In this section, the sum of nitro group substituted on carbon position of pyrazole ring in mononitropyrazoles, binitropyrazoles, and trinitropyrazoles are one, two, and three, respectively. For example, mononitropyrazole represents that only one C position in pyrazole ring is substituted by the nitro group.
2.1. Mononitropyrazoles and Their Derivatives
Mononitropyrazoles and their derivatives due to their energetic property are favored by people in many fields, such as medicine, pesticide, energetic material and so on. Among them, 3-nitropyrazole (3-NP), 4-nitropyrazole (4-NP), 1-methyl-3-nitropyrazole (3-MNP), and 1-methyl-4-nitropyrazole (4MNP) are typical examples, which are commonly used as energetic materials and intermediates for further products of other energetic materials because they contain only one nitro group and have relatively low energy. The syntheses of these compounds is often facile and can meet the development requirements of green chemistry.
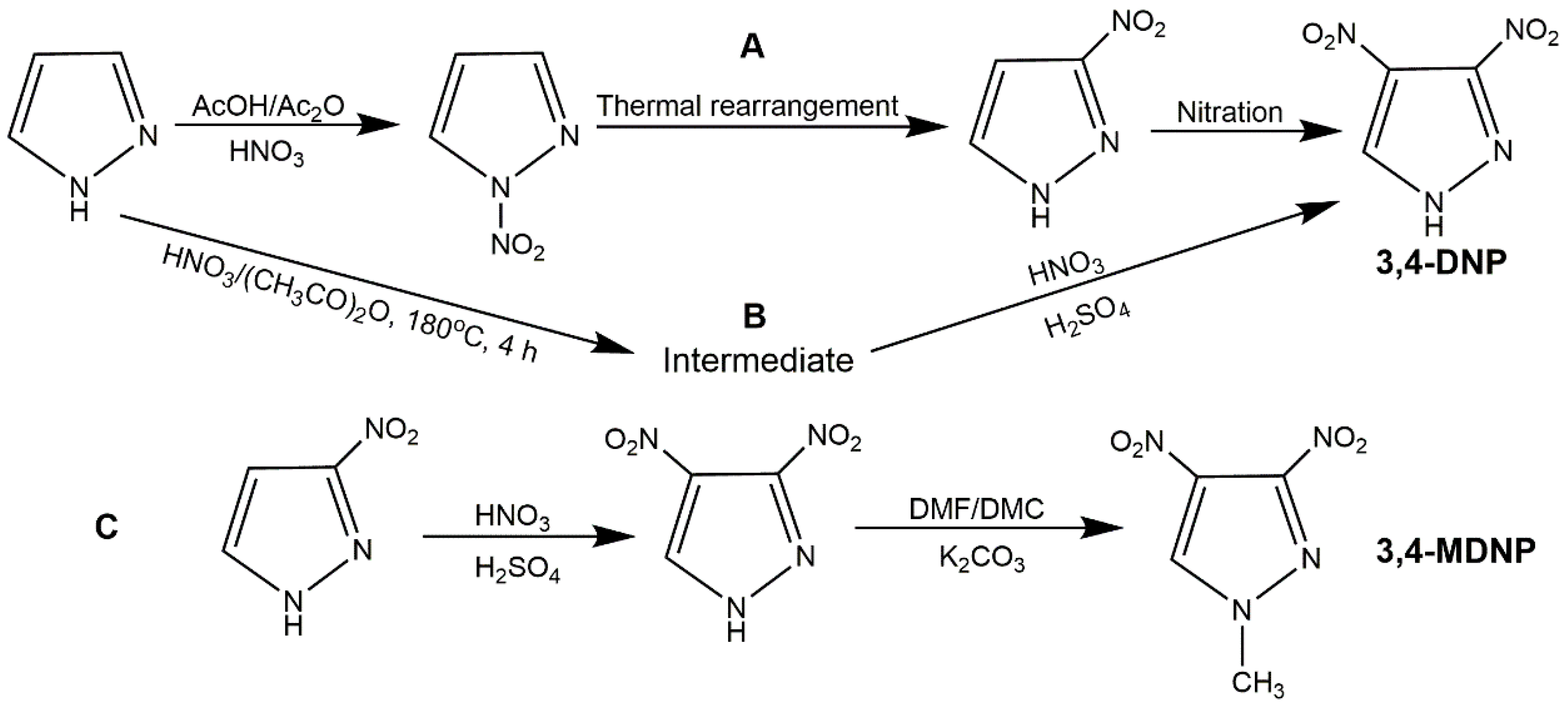
As a typical heterocyclic compound, 3-NP is an important intermediate in the synthesis of pyrazole-based compounds such as 3,4-dinitropyrazole (DNP) and other new explosives [
36,
38]. In 1970, Habraken and co-authors [
39] firstly reported synthesis of 3-NP by dissolving
N-nitropyrazole in anisole for 10 h at 145 °C. Later, Verbruggen et al. [
40] synthesized 3-NP from diazomethane and chloronitroethylene by one-step cyclization, while this reaction was high riskful due to the extremely vivacious raw materials. Nowadays, the main synthesis method of 3-NP was a two-step reaction, that is, nitration of pyrazole to obtain
N-nitropyrazole and then rearrangement of
N-nitropyrazole in organic solvent to acquire 3-NP (
Figure 1, Scheme A). The nitration agents could be HNO
3/H
2SO
4 or HNO
3/Ac
2O/HAc, and the organic solvent for rearrangement could be anisole,
n-octanol and benzonitrile [
41,
42,
43]. Among these solvents, benzonitrile was always preferred to be the rearrangement medium since anisole could require an excessively long time and
n-octanol would lead to poor-quality product. In 2014, Zhao et al. [
44] reported one convenient and green approach to synthesizing the 3-NP. They chose the oxone as the nitration agents of 3-aminopyrazole and water as the solvent (
Figure 1, Scheme B). This approach owns some advantages over the previous approach: simple operation, safety, economical reagents, the use of water as solvent, and mild conditions. As shown in
Figure 1, 3-MNP is one of the most important derivatives of 3-NP. Its synthesis is mainly accomplished by nitrated 1-methylpyrazole with various nitration agents. Katritzky et al. [
