Selective Hydrolysis of Transferrin Promoted by Zr-Substituted Polyoxometalates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrolysis of Transferrin by Zr-Substituted POMs
2.2. Interaction Between Transferrin and Zr-Substituted POMs
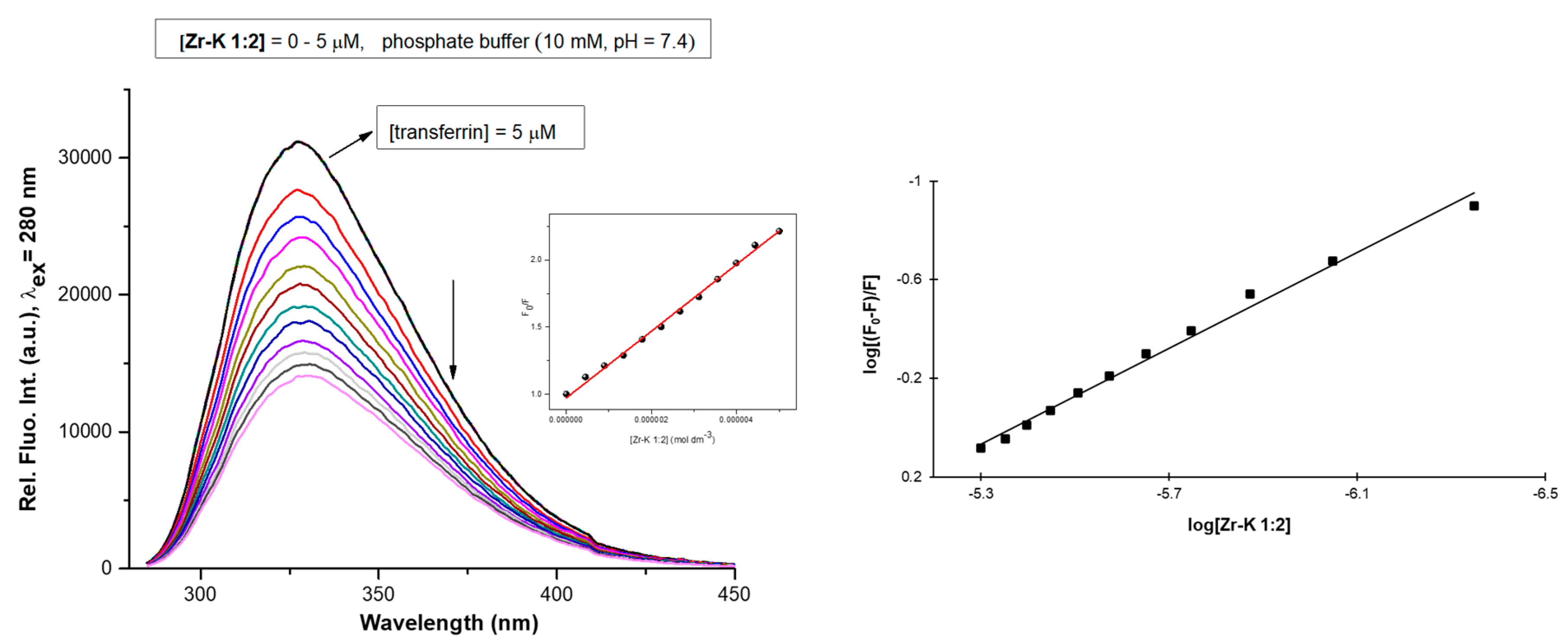
2.2.1. Tryptophan Fluorescence Quenching Studies
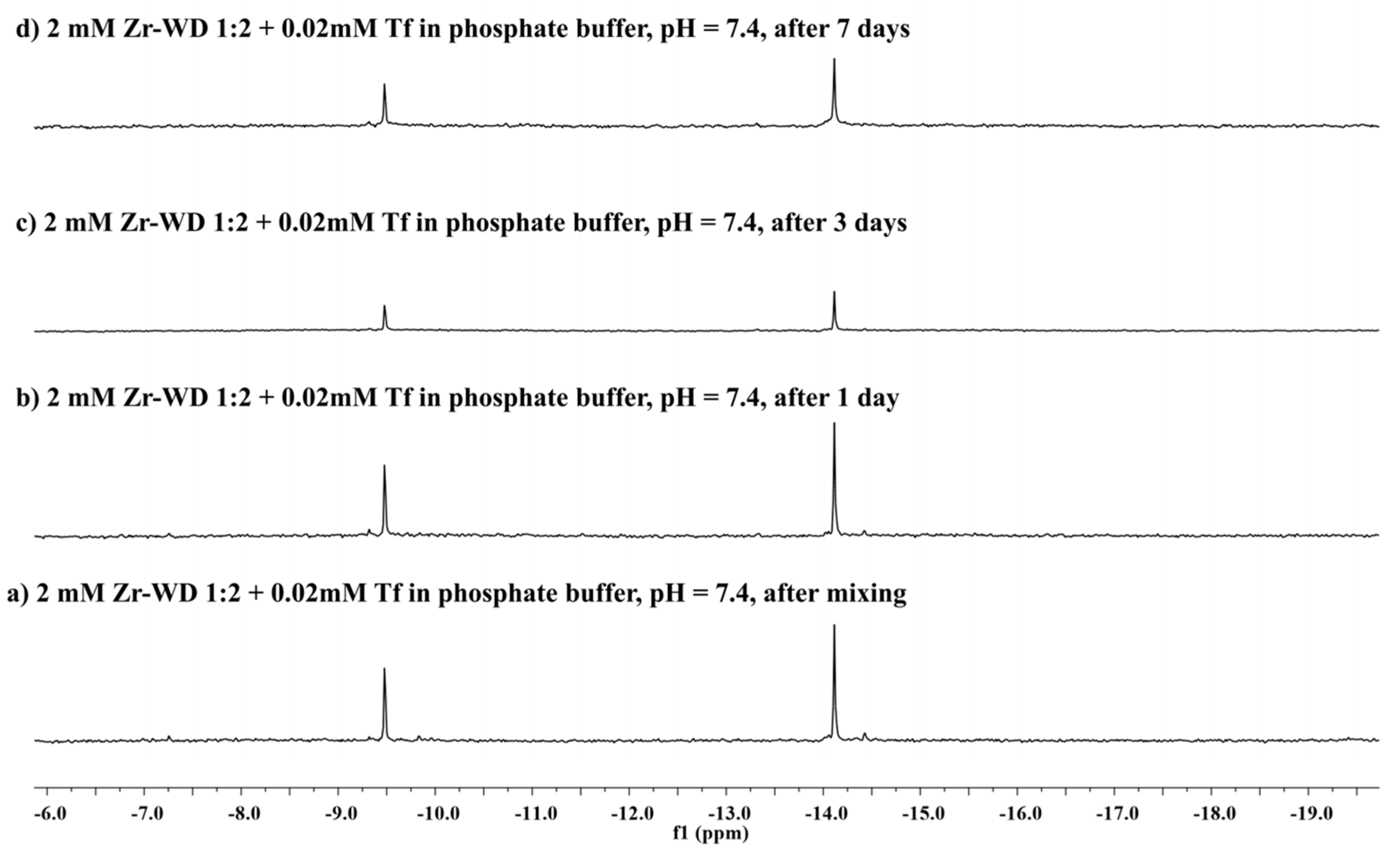
2.2.2. 31P-NMR Stability Study
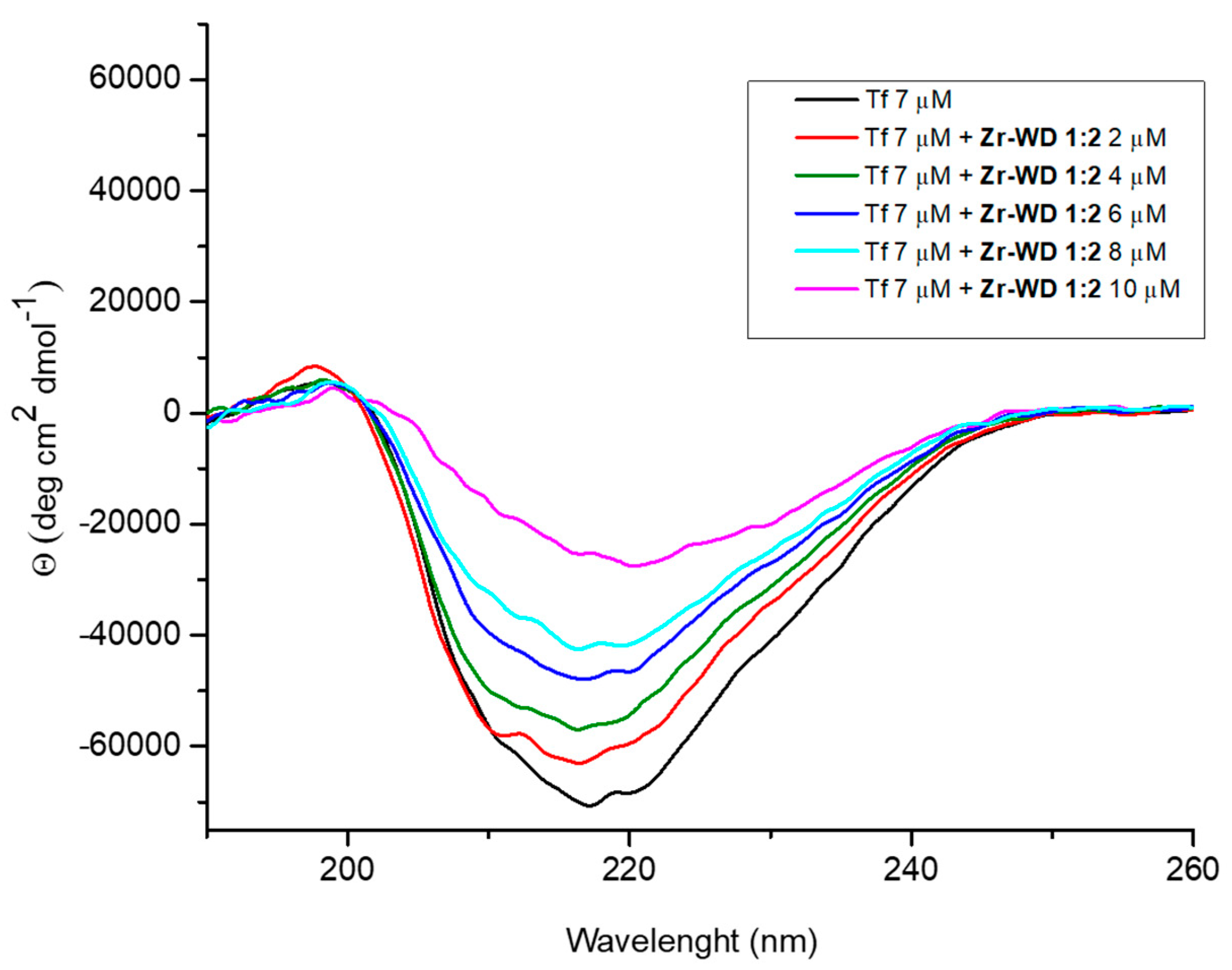
2.2.3. Circular Dichroism (CD) Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Hydrolisis Experiments
3.2.2. Fluorescence Spectroscopy
3.2.3. Circular Dichroism Spectroscopy
3.2.4. 31P-NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Su, S.-C.; Lin, C.-J.; Ting, C.-K. An effective hybrid of hill-climbing and genetic algorithm for 2D triangular protein structure prediction. Proteome Sci. 2011, 9, S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torsvik, V.; Ovreas, L.; Thingstad, T.F. Prokaryotic diversity-magnitude, dynamics, and controlling factors. Science 2002, 296, 1064–1066. [Google Scholar] [CrossRef] [Green Version]
- Piotrowski, C.; Sinz, A. Structural investigation of proteins and protein complexes by chemical cross-linking/mass spectrometry. In Integrative Structural Biology with Hybrid Methods. Advances in Experimental Medicine and Biology; Nakamura, H., Kleywegt, G., Burley, S.K., Markley, J.L., Eds.; Springer: Singapore, 2018; Volume 1105, pp. 101–121. [Google Scholar]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.-C.; Yates, J.R. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiatsiani, L.; Heck, A.J.R. Proteomics beyond trypsin. FEBS J. 2015, 282, 2612–2626. [Google Scholar] [CrossRef] [PubMed]
