Fused 1,5-Naphthyridines: Synthetic Tools and Applications
Abstract
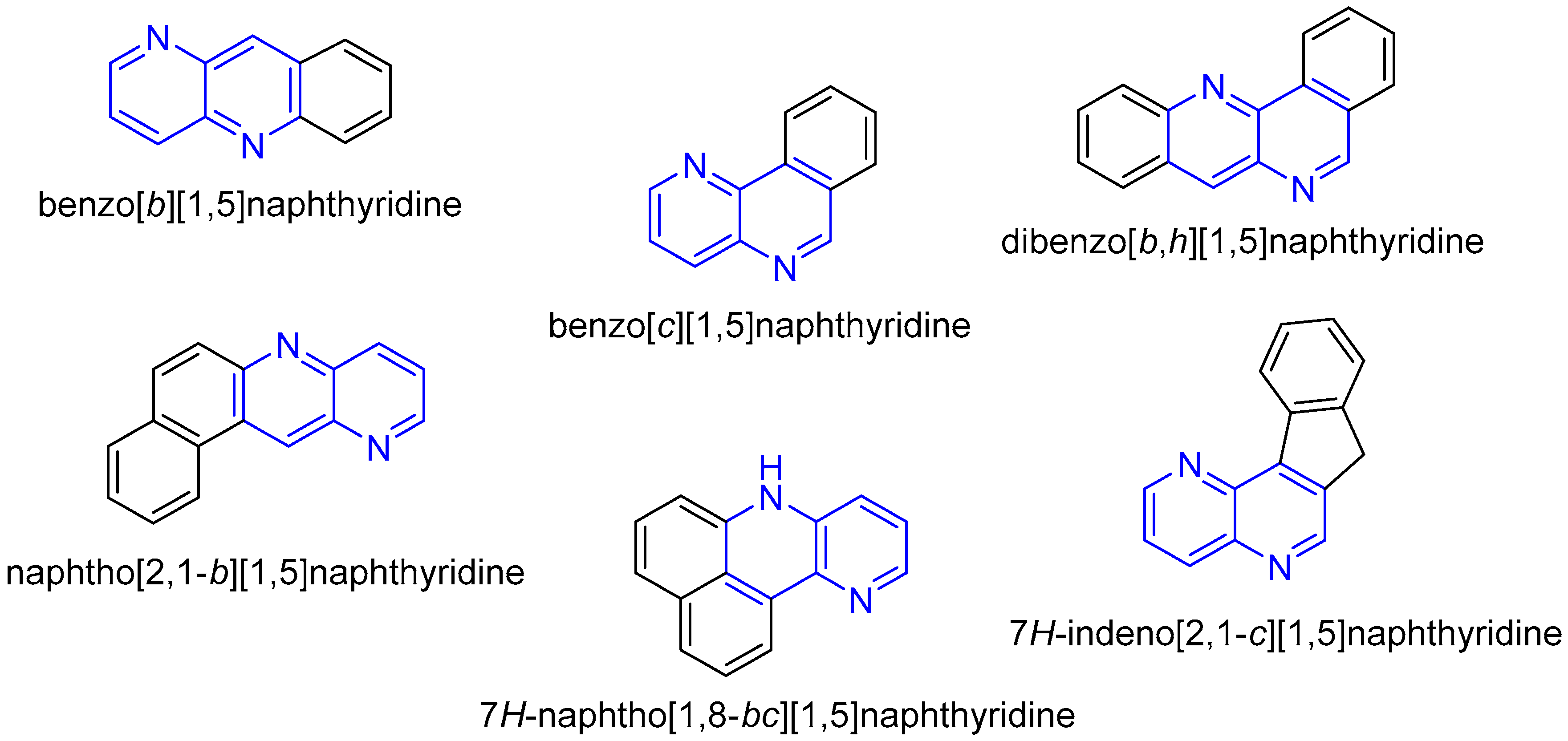
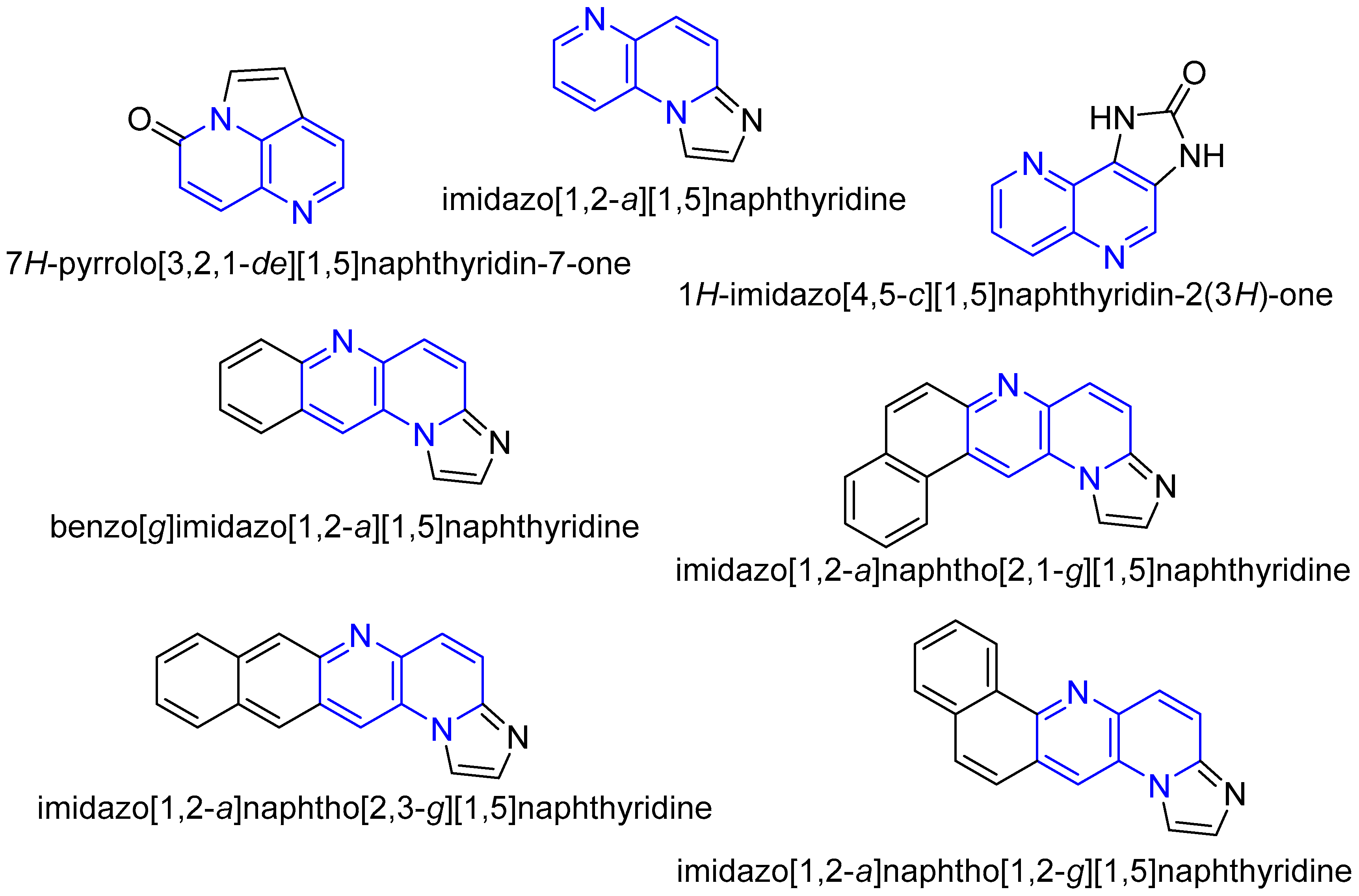
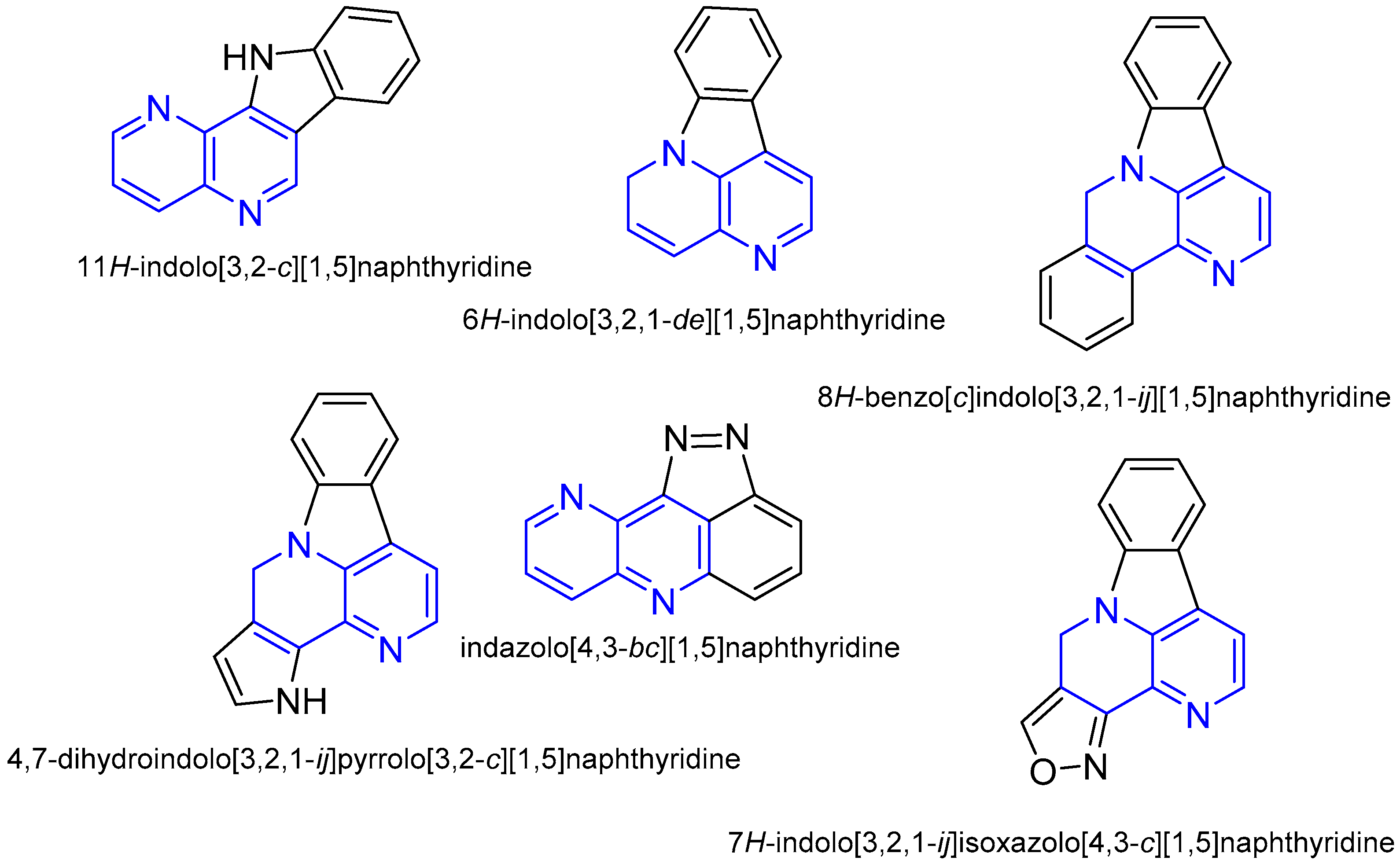
1. Introduction
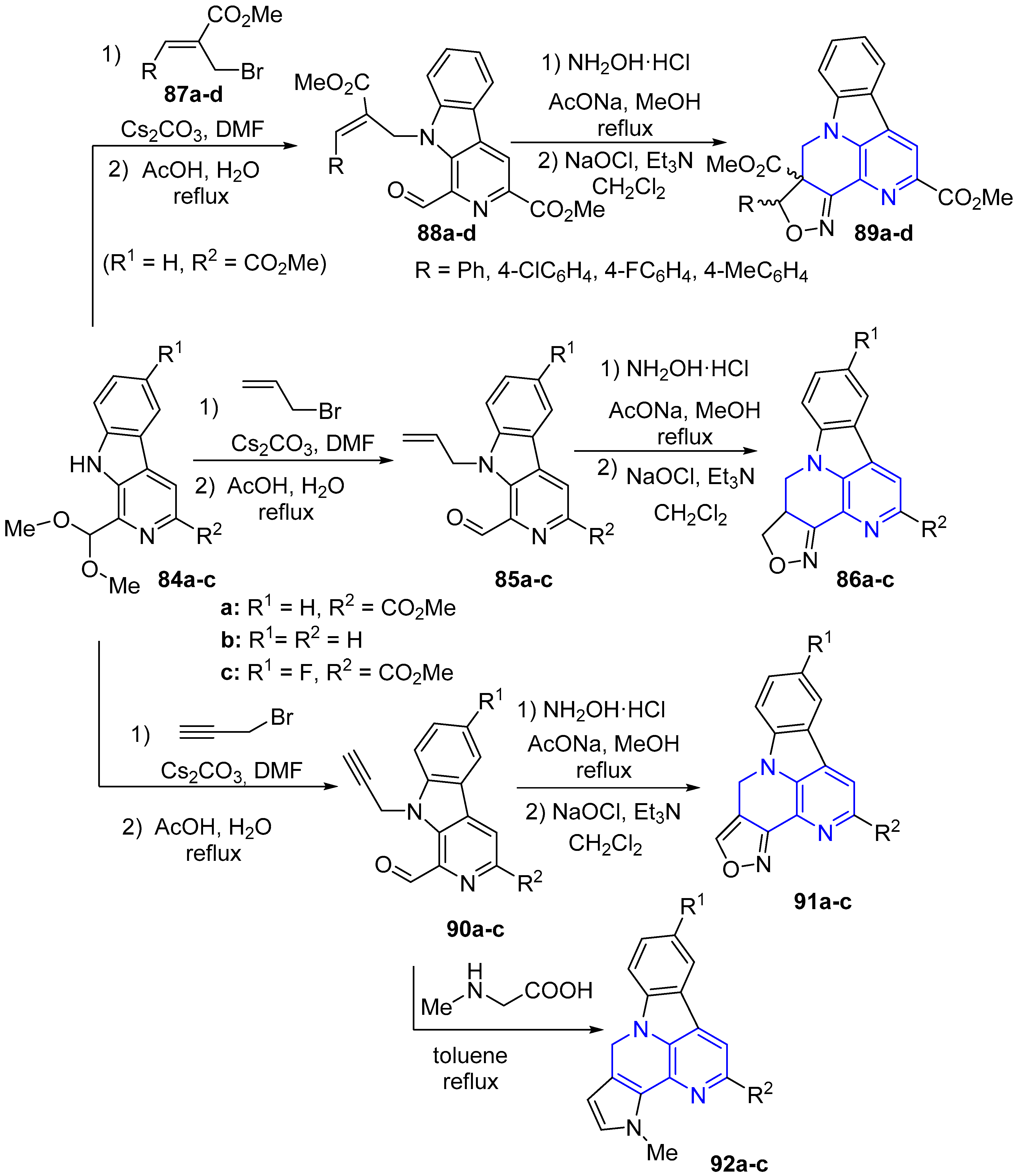
2. Synthesis of Fused 1,5-Naphthyridines
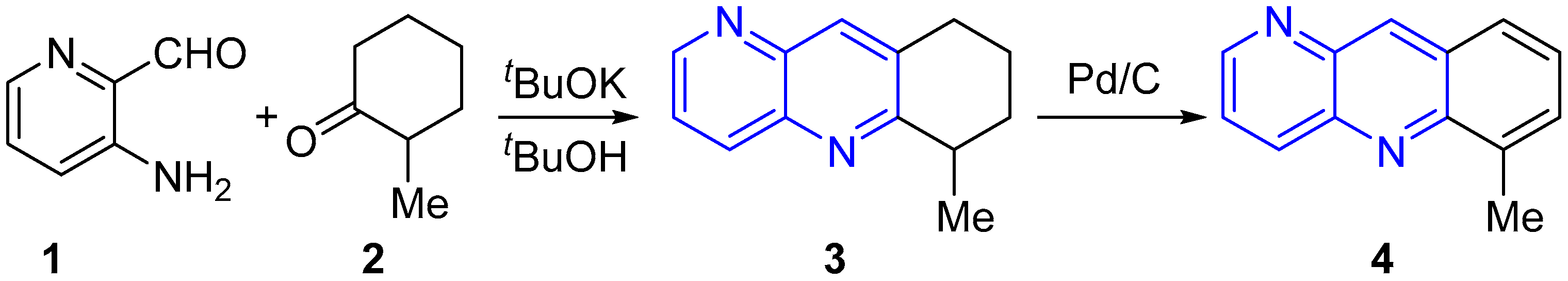
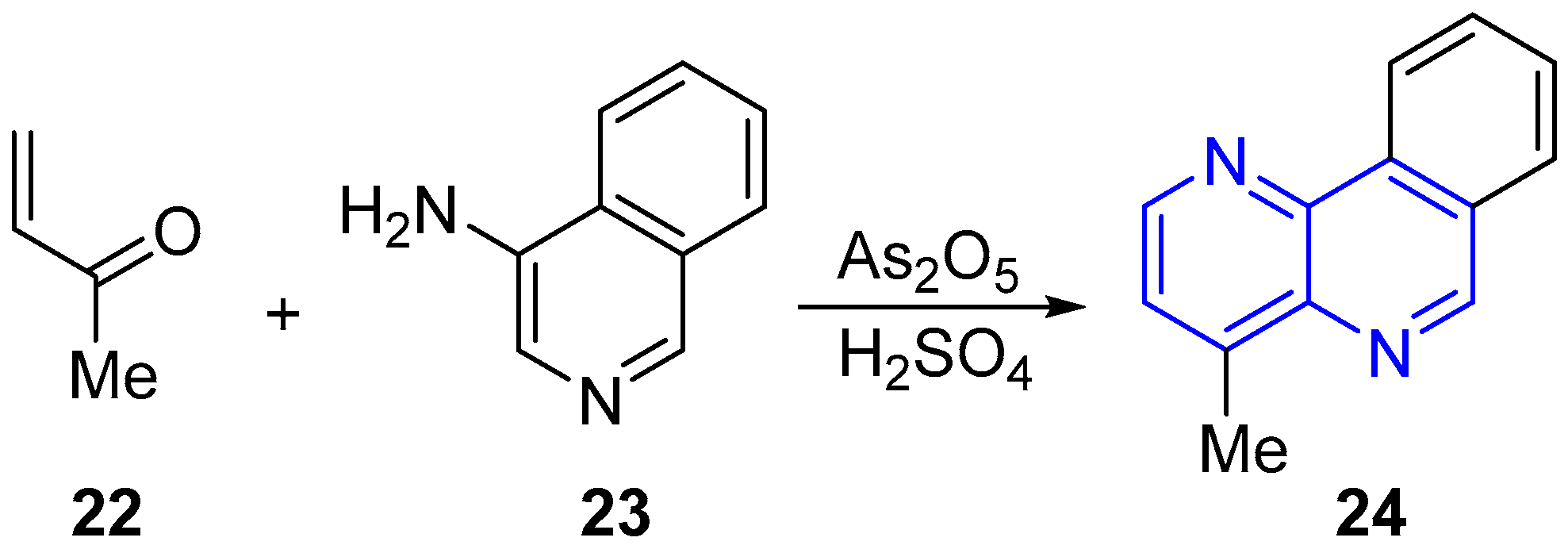
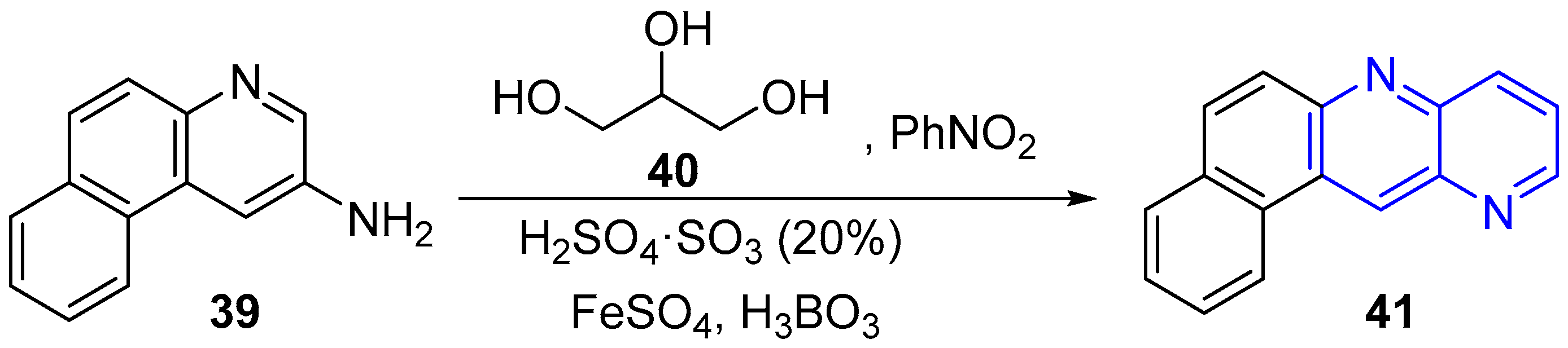
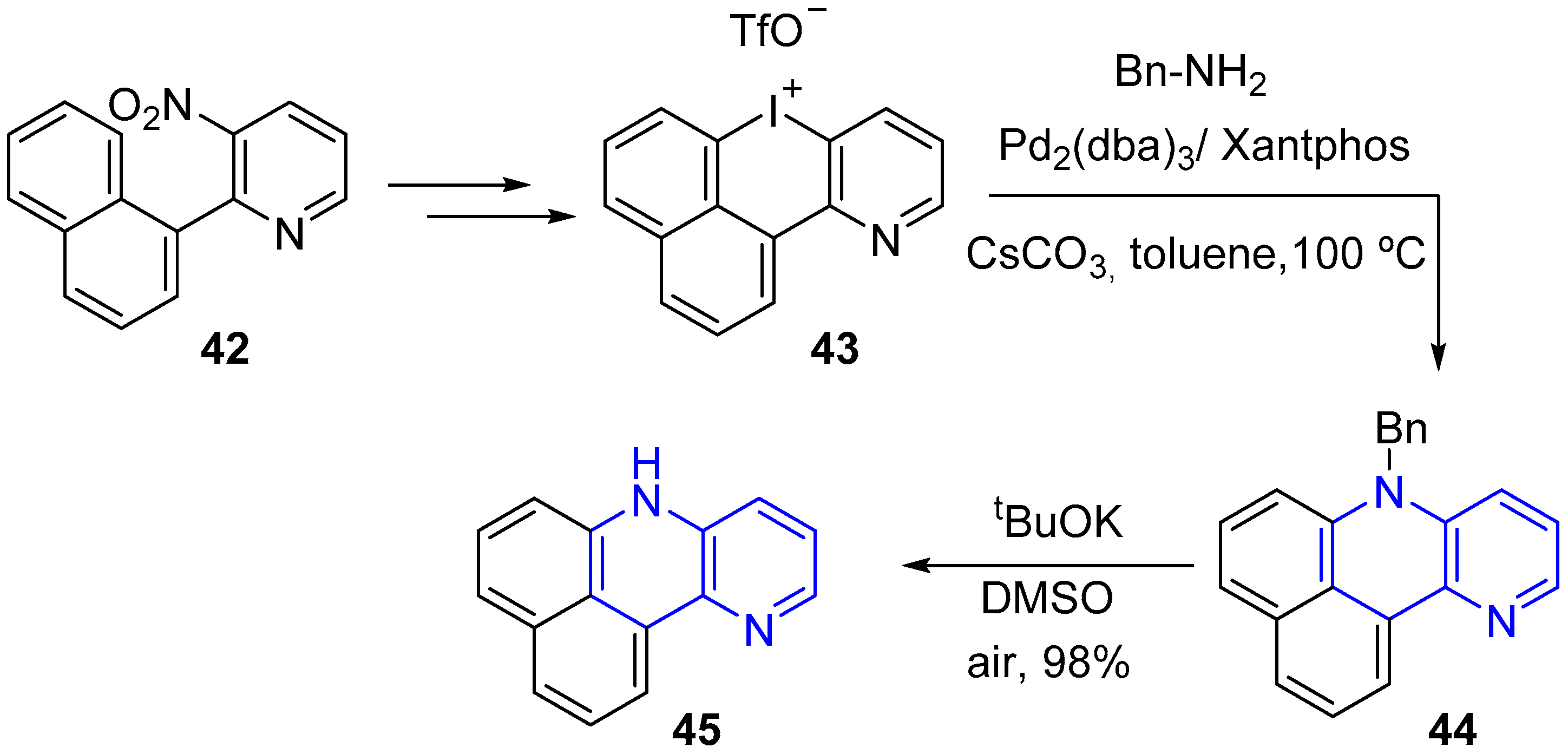
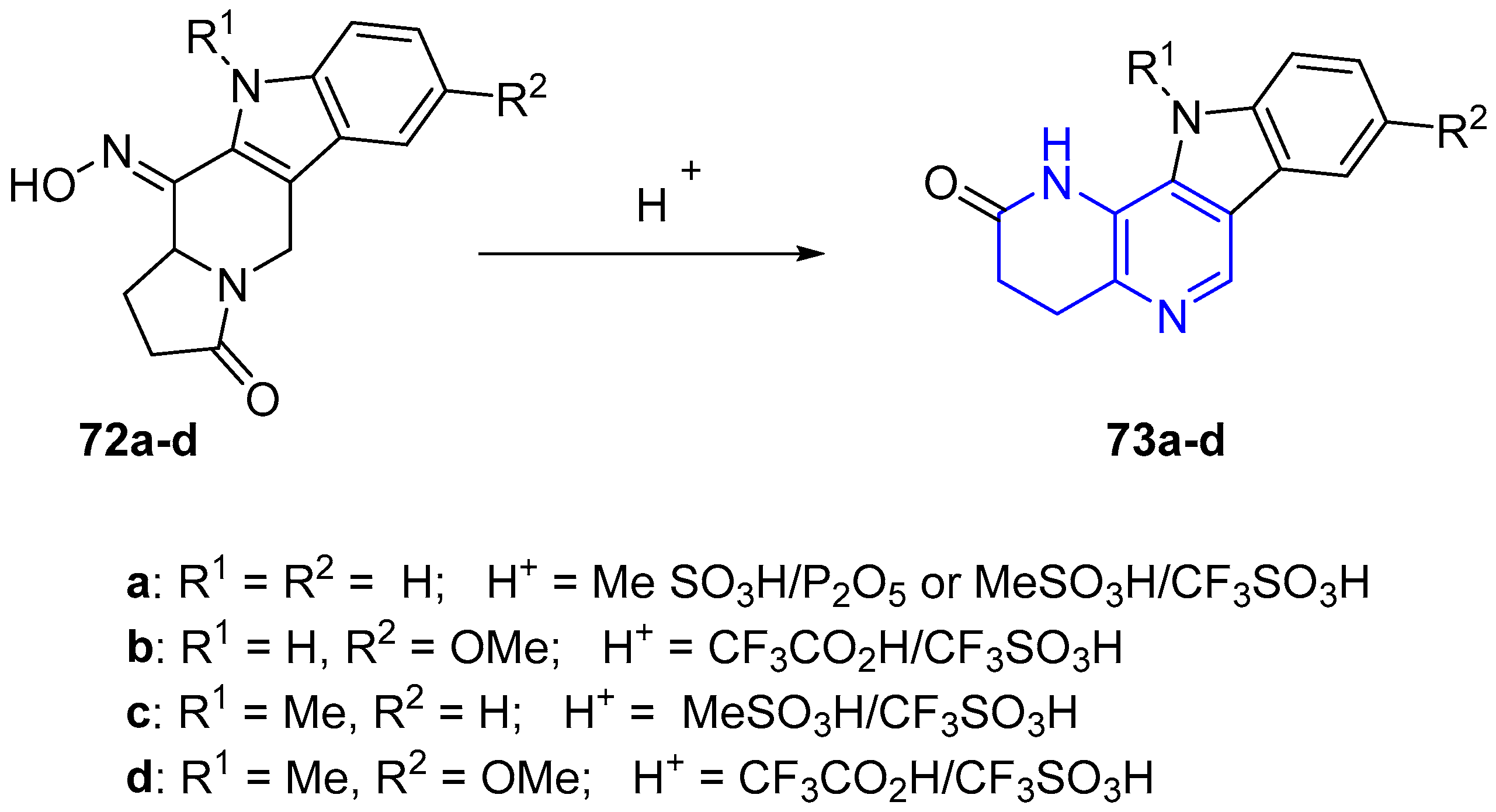
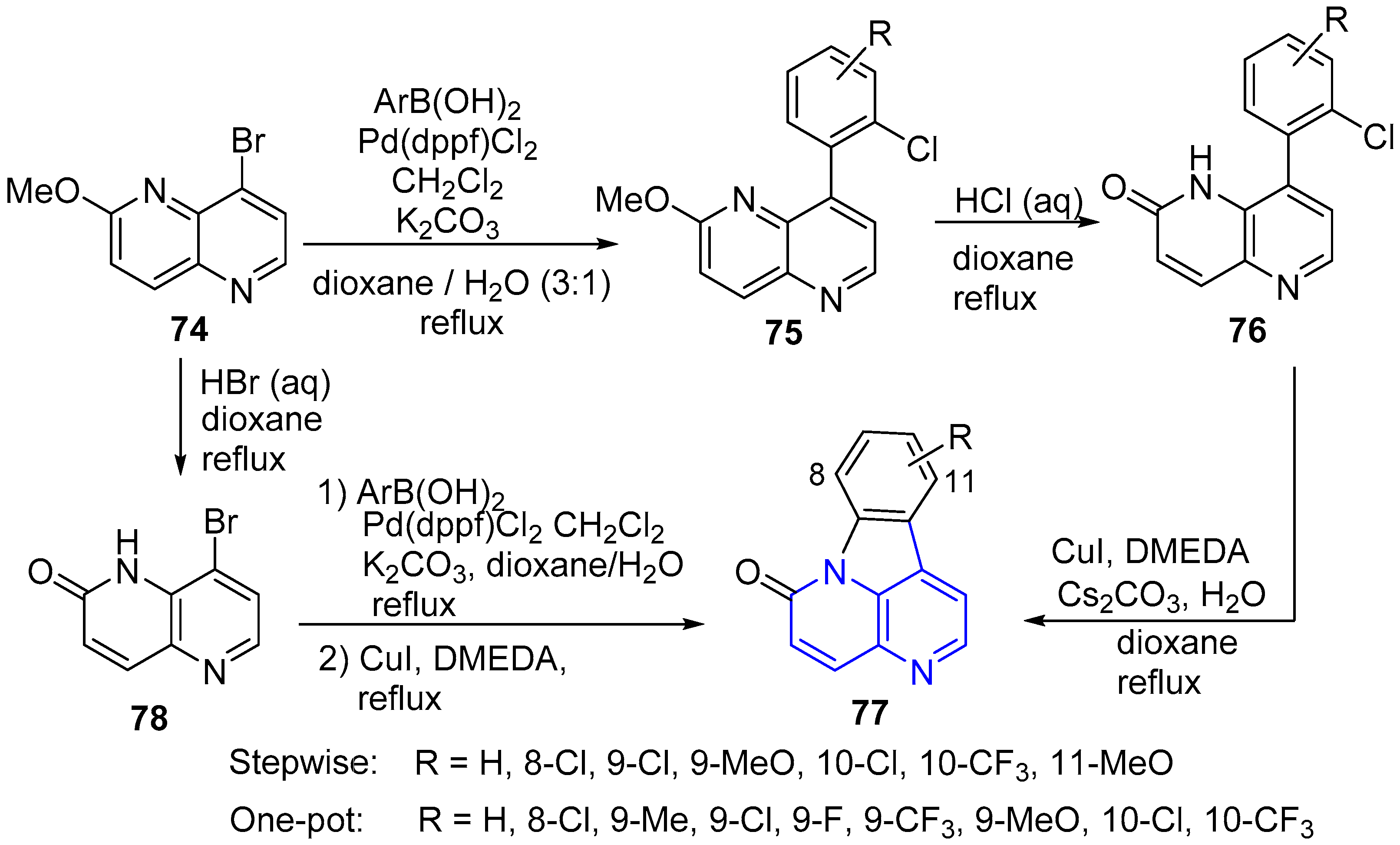
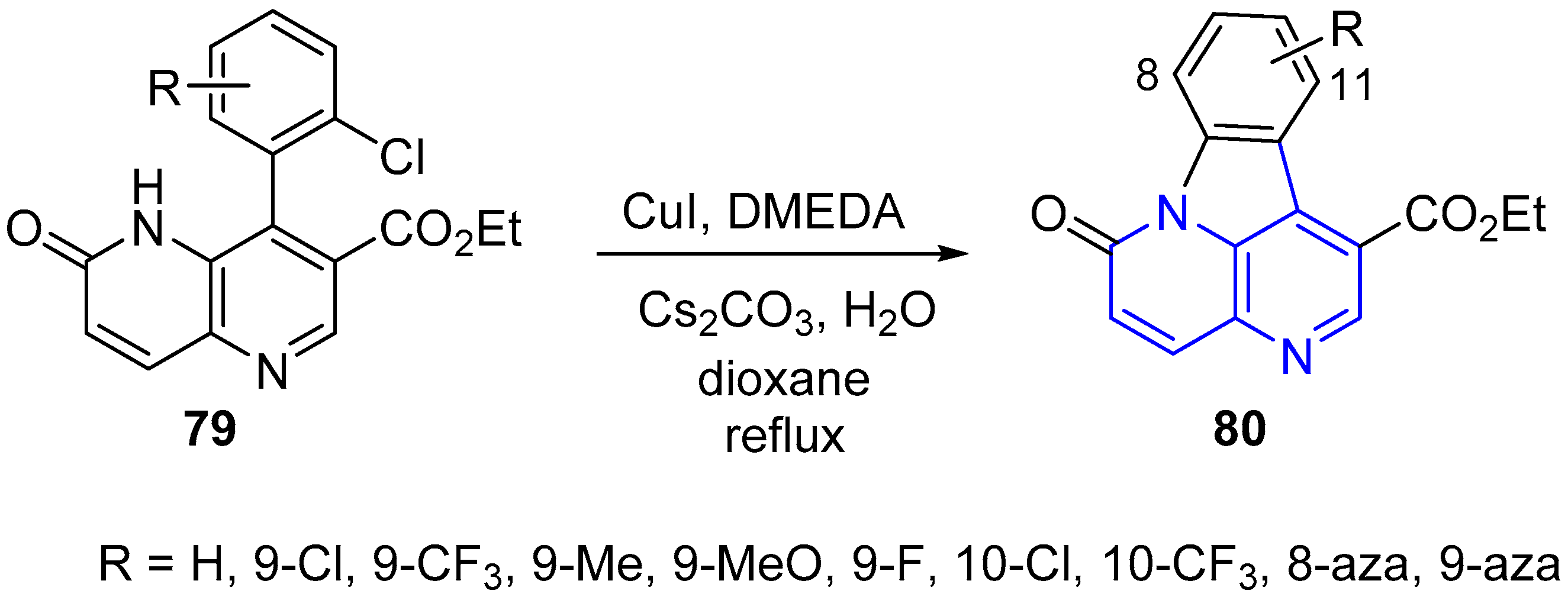
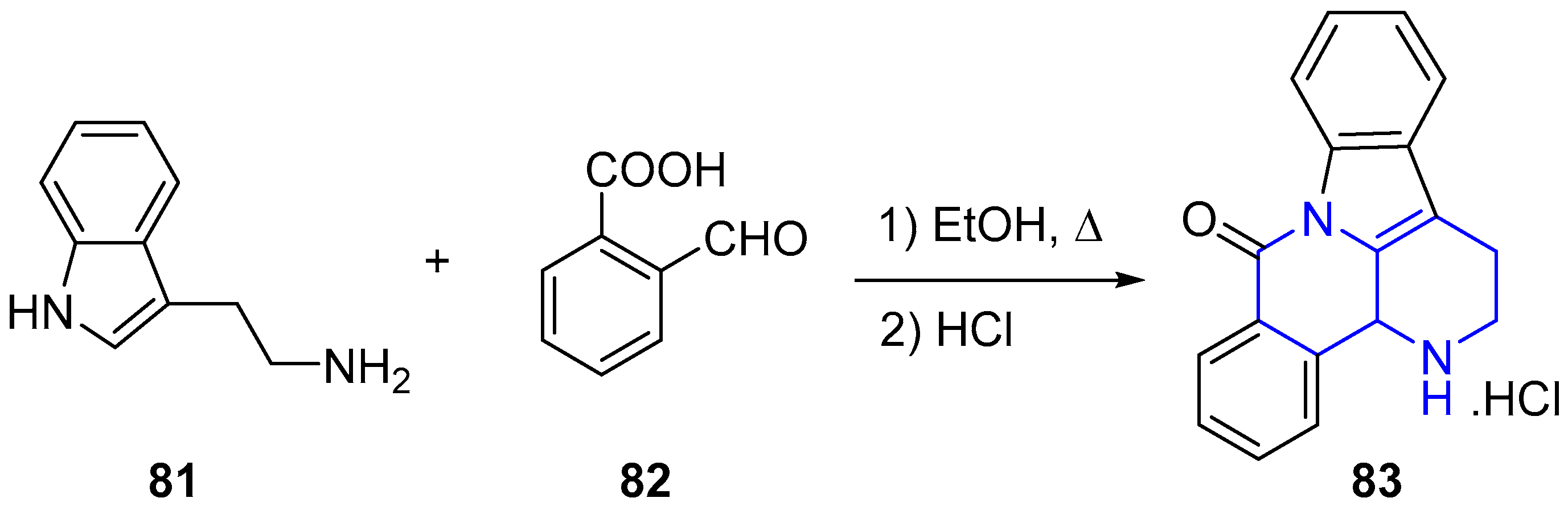
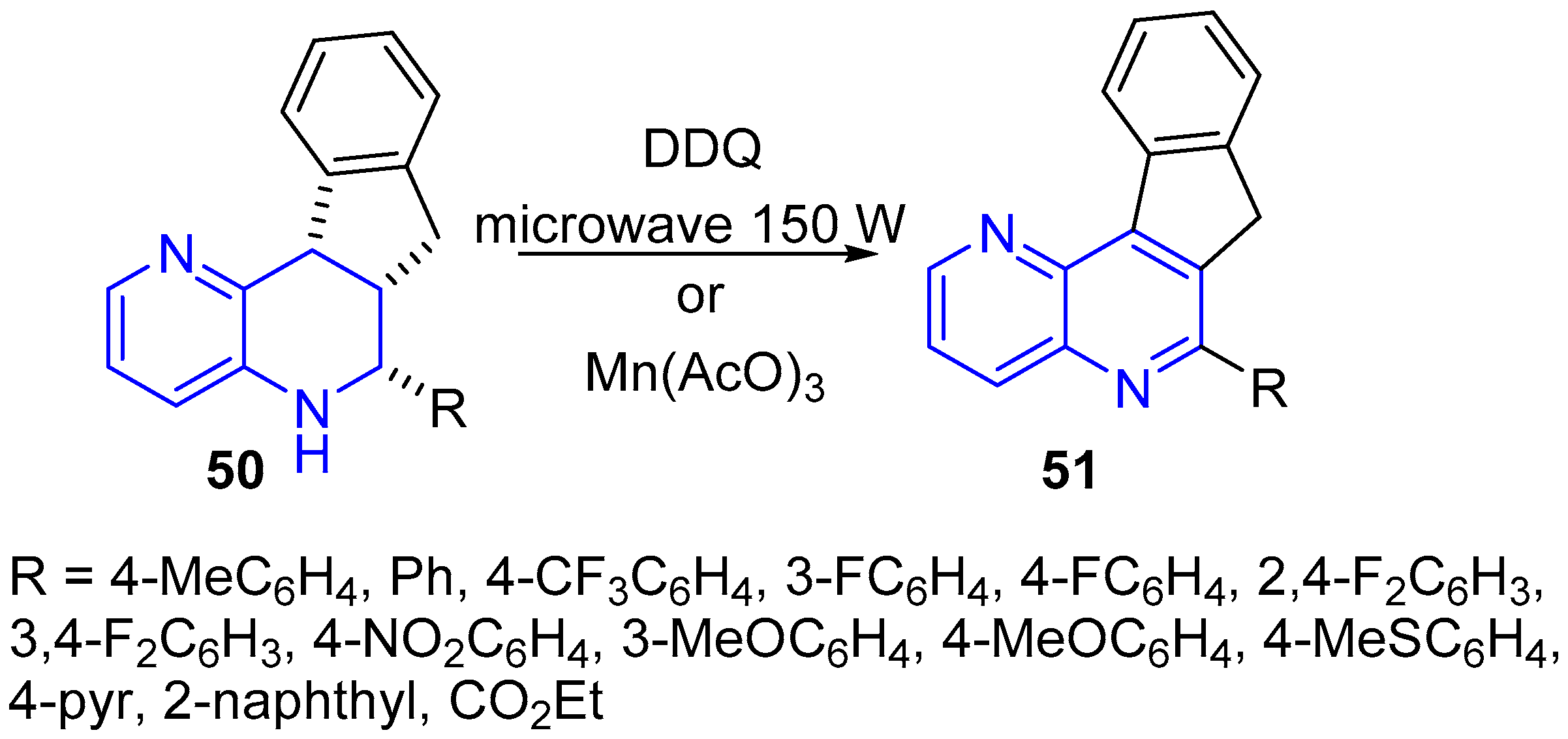
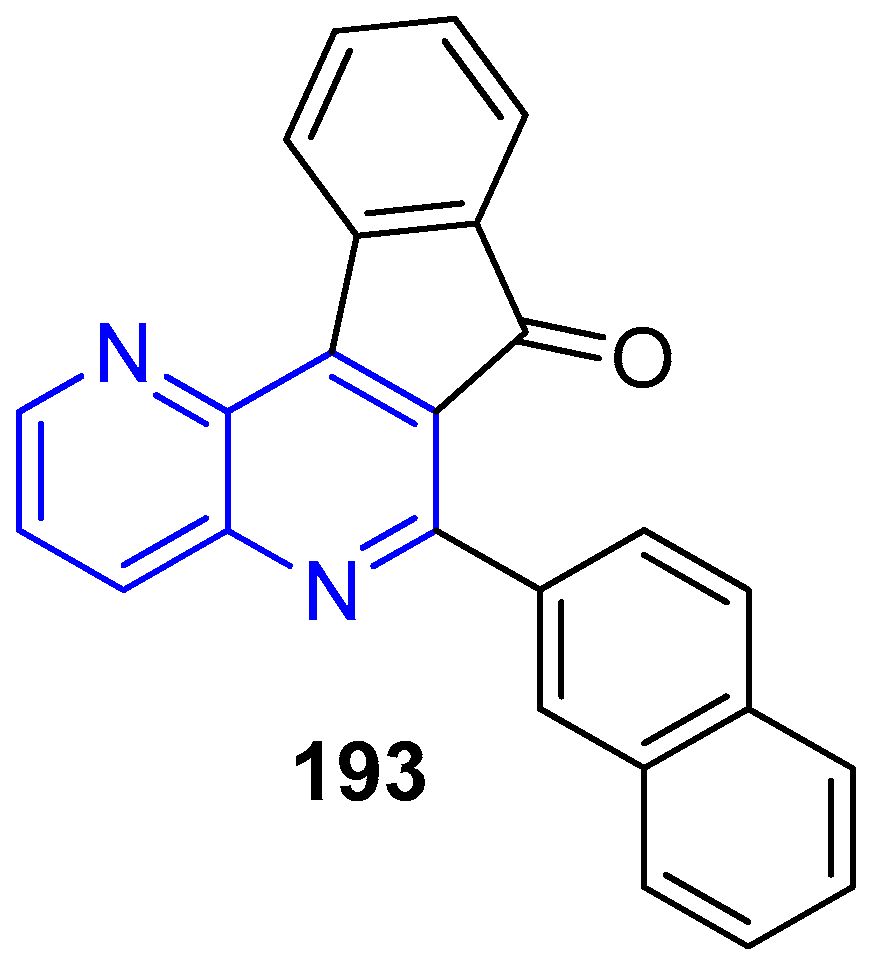
2.1. Synthesis of 1,5-Naphthyridines Fused with Carbocycles
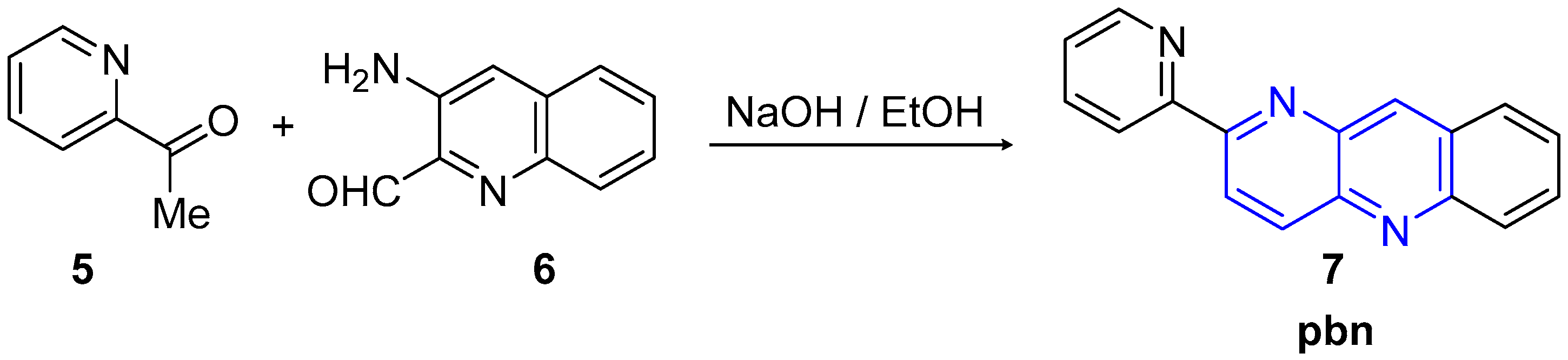
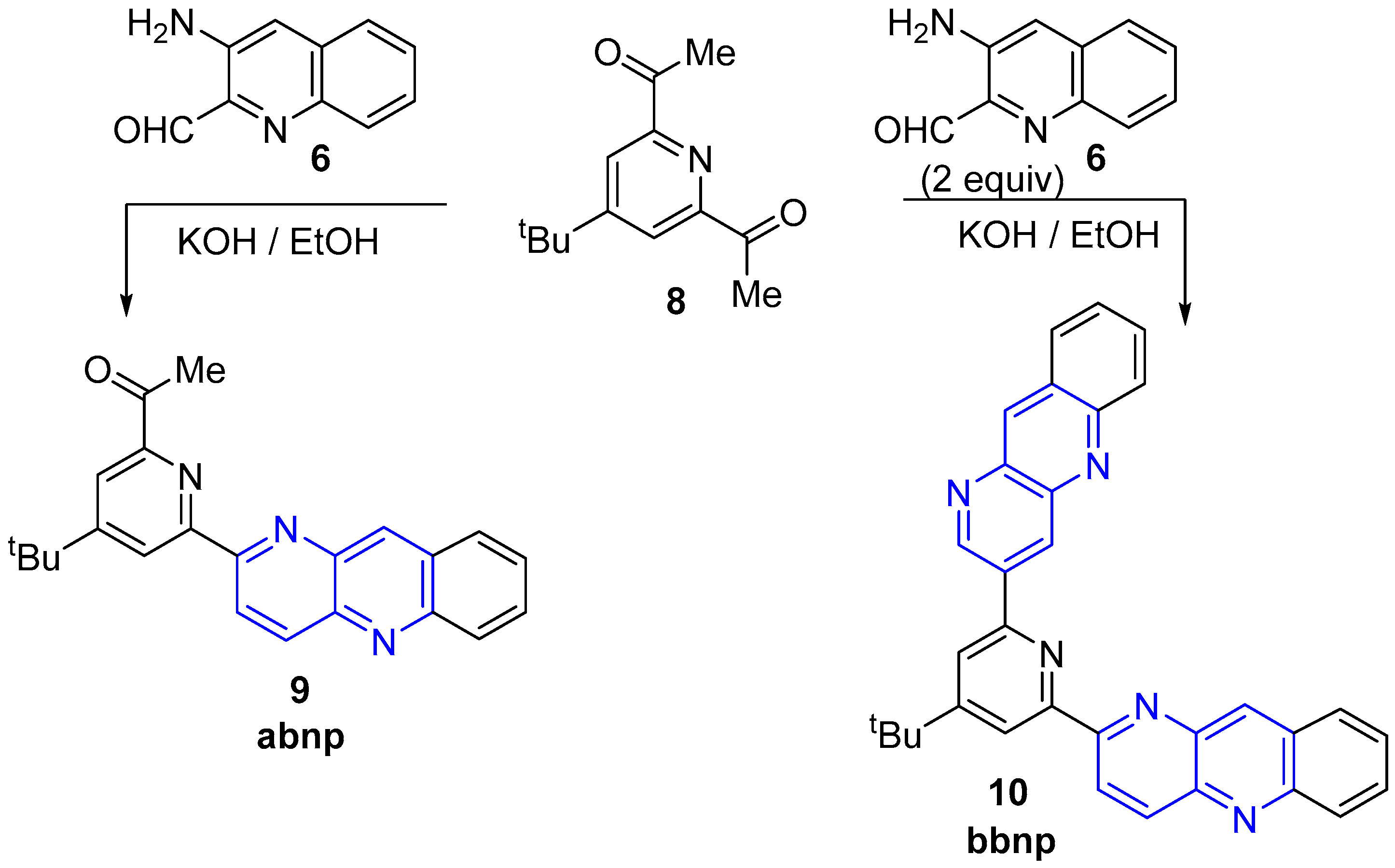
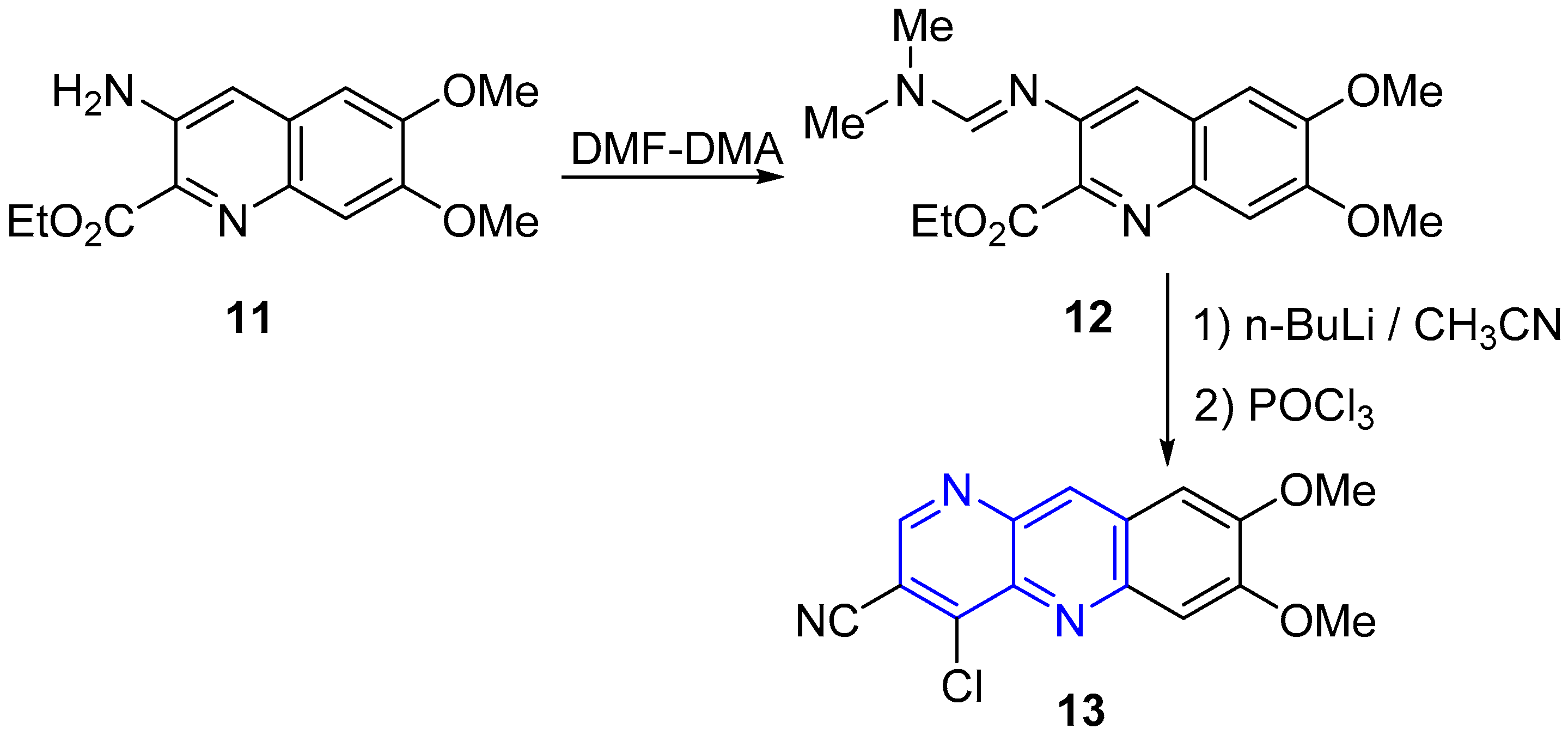
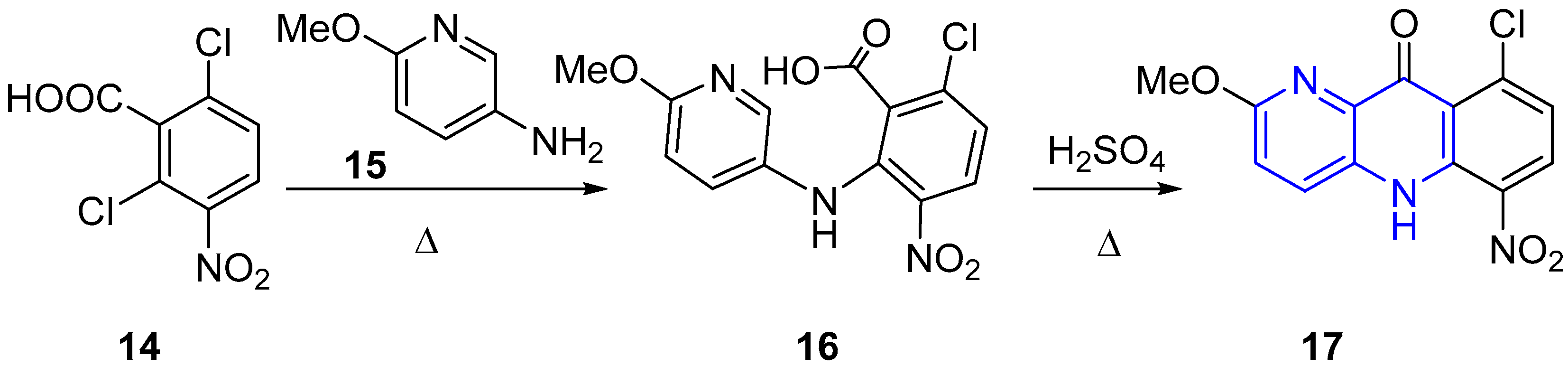
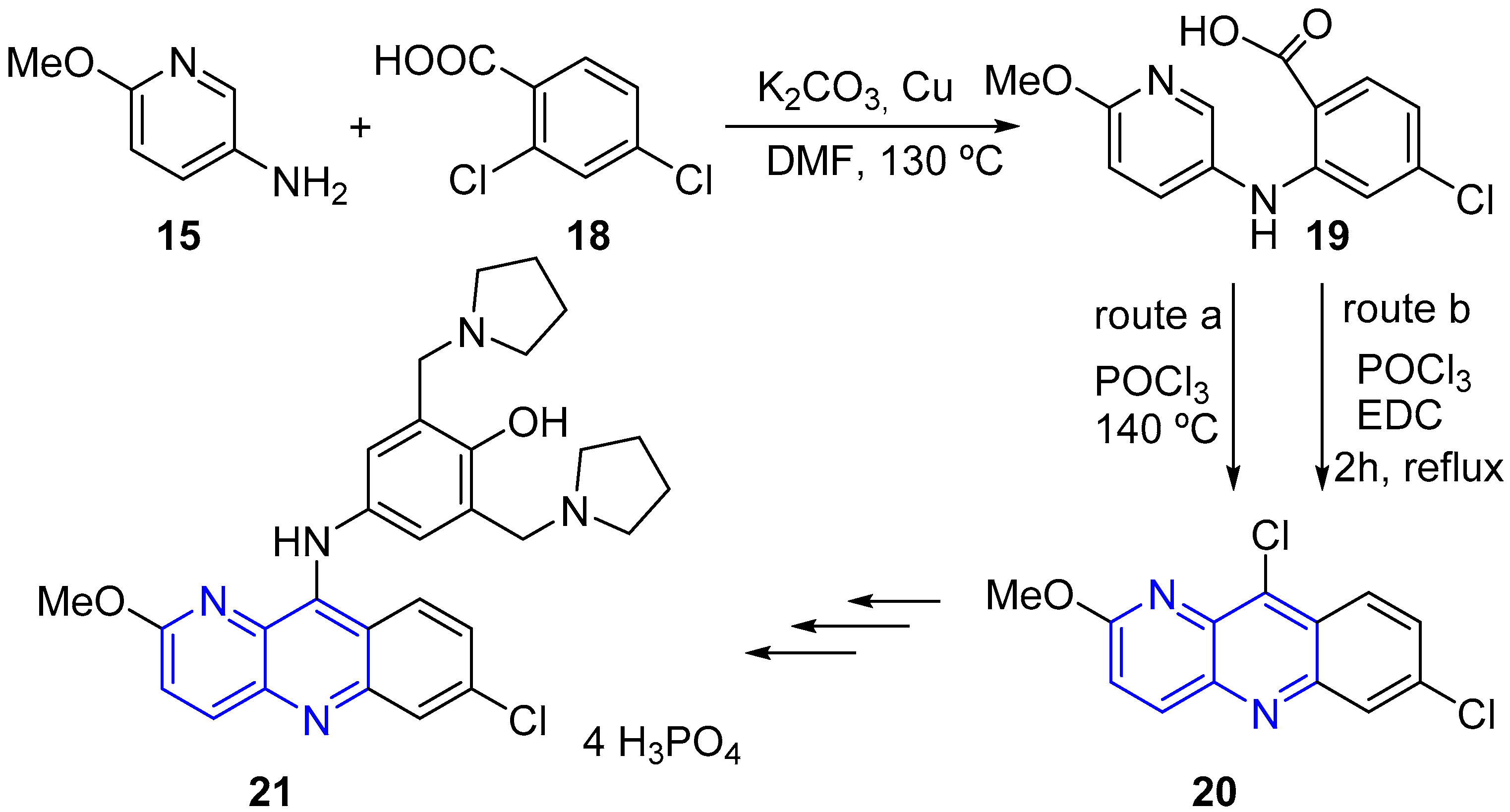
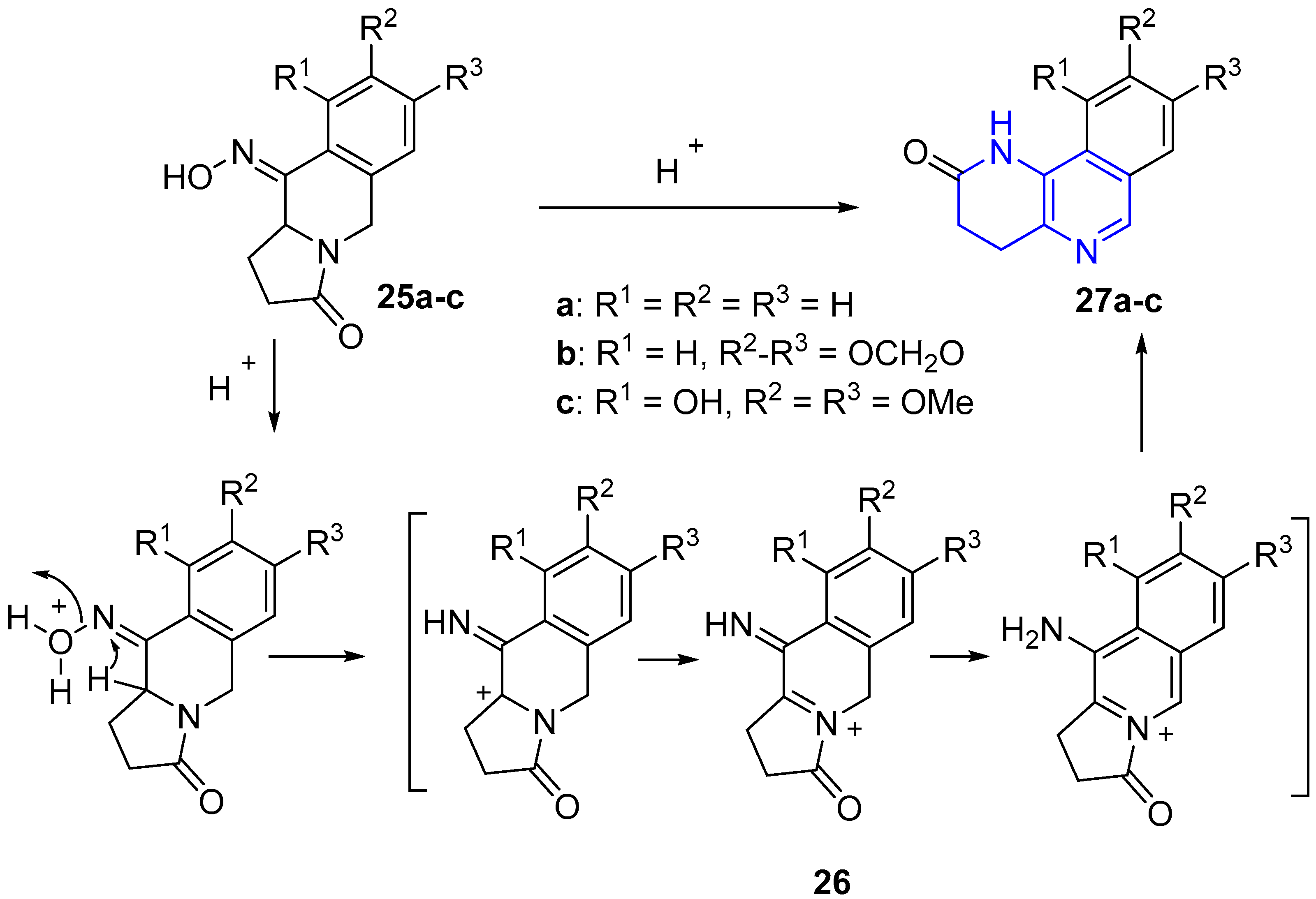
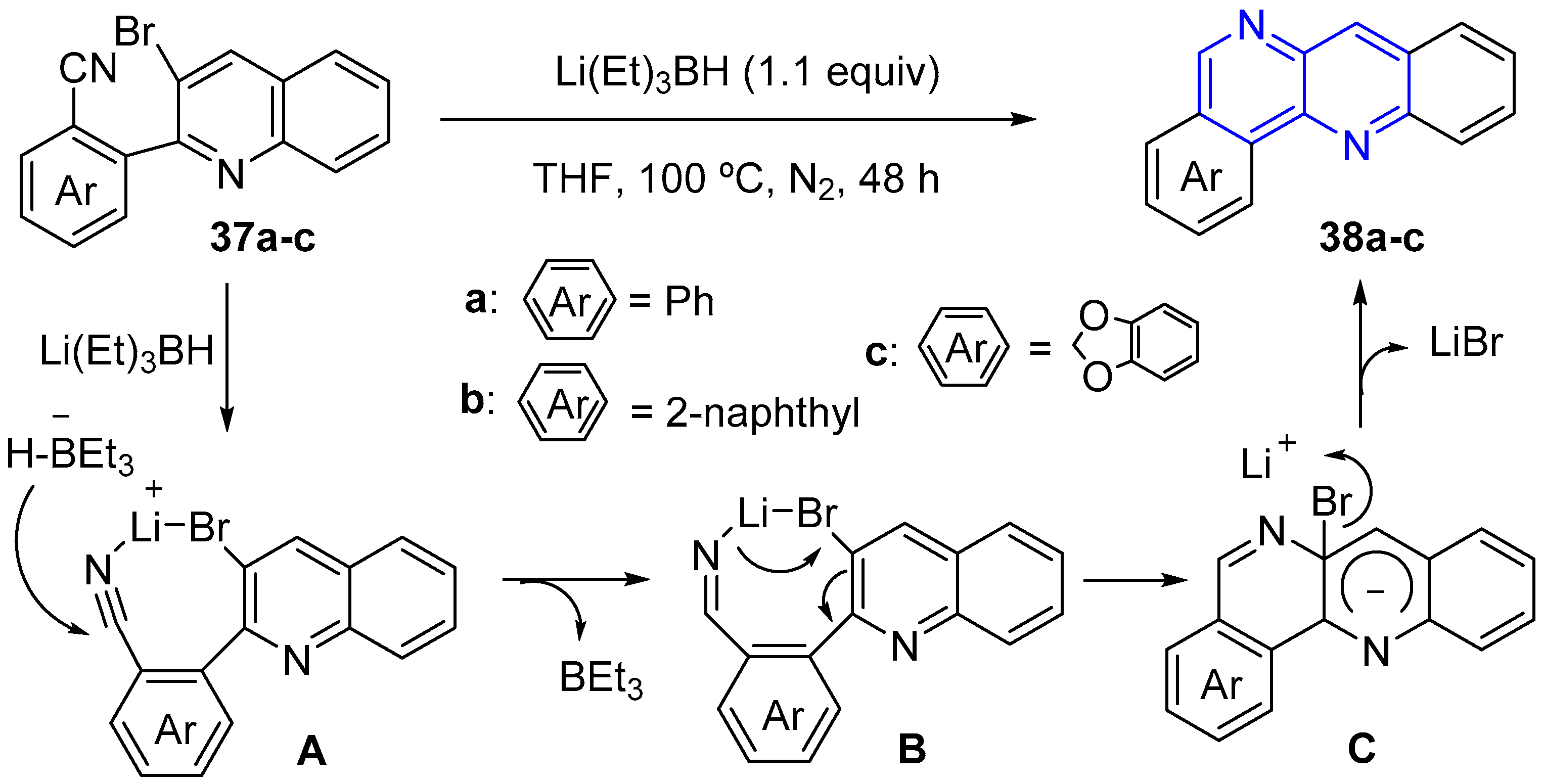
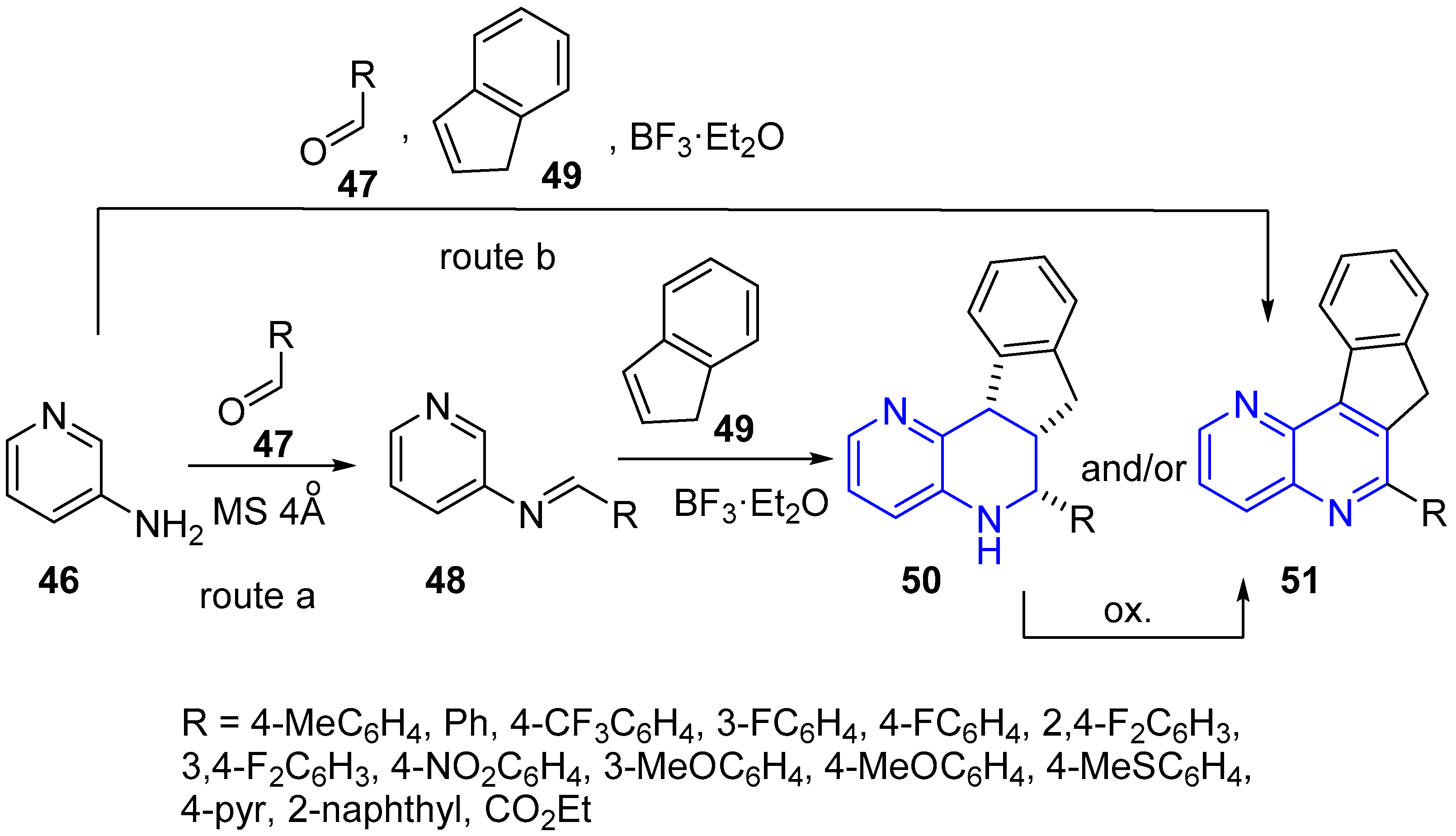
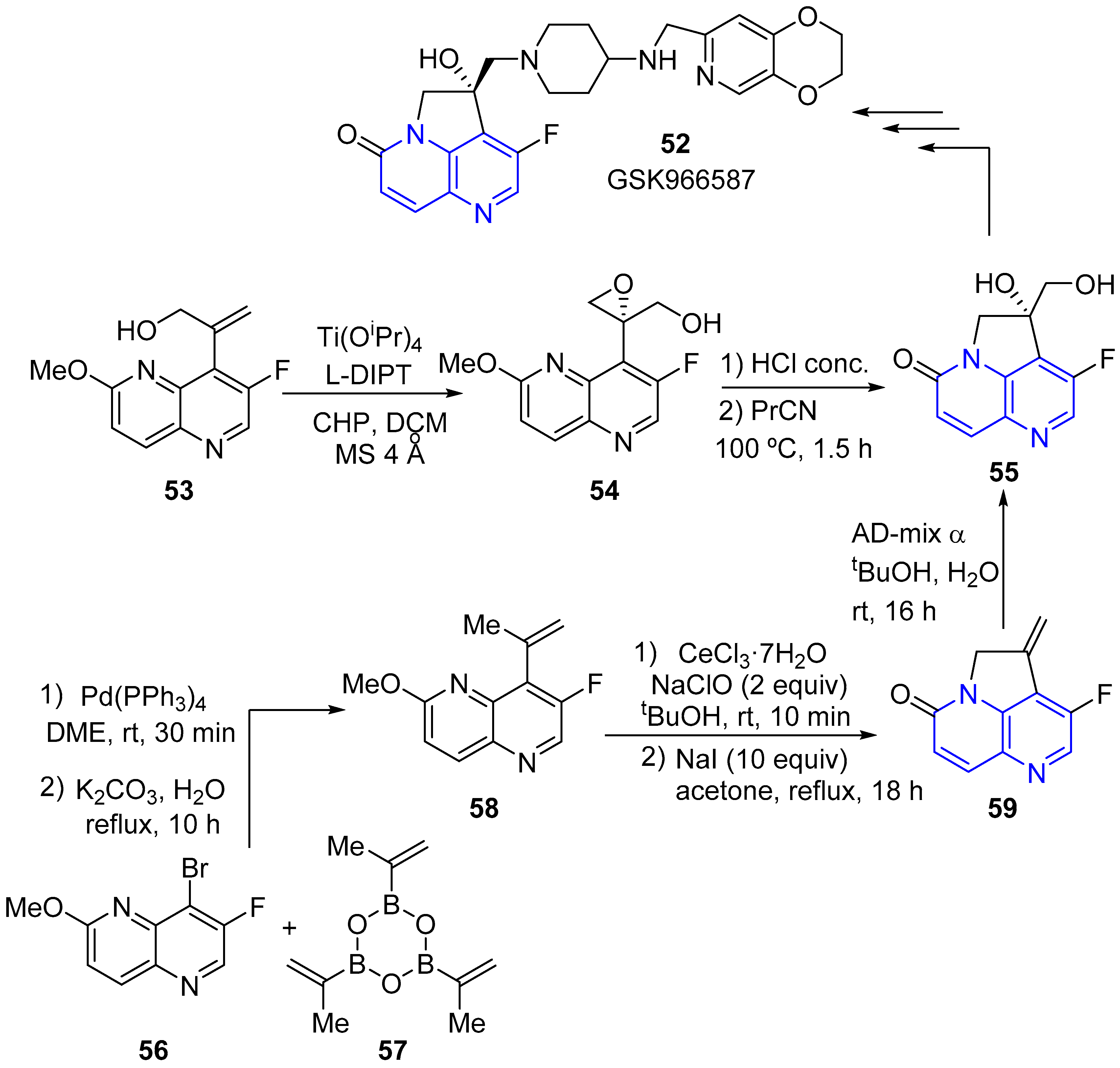
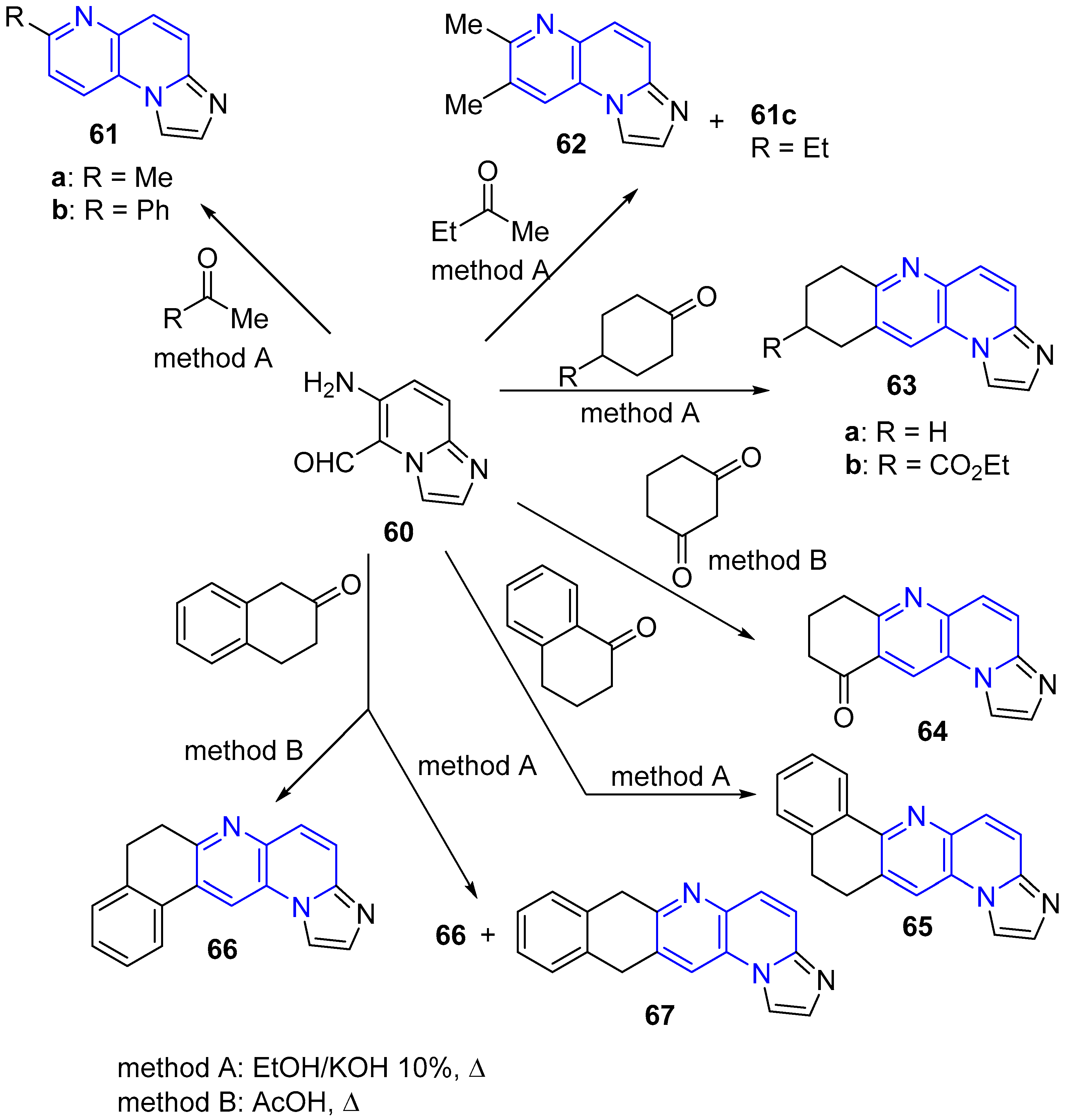
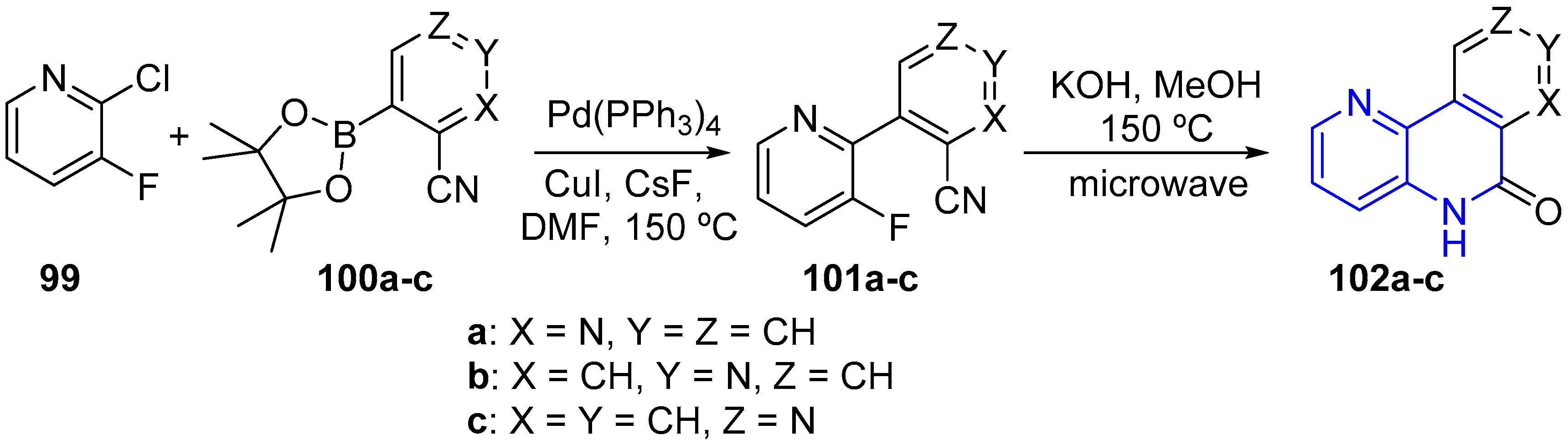
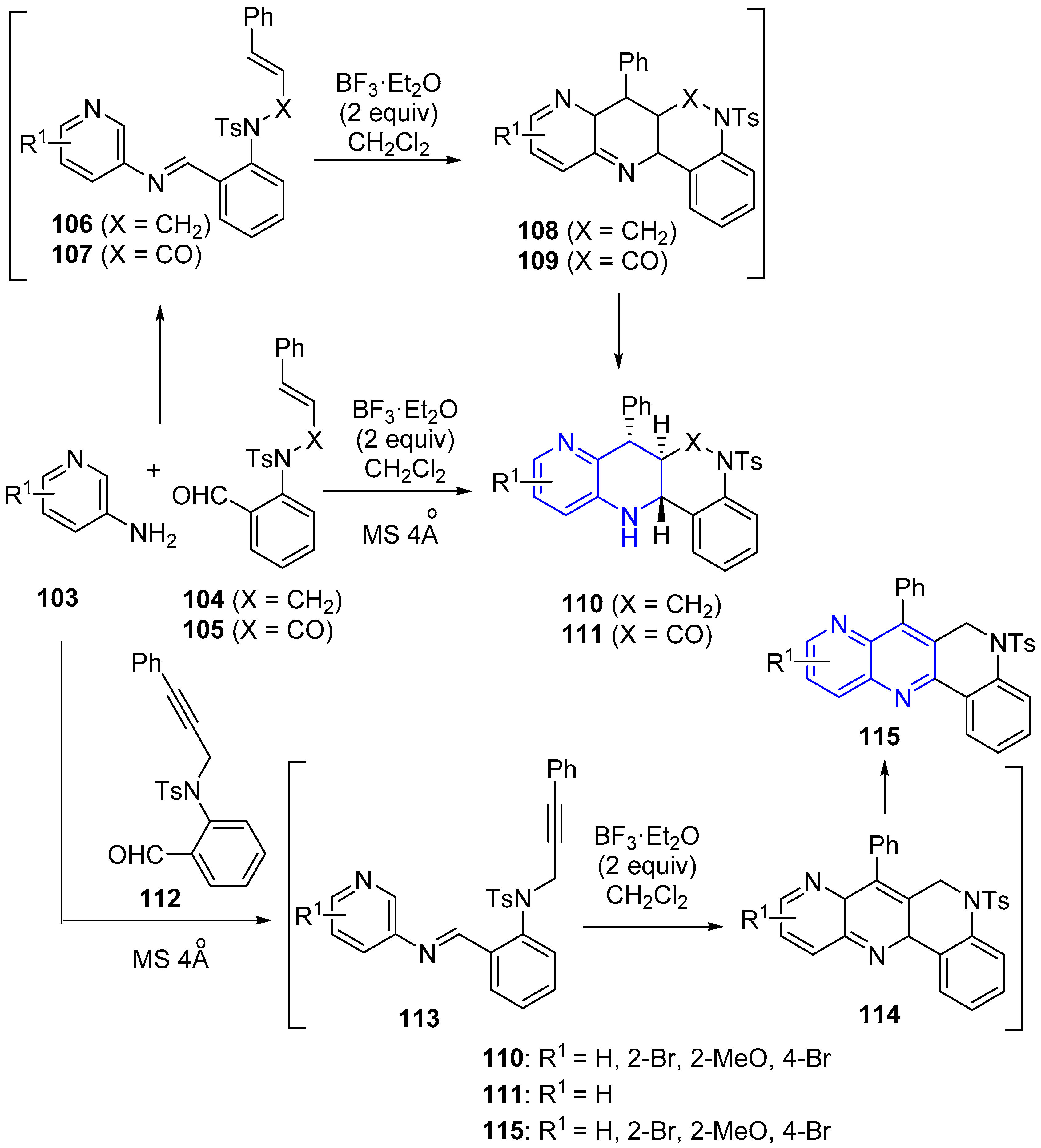
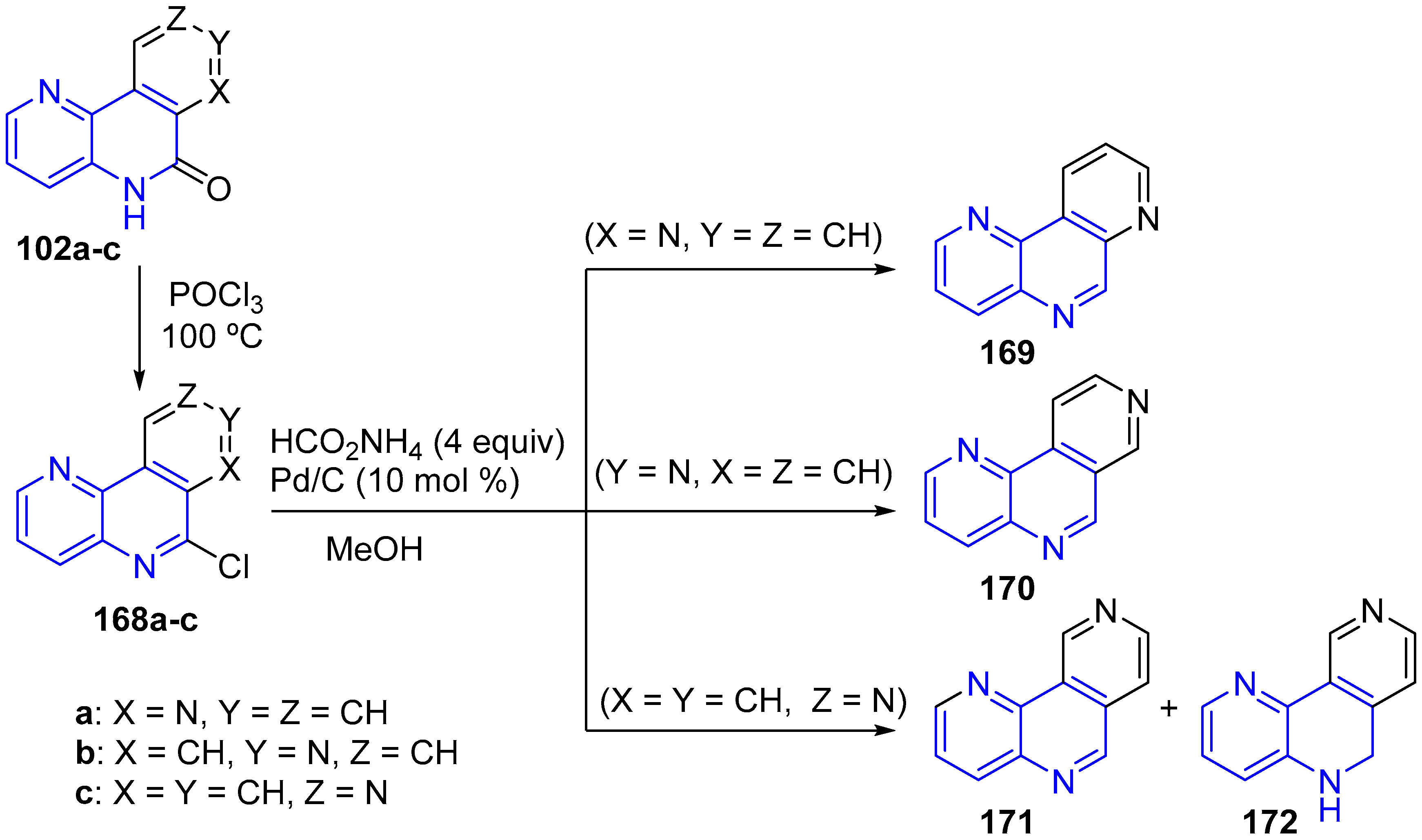
2.2. Synthesis of 1,5-Naphthyridines Fused with Nitrogen Heterocycles
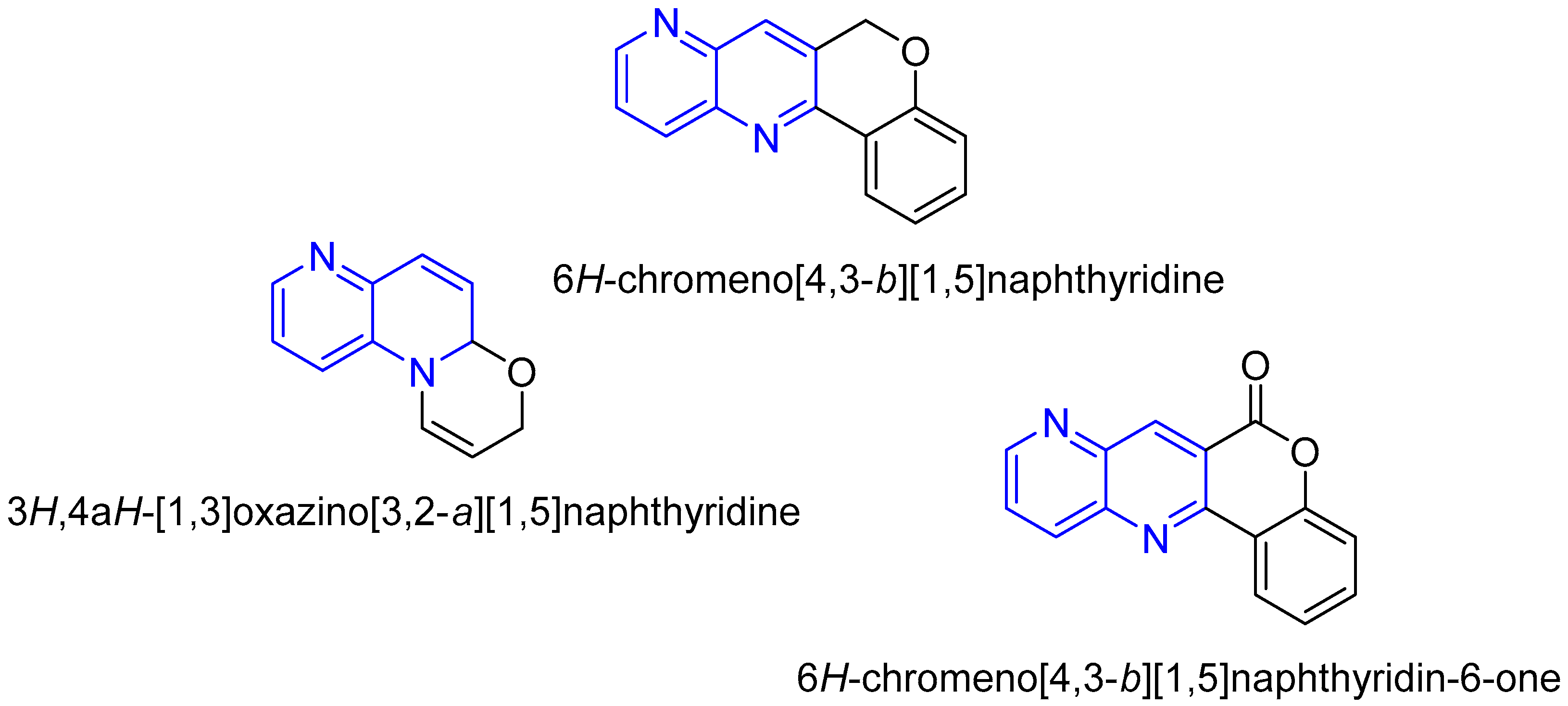
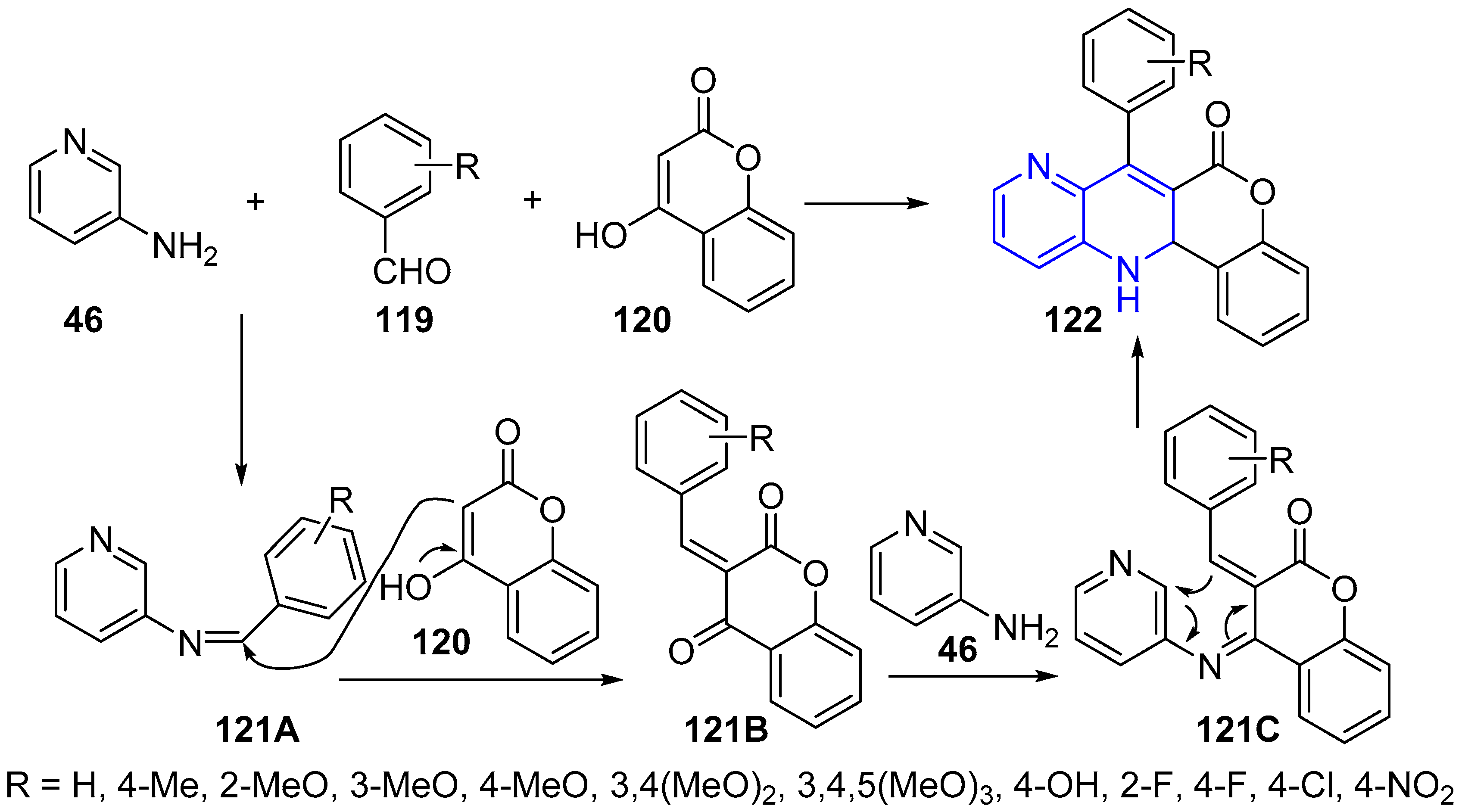
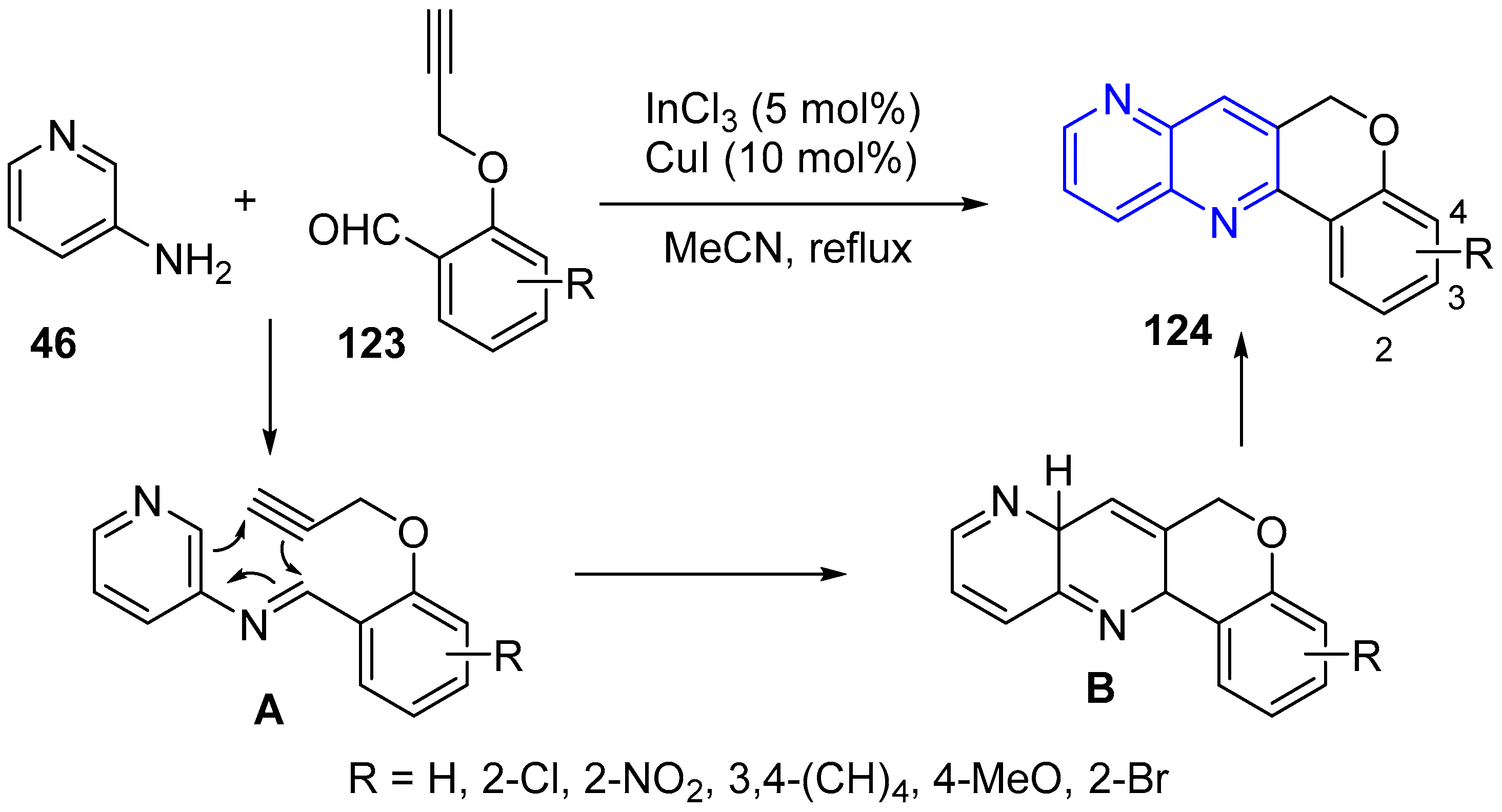
2.3. Synthesis of 1,5-Naphthyridines Fused with Oxygen-Containing Heterocycles
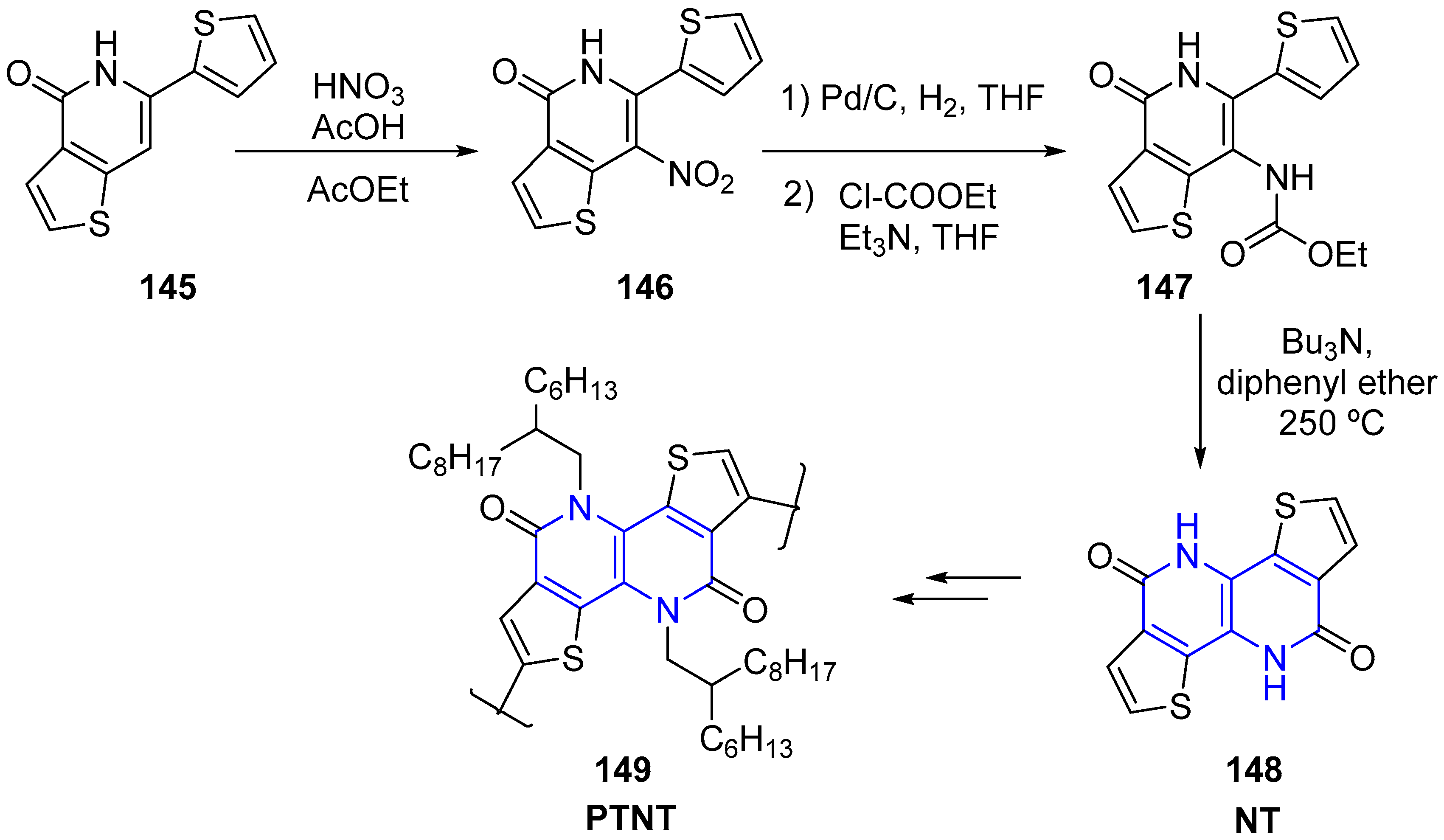
2.4. Synthesis of 1,5-Naphthyridines Fused with Sulphur-Containing Heterocycles
3. Reactivity of Fused 1,5-Naphthyridines
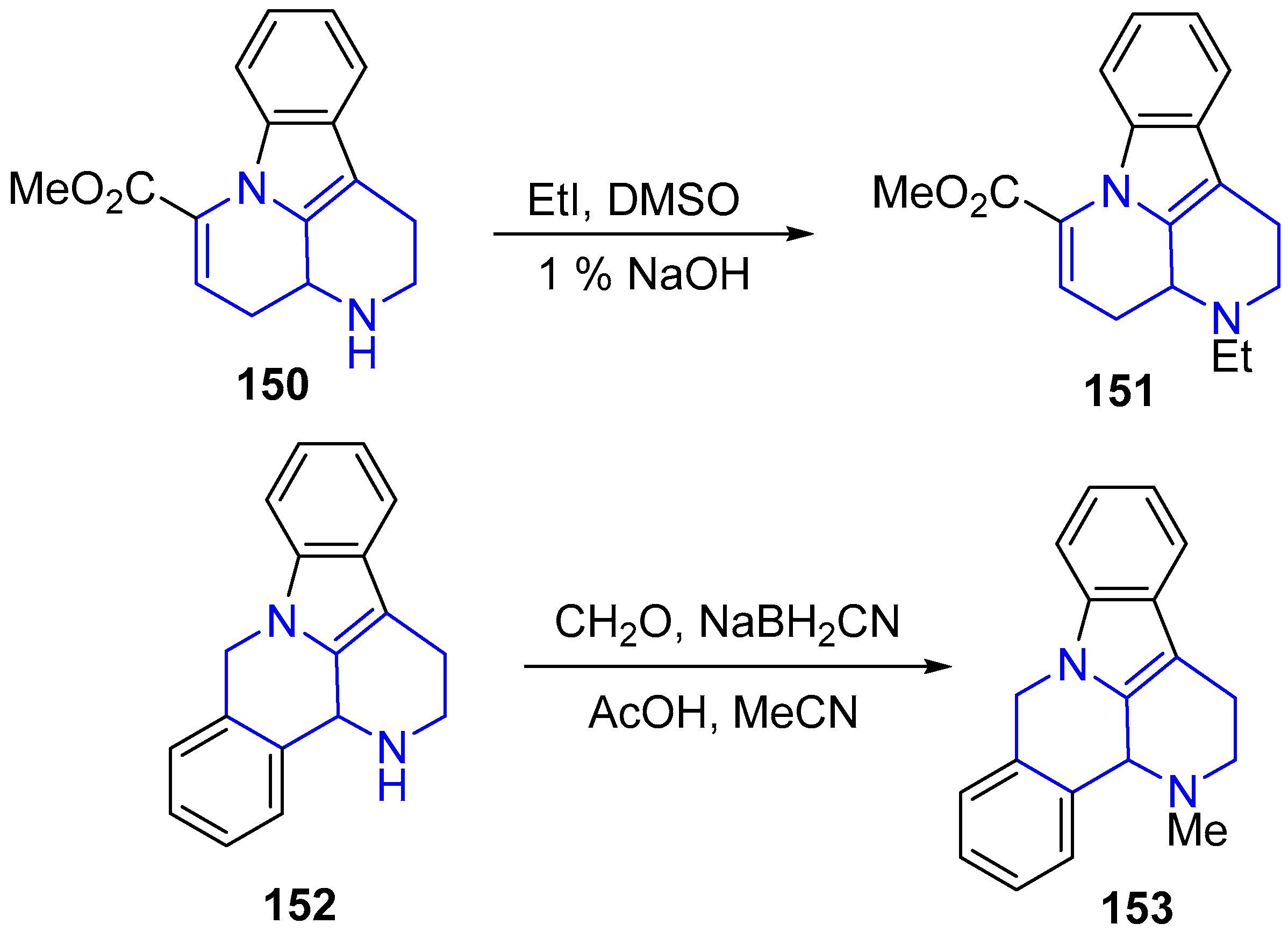
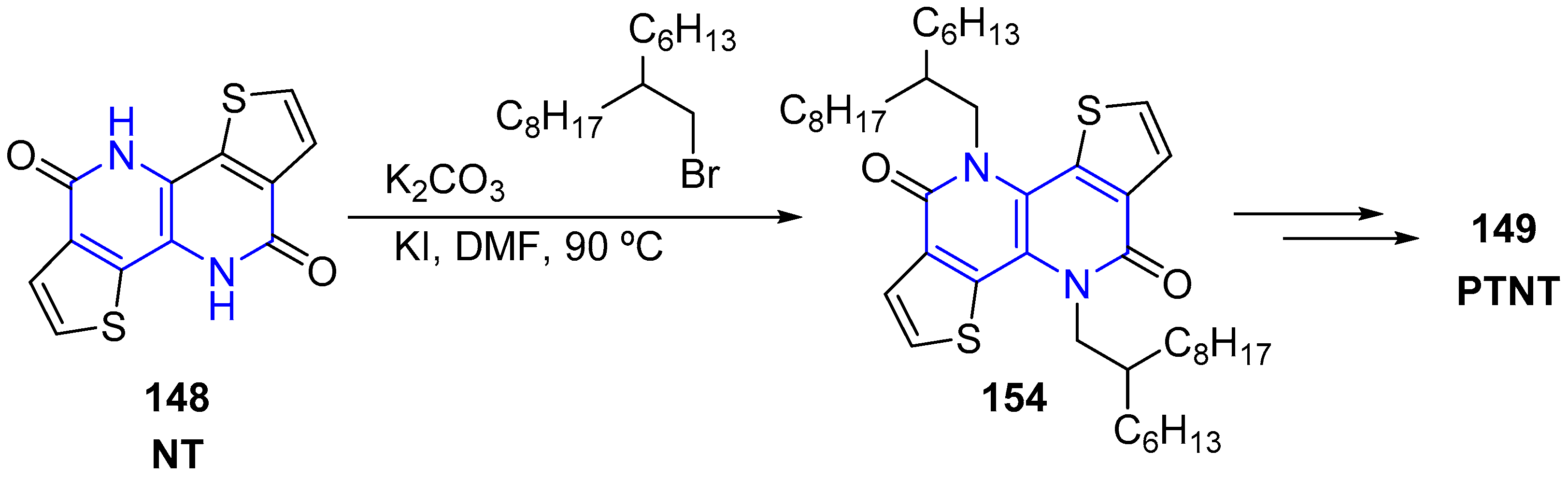
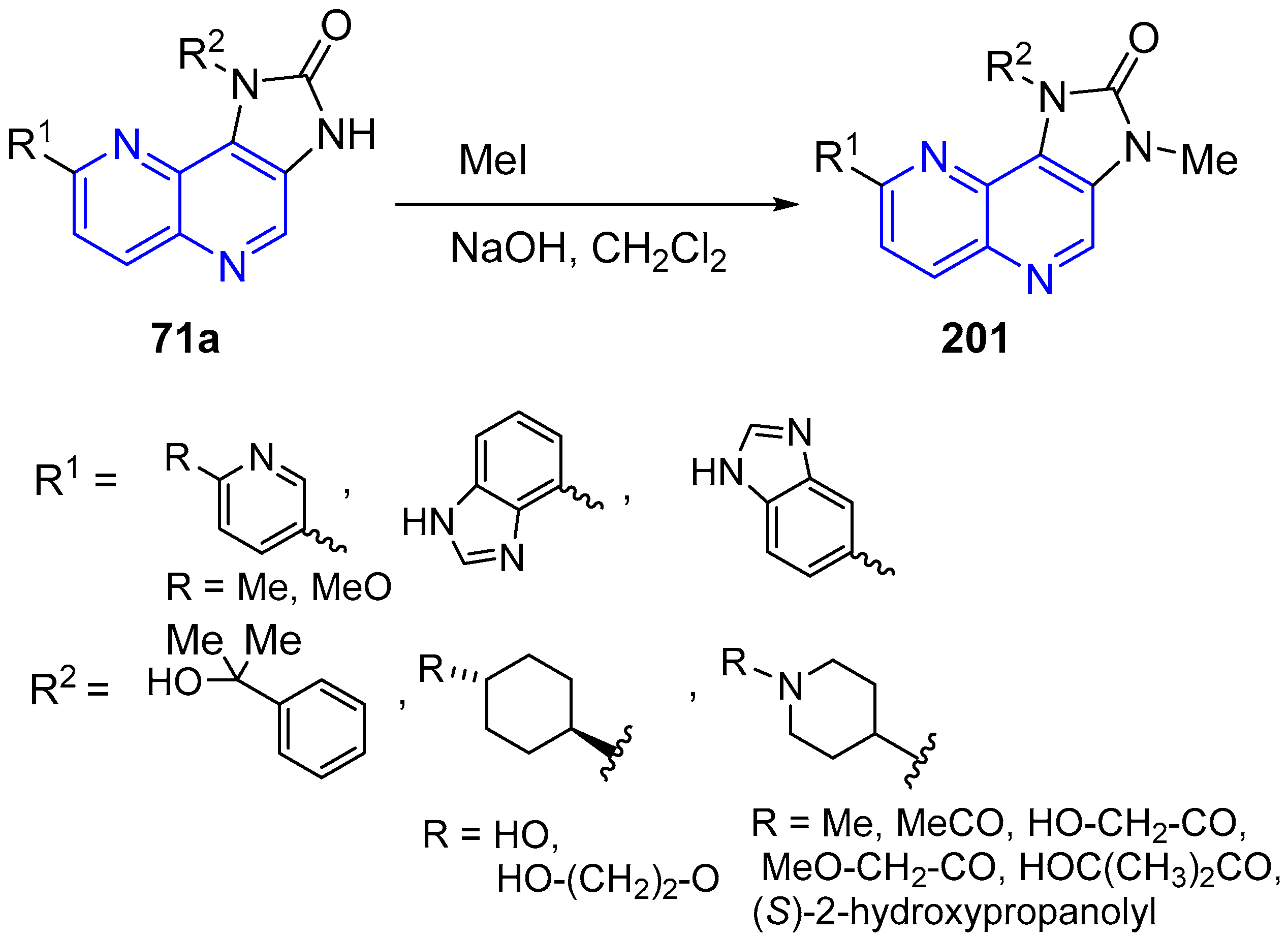
3.1. N-Alkylation
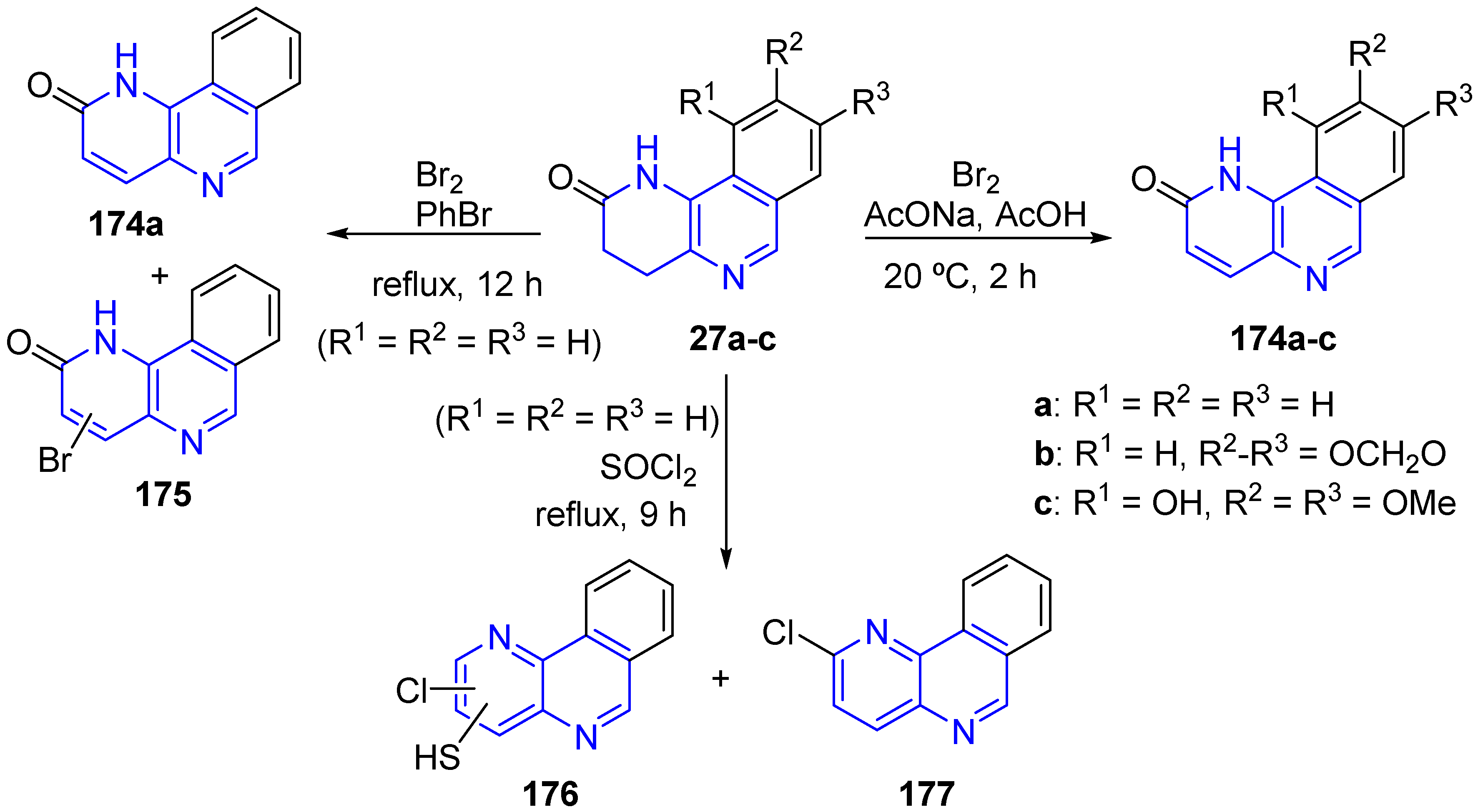
3.2. Electrophilic Substitution Reactions (SEAr)
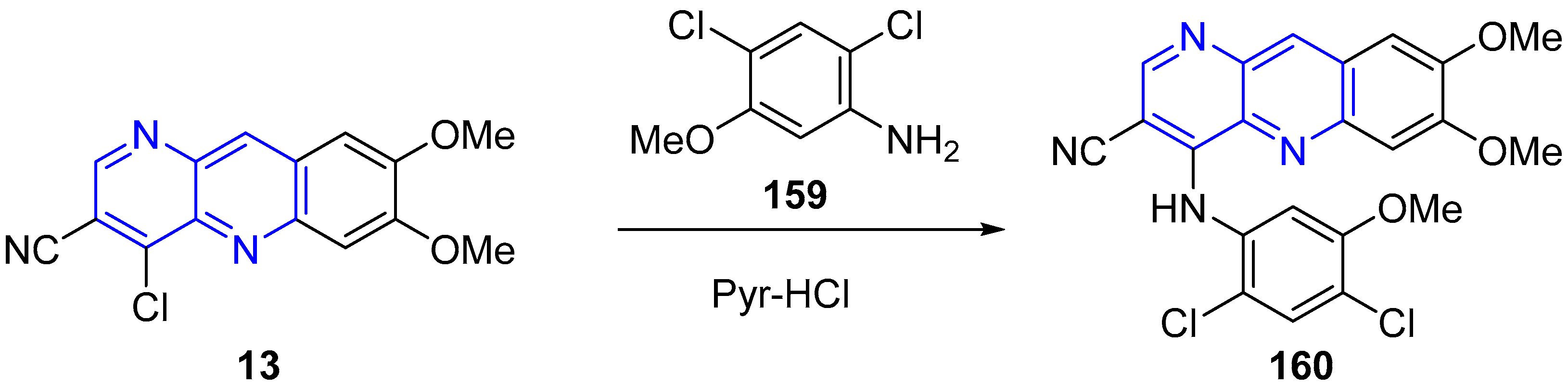
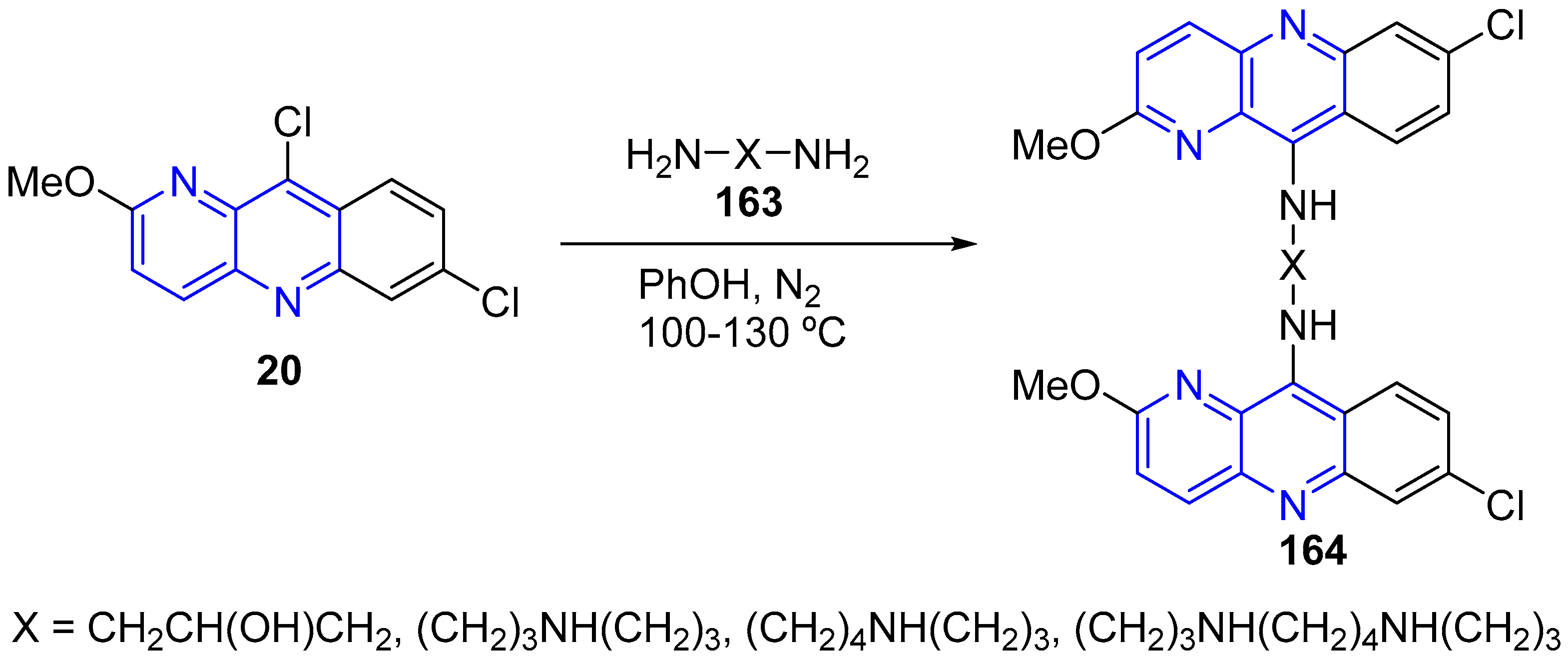
3.3. Nucleophilic Substitution Reactions (SNAr)
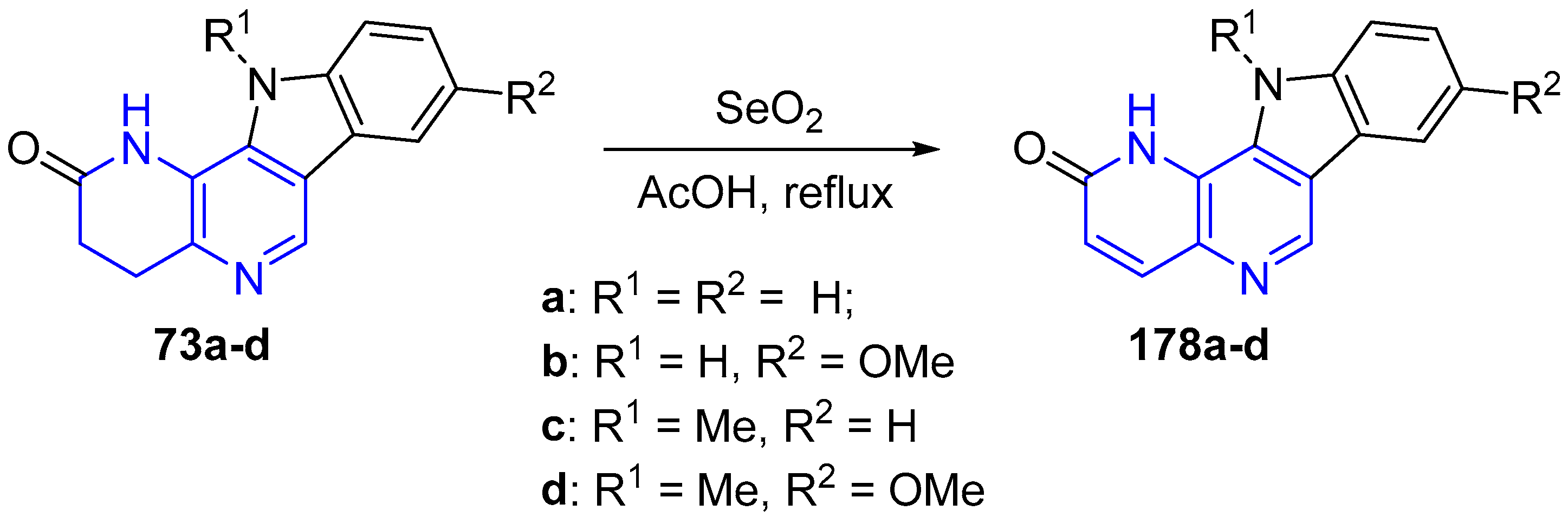
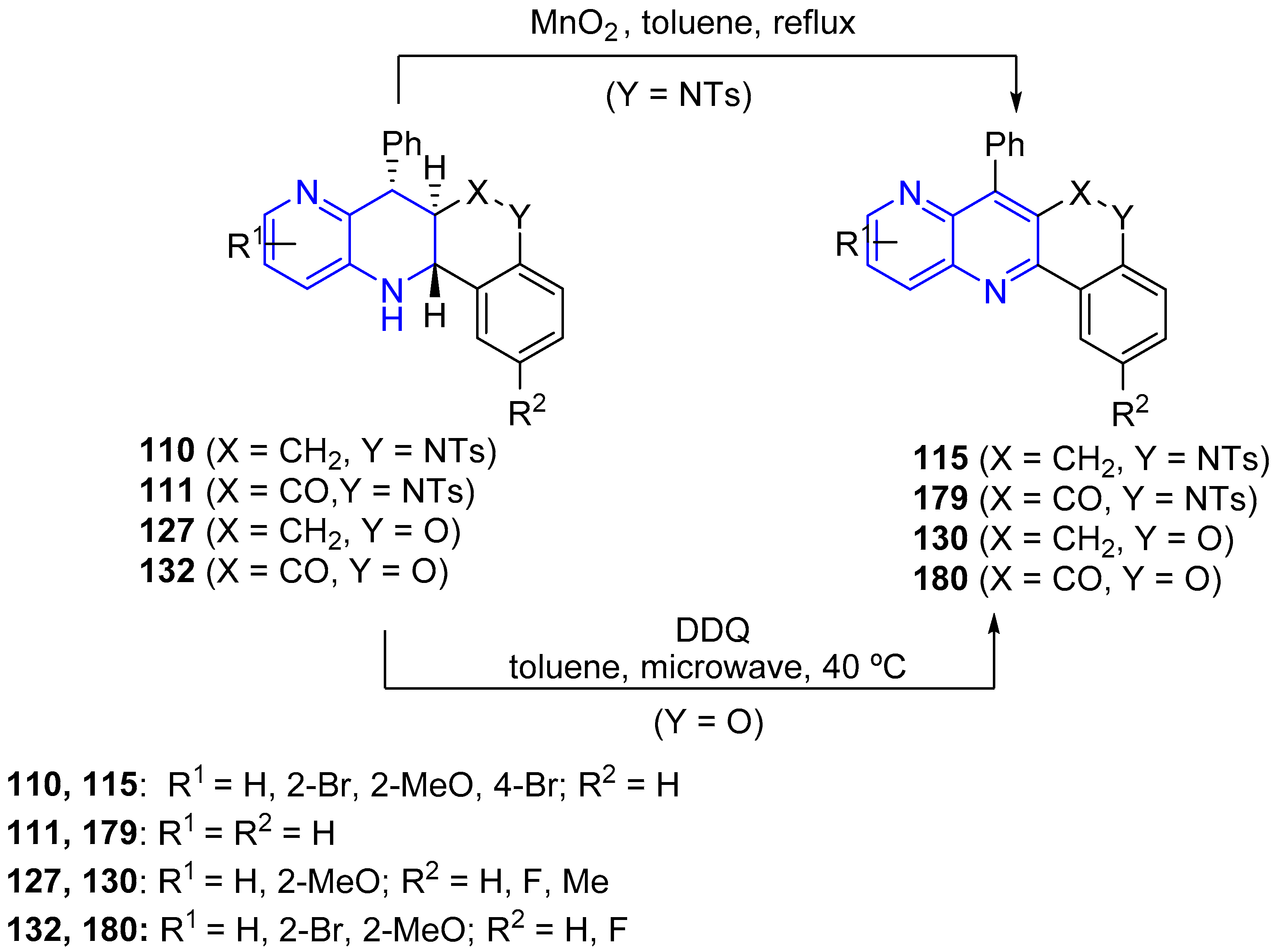
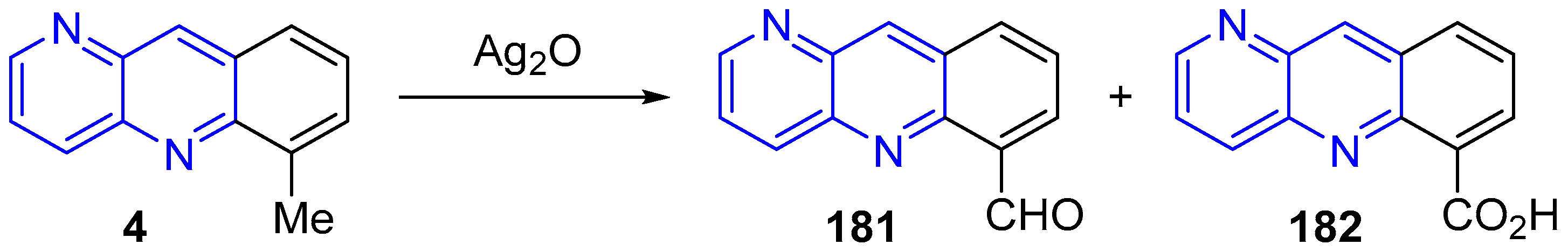
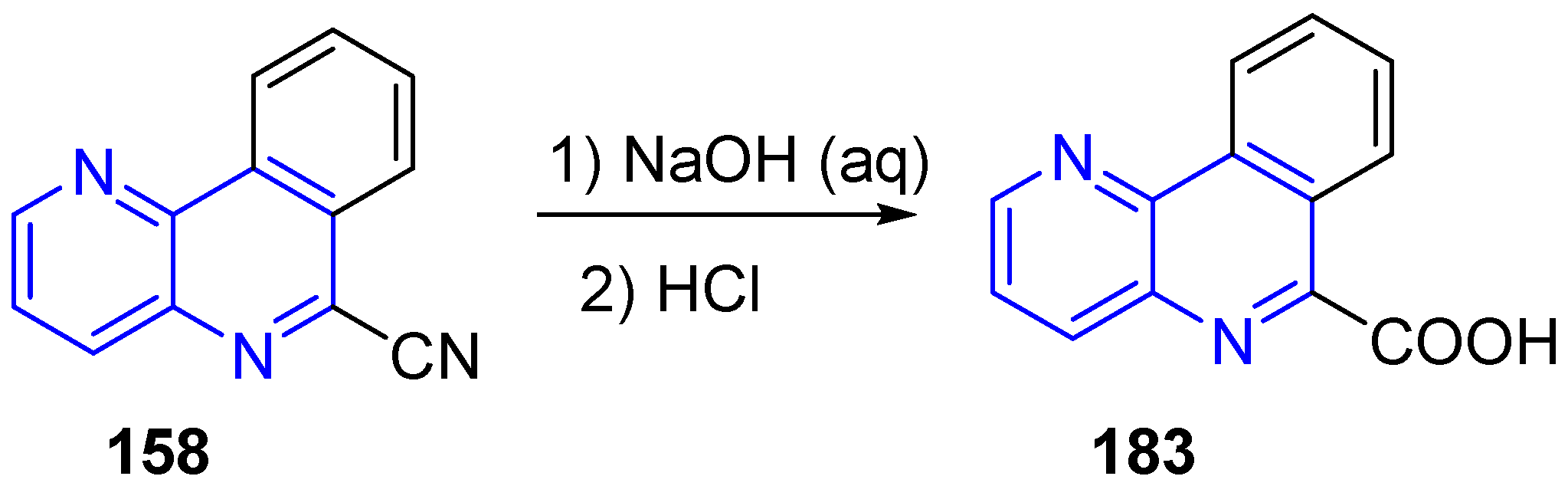
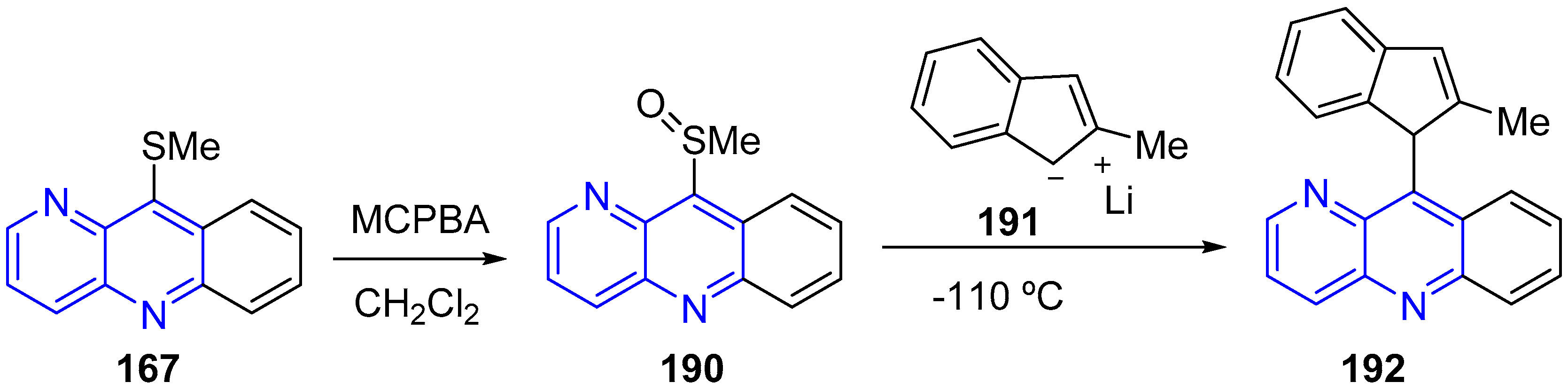
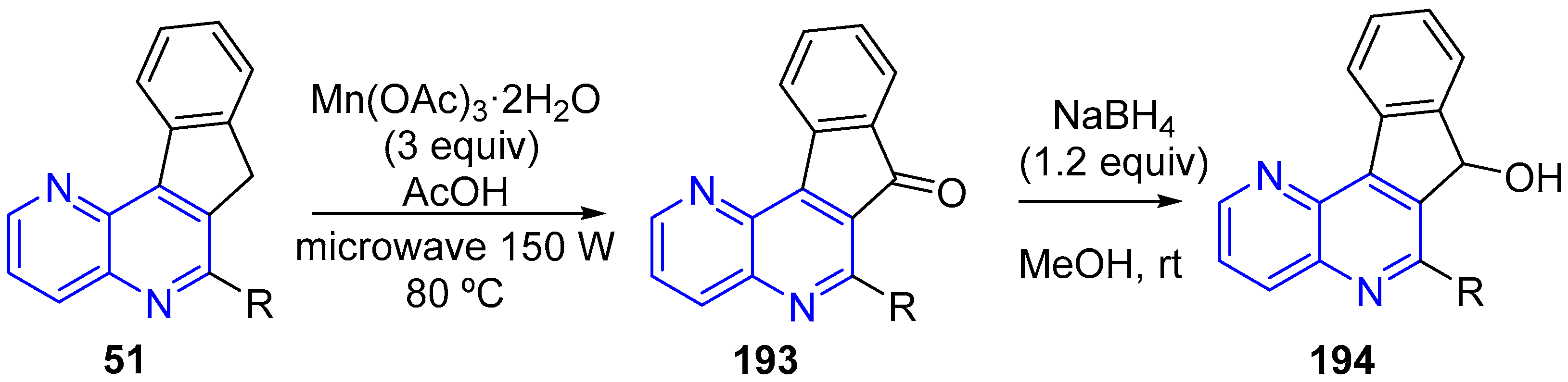
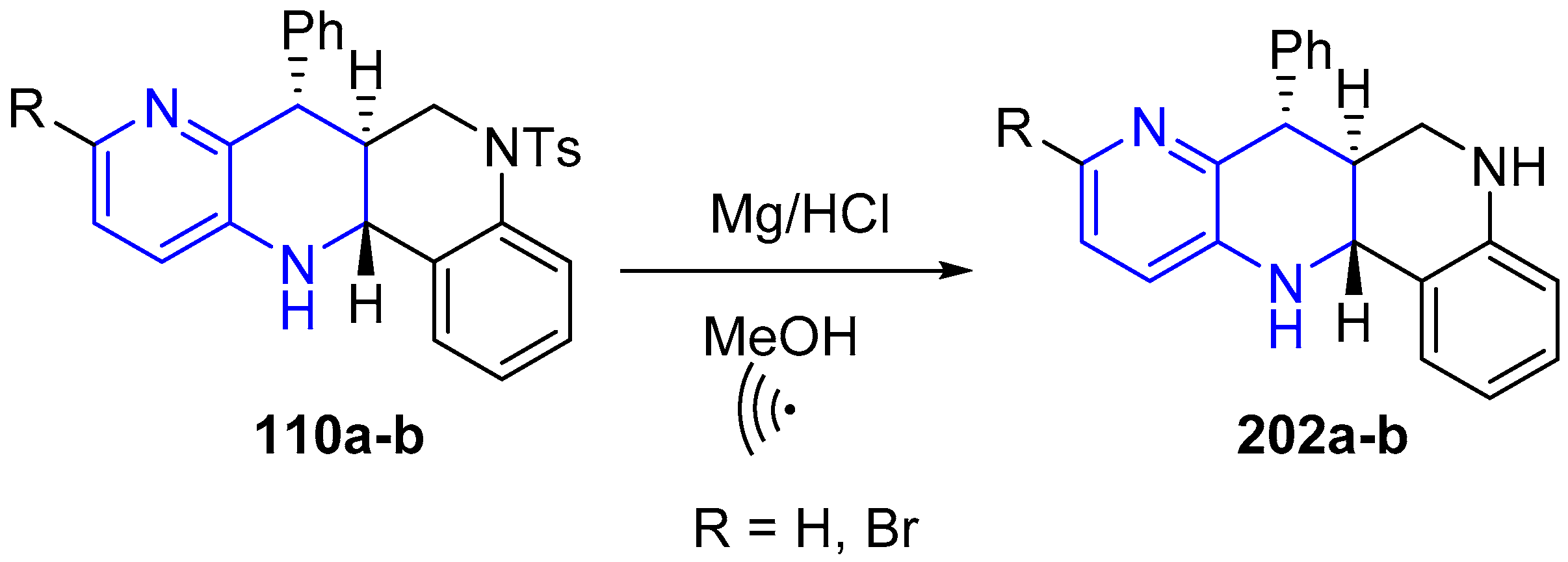
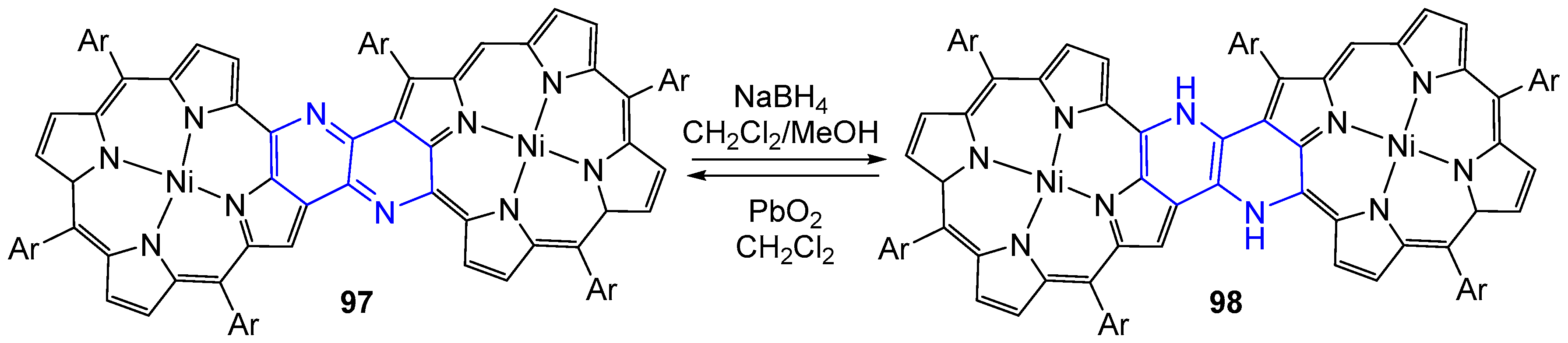
3.4. Oxidation and Reduction
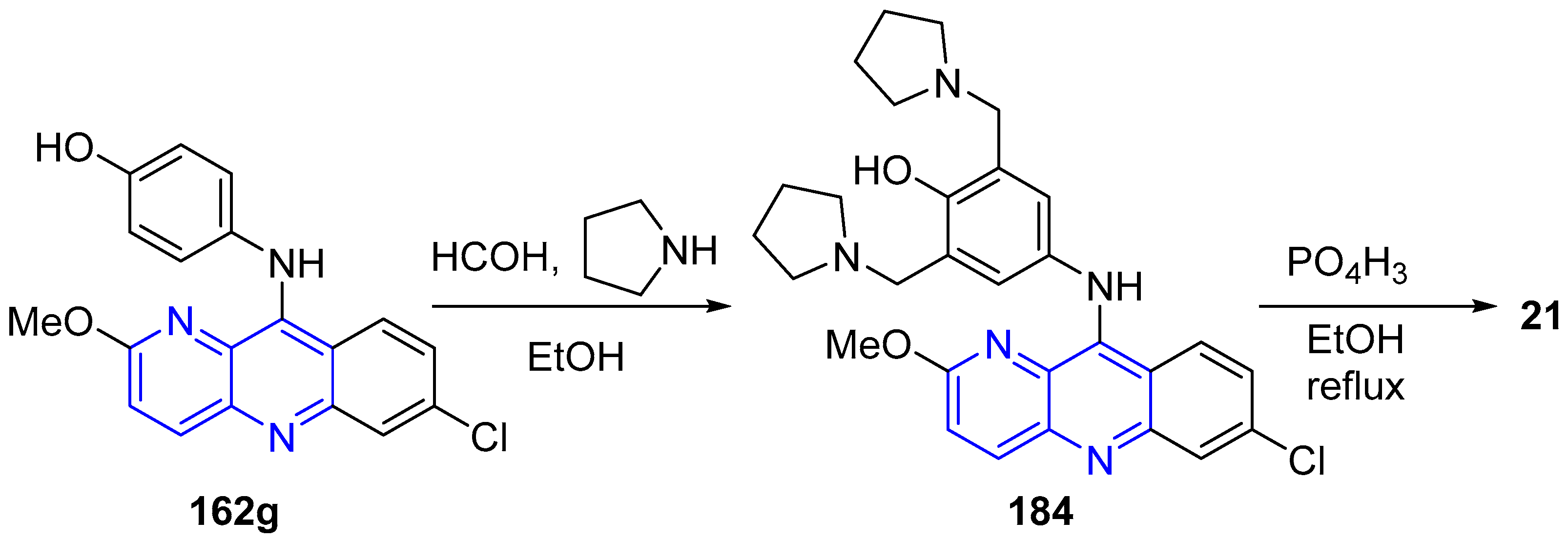
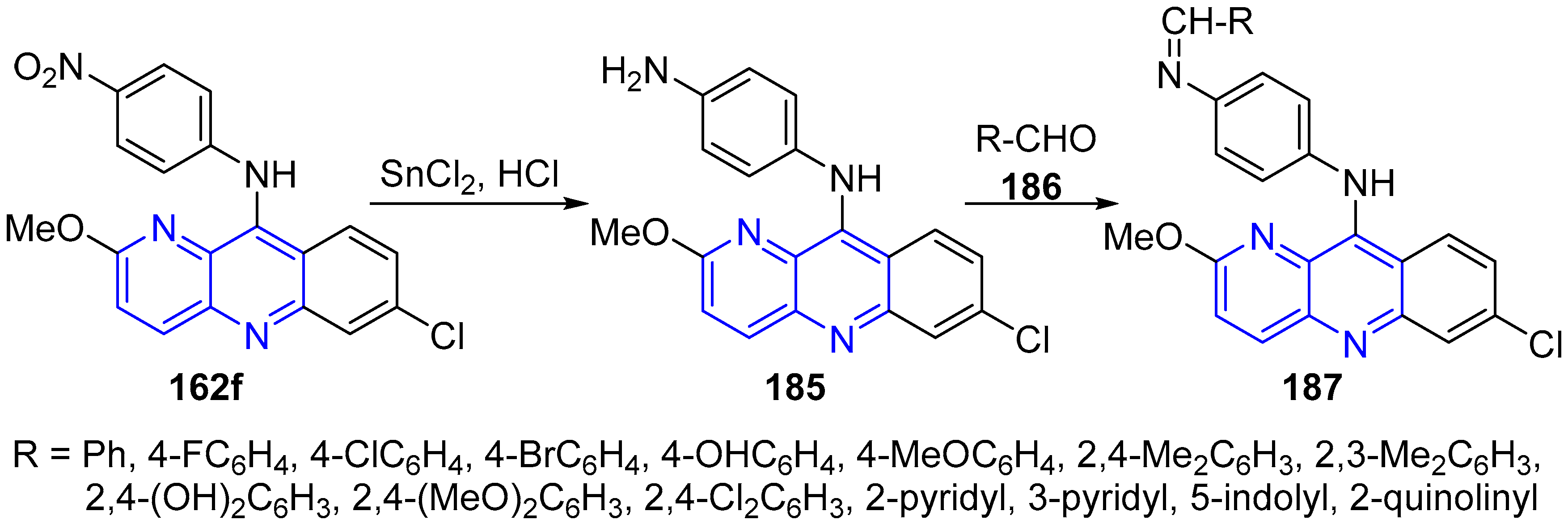
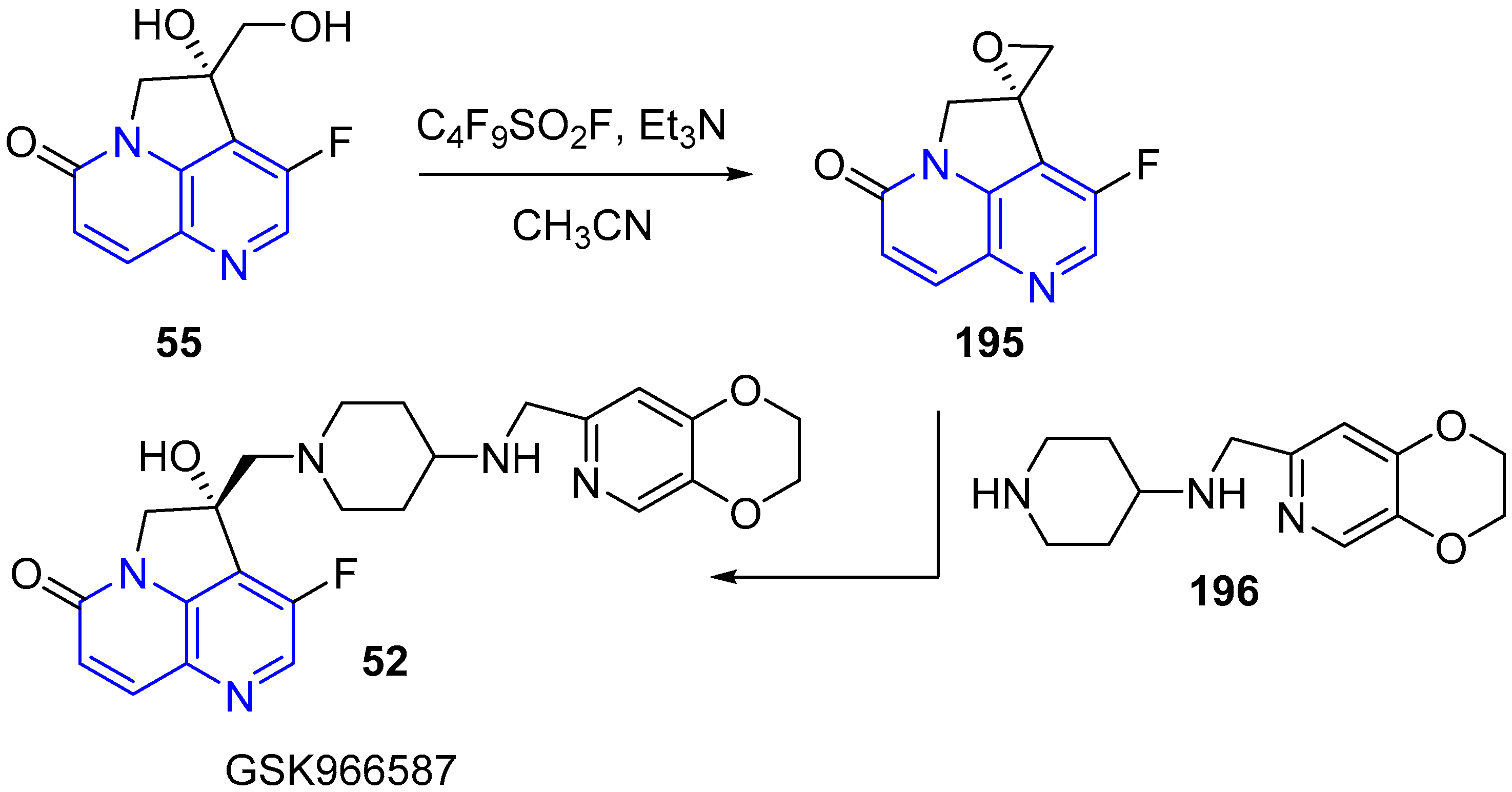
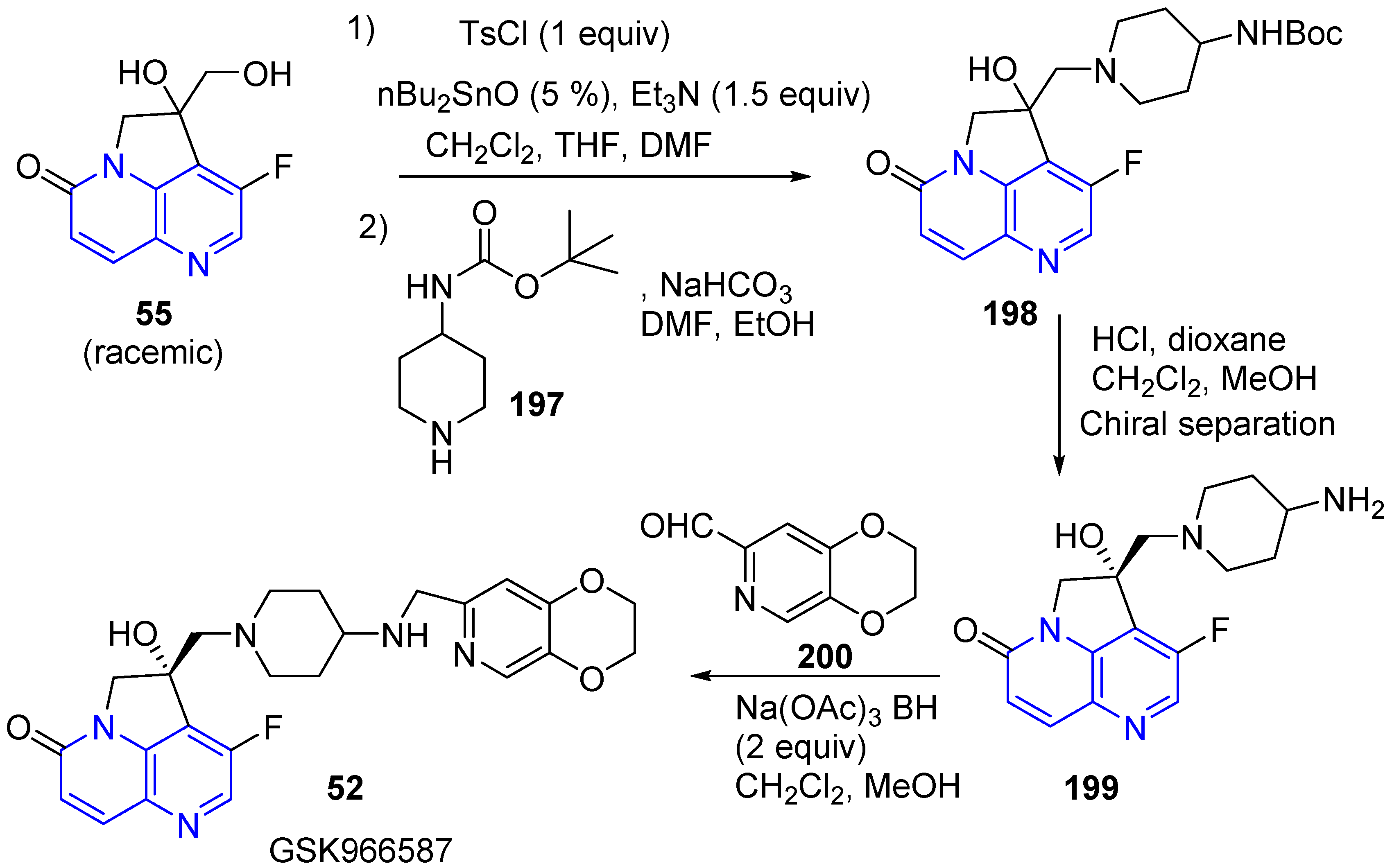
3.5. Side Chain Modifications
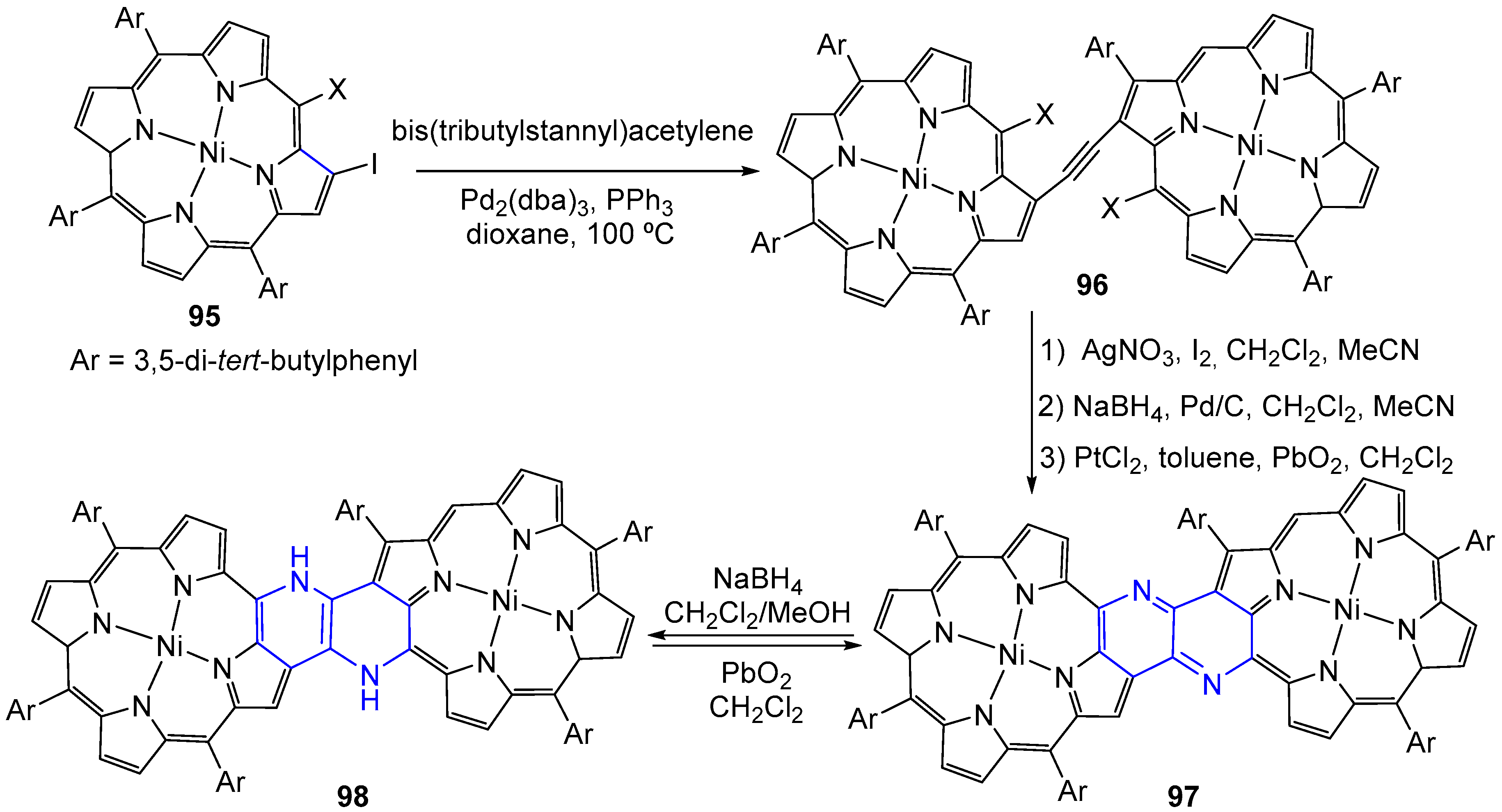
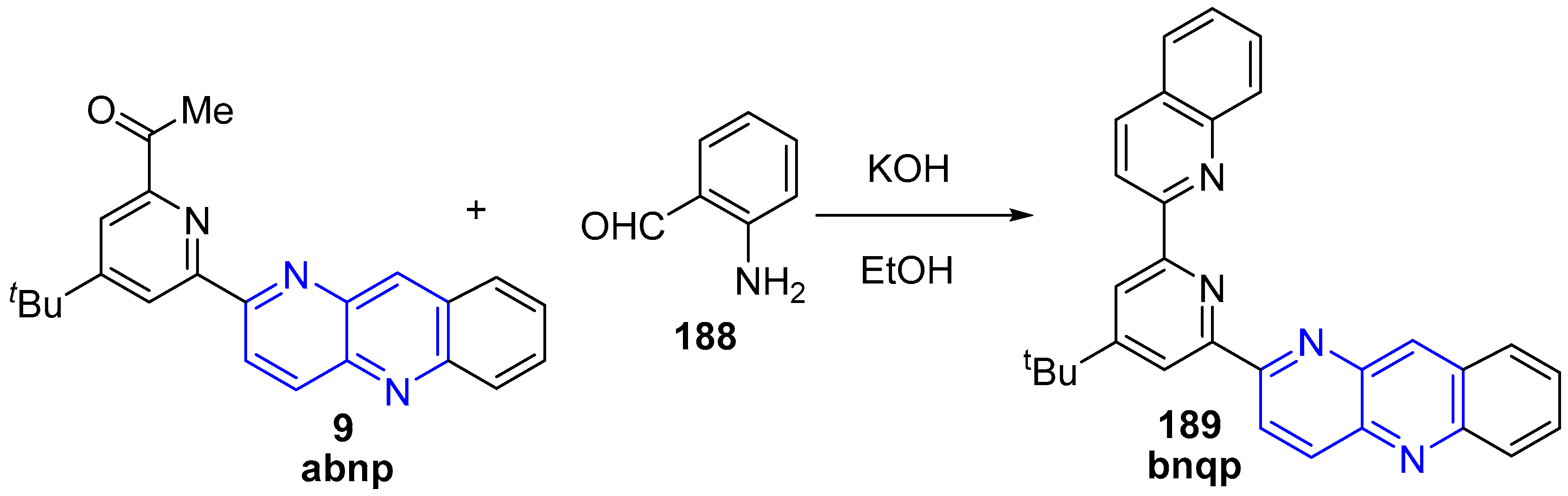
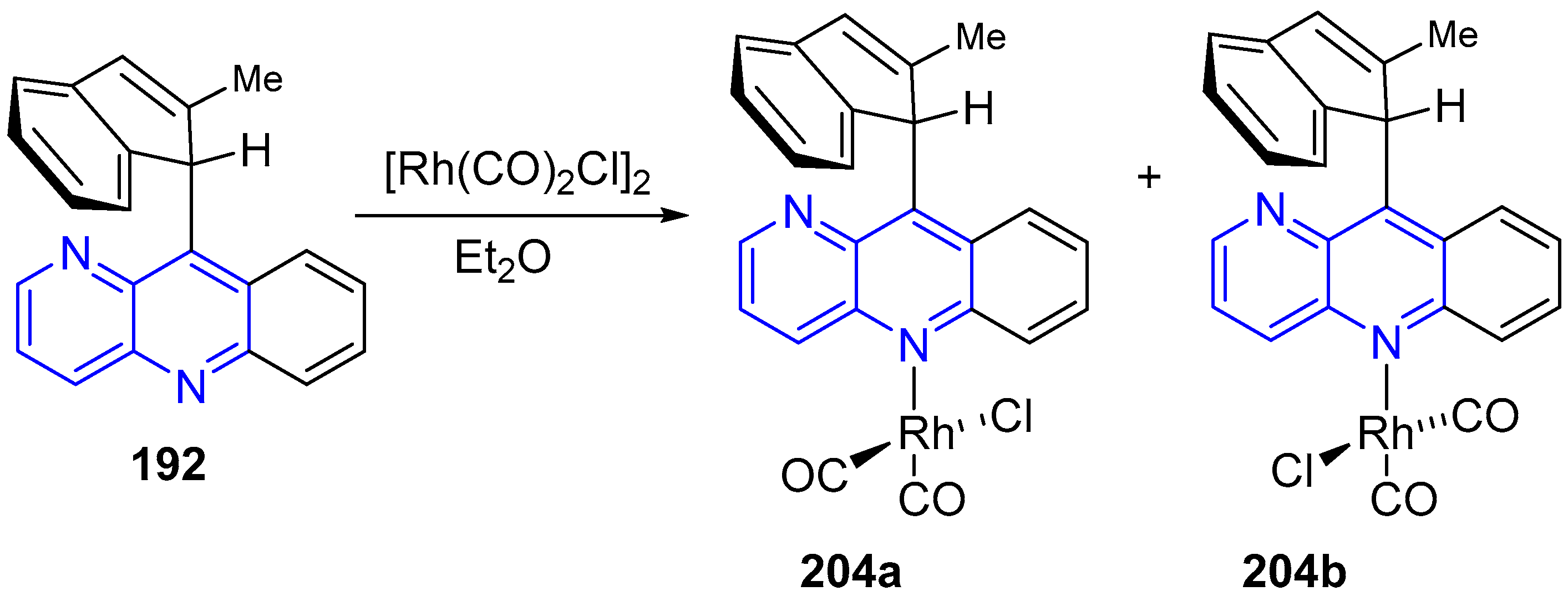
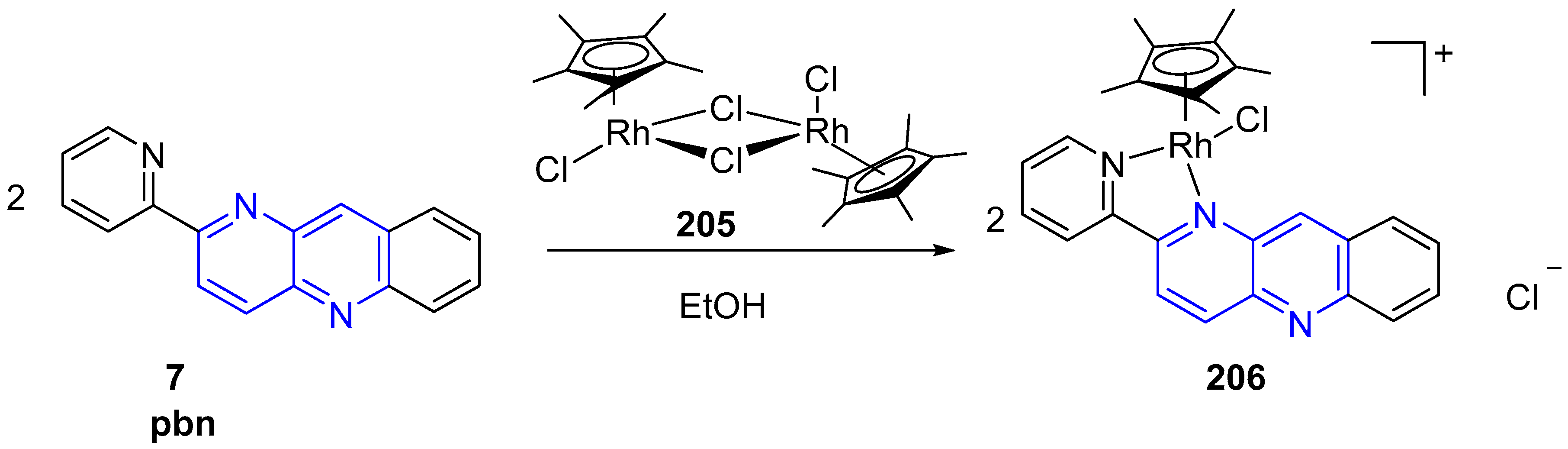