45] added 1-methylpyrazole to trifluoroacetic anhydride for 1 h in ice bath, and then concentrated nitric acid was added in the solution. After stirring for 12 h, and evaporation of trifluoroacetic anhydride and nitric acid, the 3-MNP could be obtained (
Figure 1, Scheme C). In 2013, Ravi et al. [
46] proposed that 1-methylpyrazole could reacted with silicon oxide-bismuth nitrate or silicon dioxide-sulfuric acid-bismuth nitrate in tetrahydrofuran (THF) to produce 3-MNP (
Figure 1, Scheme D), this facile route is a synthetic method of low toxicity, high efficiency, and green environmental protection. In addition, metal salts of 3-NP expand its derivatives. Li et al. [
42] prepared the metal Cu(II) salt and basic Pb salt of 3-NP, by dissolving 3-NP in NaOH solution and reacting with the CuSO
4·5H
2O solution and Pb(NO
3)
2 solution, respectively (
Figure 1, Scheme E).
4-NP is an isomer of 3-NP with melting point of 163–165 °C, density of 1.52 g/cm
3, detonation velocity of 6.68 km/s and detonation pressure of 18.81 Gpa [
47]. Similar to 3-NP, 4-NP can be obtained by nitro group rearrangement. As Rao et al. [
48] reported
N-nitropyrazole could be rearranged to 4-NP in sulfuric acid at room temperature (
Figure 2, Scheme A). Ravi et al. [
49] synthesized 4-NP in THF with 4-iodopyrazole as raw material, fuming HNO
3 as nitration agents, octahedral zeolite or silica as solid catalyst (
Figure 2, Scheme B). Li et al. [
50] reported one-pot two steps route that pyrazole could be nitrated to 4-NP by fuming HNO
3 (90%)/fuming H
2SO
4 (20%) (
Figure 2, Scheme C). 4-MNP is another important derivative of nitropyrazole with the similar performance to 3-MNP (
Table 1). In 2015, Corte et al. [
51] reported that 4-MNP could be synthesized by adding sodium hydride and iodomethane into the THF solution of 4-NP at room temperature for overnight. Ioannidis et al. [
52] improved the method by adding sodium hydride and iodomethane to the acetonitrile solution of 4-NP under nitrogen protection for 16 h. However, it is dangerous to handle sodium hydride due to its high chemical reaction activity which can easily cause combustion and explosion, limiting the further application of this method. Han et al. [
53] simplified the above method and replaced sodium hydride with potassium carbonate. They added potassium carbonate and iodomethane to the
N,
N-dimethylformamide (DMF) solution of 4-NP at 25 °C for 14 h. This method not only reduces the risk in the process, but improves the reaction yield (80–98%).