- Swaney, D.L.; Wenger, C.D.; Coon, J.J. Value of using multiple proteases for large-scale mass spectrometry-based proteomics. J. Proteome Res. 2010, 9, 1323–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristobal, A.; Marino, F.; Post, H.; Van den Toorn, H.W.P.; Mohammed, S.; Heck, A.J.R. Toward an optimized workflow for middle-down proteomics. Anal. Chem. 2017, 89, 3318–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giansanti, P.; Tsiatsiani, L.; Low, T.Y.; Heck, A.J.R. Six alternative proteases for mass spectrometry–based proteomics beyond trypsin. Nat. Protoc. 2016, 11, 993–1006. [Google Scholar] [CrossRef]
- Ly, H.G.T.; Absilis, G.; Janssens, R.; Proost, P.; Parac-Vogt, T.N. Highly amino acid selective hydrolysis of myoglobin at aspartate residues as promoted by Zirconium(IV)-substituted polyoxometalates. Angew. Chem. Int. Ed. 2015, 54, 7391–7394. [Google Scholar] [CrossRef]
- Walker, J.M. The Protein Protocols Handbook; Humana Press: Totowa, NJ, USA, 2002. [Google Scholar]
- Kaiser, R.; Metzka, L. Enhancement of cyanogen bromide cleavage yields for methionyl-serine and methionyl-threonine peptide bonds. Anal. Biochem. 1999, 266, 1–8. [Google Scholar] [CrossRef]
- Crimmins, D.L.; Mische, S.M.; Denslow, N.D. Chemical cleavage of proteins in solution. Curr. Protoc. Protein Sci. 2005, 41, 11.4.1–11.4.11. [Google Scholar] [CrossRef]
- Stroobants, K.; Moelants, E.; Ly, H.G.T.; Proost, P.; Bartik, K.; Parac-Vogt, T.N. Polyoxometalates as a novel class of artificial proteases: Selective hydrolysis of lysozyme under physiological pH and temperature promoted by a Cerium(IV) Keggin-type polyoxometalate. Chem. Eur. J. 2013, 19, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Stroobants, K.; Absillis, G.; Moelants, E.; Proost, P.; Parac-Vogt, T.N. Regioselective hydrolysis of human serum albumin by Zr(IV)-substituted polyoxotungstates at the interface of positively charged protein surface patches and negatively charged amino acid residues. Chem. Eur. J. 2014, 20, 3894–3897. [Google Scholar] [CrossRef] [PubMed]