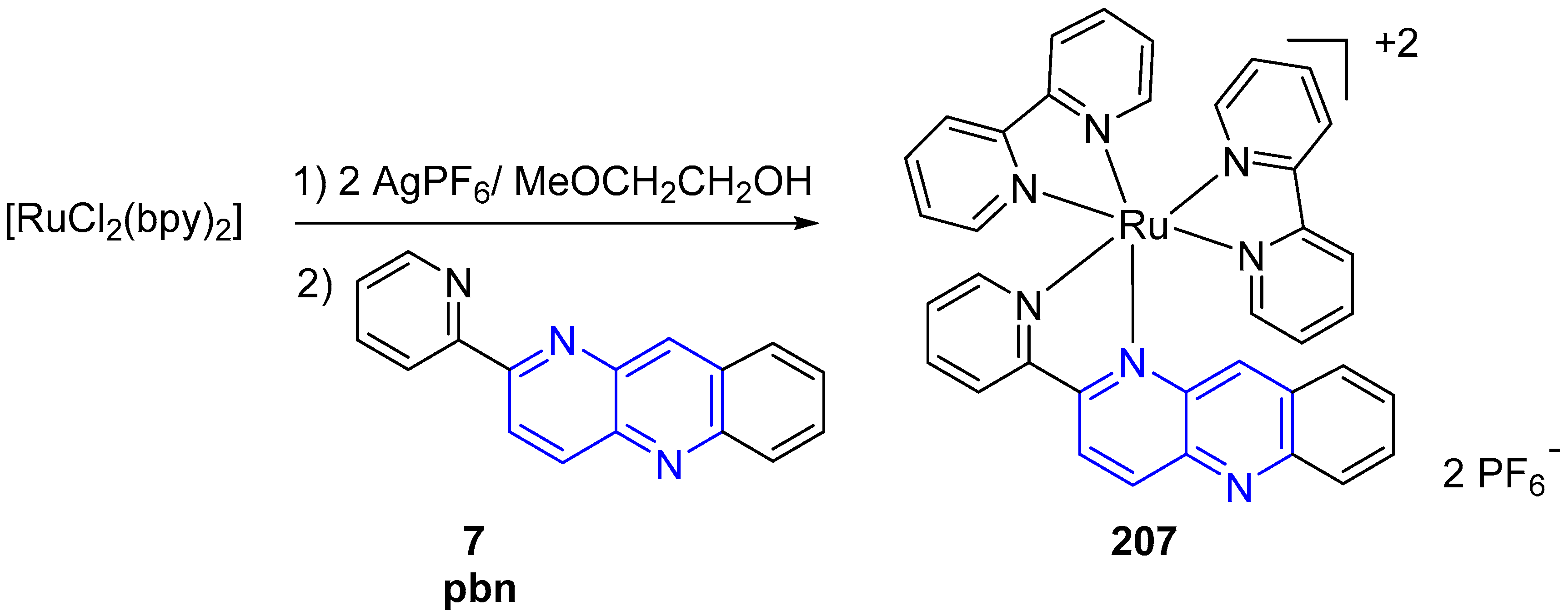
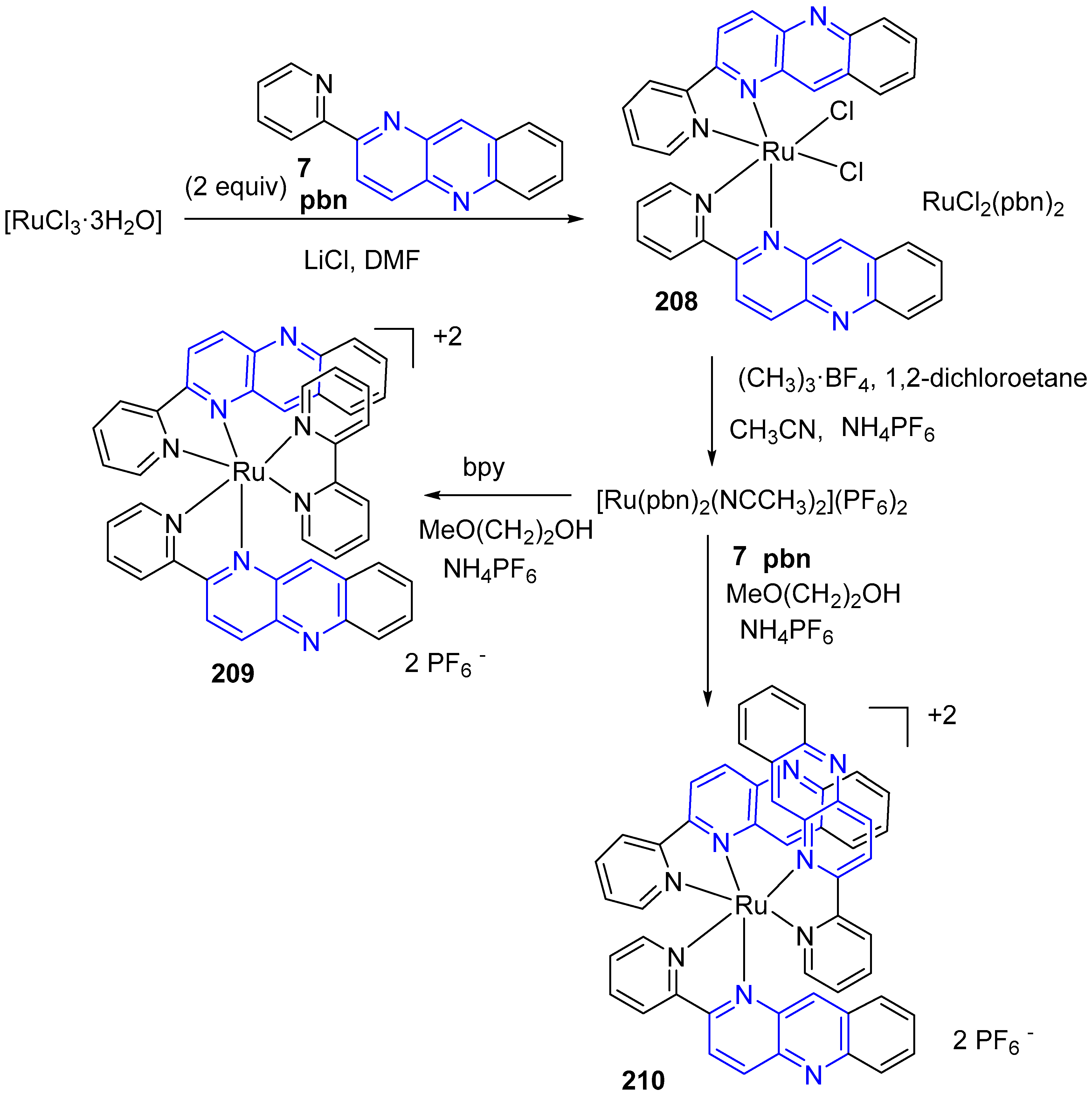
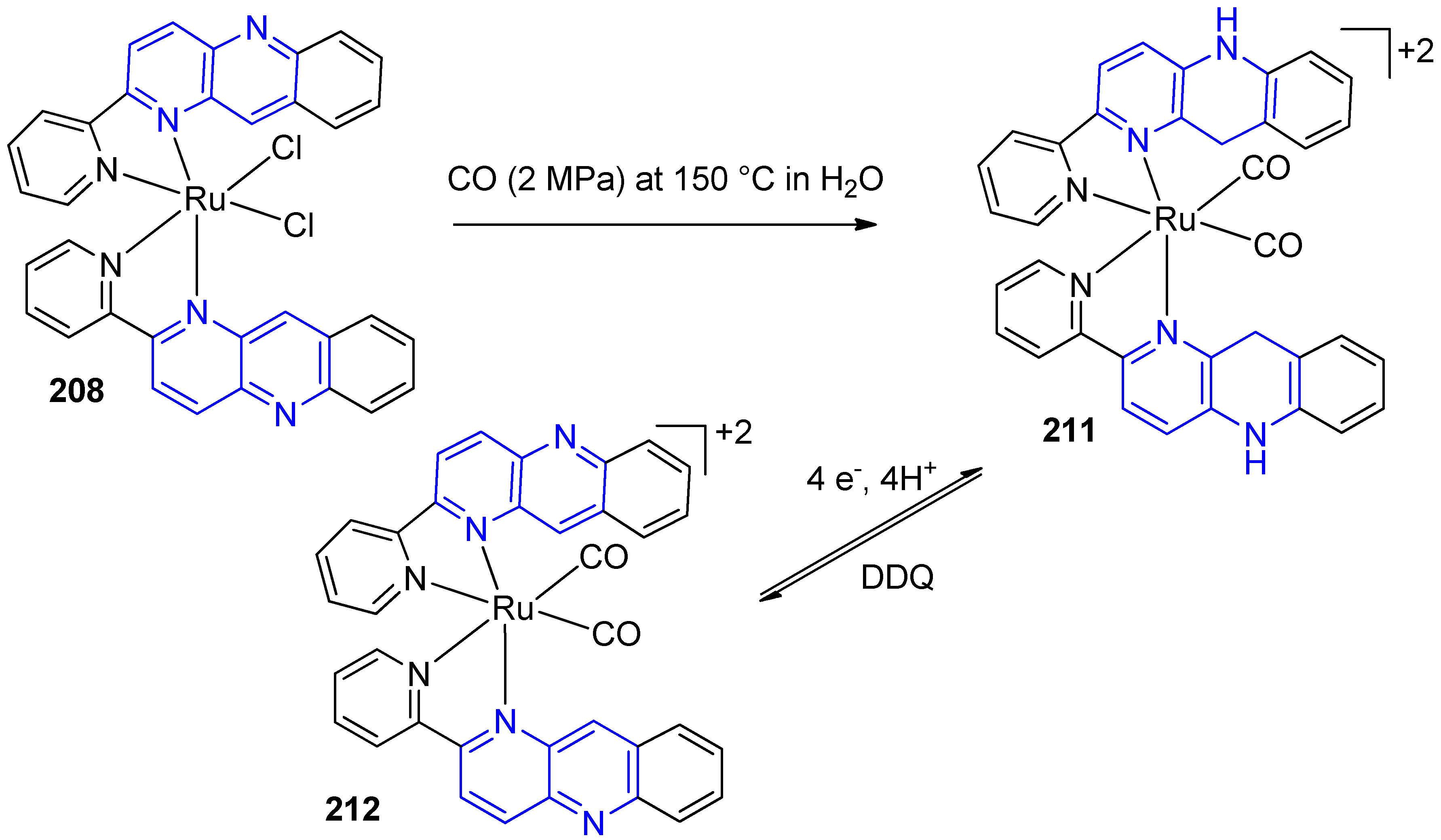
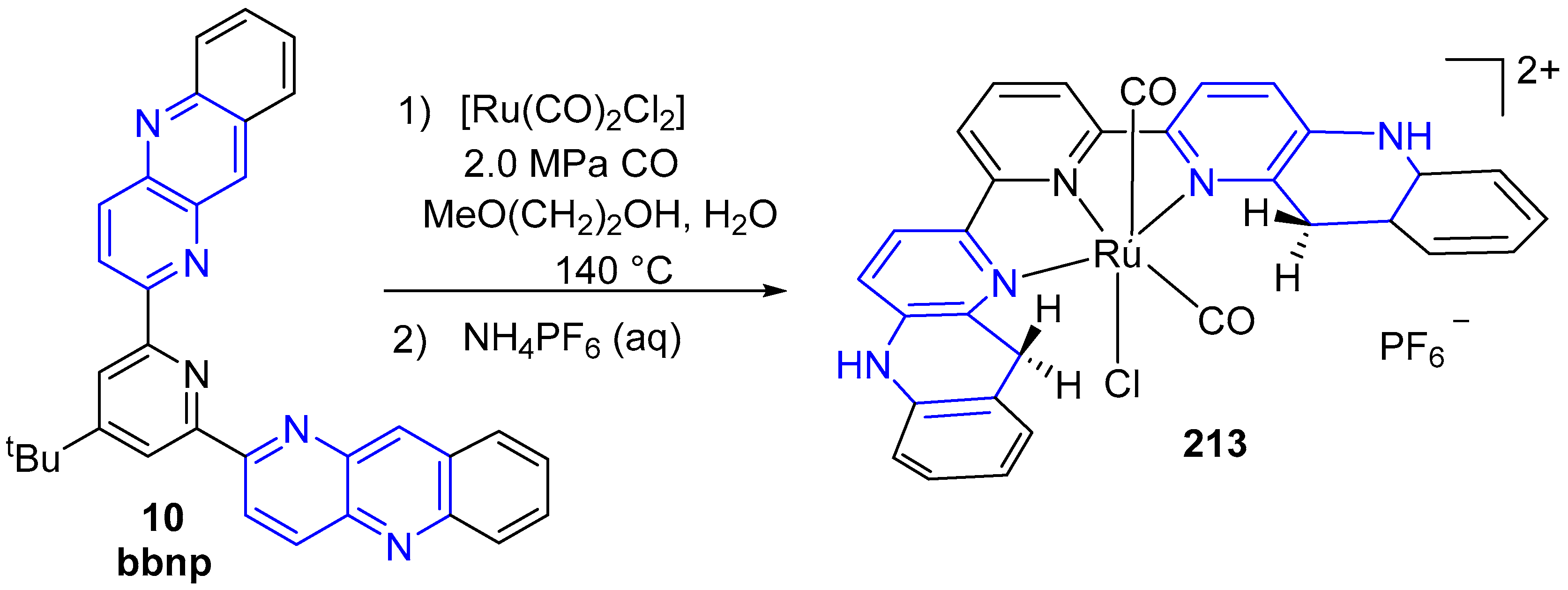
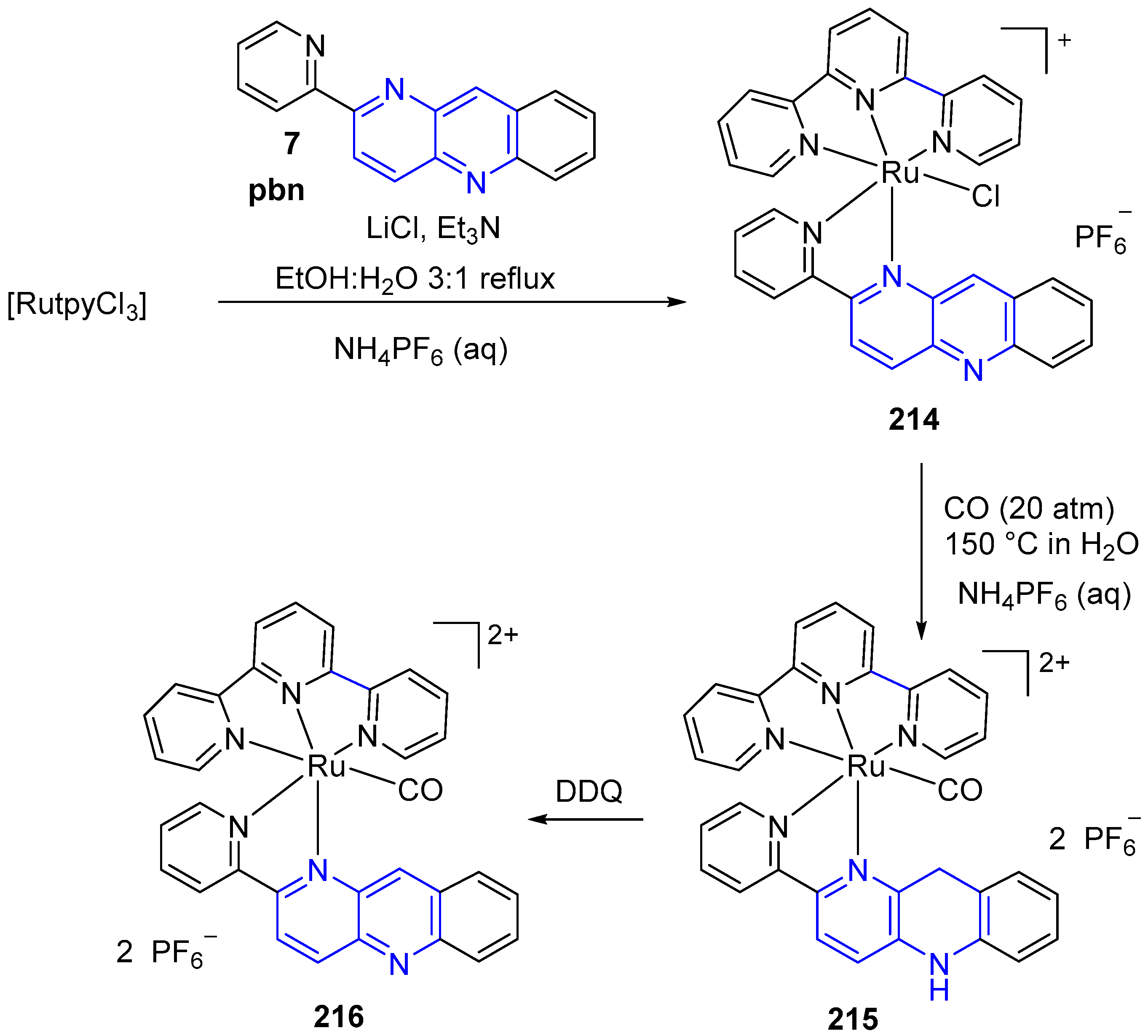
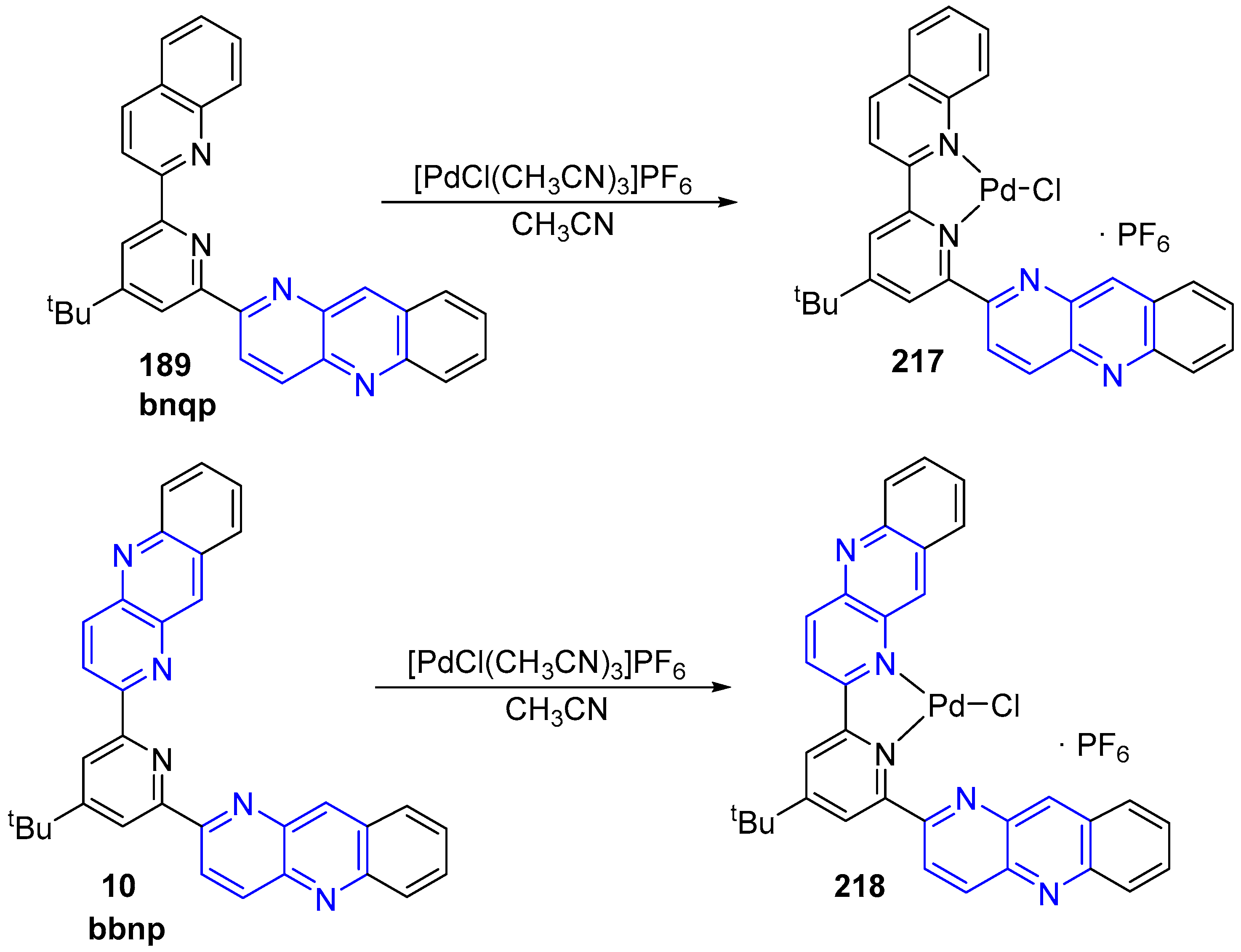
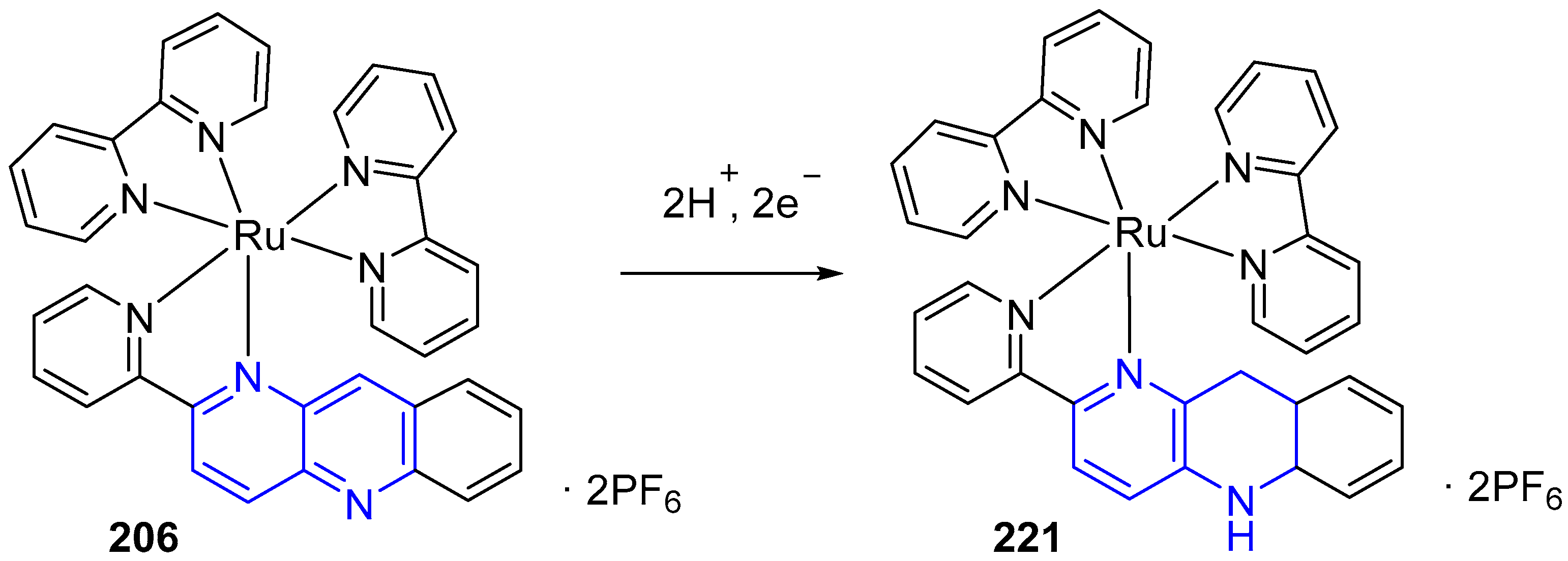
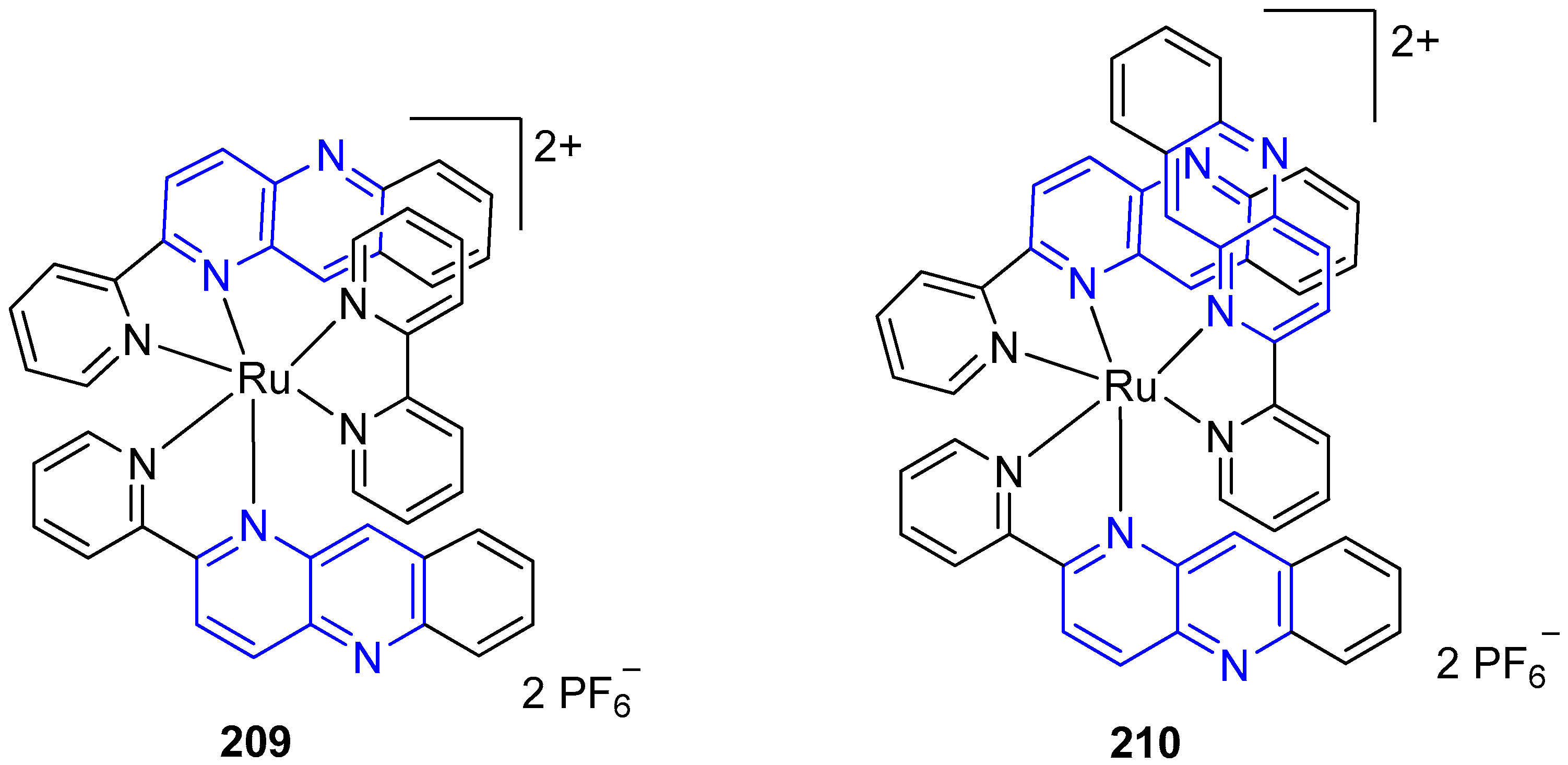
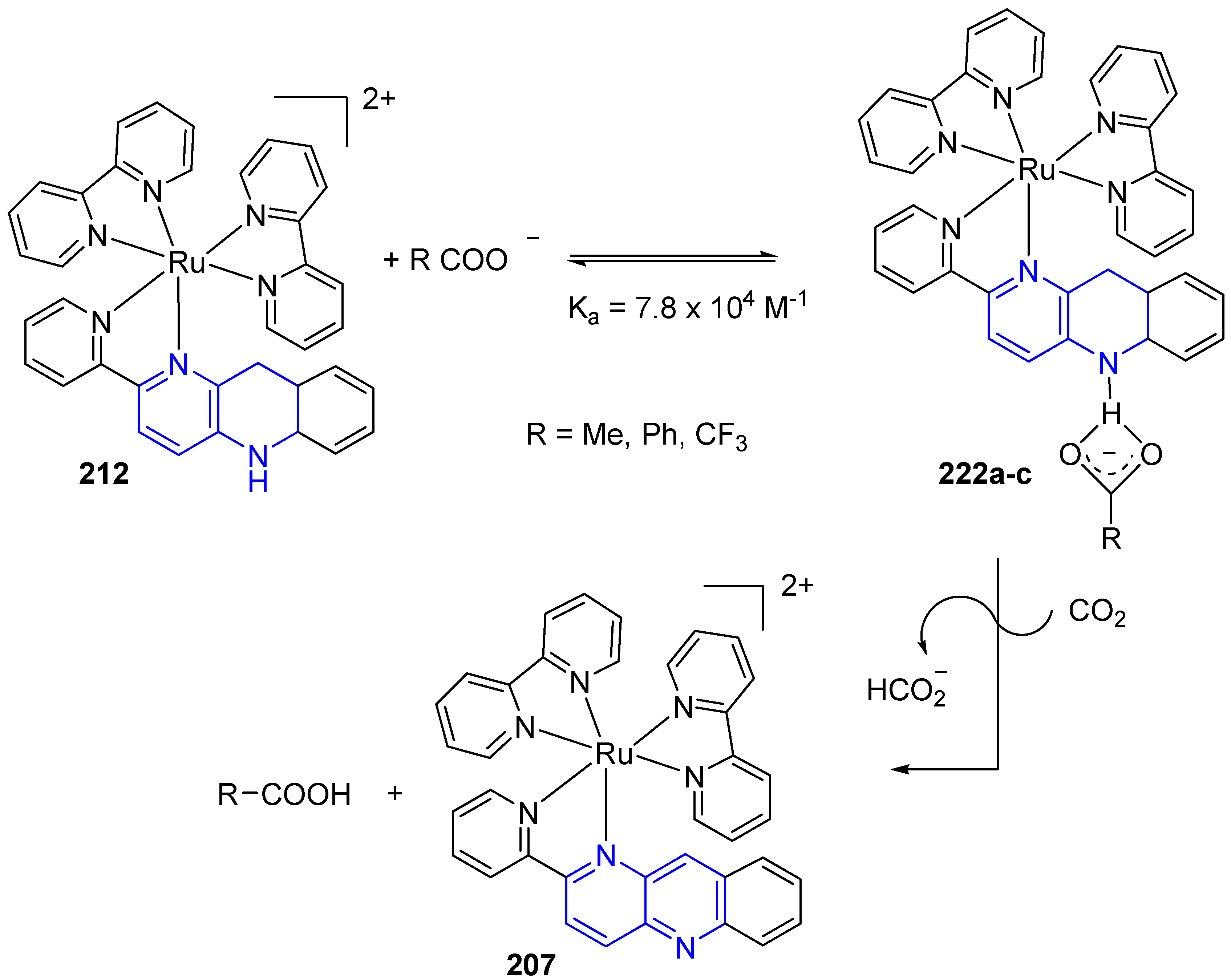
3.6. Metal Complex Formation
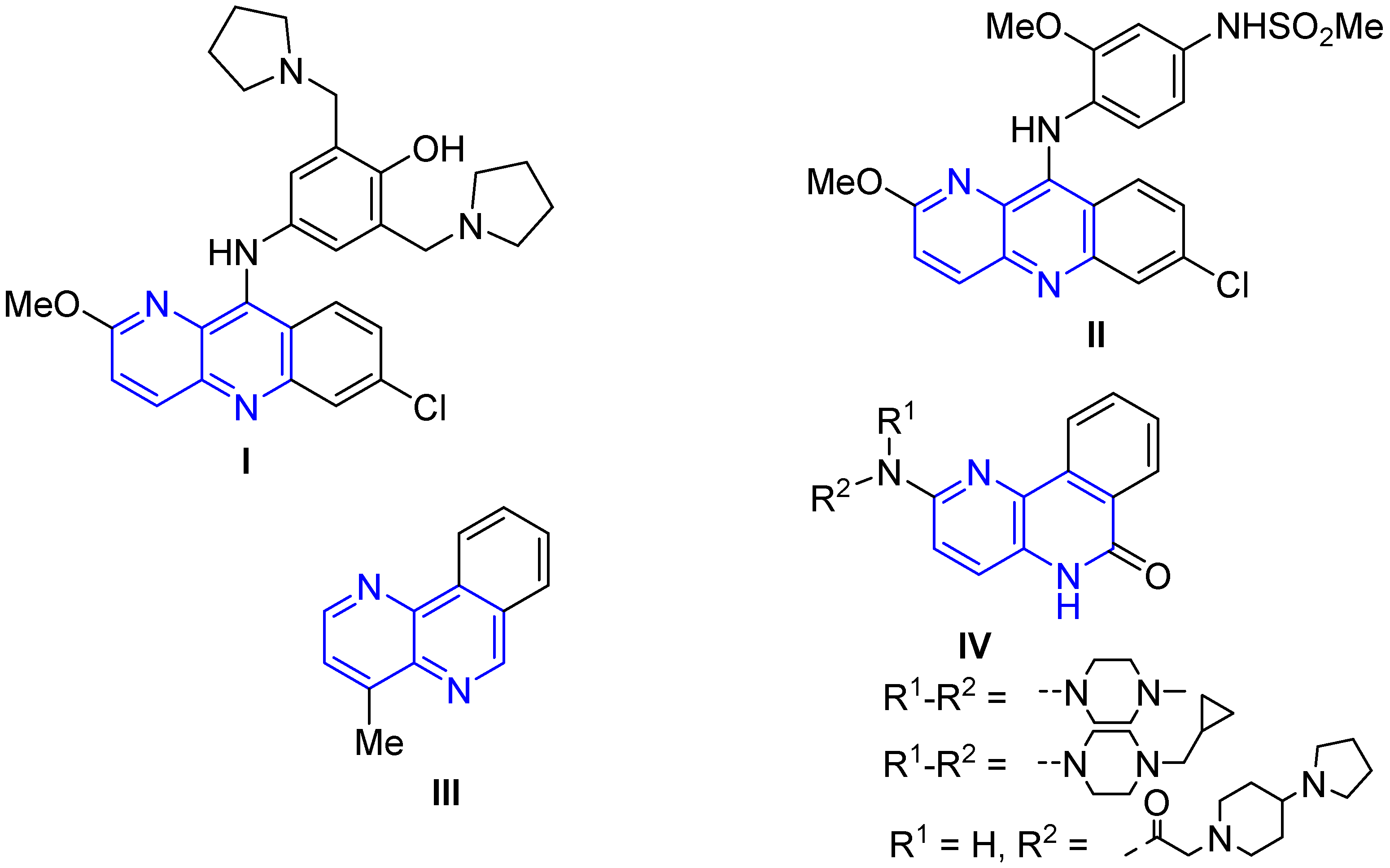
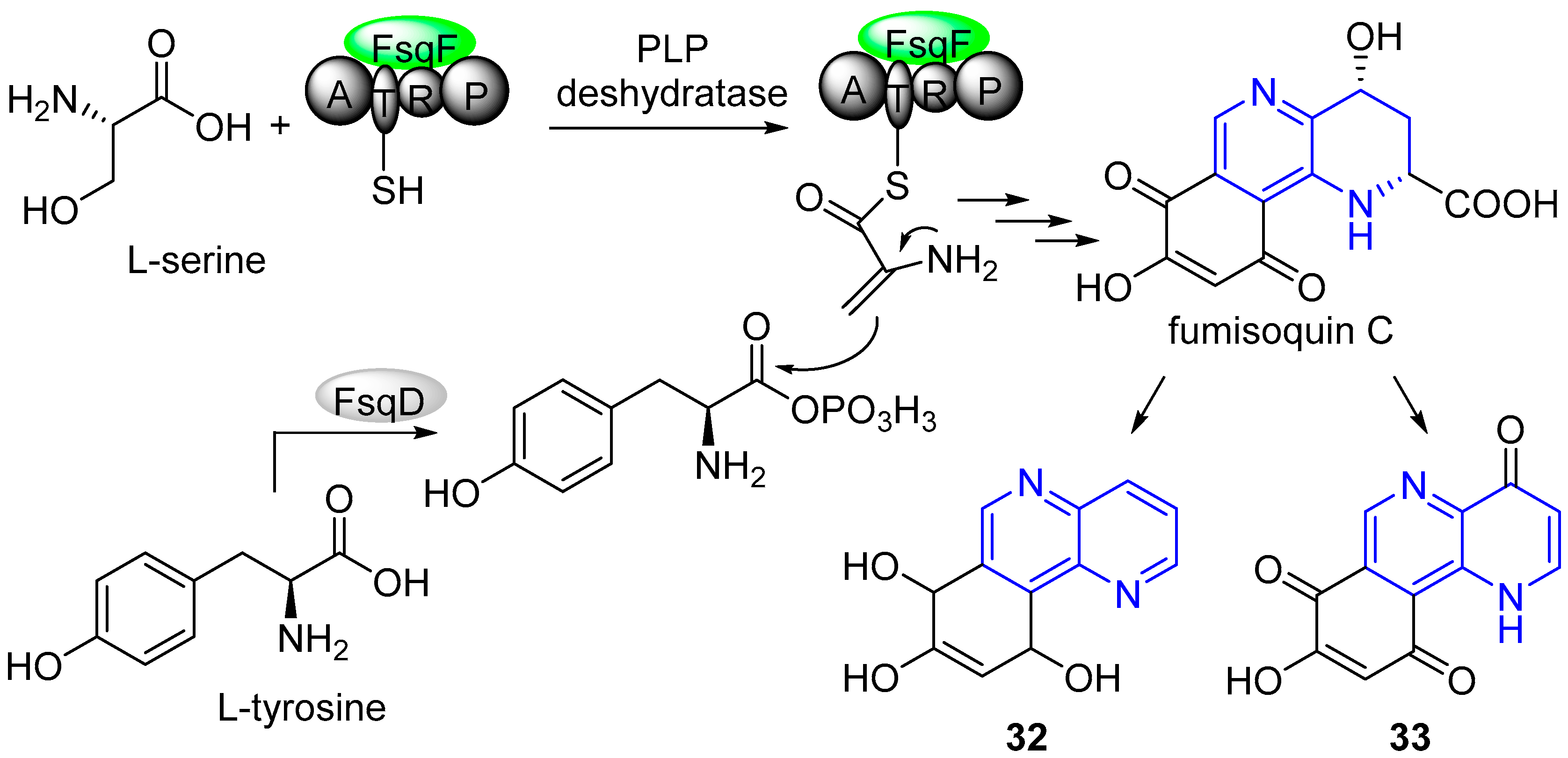
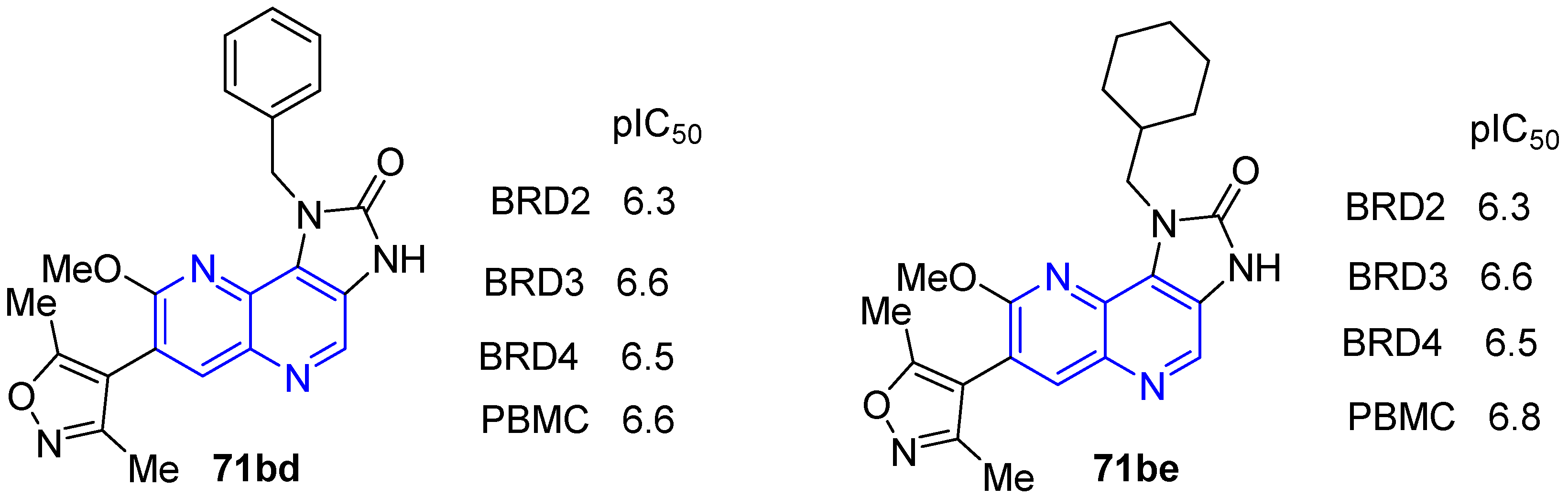
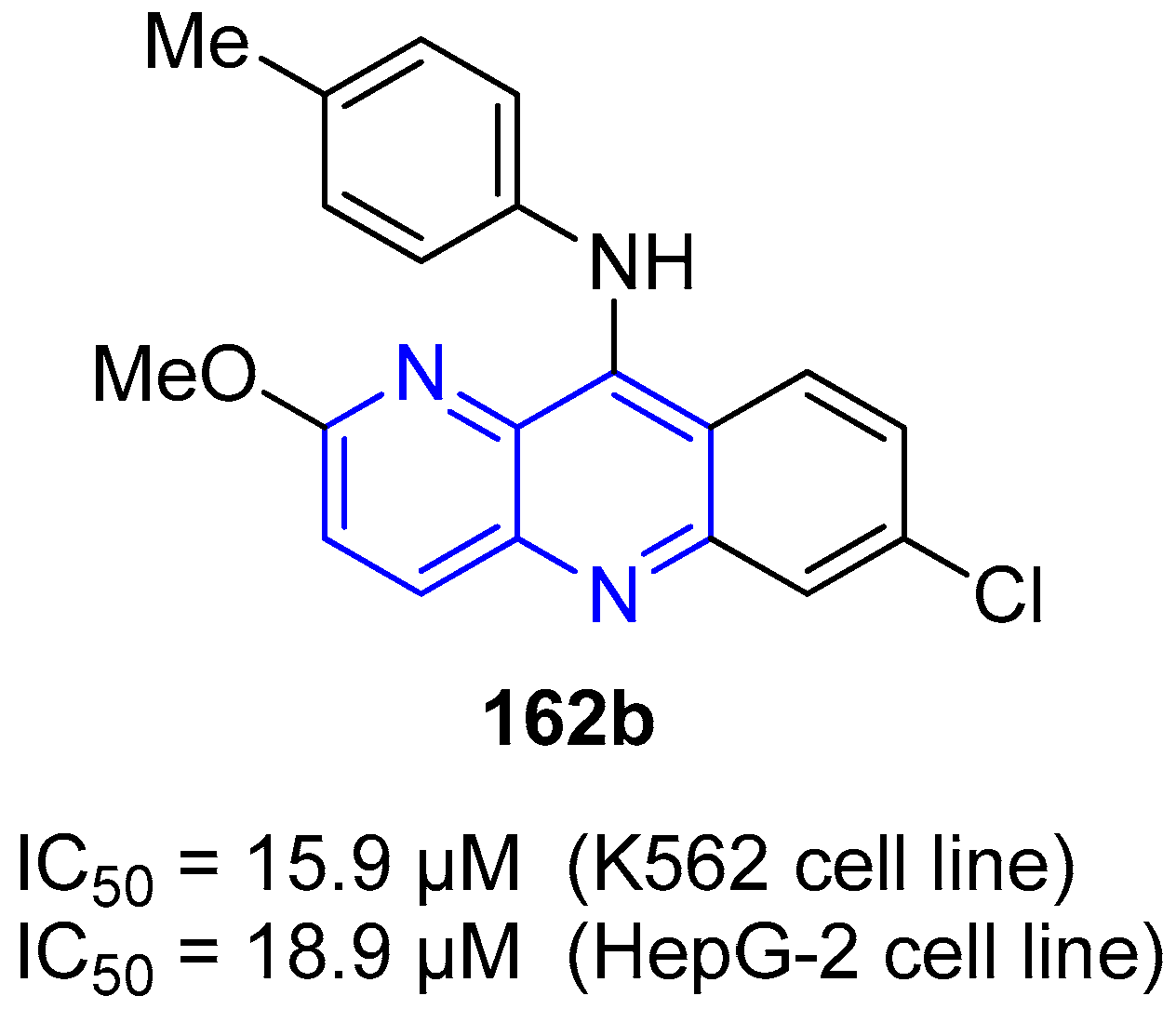
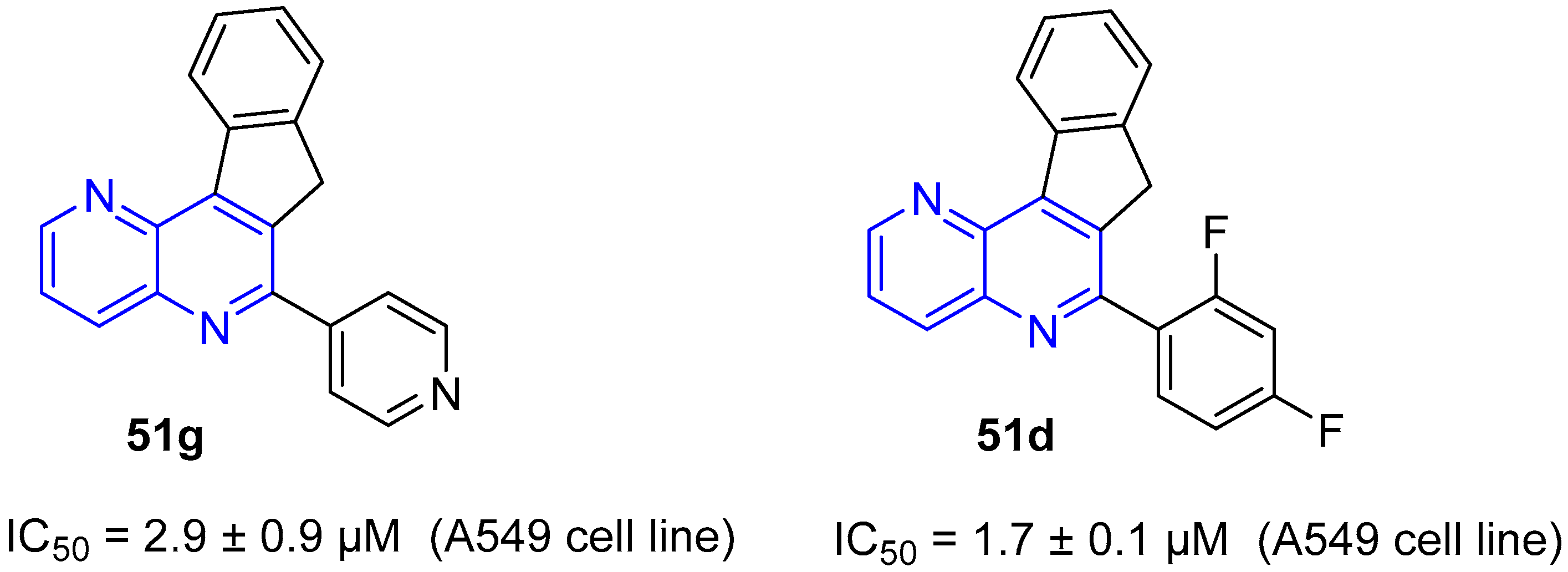
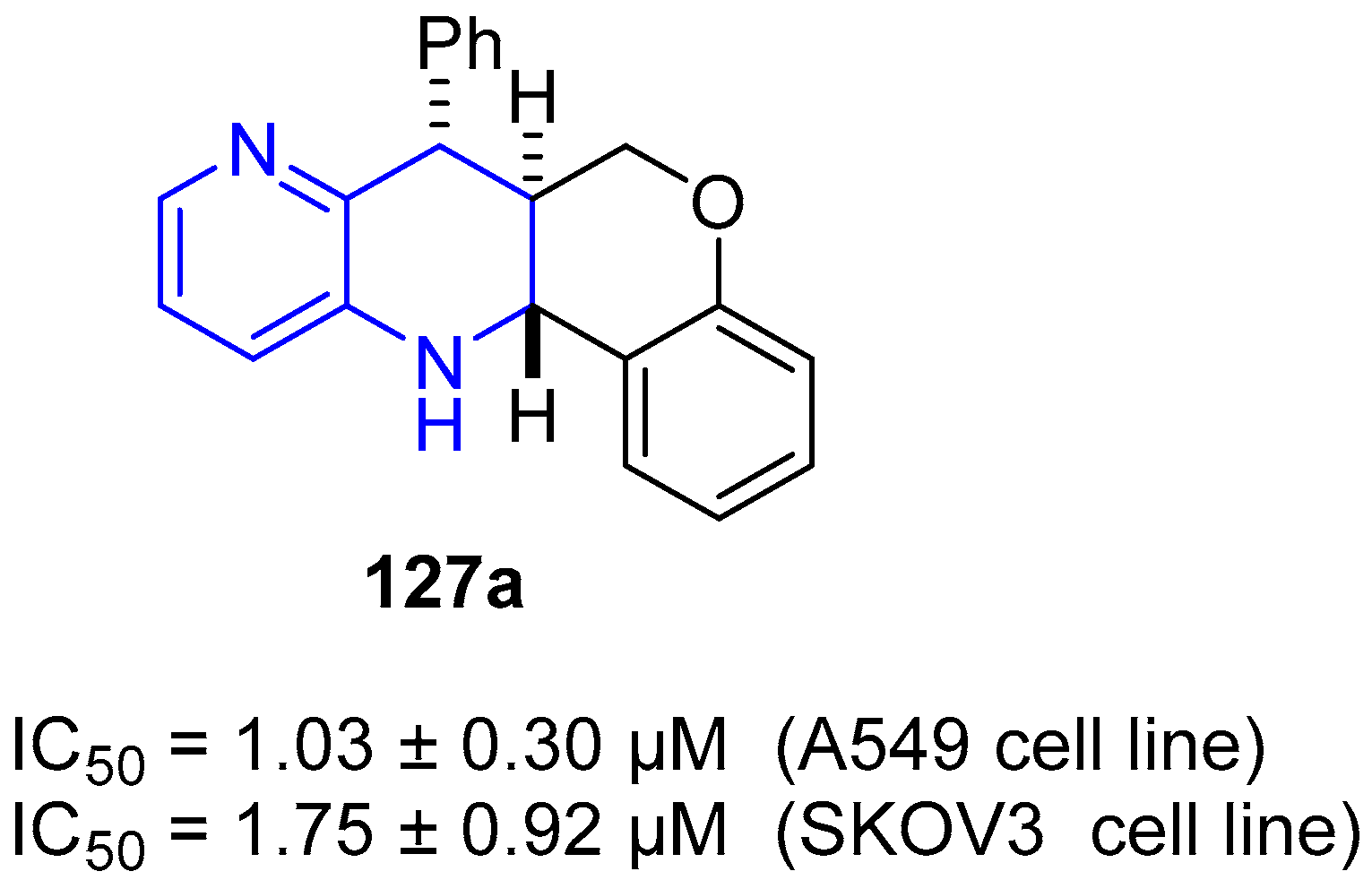
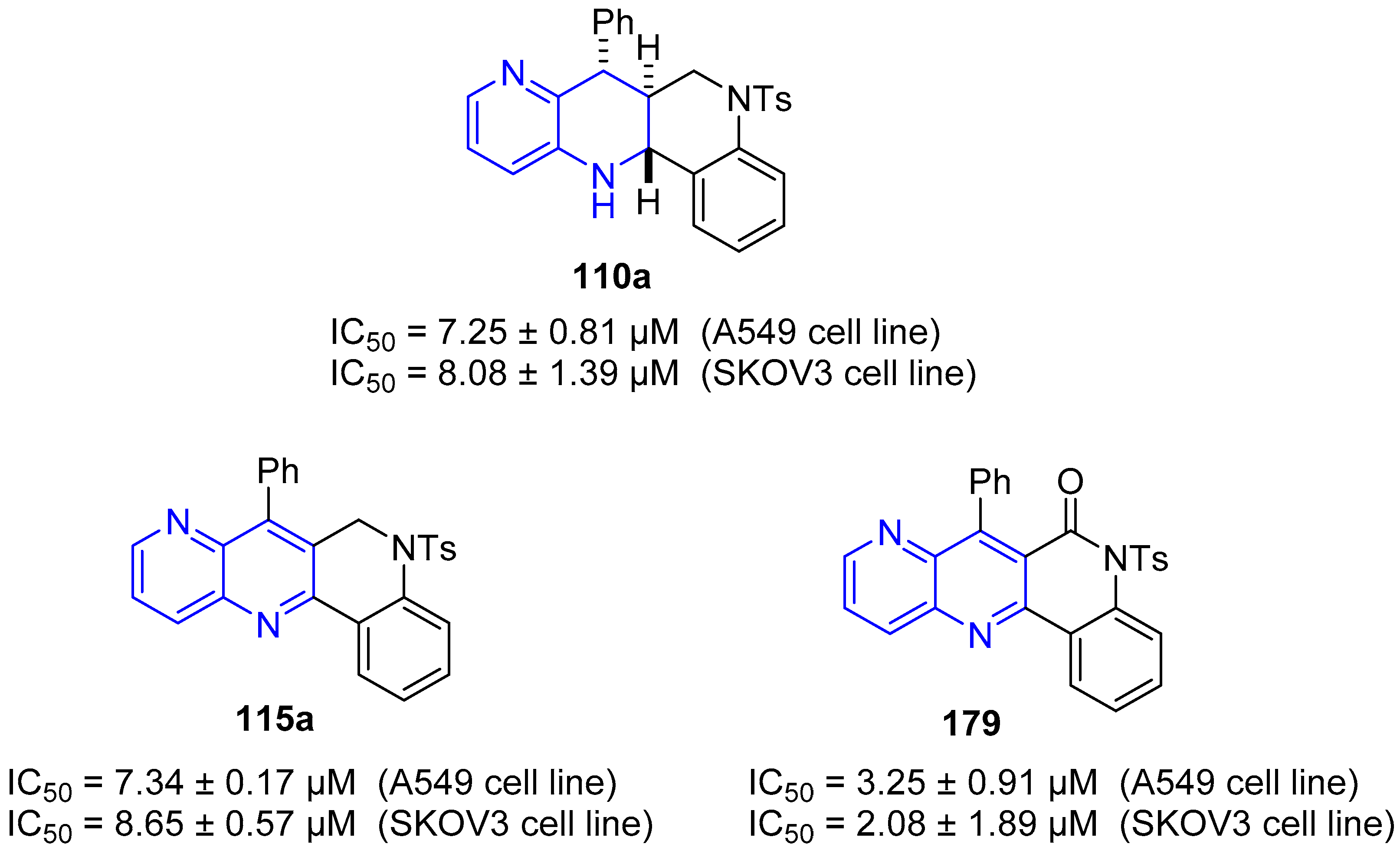
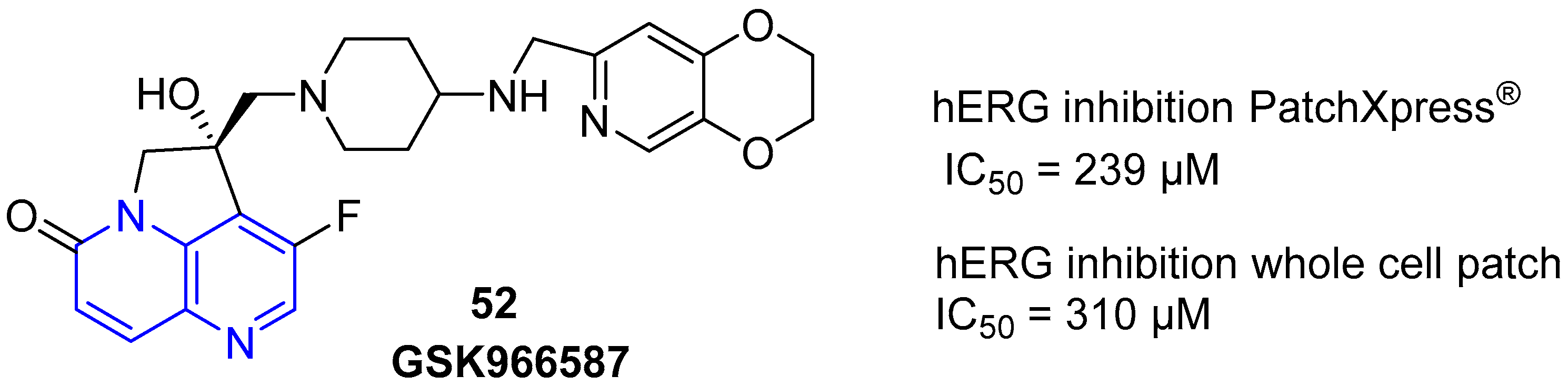
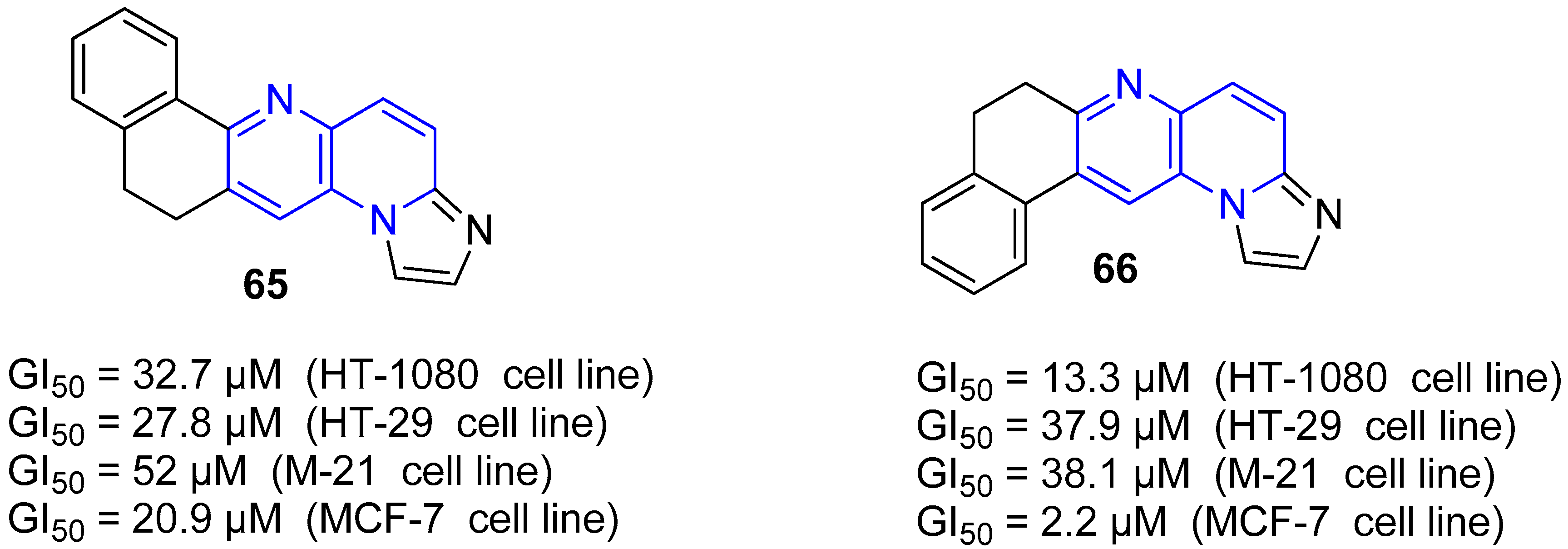
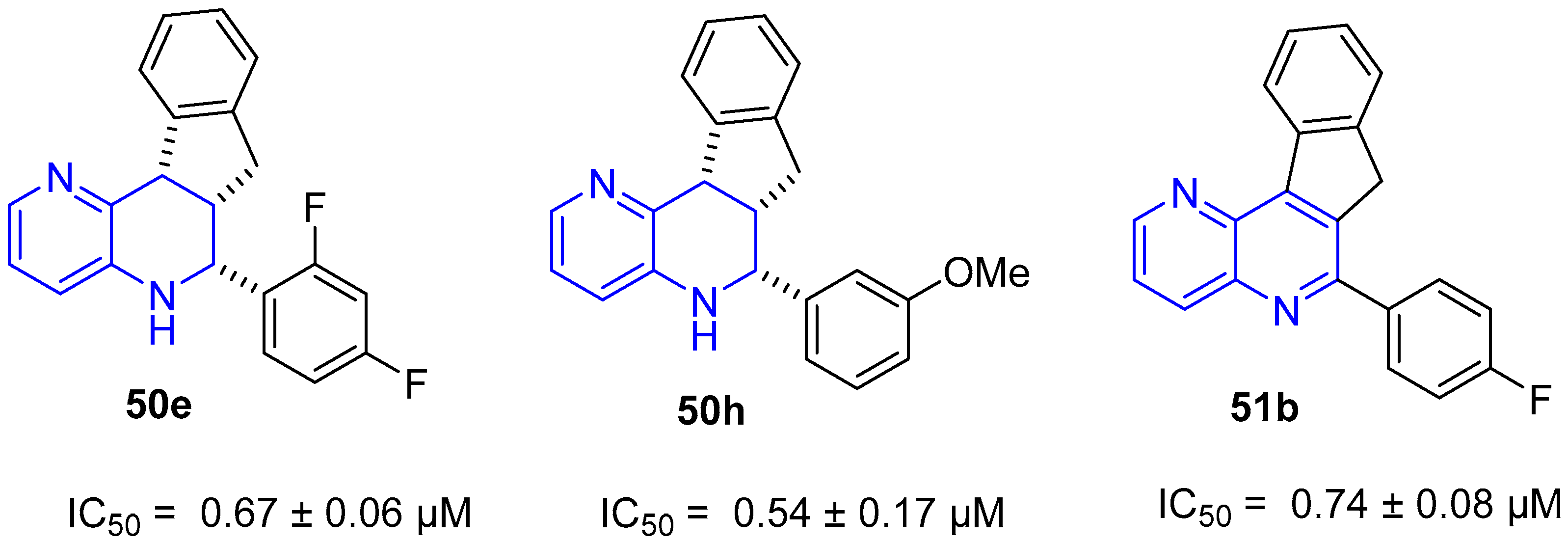
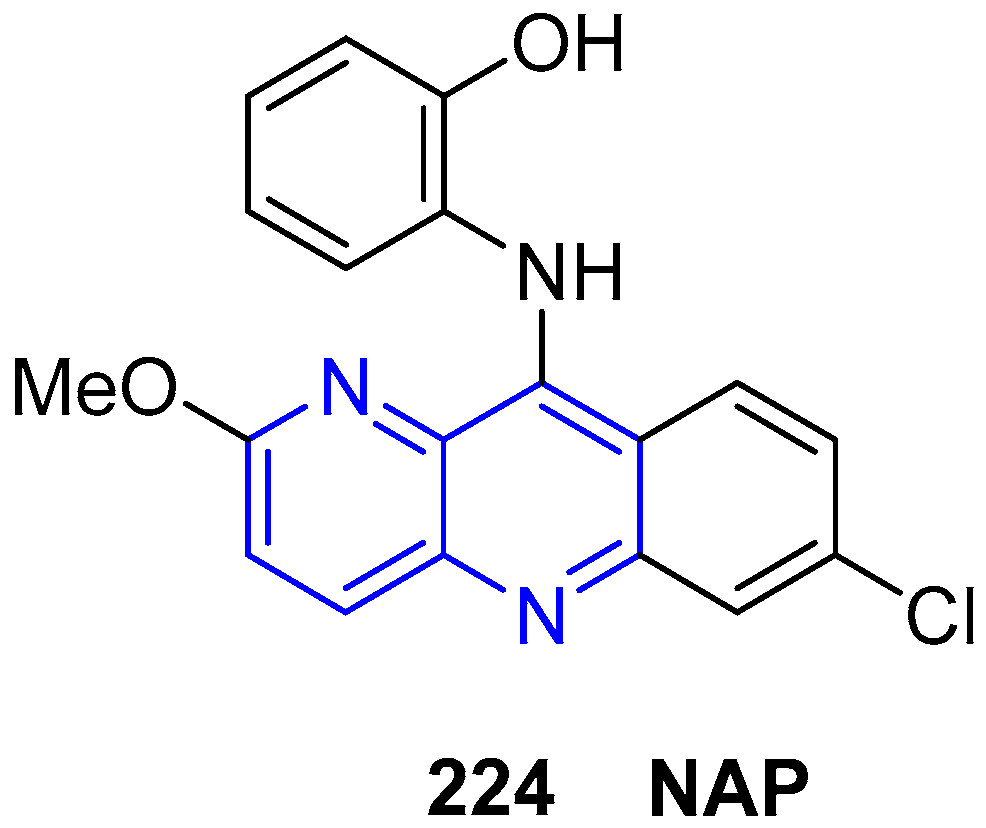
4. Biological Activity of Fused 1,5-Naphthyridines
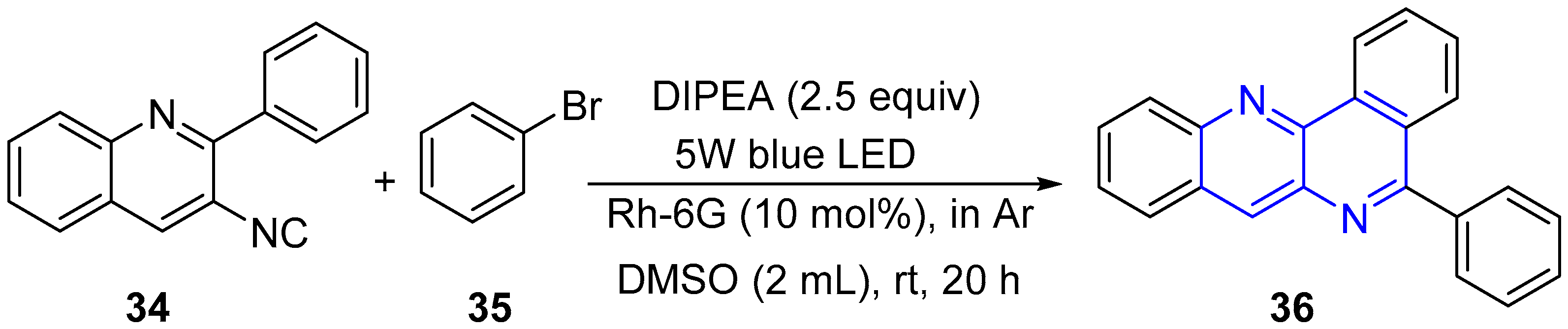
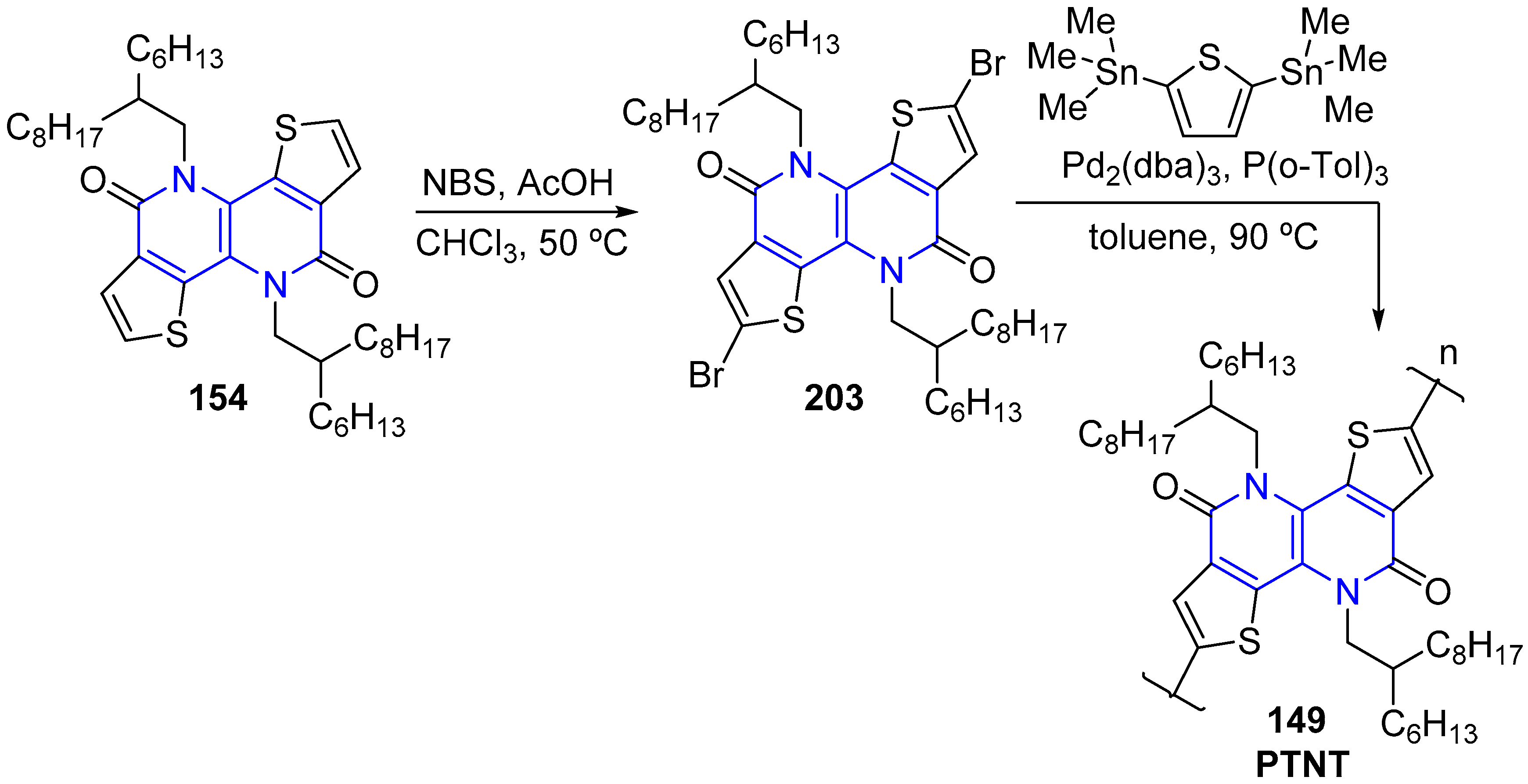
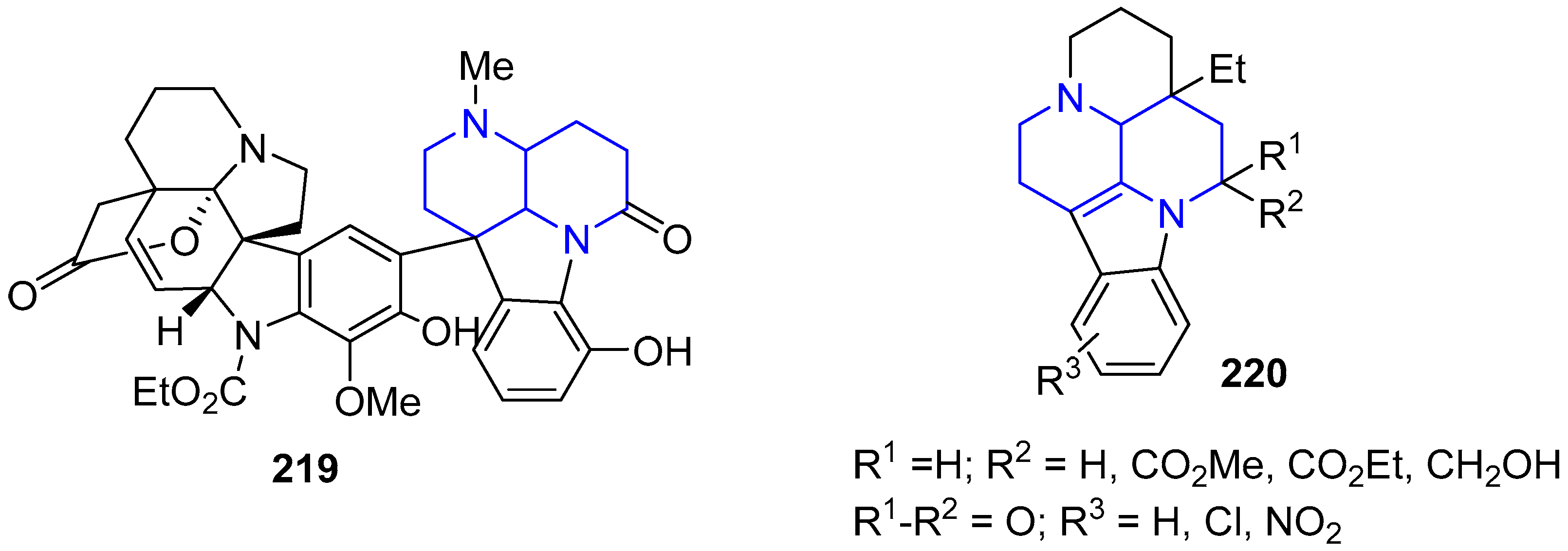
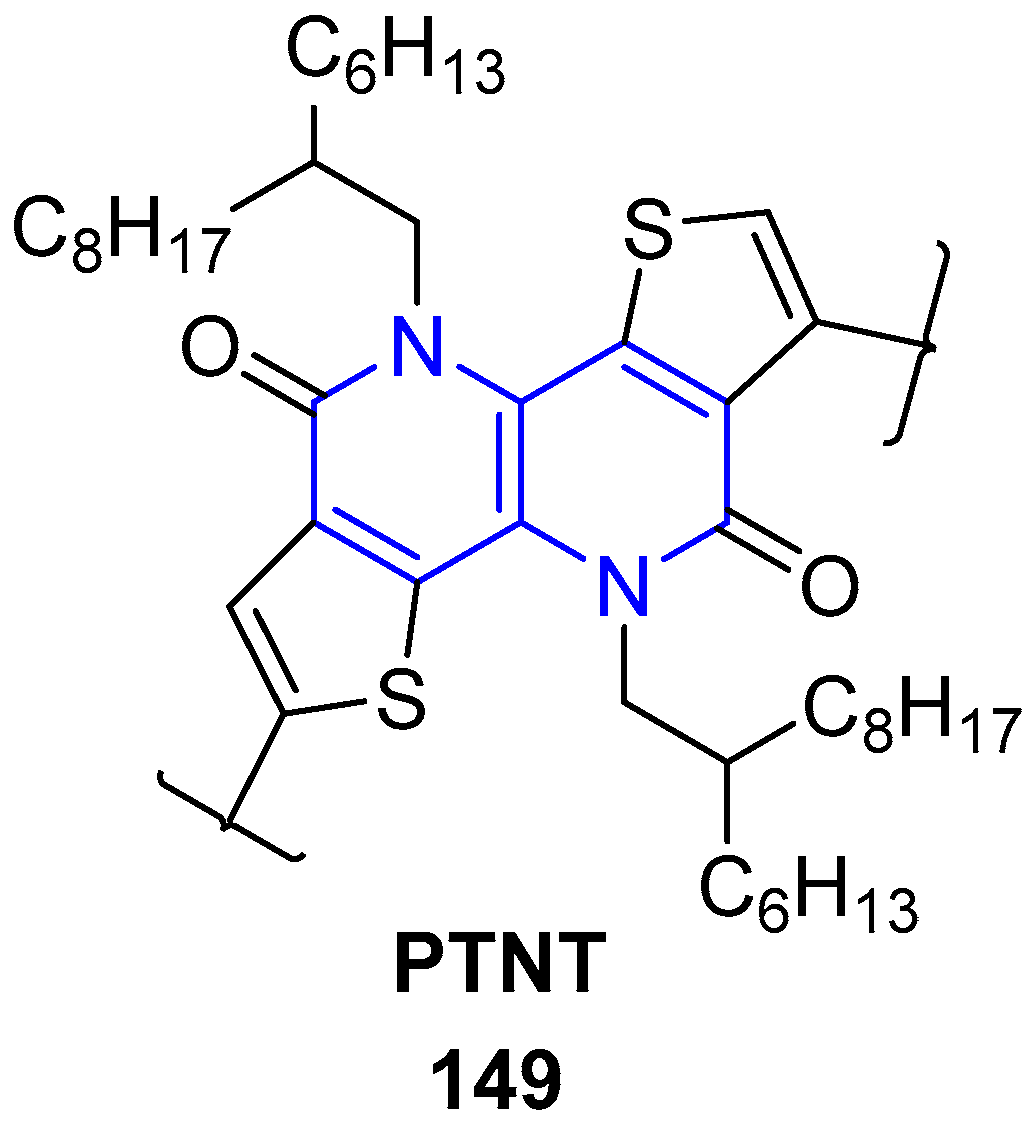
5. Other Applications of Fused 1,5-Naphthyridines
6. Conclusions
Funding
Conflicts of Interest
References
- Allen, C.F.H. The naphthyridines. Chem. Rev. 1950, 47, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, V.P.; Roman, S.V.; Dyachenko, V.D. Naphthyridines. Structure, physicochemical properties and general methods of synthesis. Russ. Chem. Rev. 2000, 69, 201–220. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Roman, S.V.; Dyachenko, V.D. Pyridopyridines. Russ. Chem. Rev. 2001, 70, 299–320. [Google Scholar] [CrossRef]
- Litvinov, V.P. Advances in the chemistry of naphthyridines. Adv. Heterocyclic Chem. 2006, 91, 189–300. [Google Scholar]
- Ivanov, A.S.; Tugusheva, N.Z.; Granik, V.G. Benzo[b]naphthyridines. Russ. Chem. Rev. 2005, 74, 915–936. [Google Scholar] [CrossRef]
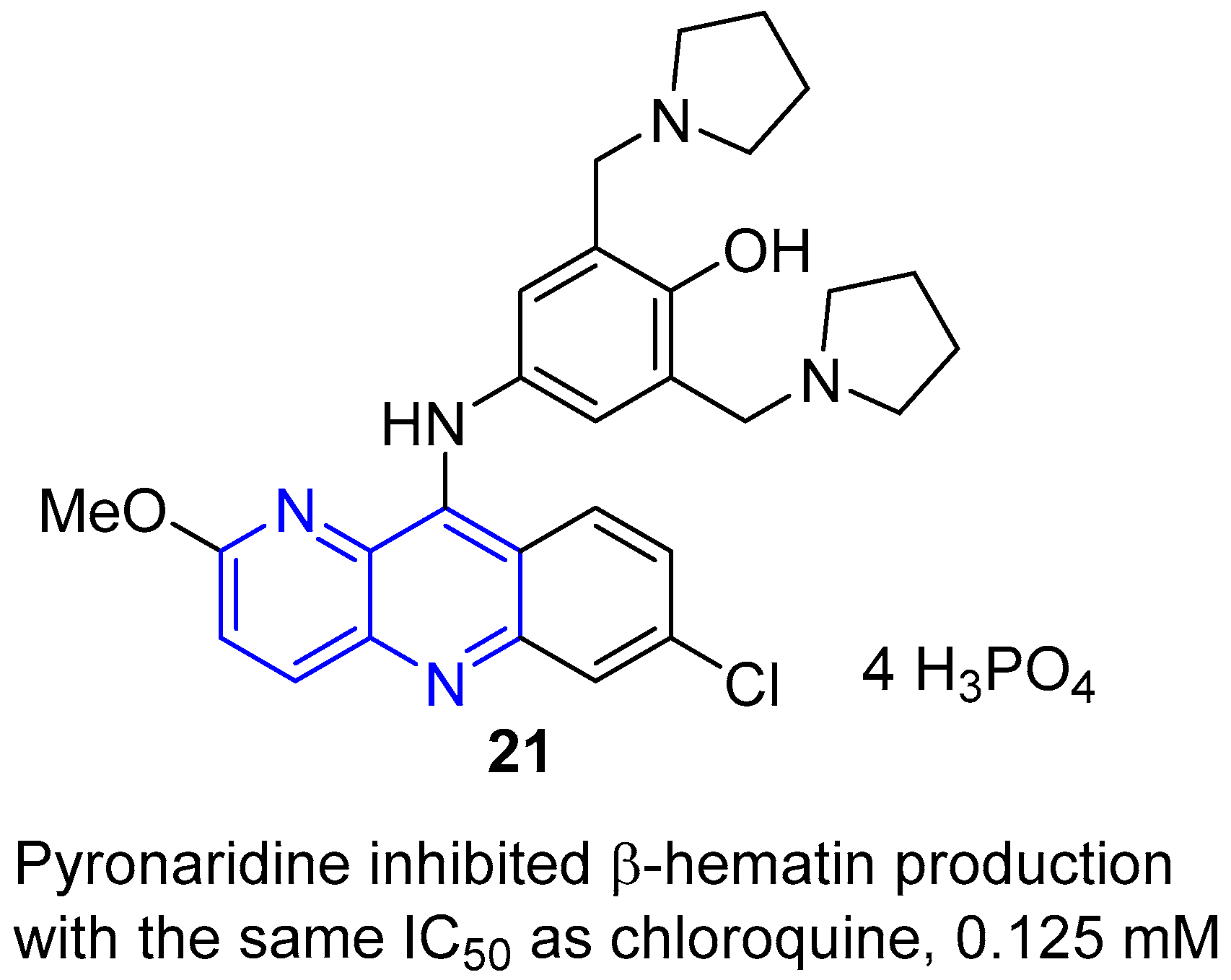
- Chen, C.; Tang, L.H.; Jantanavivat, C. Studies on a new antimalarial compound: Pyronaridine. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 7–10. [Google Scholar] [CrossRef]
- Fu, S.; Xiao, S.H. Pyronaridine: A new antimalarial drug. Parasitol. Today 1991, 7, 310–313. [Google Scholar] [CrossRef]
- Ferlin, M.; Grazia, M.; Marzano, C.; Chiarelotto, G.; Baccichetti, F.; Bordin, F. Synthesis and antiproliferative activity of some variously substituted acridine and azacridine derivatives. Eur. J. Med. Chem. 2000, 35, 827–837. [Google Scholar] [CrossRef]
- Chrzastek, L.; Mianowska, B.; Sliwa, W. Synthesis and properties of methyl-, formyl- and amino-diazaphenanthrene. Aust. J. Chem. 1994, 47, 2129–2133. [Google Scholar] [CrossRef]