Table 1 shows the energetic performances of the four typical monopyrazoles. We can see that these energetic performances of pyrazole-based compounds are not satisfying, especially the detonation properties and the nitrogen content. So, these nitropyrazoles are always used as intermediates for the preparation of novel high-performance energetic materials. Furthermore, it is also necessary to explore new high performances energetic materials based on mononitropyrazoles. For example, Deng et al. [
54] prepared 5-methyl-4-nitro-1
H-pyrazol-3(2
H)-one (MNPO) and its energetic salts, showing better performances than these above mononitropyrazoles.
The introduction of a polynitromethyl group into a heterocyclic compound is interesting for energetic field, because it can increase the oxygen content and improve the energetic properties of energetic material. Generally, the incorporation of a polynitromethyl group (trinitromethyl and dinitromethyl) to nitropyrazoles is essentially equivalent to introducing at least one -NO
2 (since one -NO
2 is used for the complete oxidation of the C atom in -CH
3) [
56]. For the trinitromethyl group, it can be incorporated into N position or C position of nitropyrazoles with different energetic properties. The N-H bond of nitropyrazole is relatively active which could provide a reaction site for functionalization easily. In 2014, Yin et al. [
57] obtained the carbon and nitrogen functionalization of nitropyrazole with
N-trinitroethylamino group (
Figure 3, Scheme A). Thereby, 4-NP reacted with NH
2OSO
3H acid and K
2CO
3 to accomplish amination, and after functionalization of amino group, the 1-amino-4-nitropyrazole underwent the Mannich reaction with trinitroethanol to get 4-nitro-
N-(2,2,2-trinitroethyl)-1
H-pyrazol-1-amine (
1). In 2015, Dalinger et al. [
58] prepared and characterized a nitropyrazole bearing a trinitromethyl moiety at N atom, 4-nitro-1-(trinitromethyl)-pyrazoles (
2). They synthesized the target compound by a destructive nitration of 4-nitro-1-acetonpyrazole with a mixture of concentrated HNO
3 and H
2SO
4 (
Figure 3, Scheme B). Although the compound 1 was successfully synthesized, the yield was very low (28%) and this process was comparatively too time-consuming (15 d). To explore new high-performance EM, several
C-trinitromethyl-substituted mononitropyrazoles have been reported. In 2018, Zhang and co-authors [
56] first synthesized the
C-trinitromethyl-substituted nitropyrazole (
Figure 4, Scheme A). The reaction of 3-pyrazolecarbaldehyde oxime with N
2O
4 produced the 3-trinitromethylpyrazole and 1-nitro-3-trinitromethylpyrazole (
3). They found that the increasing N
2O
4 concentration could improve the proportion of
3 and 3-trinitromethylpyrazole reacting with N
2O
4 also form
3, indicating N
2O
4 enable nitrate the N position of pyrazole. After the introduction of trinitromethyl group on C position, the 4-nitro-3-trinitromethylpyrazole (
4) could be obtained with fuming nitric acid and oleum by -NO
2 rearrangement of
3 or nitration of 3-trinitromethylpyrazole. In 2019, Xiong et al. [
59] further designed 3-Trinitromethyl-4-nitro-5-nitramine-1
H-pyrazole (
5). It was notable that the yield of
5 could improve with the concentration of HNO
3 increasing in the last nitration step of Scheme B (
Figure 4). For the dinitromethyl group, Semenov et al. [
60] prepared the 4-nitro-1-dinitromethylpyrazole by nitrating 4-nitro-1-acetonylpyrazole using H
2SO
4/H
2O mixture, and while the yield was low and it was not investigated as energetic material. In 2019, Pang et al. [
61] introduced the dinitromethyl group into nitropyrazole and developed the salt, hydrazinium 5-nitro-3-dinitromethyl-2
H-pyrazole (
6), according to Scheme A in
Figure 5. In 2020, Cheng et al. [
62] synthesized 3-nitro-4-dinitromethyl-2
H-pyrazole (
7) and its salts, further exploring the application of dinitromethyl group in mononitropyrazolle.
Table 2 shows the energetic properties of the polynitromethyl-substituted mononitropyrazoles and salts compared with TNT and RDX. All the density of the derivatives of mononitropyrazole was higher than TNT and close to that of RDX, especially
7a showed the highest density.