- Sap, A.; Absillis, G.; Parac-Vogt, T.N. Selective hydrolysis of oxidized insulin chain B by a Zr(IV)-substituted Wells-Dawson polyoxometalate. Dalton Trans. 2015, 44, 1539–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sap, A.; Van Tichelen, L.; Mortier, A.; Proost, P.; Parac-Vogt, T.N. Tuning the selectivity and reactivity of metal-substituted polyoxometalates as artificial proteases by varying the nature of the embedded lewis acid metal ion. Eur. J. Inorg. Chem. 2016, 32, 5098–5105. [Google Scholar] [CrossRef]
- Sap, A.; Vandebroek, L.; Goovaerts, V.; Martens, E.; Proost, P.; Parac-Vogt, T.N. Highly selective and tunable protein hydrolysis by a polyoxometalate complex in surfactant solutions: A step toward the development of artificial metalloproteases for membrane proteins. ACS Omega 2017, 2, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Quanten, T.; De Mayaer, T.; Shestakova, P.; Parac-Vogt, T.N. Selectivity and reactivity of ZrIV and CeIV substituted Keggin type polyoxometalates towards cytochrome c in surfactants solutions. Front. Chem. 2018, 6, 372. [Google Scholar] [CrossRef]
- Ly, H.G.T.; Fu, G.; Kondinski, A.; Bueken, B.; De Vos, D.; Parac-Vogt, T.N. Superactivity of MOF-808 toward Peptide Bond Hydrolysis. J. Am. Chem. Soc. 2018, 140, 6325–6335. [Google Scholar] [CrossRef]
- Moons, J.; de Azambuja, F.; Mihailovic, J.; Kozma, K.; Smiljanic, K.; Amiri, M.; Cirkovic Velickovic, T.; Nyman, M.; Parac-Vogt, T.N. Discrete Hf18 metal-oxo cluster as a heterogeneous nanozyme for site specific proteolysis. Angew. Chem. Int. Ed. 2020, 59, 9094–9101. [Google Scholar] [CrossRef]
- Loosen, A.; de Azambuja, F.; Smolders, S.; Moons, J.; Simms, C.; De Vos, D.; Parac-Vogt, T.N. Interplay between structural parameters and reactivity of Zr6-based MOFs as artificial proteases. Chem. Sci. 2020. [Google Scholar] [CrossRef]