- Ferraris, D.; Ko, Y.-S.; Pahutski, T.; Ficco, R.P.; Serdyuk, L.; Alemu, C.; Bradford, C.; Chiou, T.; Hoover, R.; Huang, S.; et al. Design and synthesis of poly ADP-ribose polymerase-1 inhibitors. 2. Biological evaluation of aza-5[H]-phenanthridin-6-ones as potent, aqueous-soluble compounds for the treatment of ischemic injuries. J. Med. Chem. 2003, 46, 3138–3151. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.-A.; Tanaka, K. Reversible hydride generation and release from the ligand of [Ru(pbn)(bpy)2](PF6)2 driven by a pbn-localized redox reaction. Angew. Chem. Int. Ed. 2005, 44, 5891–5894. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K. Metal-catalyzed reversible conversion between chemical and electrical energy designed towards a sustainable society. Chem. Rec. 2009, 9, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Tannai, H.; Koizumi, T.-A.; Tanaka, K. Palladium(II) complexes bearing the terpyridine-type tridentate ligand with benzo[b]-1,5-naphthyridin-2-yl groups. Inorg. Chim. Acta 2007, 360, 3075–3082. [Google Scholar] [CrossRef]
- Wang, Y.D.; Boschelli, D.H.; Johnson, S.; Honores, E. A facile one-pot synthesis of 2-substituted-3-aminoquinolines: Preparation of benzo [b] naphthyridine-3-carbonitriles. Tetrahedron 2004, 60, 2937–2942. [Google Scholar] [CrossRef]
- Magnano, A.; Sparapani, S.; Lucciarini, R.; Michela, M.; Amantini, C.; Santoni, G.; Antonini, I. Synthesis and biological evaluation of indazolo[4,3-bc][1,5]naphthyridines (10-aza-pyrazolo[3,4,5-kl]acridines): A new class of antitumor agents. Bioorg. Med. Chem. 2004, 12, 5941–5947. [Google Scholar] [CrossRef]
- Luan, X.; Gao, C.; Sun, Q.; Tan, C.; Liu, H.; Jin, Y.; Jiang, Y. Novel synthetic azaacridine analogues as topoisomerase 1 inhibitors. Chem. Lett. 2011, 40, 728–729. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, S.K.; Cho, J.H.; Yoon, S.S. Improved manufacturing process for pyronaridine tetraphosphate. Bull. Korean Chem. Soc. 2014, 35, 521–524. [Google Scholar] [CrossRef]
- Akue-Gedu, R.; Couturier, D.; Henichart, J.-P.; Rigo, B.; Sanz, G.; Van Hijfte, L.; Bourry, A. Studies on pyrrolidinones. Synthesis of fused 1,5-naphthyridines. Tetrahedron 2012, 68, 5644–5654. [Google Scholar] [CrossRef]
- Read, M.L.; Gundersen, L.L. Synthesis of phenanthridine derivatives by microwave-mediated cyclization of o-furyl(allylamino)arenes. J. Org. Chem. 2013, 78, 1311–1316. [Google Scholar] [CrossRef]
- Baccile, J.A.; Spraker, J.E.; Le, H.H.; Brandenburger, E.; Gomez, C.; Bok, J.W.; Macheleidt, J.; Brakhage, A.A.; Hoffmeister, D.; Keller, N.P.; et al. Plant-like biosynthesis of isoquinoline alkaloids in Aspergillus fumigatus. Nat. Chem. Biol. 2016, 12, 419–424. [Google Scholar] [CrossRef]
- Li, X.; Liang, D.; Huang, W.; Sun, H.; Wang, L.; Ren, M.; Wang, B.; Ma, Y. Metal-free photocatalyzed cross coupling of aryl (heteroaryl) bromides with isonitriles. Tetrahedron 2017, 73, 7094–7099. [Google Scholar] [CrossRef]
