1392 m, 1323 s, 1273 m, 1211 m,1172 m, 1064 m, 1026 m, 978 s, 821 m, 756 s, 709 m, 687 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 2.22 (s, 3H, CH3); 5.90 (s, 1H, H4), 7.46–8.20 (m, 10H, Ar-H); UV-Vis (λmax, nm): 395; Anal. Calculated for C22H14I3ClN4O4Te: C 27.58, H 1.50, N 5.95, Found: C 28.10, H 1.51, N 6.01%.
(2-(3-(4-Methoxyphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)tellurium triiodide (21)
Yellowish brown crystalline solid; 70 Yield: %; M.p.: 113–105 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 22; Rf(ethyl acetate-n-hexane) = 65; FTIR (KBr) cm−1: 3059 w, 2905 w, 2835 w, 1598 s, 1570 s, 1515 m, 1465 m, 1428 s, 1327 s, 1265 m, 1227 m, 1192 m, 1117 m, 1013 s, 915 m, 820 m, 755 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 3.8 (s, 6H, 2OCH3), 5.81 (s, 2H, H4, and H4′), 7.43–8.22 (m, 20H, Ar–H); UV-Vis (λmax, nm): 391; Anal. Calculated for C22H14I3ClN4O4Te: C 27.58, H 1.47, N 5.85, Found: C 27.62, H 1.41, N 6.94%.
3.2.8. General Method for the Preparation of Diaryl Tellurium Dichlorides
Bis[(2-(3-(4-substitutedphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium dichlorides 22–24
Thionyl chloride (0.12 g, 1.00 mmol) in 15 mL of ethanol was added drop wise to an ethanolic solution of diaryl tellurides compounds (i.e., compounds 13, 14, or 15) (1.00 mmol) with stirring at room temperature for 30 minutes. A yellow precipitate was formed immediately. Recrystallization by ethanol gave a yellow solid of compounds 22–24.
Bis[(2-(3-(4-bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium dichloride (22)
Yellowish orange crystalline solid; Yield: 83%; M.p.: 187–180 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 20; Rf = 0.60 (ethyl acetate-n-hexane); FTIR (KBr) cm−1: 3063 w, 1660 s, 1609 s, 1597, 1465 m, 1438 m, 1323 s, 1273 m, 1311 m, 1272 m, 1211 s, 1181 m, 1157 m, 1041 s, 986 s, 860 s, 790 m, 756 m, 719 m, 667 m, 655 m, 578 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 5.95 (s, 2H, H4, and H4′), 7.46–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 385; Anal. Calculated for C42H22Br2Cl4N8O8Te: C 42.18, H 1.85, N 9.37 Found: C 42.20, H 1.87, N 9.37%.
Bis[(2-(3-(4-methylphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium dichloride (23)
Yellow crystalline solid; Yield: 73%; M.p.: 104–106 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 21; Rf (ethyl acetate-n-hexane) = 0.58; FTIR (KBr) cm−1: 3063 w, 2927, 1600 s, 1570 s, 1485 s, 1438 m, 1323 s, 1273 m, 1211 m, 1172 m, 1064 s, 1007 m, 976 s, 820 m, 756 m, 709 m, 687 m, 582 m, 536 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 2.06 (s, 6H, 2CH3); 5.92 (s, 2H, H4, and H4′), 7.45–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 385; Anal. Calculated for C44H28Cl4N8O8Te: C 49.57, H 2.65, N 10.51, Found: C 49.61, H 2.69, N 10.58%.
Bis[(2-(3-(4-methoxyphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium dichloride (24)
Light-yellowish crystalline solid; Yield: 78%; M.p.: 181–183 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 26; Rf (ethyl acetate-n-hexane) = 0.60; FTIR (KBr) cm−1: 3063 w, 2928 w, 2843 w, 1604 s, 1570 s, 1521 m, 1465 m, 1431 m, 1330 s, 1261 m, 1226 m, 1180 m, 1033 m, 1014 m, 976 s, 829 m, 758 m, 682 m, 578 m; 1H NMR (500 MHz, DMSO-d6, δ /ppm): 3.86 (s, 6H, 2OCH3), 5.80 (s, 2H, 2H4), 7.45–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 385; Anal. Calculated for C44H28Cl4N8O10Te: C 48.12, H 2.57, N 10.20, Found: C 48.18, H 2.62, N 10.20%.
3.2.9. General Method for the Preparation of Diaryl Tellurium Diiodides
Bis[(2-(3-(4-substitutedphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium diiodides 25–27
A solution of iodine (0.10 g, 0.78 mmol) in 10 mL of ethanol added to a solution of diaryl tellurides compounds 13, 14, or 15 (0.78 mmol) in 20 mL ethanol with stirring at room temperature for 30 min gave a brown solid of compounds 25, 26, and 27, respectively.
Bis[(2-(3-(4-bromophenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium diiodides (25)
Yellowish brown crystalline solid; Yield: 73%; M.p.: 113–115 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 27; Rf (ethyl acetate-n-hexane) = 0.66; FTIR (KBr) cm−1: 3059 w, 1604 s, 1523 m, 1469 m, 1438 m, 1334 s, 1311 m, 1269 m, 1211 m, 1010 m, 972 m, 860 m, 748 m, 721 m, 686 m, 655; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 5.95 (s, 2H, H4 and H4′), 7.46–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 390; Anal. Calculated for C42H22I2Br2Cl2N8O8Te: C 27.52, H 1.68, N 5.84 Found: C 27.60, H 1.71, N 5.91%.
Bis[(2-(3-(4-methylphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium diiodides (26)
Yellowish brown crystalline solid; Yield: 77%; M.p.: 80–82 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 21; Rf(ethyl acetate-n-hexane) = 0.66; FTIR (KBr) cm−1: 3059 m, 2920 w, 1604 s, 1523 m, 1462 m, 1438 m, 1334, 1311 s, 1269 m, 1211 s, 1037 m, 1010 m, 972 m, 860, 748 s, 721 m, 585 m, 655 m, 578 m, 528 m, 447 m; 1H NMR (500 MHz, DMSO-d6, δ /ppm): 2.06 (s, 6H, 2CH3); 5.92 (s, 2H, H4, and H4′), 7.45–8.20 (m, 20H, Ar–H); UV-Vis (λmax, nm): 395; Anal. Calculated for C44H28I2Cl2N8O8Te: C 36.59, H 1.61, N 8.13, Found: C 37.04, H 1.66, N 8.15 %.
Bis[(2-(3-(4-methoxyphenyl)-5-(2-chlorophenyl)-1H-pyrazol-1-yl)-3,5-dinitrophenyl)]tellurium diiodides (27)
Yellowish brown crystalline solid; Yield: 66%; M.p.: 93–95 °C; Molar conductance (Λm, ohm−1 cm−1 mol−1): 19; Rf (ethyl acetate-n-hexane) = 0.60; FTIR (KBr) cm−1: 3056 w, 2928 w, 2840 w, 1600 s, 1572 s, 1521 m, 1465 m, 1430 m, 1334 s, 1260 m, 1226 m, 1180 m, 1032 m, 1016 m, 976 s, 829 m, 758 m, 682 m, 577 m; 1H NMR (500 MHz, DMSO-d6, δ/ppm): 3.83 (s, 6H, 2OCH3), 5.82 (s, 2H, H4, and H4′), 7.46–8.22 (m, 20H, Ar–H); UV-Vis (λmax, nm): 390; Anal. Calculated for C44H28I4Cl2N8O10Te: C 42.31, H 2.26, N 8.97, Found: C 42.39, H 2.31, N 9.02%.