3 and
5 owned the desirable detonation properties, while exhibited poor safety. It was notable that
C-trinitromethyl-substituted derivatives owned higher heat of formation than those of
N-trinitromethyl-substituted derivatives, and the derivatives with dinitromethyl group owned lower heat of formation than derivatives with trinitromethyl group. Most of the neutral derivatives hold low decomposition temperatures owing to the instability of the polynitromethyl moiety. Compound
4 had the highest decomposition temperature possibly because of the strong intermolecular hydrogen bonding interactions. By comparing
4 and
5, we can see the nitramino group could further increase the power with low sensitivities. For the salts of compound
7,
7d with high detonation properties (comparing with RDX) and low sensitivities could serve as a promising candidate as a new high energy density oxidizer.
Connecting nitropyrazoles with nitrogen-rich compounds (including tetrazole, triazole, furazan, tetrazine, triazine, and others) has attracted more interest in many fields, it also be an effective approach to increasing the content of nitrogen and getting new high-performance energetic materials. In 2015, Yin et al. [
63] synthesized energetic salts based on
N-methyl 6-nitropyrazolo[3,4-
d][1,2,3]triazol-3(4
H)-olate in a similar manner exhibiting good detonation performance with relatively low sensitivities. In 2016, Dalinger et al. [
64] synthesized and investigated systematically a series of 1- and 5-(pyrazolyl)tetrazole amino and nitro derivatives which could be components of dyes and luminophores, and high-energy materials. Some of them were always used as intermediates due to their poor energetic properties. In 2017, Zyuzin et al. [
65] introduced the 2,2-bis(methoxy-
NNO-azoxy)ethyl group to nitropyrazoles to increase the hydrogen content for some special application (gun propellants, solid rocket propellants and others). The derivatives of 3-NP and 4-NP showed high heat of formation, while the oxygen balances and calculated detonation velocity were not ideal. Then, Zyuzin et al. [
66] further introduced the trinitromethyl moiety owning the most oxygen-rich block into the combination of tetrazole and pyrazole rings to obtain oxygen-balanced energetic materials with high nitrogen content (
8–
11) (
Figure 6). In 2019, Tang et al. [
67] developed several compounds and salts based 3,5-diamino-4-nitropyrazole functionalizing the with tetrazole group and triazine group (
12–
15) (
Figure 7). As shown in
Table 3, all the compounds had high density, high nitrogen content and good detonation properties, while the thermal stability of
12–
15 was better than that of
8–
11. In particular, the derivatives
12–
15 showed excellent insensitivities. In addition, most compounds owned positive and high heat of formation, but the presence of water molecules in
13a result in its negative heat of formation. Considering the low sensitivities, good detonation properties, and high thermal stabilities, these derivatives with nitrogen-rich groups may be the candidates of insensitive high energetic materials.
Moreover, nitrogen-rich heterocycles with a nitramino moiety could exhibit better performance than the corresponding nitro-substituted analogs as above mentioned [
59,
68]. In 2019, Shreeve and her group [
69] reported a green synthetic route for high-performance nitramino nitropyrazoles.
Figure 8 depicted the synthesis of corresponding derivatives, among them the 3,5-dinitramino-4-nitropyrazole (
16) was quite sensitive to mechanical stimulation. From
Table 4, the compound
16b showed promising properties with a high density (1.87 g·cm
−3), good detonation properties (
D of 9.58 km·s
−1 and
P of 38.5 GPa), decomposition temperature of 194 °C, and acceptable sensitivities. Xu et al. [
70] introduced nitramino and triazole groups into mononitropyrazole to construct multiple hydrogen bonds (
17), and synthesized the 4-nitro-3,5-bis(1
H-1,2,4-triazol-3-nitramino)-1
H-pyrazole (
19) and its ionic derivatives (
19a–
i) as shown in
Figure 9.
Table 4 also showed their energetic properties. Compound
17 had the highest decomposition temperature (353.6 °C) and excellent low sensitivity (IS > 40, FS > 360), indicating it could be used as heat-resistant insensitive explosive. The compounds (
18–
19i) exhibited moderate detonation properties, high positive heat of formation and ideal insensitivities which had great potential application in green and safe energetic materials. Ma et al. [
71] also fused nitropyrazole with triazine and nitramino groups, and prepared a series of salts based on compounds
20 and
21 (
Figure 10). These compounds owned high thermal stability and excellent insensitive properties because of the existence of triazine ring.