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Rompel, A. The use of polyoxometalates in protein crystallography—An attempt to widen a well-known bottleneck. Coord. Chem. Rev. 2015, 299, 22–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krivosudský, L.; Roller, A.; Rompel, A. Tuning the interactions of decavanadate with thaumatin, lysozyme, proteinase K and human serum proteins by its coordination to a pentaaquacobalt(ii) complex cation. New J. Chem. 2019, 43, 17863–17871. [Google Scholar] [CrossRef] [Green Version]
- Bijelic, A.; Dobrov, A.; Roller, A.; Rompel, A. Binding of a Fatty Acid-Functionalized Anderson-Type Polyoxometalate to Human Serum Albumin. Inorg. Chem. 2020, 59, 5243–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hungerford, G.; Hussain, F.; Patzke, G.R.; Green, M. The photophysics of europium and terbium polyoxometalates and their interaction with serum albumin: A time-resolved luminescence study. Phys. Chem. Chem. Phys. 2010, 12, 7266–7275. [Google Scholar] [CrossRef]
- Zhang, G.; Keita, B.; Craescu, C.T.; Miron, S.; de Oliveira, P.; Nadjo, L. Molecular Interactions between Wells-Dawson Type Polyoxometalates and Human Serum Albumin. Biomacromolecules 2008, 9, 812–817. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, Y.; Zhang, G.; Yao, J.; Bassil, B.S.; Kortz, U.; Keita, B.; de Oliveira, P.; Nadjo, L.; Craescu, C.T.; et al. Molecular Interaction between a Gadolinium–Polyoxometalate and Human Serum Albumin. Eur. J. Inorg. Chem. 2009, 34, 5189–5193. [Google Scholar] [CrossRef]
- Stroobants, K.; Goovaerts, V.; Absillis, G.; Bruylants, G.; Moelants, E.; Proost, P.; Parac-Vogt, T.N. Molecular origin of the hydrolytic activity and fixed regioselectivity of a Zr(IV)-substituted polyoxotungstate as artificial protease. Chem. Eur. J. 2014, 20, 9567–9577. [Google Scholar] [CrossRef] [Green Version]