- Chen, W.-L.; Chen, C.-Y.; Chen, Y.-F.; Hsieh, J.-C. Hydride-induced anionic cyclization: An efficient method for the synthesis of 6-H-phenanthridines via a transition-metal-free process. Org. Lett. 2015, 17, 1613–1616. [Google Scholar] [CrossRef] [PubMed]
- Letessier, J.; Geffe, M.; Schollmeyer, D.; Detert, H. Synthesis of a naphtho-pyrido-annulated iodonium salt and Pd-catalyzed transformation to 7H-naphtho [1, 8-bc][1,5]naphthyridine. Synthesis 2013, 45, 3173–3178. [Google Scholar] [CrossRef]
- Alonso, C.; Fuertes, M.; Gonzalez, M.; Rubiales, G.; Tesauro, C.; Knudsen, B.R.; Palacios, F. Synthesis and biological evaluation of indeno[1,5]naphthyridines as topoisomerase I (TopI) inhibitors with antiproliferative activity. Eur. J. Med. Chem. 2016, 115, 179–190. [Google Scholar] [CrossRef]
- Tejeria, A.; Perez-Pertejo, Y.; Reguera, R.M.; Balana-Fouce, R.; Alonso, C.; Fuertes, M.; Gonzalez, M.; Rubiales, G.; Palacios, F. Antileishmanial effect of new indeno-1,5-naphthyridines, selective inhibitors of Leishmania Infantum type IB DNA topoisomerase. Eur. J. Med. Chem. 2016, 124, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Palacios, F.; Alonso, C.; Arrieta, A.; Cossío, F.P.; Ezpeleta, J.M.; Fuertes, M.; Rubiales, G. Lewis acid activated aza-Diels-Alder reaction of N-(3-pyridyl)aldimines: An experimental and computational study. Eur. J. Org. Chem. 2010, 2010, 2091–2099. [Google Scholar] [CrossRef]
- Voight, E.A.; Yin, H.; Downing, S.V.; Calad, S.A.; Matsuhashi, H.; Giordano, I.; Hennessy, A.J.; Goodman, R.M.; Wood, J.L. Target-directed synthesis of antibacterial drug candidate GSK966587. Org. Lett. 2010, 12, 3422–3425. [Google Scholar] [CrossRef]
- Miles, T.J.; Hennessy, A.J.; Bax, B.; Brooks, G.; Brown, B.S.; Brown, P.; Cailleau, N.; Chen, D.; Dabbs, S.; Davies, D.T.; et al. Novel hydroxyl tricyclics (e.g., GSK966587) as potent inhibitors of bacterial type IIA topoisomerases. Bioorg. Med. Chem. Lett. 2013, 23, 5437–5441. [Google Scholar] [CrossRef]
- Andaloussi, M.; Moreau, E.; Masurier, N.; Lacroix, J.; Gaudreault, R.C.; Chezal, J.-M.; El Laghdach, A.; Canitrot, D.; Debiton, E.; Teulade, J.-C.; et al. Novel imidazo[1,2-a]naphthyridinic systems (part 1): Synthesis, antiproliferative and DNA-intercalating activities. Eur. J. Med. Chem. 2008, 43, 2505–2517. [Google Scholar] [CrossRef]
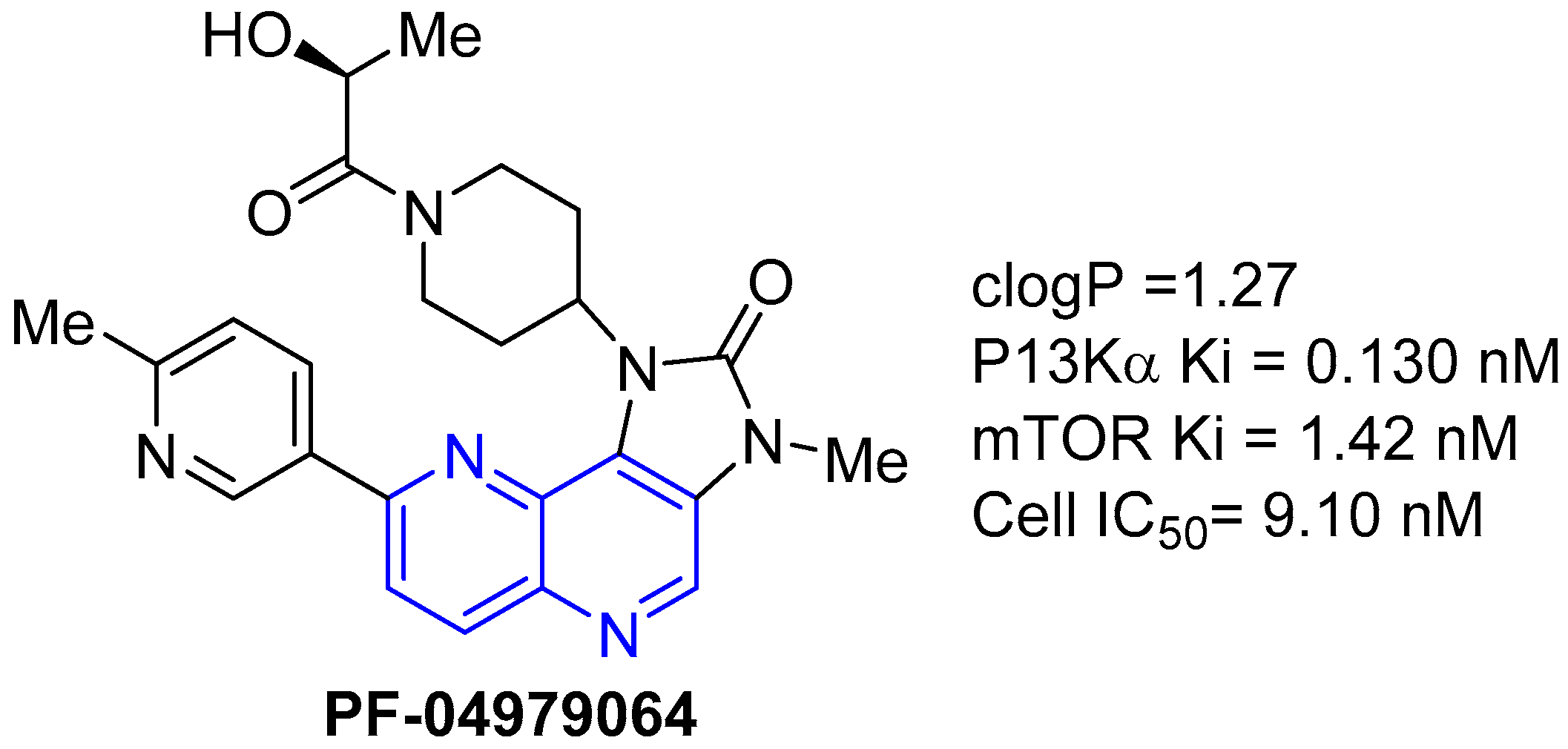
- Cheng, H.; Li, C.; Bailey, S.; Baxi, S.M.; Goulet, L.; Guo, L.; Hoffman, J.; Jiang, Y.; Johnson, T.O.; Johnson, T.W.; et al. Discovery of the highly potent PI3K/mTOR dual inhibitor PF-04979064 through structure-based drug design. ACS Med. Chem. Lett. 2013, 4, 91–97. [Google Scholar] [CrossRef]
- Mirguet, O.; Lamotte, Y.; Chung, C.-W.; Bamborough, P.; Delann, D.; Bouillot, A.; Gellibert, F.; Krysa, G.; Lewis, A.; Witherington, J.; et al. Naphthyridines as novel BET family bromodomain inhibitors. Chem. Med. Chem. 2014, 9, 580–589. [Google Scholar] [CrossRef]