In summary, most of mononitropyrazoles and their derivatives owned relatively low thermal properties and detonation properties. They are always used as intermediates for novel complicated energetic materials. The introduction of polynitromethyl group can improve the oxygen balance efficiently, while have a little influence on the heats of formation. The nitramino group and nitrogen-rich heterocyclic can enhance the detonation properties, improve the safety, and increase the heats of formation of mononitropyrazoles. The choice of solvent and nitrification in synthesis routes should be more environmental and facile.
2.2. Dinitropyrazoles and Their Derivatives
Dinitropyrazoles own higher density and better detonation performance than mononitropyrazoles attributing to one more nitro group. The typical dinitropyrazoles include 3,4-dinitropyrazole (3,4-DNP), 3,5-dinitropyrazole (3,5-DNP), 1-methyl-3,4-dinitropyrazole (3,4-MDNP), 1-methyl-3,5-dinitropyrazole (3,5-MDNP), and 4-amino-3,5-dinitropyrazole (LLM-116).
3,4-DNP is a kind of white crystal, possessing higher density (1.87 g·cm
−3), lower melting point (86–88 °C), higher decomposition temperature (285 °C), higher detonation velocity (8.1 km·s
−1) and detonation pressure (29.4 GPa) than TNT. This compound was first reported by Biffin’s team in 1966 [
72]. In an earlier study, pyrazole, 4-NP, 3-nitro-4-cyanopyrazole and other raw materials have been investigated to prepare 3,4-DNP, while most of the methods did not satisfied industrialization due to complex process, high production cost or low yield [
45,
55,
73,
74,
75,
76]. At present, the three-step synthetic route as shown in
Figure 11 (Scheme A), and the two-step route (Scheme B) are the most widely used [
77,
78,
79,
80]. 3,4-MDNP is a typical thermal stability nitropyrazole, exhibiting stable thermodynamic state at 300 °C. Its melting point and density are lower than those of 3,4-DNP (20–23 °C, 1.67 g·cm
−3), and 3,4-DNP shows low detonation velocity (7.76 km s
−1) and detonation pressure (25.57 GPa) due to the introduction of methyl group. It has potential application in liquid explosive, which can reduce the melting point of liquid phase carrier in castable explosive [
32]. Recently, Ravi et al. [
73] had synthesized 3,4-MDNP by nitrating 1-methylpyrazole or 1-methyl-3-nitropyrazole with montmorillonite (K-10) and Bi(NO
3)
3, while this method was high cost and the products were difficult to separate. Li et al. [
81] reacted 3,4-DNP and dimethyl carbonate (DMC) in DMF with K
2CO
3 as catalyst, then, his group further synthesized 3,4-MDPN with 3-NP as raw material (
Figure 11, Scheme C) [
82]. In this method, DMC was used as methylation agent and the yield of methylation was high (95.6%), which could meet the requirement of green chemistry. As 3,5-DNP with a melting point of 173–174 °C and density of 1.80 g·cm
−3, the decomposition temperature of 316.8 °C owns higher detonation properties than 3,4-DNP (7.76 km·
−1 and 25.57 GPa). Moreover, 3,5-DNP is relatively stable because of the symmetrical molecular distribution, it can be used as a simple explosive or as a key intermediate in the synthesis of insensitive explosives [
55]. Generally, the starting materials for preparing 3,5-DNP could be pyrazole and 3-NP. Wang et al. [
83] nitrated 3-NP to get 1,3-dinitropyrazole, then 1,3-dinitropyrazole was reacted with NH
3 in PhCN to produce the ammonium salt of 3,5-DNP. After neutralization with hydrochloric acid, the 3,5-DNP could be obtained (
Figure 12, Scheme A). Liu et al. [
28] also nitrated 3-NP, and rearranged 1,3-dinitropyrazole to get 3,5-DNP (
Figure 12, Scheme B). For pyrazole as starting material, 3,5-DNP was always prepared by a four-step route (nitration of pyrazole, rearrangement of
N-nitropyrazole, nitration of 3-NP, and rearrangement of 1,3-dinitropyrazole). 3,5-MDNP owns the similar energetic properties with 3,4-MDNP, while it has a higher melting point (about 60 °C). Moreover, 3,5-MDNP could be synthesized by methylation of 3,5-DNP [
84]. However, most methylation agents were extremely toxic, thus searching for a green methylation agent would be the key factor.