- Goovaerts, V.; Stroobants, K.; Absillis, G.; Parac-Vogt, T.N. Understanding the regioselective hydrolysis of human serum albumin by Zr(IV)-substituted polyoxotungstates using tryptophan fluorescence spectroscopy. Inorganics 2015, 3, 230–245. [Google Scholar] [CrossRef] [Green Version]
- Ly, H.G.T.; Parac-Vogt, T.N. Spectroscopic study of the interaction between horse heart myoglobin and Zirconium(IV)-substituted polyoxometalates as artificial proteases. ChemPhysChem 2017, 18, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Vandebroek, L.; Van Meervelt, L.; Parac-Vogt, T.N. Direct observation of the Zr(IV) interaction with the carboxamide bond in a noncovalent complex between Hen Egg White Lysozyme and a Zr-substituted Keggin polyoxometalate. Acta Cryst. C 2018, C74, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, R.T.; Moore, S.A.; Chen, J.; Anderson, B.F.; Baker, H.; Luo, Y.; Bewley, M.; Smith, C.A.; Murphy, M.E.; Wang, Y.; et al. Two high-resolution crystal structures of the recombinant N-Lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry 1998, 37, 7919–7928. [Google Scholar] [CrossRef]
- Vincent, J.B.; Love, S. The binding and transport of alternative metals by transferrin. Biochim. Biophys. Acta 2012, 1820, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Collins-Wildman, D.L.; Kim, M.; Sullivan, K.P.; Plonka, A.M.; Frenkel, A.I.; Musaev, D.G.; Hill, C.L. Buffer-induced acceleration and inhibition in polyoxometalate—Catalyzed organophosphorus ester hydrolysis. ACS Catal. 2018, 8, 7068–7076. [Google Scholar] [CrossRef]
- Moons, J.; Van Rompuy, L.S.; Rodriguez, A.; Abdelhameed, S.A.M.; Simons, W.; Parac-Vogt, T.N. Hydrolysis of transferrin promoted by a cerium(IV)-Keggin polyoxometalate. Polyhedron 2019, 170, 570–575. [Google Scholar] [CrossRef]