- Sravanthi, T.V.; Manju, S.L. Indoles-a promising scaffold for drug development. Eur. J. Pharmaceut. Sci. 2016, 91, 1–10. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef]
- Gribble, G.W. Indole Ring Synthesis: From Natural Products to Drug Discovery; John Willey & Sons, Ltd.: Chichester, UK, 2016; p. 704. [Google Scholar]
- Maestro, A.; Martín-Encinas, E.; Alonso, C.; Martinez de Marigorta, E.; Rubiales, G.; Vicario, J.; Palacios, F. Synthesis of novel antiproliferative hybrid bis-(3-indolyl)methane phosphonate derivatives. Eur. J. Med. Chem. 2018, 58, 874–883. [Google Scholar] [CrossRef]
- Gollner, A.; Koutentis, P.A. Two-step total syntheses of canthin-6-one alkaloids: New one-pot sequential Pd-catalyzed Suzuki-Miyaura coupling and Cu-catalyzed amidation reaction. Org. Lett. 2010, 12, 1352–1355. [Google Scholar] [CrossRef]
- Ioannidou, H.A.; Martin, A.; Gollner, A.; Koutentis, P.A. Three-step synthesis of ethyl canthinone-1-carboxylates from ethyl 4-bromo-6-methoxy-1,5-naphthyridine-3-carboxylate via a Pd-catalyzed Suzuki-Miyaura coupling and a Cu-catalyzed amidation reaction. J. Org. Chem. 2011, 76, 5113–5122. [Google Scholar] [CrossRef]
- Singh, V.; Hutait, S.; Biswas, S.; Batra, S. Versatility of substituted 1-formyl-9H-β-carbolines for the synthesis of new fused β-carbolines via intramolecular 1,3-dipolar cycloaddition. Eur. J. Med. Chem. 2010, 3, 531–539. [Google Scholar]
- Fujimoto, K.; Osuka, A. A 1,5-naphthyridine-fused porphyrin dimer: Intense NIR absorption and facile redox interconversion with its reduced congener. Chem. Eur. J. 2018, 24, 6530–6533. [Google Scholar] [CrossRef]
- Yet, L. Chapter 6.1—Six-membered ring systems: Pyridine and benzo derivatives. In Progress in Heterocyclic Chemistry; Gordon, W.G., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 30, pp. 311–355. [Google Scholar]
- Chiacchio, M.A.; Iannazzo, D.; Romeo, R.; Giofre, S.V.; Legnani, L. Pyridine and pyrimidine derivatives as privileged scaffolds in biologically active agents. Curr. Med. Chem. 2019, 26, 7166–7195. [Google Scholar] [CrossRef]
- Singh, N.; Kolli, M.K.; Kumari, M. Steroidal heterocycles: A review. Res. Rev.: J. Pharm. Sci. 2018, 9, 27–35. [Google Scholar]
- Navneetha, O.; Deepthi, K.; Rao, A.M.; Jyostna, T. Saritha. A review on chemotherapeutic activities of quinoline. Int. J. Pharm. Chem. Biol. Sci. 2017, 7, 364–372. [Google Scholar]
- Shang, X.-F.; Morris-Natschke, S.L.; Liu, Y.-Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.-C.; Yang, G.-Z.; Lee, K.-H. Biologically active quinoline and quinazoline alkaloids. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef] [PubMed]
- Cailly, T.; Fabis, F.; Legay, R.; Oulyadi, H.; Rault, S. The synthesis of three new heterocycles: The pyrido[4,3 or 3,4 or 2,3-c]-1,5-naphthyridines. Tetrahedron 2007, 63, 71–76. [Google Scholar] [CrossRef]