LLM-116 is a powerful and insensitive explosive, its energy is 90% of HMX and its impact sensitivity is extremely low [
55,
85]. It was first synthesized by the Lawrence Livermore National Laboratory (LLNL) in 2001, and many studies were performed to assess its synthesis in the following years. Wang et al. [
86] utilized vicarious nucleophilic substitution (VNS) of 3,5-DNP and trimethylhydrazine iodideto (TMHI) to prepare LLM-116 with a yield of 60%, while the toxic TMHI was the main factors restricting wide application of this method. In 2014, Stefan et al. [
87] developed four synthetic routes of LLM-116, using 4-NP, 3,5-dimethylpyrazole, 3,5-DNP and 4-chloropyrazole as starting materials, respectively (
Figure 13, Scheme A–D).
Table 5 shows the comparison of the four routes. The synthesis of Scheme D was simple and its yield was high, which was suitable for industrialization. Zhang et al. [
88] also used 4-chloropyrazole as a starting material to synthesize LLM-116 with an overall yield of 65%.
In addition, 4-Chloro-3,5-dinitropyrazole was a useful intermediate in the preparation of various 3,5-DNP [
89], owning good reactivity towards nucleophiles. He et al. [
90] synthesized a series of 3,5-DNP derivatives based on 4-chloro-3,5-dinitropyrazole and 1-methyl-4-chloro-3,5-dinitropyrazole shown in
Figure 14. From
Table 6, all compounds exhibited better detonation properties than those of TNT, and these compounds owned better IS than RDX except compound
33. Compounds
26 and
28 had an especially good balance between physical properties and detonation properties as well as excellent insensitivity, making them potential replacement of RDX.
Energetic salts often possess superior properties comparing with non-ionic species since they always show lower vapor pressures, lower impact and friction sensitivities, and enhanced thermal stabilities [
19]. In addition to the derivatives mentioned above, Klapötke group [
26] developed the ionic salts of 3,4-DNP and 3,5-DNP shown in
Figure 15, and these salts were extremely insensitive in
Table 7. Comparing with 3,4-DNP,
36 and
38 owned much lower decomposition temperatures, similar to that of
37,
39 and 3,5-DNP. Zhang et al. [
91] developed the ionic salts of LLM-116 with several nitrogen-rich cations as shown in
Figure 16. These compounds showed extraordinary insensitivity to impact (>60 J), as the detonation properties of
40i and
41k were comparable to those of TATB (31.15 GPa, 8.11 km·s
−1) (
Table 7).
N-oxidation of nitrogen-rich heterocycles including transformation of amino group to nitroso, azoxy, or nitro groups is another approach to designing HEDMs, which opens new avenues for the development of HEDMs [
92,
93]. The efforts to developing
N-oxidation of dinitropyrazoles have been made recently. Bölter et al. [
94] introduced -OH on
N atom of 3,4-DNP and 3,5-DNP, and obtained several salts (
Figure 17, Scheme A). From
Table 8, these compounds were less sensitive than RDX, and did not exhibited excellent detonation properties. Yin et al. [
95] synthesized a family of 4-amino-3,5-dinitro-1
H-pyrazol-1-ol (
44) and its ionic derivatives (
44a–
f) (
Figure 17, Scheme B). Except
44·H
2O, all the compounds (
44a–
f, and
45) with thermal decomposition temperatures (169–216 °C) shown good balance between detonation properties and insensitive properties as shown in
Table 8. Zhang et al. [
96] synthesized the 4-nitramino-3,5-dinitropyrazole by nitrating the -NH
2 of LLM-116, and prepared several energetic salts which exhibited good insensitivity and moderate detonation properties.
As mentioned above, polynitromethyl are considered to be more favorable groups to give remarkable improvements in densities and detonation properties of energetic materials. Especially the
N-trinitroethylamination of nitropyrazole is more available since it is stable to be handled safely. The
N-trinitroethylamination of dinitropyrazole was firstly proposed by Shreeve team [
57]. They obtained several
N-amino-dinitropyrazoles firstly, then these compounds underwent Mannich reactions with trinitroethanol to acquire the corresponding derivatives (
46–
50) (
Figure 18, Scheme A). It was noteworthy that 1-amino-3,5-dinitropyrazole and 1-amino-3,4-dinitro-5-cyanopyrazole failed to get the corresponding compounds due to the electron-withdrawing effect of substituent groups bonded to dinitropyrazole ring. In addition, they employed an alternative synthetic method to obtain 1,5-diamino-3,4-dinitropyrazole (
51) (
Figure 18, Scheme B) because attempted amination of this compound using TsONH
2 acid or NH
2OSO
3H failed. From
Table 9, although the azido-functionalized dinitropyrazole (
47) decomposed at 121 °C, compound
46 and
51 had high decomposition temperatures, and
47 and
50–
52 owned higher density than RDX. These indicated the introduction of an -NH
2 could enhance density. In addition,
N-trinitroethylamination of dinitropyrazole (
48–
50 and
52) shown high HOF and good detonation properties.