- MacGillivray, R.T.A.; Mendez, E.; Sinha, S.K.; Sutton, M.R.; Lineback-Zins, J.; Brew, K. The complete amino acid sequence of human serum transferrin. Proc. Natl. Acad. Sci. USA 1982, 79, 2504–2508. [Google Scholar] [CrossRef] [Green Version]
- Goovaerts, V.; Stroobants, K.; Absillis, G.; Parac-Vogt, T.N. Eu(III) luminescence and tryptophan fluorescence spectroscopy as a tool for understanding interactions between hen egg white lysozyme and metal-substituted Keggin type polyoxometalates. J. Inorg. Biochem. 2015, 150, 72–80. [Google Scholar] [CrossRef]
- Goovaerts, V.; Stroobants, K.; Absillis, G.; Parac-Vogt, T.N. Molecular interactions between serum albumin proteins and Keggin type polyoxometalates studied using luminescence spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 18378–18387. [Google Scholar] [CrossRef]
- Luong, T.K.N.; Shestakova, P.; Mihaylov, T.T.; Absillis, G.; Pierloot, K.; Parac-Vogt, T.N. Multinuclear diffusion NMR spectroscopy and DFT modeling: A powerful combination for unraveling the mechanism of phosphoester bond hydrolysis catalyzed by metal-substituted polyoxometalates. Chem. Eur. J. 2015, 21, 4428–4439. [Google Scholar] [CrossRef]
- Vandebroek, L.; De Zitter, E.; Ly, H.G.T.; Conić, D.; Mihaylov, T.; Sap, A.; Proost, P.; Pierloot, K.; Van Meervelt, L.; Parac-Vogt, T.N. Protein-assisted formation and stabilization of catalytically active polyoxometalate species. Chem. Eur. J. 2018, 24, 10099–10108. [Google Scholar] [CrossRef] [PubMed]
- Sap, A.; De Zitter, E.; Van Meervelt, L.; Parac-Vogt, T.N. Structural characterization of the complex between hen egg-white lysozyme and Zr(IV)-substituted Keggin polyoxometalate as artificial protease. Chem. Eur. J. 2015, 21, 11692–11695. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Li, H.; Sadler, P.J. Transferrin as a metal ion mediator. Chem. Rev. 1999, 99, 2817–2842. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.L.S.; Miles, A.J.; Whitmore, L.; Wallace, B.A. Distinct circular dichroism spectroscopic signatures of polyproline II and unordered secondary structures: Applications in secondary structure analyses. Protein Sci. 2014, 23, 1765–1772. [Google Scholar] [CrossRef] [Green Version]
- Kato, C.N.; Shinohara, A.; Hayashi, K.; Nomiya, K. Syntheses and X-ray crystal structures of Zirconium(IV) and Hafnium(IV) complexes containing monovacant Wells-Dawson and Keggin polyoxotungstates. Inorg. Chem. 2006, 45, 8108–8119. [Google Scholar] [CrossRef] [PubMed]
- Contant, R.; Klemperer, W.G.; Yaghi, O. Chapter 3: Early transition metal poly oxoanions 18. Potassium Octadecatungstodiphosphates(V) and related lacunary compounds. In Inorganic Syntheses; Ginsberg, Ed.; John Wiley & Sons: New York, NY, USA, 1990; Volume 27, pp. 104–107. [Google Scholar]
- Carabineiro, H.; Villanneau, R.; Carrier, X.; Herson, P.; Lemos, F.; Ribeiro, F.R.; Proust, A.; Che, M. Zirconium-substituted isopolytungstates: Structural models for zirconia-supported tungsten catalysts. Inorg. Chem. 2006, 45, 1915–1923. [Google Scholar] [CrossRef]
- Gaunt, A.J.; May, I.; Collison, D.; Holman, K.T.; Pope, M.T. Polyoxometal cations within polyoxometalate anions. Seven-coordinate uranium and zirconium heteroatom groups in [(UO2)12(μ3-O)4(μ2-H2O)12(P2W15O56)4]32− and [Zr4(μ3-O)2(μ2-OH)2(H2O)4(P2W16O59)2]14−. J. Mol. Struct. 2003, 656, 101–106. [Google Scholar] [CrossRef]
- Cleveland, D.W.; Fischer, S.G.; Kirschner, M.W.; Laemmli, U.K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J. Biol. Chem. 1977, 252, 1102–1106. [Google Scholar]
- Westermeier, R. Sensitive, quantitative, and fast modifications for coomassie blue staining of polyacrylamide gels. Proteomics 2006, 6, 61–64. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Zr-L 1:1, Zr-L 2:2, Zr-K 1:2, Zr-K 2:2, Zr-WD 1:2 and Zr-WD 4:2 are available from the authors. |