- Martín-Encinas, E.; Selas, A.; Tesauro, C.; Rubiales, G.; Knudsen, B.R.; Palacios, F.; Alonso, C. Synthesis of novel hybrid quinolino[4,3-b][1,5]naphthyridines and quinolino[4,3-b][1,5]naphthyridin-6(5H)-one derivatives and biological evaluation as topoisomerase I inhibitors and antiproliferatives. Eur. J. Med. Chem. 2020, 195, 112292–112304. [Google Scholar] [CrossRef]
- For excellent recent review see: Costa, M.; Dias, T.A.; Brito, A.; Proenca, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar]
- For excellent recent review see: Pratap, R.; Ram, V.J. Natural and synthetic chromenes, fused chromenes, and versatility of dihydrobenzo[h]chromenes in organic synthesis. Chem. Rev. 2014, 114, 10476–10526. [Google Scholar]
- Belyaeva, K.V.; Nikitina, L.P.; Afonin, A.V.; Vashchenko, A.V.; Muzalevskiy, V.M.; Nenajdenko, V.G.; Trofimov, B.A. Catalyst-free 1:2 annulation of quinolines with trifluoroacetylacetylenes: An access to functionalized oxazinoquinolines. Org. Biomol. Chem. 2018, 16, 8038–8041. [Google Scholar] [CrossRef]
- Wang, D.; Wang, D.L.; Shi, X.C.; Qian, J.H. A facile one-pot synthesis of chromeno[4,3-b][1,5]naphthyridines. Heterocycles 2016, 92, 2141–2144. [Google Scholar] [CrossRef]
- Maji, P.K.; Mahalanobish, A. Efficient one pot synthesis of chromenonaphthyridine derivatives by CuI/InCl3 catalyzed aza Diels-Alder reaction. Heterocycles 2018, 96, 43–49. [Google Scholar] [CrossRef]
- Martin-Encinas, E.; Rubiales, G.; Knudssen, B.R.; Palacios, F.; Alonso, C. Straightforward synthesis and biological evaluation as topoisomerase I inhibitors and antiproliferative agents of hybrid chromeno[4,3-b][1,5]naphthyridines and chromeno[4,3-b][1,5]naphthyridin-6-ones. Eur. J. Med. Chem. 2019, 178, 752–766. [Google Scholar] [CrossRef]
- Bisagni, E.; Landras, C.; Thirot, S.; Huel, C. A convenient way to dibenzo[c,h]-1,5-naphthyridines (11-aza-benzo[c]phenanthridines). Tetrahedron 1996, 52, 10427–10440. [Google Scholar] [CrossRef]
- Kroon, R.; Diaz de Zerio-Mendaza, A.; Himmelberger, S.; Bergqvist, J.; Bäcke, O.; Faria, G.C.; Gao, F.; Obaid, A.; Zhuang, W.; Gedefaw, D.; et al. New tetracyclic lactam building block for thick, broad-bandgap photovoltaics. J. Am. Chem. Soc. 2014, 136, 11578–11581. [Google Scholar] [CrossRef]
- Sliwa, W.; Mlochowski, J. Synthesis of benzo[h]naphthyridine and 1,9-and 2,7-phenanthroline N-oxides. Roczniki Chemii 1976, 50, 695–698. [Google Scholar]
- Bachowska, B. A convenient route to cyano derivatives of benzonaphthyridines. Chem. Heterocycl Com+ 2011, 47, 332–335. [Google Scholar] [CrossRef]
- Duan, L.P.; Wen, A.D.; Wu, N.B.; Tao, Y.; Zhang, H.B. Synthesis and anti-intestinal nematode activity of variously substituted benzonaphthyridine derivatives. Molecules 2011, 16, 1593–1602. [Google Scholar] [CrossRef]