N-trinitromethyl moiety was introduced by Dalinger’s team [
58], they synthesized 3,4-dinitro--1-(trinitromethyl)-pyrazoles (
53) and 3,5-dinitro-1-(trinitromethyl)-pyrazoles (
54) with excellent physical and computational properties as shown in
Figure 19. They were a little less insensitive than the RDX and PETN, similar to
N-trinitroethylamination dinitropyrazoles shown in
Table 9. Fluorine and fluorinated functional groups are importantly promising substituents in the field of energetic materials [
97]. C(NO
2)
2F and C(NO
2)
2NF
2 moieties bring high energy, maintaining high density and good thermal property were incorporated into dinitropyrazole by fluorinated compound
55 (
Figure 19, Scheme C). The two compounds had high density (≥1.92 g·cm
−3), good oxygen balance (+2.55% for
57 and 0% for
56), and high detonation pressure and velocity [
98].
Dinitropyrazoles bearing other heterocycles are also interesting and notable. To obtain the melt-castable explosives with good compatibility, improved oxygen balance and moderate detonation properties, compound
58 incorporating both
N-trinitromethyl and
C-methyl substituents in addition to nitro groups was synthesized by Sheremetev’s group [
99] (
Figure 20). This low melting temperature compound has been proved to own higher detonation pressure and velocity values than those of others melt-castable energetic heterocycles bearing methyl group, which provided feasible route to castable energetic materials. In addition, introduction of polynitrogen heterocycle and formation of energetic salts are main methods to improve the thermal stability of explosives [
100]. In 2016, a heat-resistant energetic material, compound
59 bearing triazole ring, was synthesized using 5-amino-3-nitro-1
H-1,2,4-triazole (ANTA) and 3,4,5-trinitrated-1
H-pyrazole (TNP), and several salts based on it were developed by Zhou et al. [
101] (
Figure 21, Scheme A). As shown in
Table 10, compound
59 had high decomposition temperature (270 °C) and high positive HOF (833 kJ·mol
−1). All the salts showed good thermal stability, excellent insensitivity, and good detonation properties. In particular, the guanidinium salt
59d exhibited the best thermal stability superior than that of most explosives. Considering thermal stability and energetic properties, compounds
59 and
59d could be used as heat-resistant explosives and it was possible that these compounds can be applied as heat-resistant materials. Afterwards, their group reported a family of unsymmetrical
N-bridged dinitropyrazoles synthesized by TNP and 5-amino-1
H-tetrazole (ATZ) and its organic salts (
Figure 21, Scheme B). Several compounds (
60,
60b, and
60c) with high N contents exhibited superior detonation velocities but inferior detonation pressures compared to HMX and insensitivities to impact (IS > 40 J) and friction (FS > 360 N) comparable to those of TATB (
Table 10), which could be promising insensitive HEDMs for practical application.
In summary, some dinitropyrazoles and derivatives exhibit low melting points and high decomposition temperatures as well as good detonation, which can make them competitive candidates for a castable explosive. To further improve the performance of dinitropyrazole-based energetic materials, a combination of several functional groups should be better, for example, the combination of nitramine and polynitrogen heterocyclic which can endow them with high thermal stability and good detonation performance.