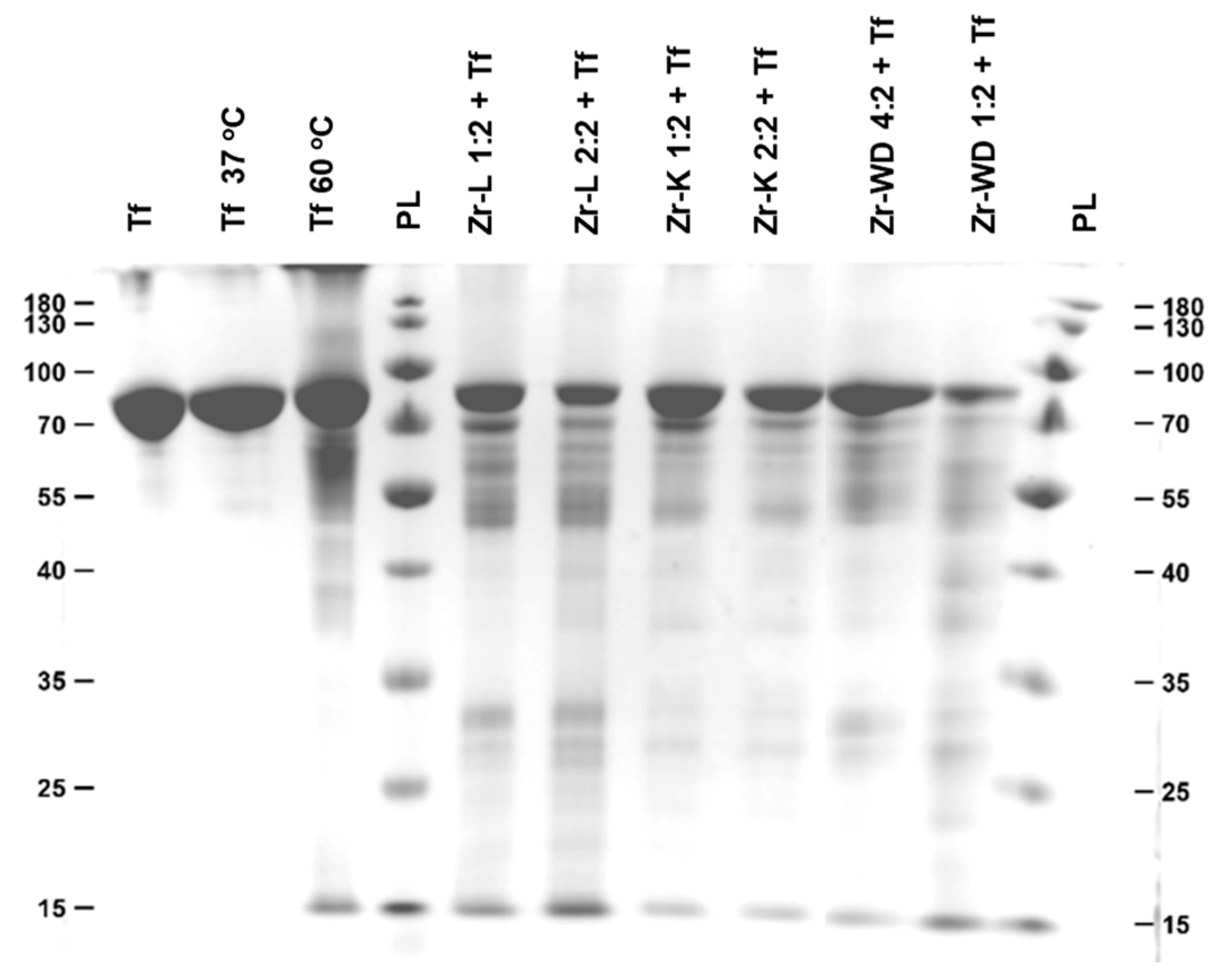
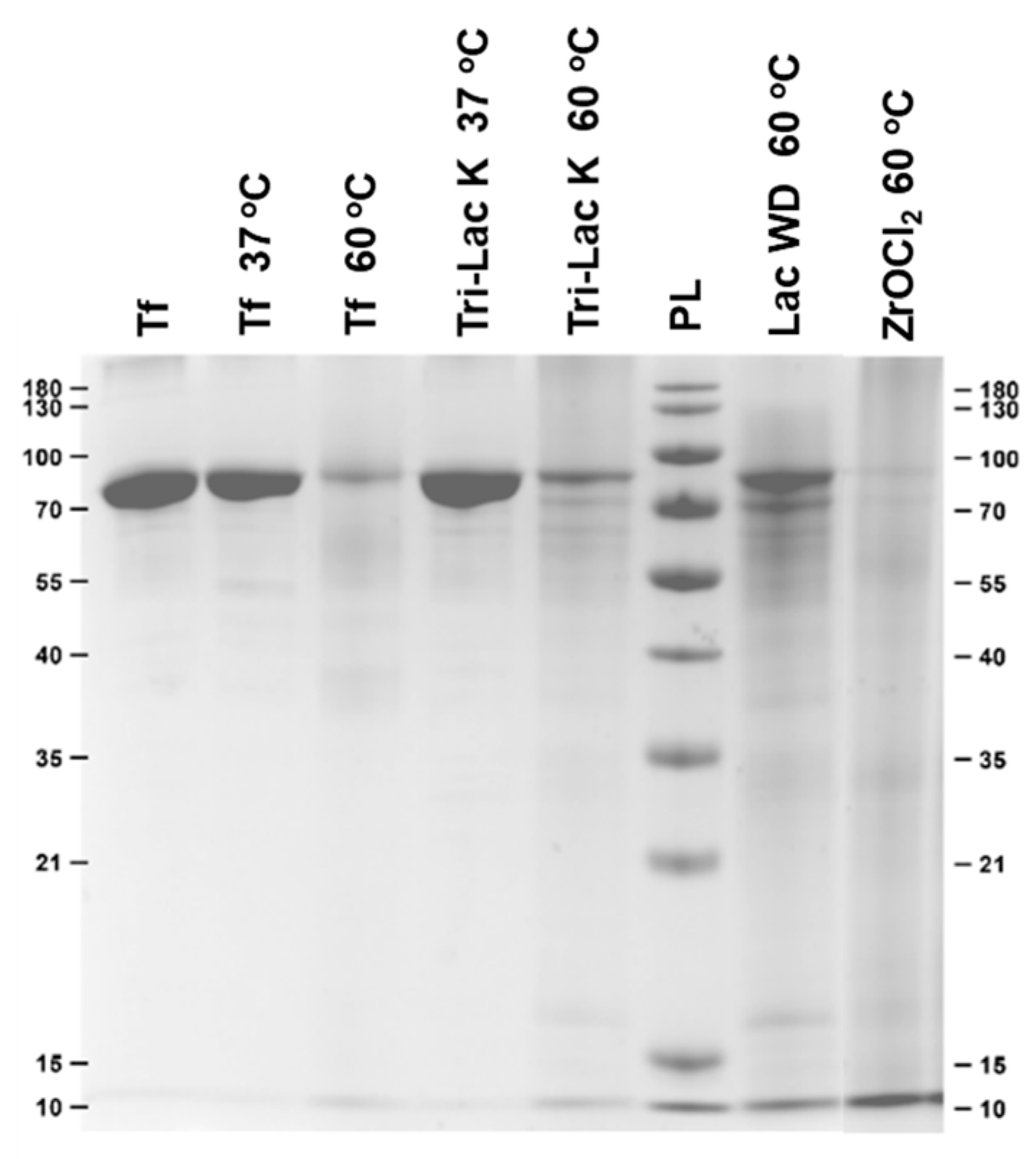
| POM | Hydrolysis Efficiencies (%) | |
|---|---|---|
| After 3 Days | After 7 Days | |
| Zr-WD 1:2 | 79 | 97 |
| Zr-WD 4:2 | 64 | 92 |
| Zr-K 2:2 * | 45 | 82 |
| Zr-K 1:2 * | 48 | 73 |
| Zr-L 1:1 | 48 | 80 |
| Zr-L 2:2 | 50 | 81 |
| Tri-Lac K 37 °C | / | 0 |
| Tri-Lac K 60 °C | / | ~4 |
| Lac WD 60 °C | / | ~6 |
| POM | Hypochromic Effect (%) | n | Ka (M−1) | R2 | Hydrolysis (%) After 7 Days |
|---|---|---|---|---|---|
| Zr-WD 1:2 | 83.04 | 1.52 | 5.71 × 108 | 0.9920 | 97 |
| Zr-WD 4:2 | 80.92 | 1.30 | 3.02 × 107 | 0.9805 | 92 |
| Zr-K 2:2 * | 66.92 | 1.13 | 2.17 × 106 | 0.9878 | 82 |
| Zr-K 1:2 * | 53.75 | 0.97 | 1.67 × 105 | 0.9921 | 73 |
| Zr-L 1:1 | 36.60 | 0.97 | 8.22 × 104 | 0.9975 | 80 |
| Zr-L 2:2 | 30.84 | 0.85 | 1.61 × 104 | 0.9721 | 81 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Rompuy, L.S.; Savić, N.D.; Rodriguez, A.; Parac-Vogt, T.N. Selective Hydrolysis of Transferrin Promoted by Zr-Substituted Polyoxometalates. Molecules 2020, 25, 3472. https://doi.org/10.3390/molecules25153472
Van Rompuy LS, Savić ND, Rodriguez A, Parac-Vogt TN. Selective Hydrolysis of Transferrin Promoted by Zr-Substituted Polyoxometalates. Molecules. 2020; 25(15):3472. https://doi.org/10.3390/molecules25153472
Chicago/Turabian StyleVan Rompuy, Laura S., Nada D. Savić, Alvaro Rodriguez, and Tatjana N. Parac-Vogt. 2020. "Selective Hydrolysis of Transferrin Promoted by Zr-Substituted Polyoxometalates" Molecules 25, no. 15: 3472. https://doi.org/10.3390/molecules25153472
APA StyleVan Rompuy, L. S., Savić, N. D., Rodriguez, A., & Parac-Vogt, T. N. (2020). Selective Hydrolysis of Transferrin Promoted by Zr-Substituted Polyoxometalates. Molecules, 25(15), 3472. https://doi.org/10.3390/molecules25153472