- Sieb, D.; Schuhen, K.; Morgen, M.; Herrmann, H.; Wadepohl, H.; Lucas, N.T.; Baker, R.W.; Enders, M. Synthesis and complexation behavior of indenyl and cyclopentadienyl ligands functionalized with a naphthyridine unit. Organometallics 2012, 31, 356–364. [Google Scholar] [CrossRef]
- Kobayashi, K.; Koizumi, T.-A.; Ghosh, D.; Kajiwara, T.; Kitagawa, S.; Tanaka, K. Electrochemical behavior of a Rh(pentamethylcyclopentadienyl) complex bearing an NAD+/NADH-functionalized ligand. Dalton Trans. 2018, 47, 5207–5216. [Google Scholar] [CrossRef]
- Fukushima, T.; Wada, T.; Ohtsu, H.; Tanaka, K. Photoinduced four- and six-electron reduction of mononuclear ruthenium complexes having NAD+ analogous ligands. Dalton Trans. 2010, 39, 11526–11534. [Google Scholar] [CrossRef]
- Fukushima, T.; Ghosh, D.; Kobayashi, K.; Ohtsu, H.; Kitagawa, S.; Tanaka, K. Four-electron reduction of a new ruthenium dicarbonyl complex having two NAD model ligands through decarboxylation in water. Inorg. Chem. 2016, 55, 11613–11616. [Google Scholar] [CrossRef]
- Ohtsu, H.; Fujii, S.; Tsuge, K.; Tanaka, K. Novel synthesis of a four-electron-reduced ruthenium(II) NADH-type complex under water-gas-shift reaction conditions. Dalton Trans. 2016, 45, 16130–16133. [Google Scholar] [CrossRef]
- Ghosh, D.; Fukushima, T.; Kobayashi, K.; Sen, S.; Kitagawa, S.; Kato, T.; Tanaka, K. Base assisted C–C coupling between carbonyl and polypyridyl ligands in a Ru-NADH-type carbonyl complex. Dalton Trans. 2017, 46, 4373–4381. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Tough, D.F. Bromodomains: A new target class for small molecule drug discovery. Drug Discov. Today Ther. Strateg. 2012, 9, e111–e120. [Google Scholar] [CrossRef]
- Auparakkitanon, S.; Chapoomram, S.; Kuaha, K.; Chirachariyavej, T.; Wilairat, P. Targeting of hematin by the antimalarial pyronaridine. Antimicrob. Agents Chemother. 2006, 50, 2197–2200. [Google Scholar] [CrossRef] [PubMed]
- Polyansky, D.; Cabelli, D.; Muckerman, J.T.; Fujita, E.; Koizumi, T.A.; Fukushima, T.; Wada, T.; Tanaka, K. Photochemical and radiolytic production of an organic hydride donor with a RuII complex containing an NAD+ model ligand. Angew. Chem., Int. Ed. 2007, 46, 4169–4172. [Google Scholar] [CrossRef] [PubMed]
- Polyansky, D.E.; Cabelli, D.; Muckerman, J.T.; Fukushima, T.; Tanaka, K.; Fujita, E. Mechanism of hydride donor generation using a Ru(II) complex containing an NAD+ model ligand: Pulse and steady-state radiolysis studies. Inorg. Chem. 2008, 47, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Fujita, E.; Muckerman, J.T.; Polyansky, D.E.; Wada, T.; Tanaka, K. Photochemical stereospecific hydrogenation of a Ru complex with an NAD+/NADH-type ligand. Inorg. Chem. 2009, 48, 11510–11512. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, H.; Tanaka, K. Drastic difference in the photo-driven hydrogenation reactions of ruthenium complexes containing NAD model ligands. Chem. Commun. 2012, 48, 1796–1798. [Google Scholar] [CrossRef]