2.3. Trinitropyrazole and Its Derivatives
TNP is the unique pyrazole compound by total carbon nitrification [
102]. This compound owns good thermal stability (260–350 °C of
Td) and chemical stability, and shows high detonation velocity (9.0 km·s
−1) and detonation pressure (37.09 GPa). Wu et al. reviewed the synthesis of TNP in recent years in detail [
102], including direct nitration methods, amino oxidation method, amino diazotization method, iodo nitrification method and microwave rearrangement method. The typical synthesis of TNP is the oxidation of LLM-116 rather than 5-amino-3,4-dinitropyrazole, and this is partly because the amino group in LLM-116 has higher electron cloud density and steric hindrance than amino group in 5-amino-3,4-dinitropyrazole, which can promote the intermolecular oxidation reaction and avoid the occurrence of intermolecular side reaction effectively, and partly because the “NO
2-NH
2-NO
2” framework in LLM-116 makes it more stable and easier to synthesize. In addition, the nitrification of 3,5-DNP is another typical synthesis route of TNP. Traditional oxidation methods have the following defects: harsh reaction conditions, poor selectivity, by-products, high risk factor, expensive metal catalyst and toxic organic solvent. Although the synthesis of TNP with LLM-116 and 3,5-DNP as starting materials are mature, the synthesis of LLM-116 and 3,5-DNP are complicated. It is necessary to explore novel synthesis method. Zhao et al. [
44] used LLM-116 as starting material, water as solvent, and KHSO
5 as oxidant to synthesize TNP. Ravi et al. [
103] put forward the nitration system of metal nitrate and studied the process of nitration to TNP. These two methods are promising to prepare TNP.
Moreover, 1-methyl-3,4,5-trinitropyrazole (MTNP), a derivative of TNP, is an insensitive energetic material with 91.5 °C of melting point, 248–280 °C of decomposition temperature, 8.65 km·s
−1 of detonation velocity, and 33.7 GPa of detonation pressure [
104]. Ravi et al. [
103] added K-10 and TNP to bismuth impregnated in THF to obtain MTNP (
Figure 22, Scheme A). There were also many routes to synthesize MTNP. Dalinger et al. [
105,
106] dissolved TNP in NaHCO
3 aqueous solution with Me
2SO
4 as methylation reagent to acquire MTNP (
Figure 22, Scheme B). Guo et al. [
107] synthesized MTNP from 1-methyl-pyrazole by one-step method with nitric acid and fuming sulfuric acid (
Figure 22, Scheme C). Among these methods, selection of highly efficient catalytic synthesis process and low toxicity methylation reagent are the trend in MTNP synthesis. In addition, 1-amino-3,4,5-trinitropyrazole (ATNP) is also a derivative of TNP with excellent detonation properties (
D = 9.17 km·s
−1 and
P = 40.9 GPa) and thermal stability [
108]. This was reported by Herve et al. [
93], and the synthesis route is shown in Scheme D of
Figure 22 (Pic-
O-NH
2 = 2,4,6-trinitrophenyl-
O-hydroxylamine) with a yield of 26%.
The N-H bond in TNP is easy to neutralize with alkali or react with metal salts forming energetic salts due to the stereoscopic structure and spatial effect of pryazole ring. These energetic salts further broaden the application of TNP. Zhang et al. [
109] prepared a series of energetic salts of TNP based on nitrogen-rich cations (
61a–
m) (
Figure 23, Scheme A), all the salts showed poorer densities and detonation properties than TNP (
Table 11), but they owned good thermal stability and excellent insensitivity. Drukenmuller et al. [
110] reported the synthesis of alkali and earth alkali trinitropyrazolate (
62a–
d) (
Figure 23, Scheme B), compound
62d exhibited predominantly decomposition temperatures (
Table 11). They also prepared pyrotechnic formulations using
62c and
62d, which showed good color properties and low sensitivity as well as high
Td. In addition, Shreeve’s group [
111] synthesized 3,4,5-trinitropyrazole-1-ol (
63) and its nitrogen-rich salts (
63a–
g) (
Figure 24) the corresponding properties are shown in
Table 11. Compound
63 with its high oxygen content (51.13%) could be the green replacement of the currently used oxidizer (NH
4ClO
4), while the high IS (1 J) restricted its application. Compound
63a–
g with acceptable impact sensitivities and detonation performance could be useful energetic materials.
Polynitrogen heterocycle linking to TNP is a promising method to reach a balance between the energetic and physical properties of TNP, while there are a few references about it. Shreeve et al. [
112] reported the synthesis of asymmetric
N,
N′-ethylene bridged 5-aminotetrazole and TNP moieties. They prepared 1-(2-(3,4,5-trinitro-1
H-pyrazol-1-yl)ethyl)-1
H-tetrazol-5-amine and 1-(3-(3,4,5-Trinitro-1
H-pyrazol-1-yl)propyl)-1
H-tetrazol-5-amine, and the two compounds were excellent insensitive and moderate powerful. In addition, they synthesized 5-((3,4,5-trinitro-1
H-pyrazol-1-yl)methyl)-1
H-tetrazole by
N-methylene-
C bridging TNP and tetrazole, which showed outstanding detonation properties and moderate insensitivity [
113].