- Doherty, M.D.; Grills, D.C.; Muckerman, J.T.; Polyansky, D.E.; Fujita, E. Toward more efficient photochemical CO2 reduction: Use of scCO2 or photogenerated hydrides. Coord. Chem. Rev. 2010, 254, 2472–2482. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tanaka, K. Approach to multi-electron reduction beyond two-electron reduction of CO2. Phys. Chem. Chem. Phys. 2014, 16, 2240–2250. [Google Scholar] [CrossRef]
- Ohtsu, H.; Tsuge, K.; Tanaka, K. Remarkable accelerating and decelerating effects of the bases on CO2 reduction using a ruthenium NADH model complex. J. Photoch. Photobio. A 2015, 313, 163–167. [Google Scholar] [CrossRef]
- Ghosh, D.; Kobayashi, K.; Kajiwara, T.; Kitagawa, S.; Tanaka, K. Catalytic hydride transfer to CO2 using Ru-NAD-type complexes under electrochemical conditions. Inorg. Chem. 2017, 56, 11066–11073. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.; Shukla, A.; Sobus, J.; Sharma, A.; Gedefaw, D.; Andersson, G.G.; Andersson, M.R.; Lo, S.C.; Namdas, E.B. High-speed OLEDs and area-emitting light-emitting transistors from a tetracyclic lactim semiconducting polymer. Adv. Optical Mater. 2018, 6, 1800768. [Google Scholar] [CrossRef]
- Pan, X.; Sharma, A.; Gedefaw, D.; Kroon, R.; Diaz de Zerio-Mendaza, A.; Holmes, N.P.; Kilcoyne, A.L.D.; Barr, M.G.; Fahy, A.; Marks, M.; et al. Environmentally friendly preparation of nanoparticles for organic photovoltaics. Org. Electron. 2018, 59, 432–440. [Google Scholar] [CrossRef]
- Kocak, G.; Gedefaw, D.; Andersson, M.R.; Gedefaw, D. Optimizing polymer solar cells using non-halogenated solvent blends. Polymers 2019, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Feron, K.; Cave, J.M.; Thameel, M.N.; O’Sullivan, C.; Kroon, R.; Andersson, M.R.; Zhou, X.; Fell, C.J.; Belcher, W.J.; Walker, A.B.; et al. Utilizing energy transfer in binary and ternary bulk heterojunction organic solar cells. ACS Appl. Mater. Interfaces 2016, 8, 20928–20937. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.B.; Tesauro, C.; Gonzalez, M.; Kristoffersen, E.L.; Alonso, C.; Rubiales, G.; Coletta, A.; Froehlich, R.; Stougaard, M.; Ho, Y.P.; et al. Advantages of an optical nanosensor system for the mechanistic analysis of a novel topoisomerase I targeting drug: A case study. Nanoscale 2017, 9, 1886–1895. [Google Scholar] [CrossRef]
- Dai, Q.; Gao, C.; Liu, Y.; Liu, H.; Xiao, B.; Chen, C.; Chen, J.; Yuan, Z.; Jiang, Y. Highly sensitive and selective “naked eye” sensing of Cu(II) by a novel acridine-based sensor both in aqueous solution and on the test kit. Tetrahedron 2018, 74, 6459–6464. [Google Scholar] [CrossRef]











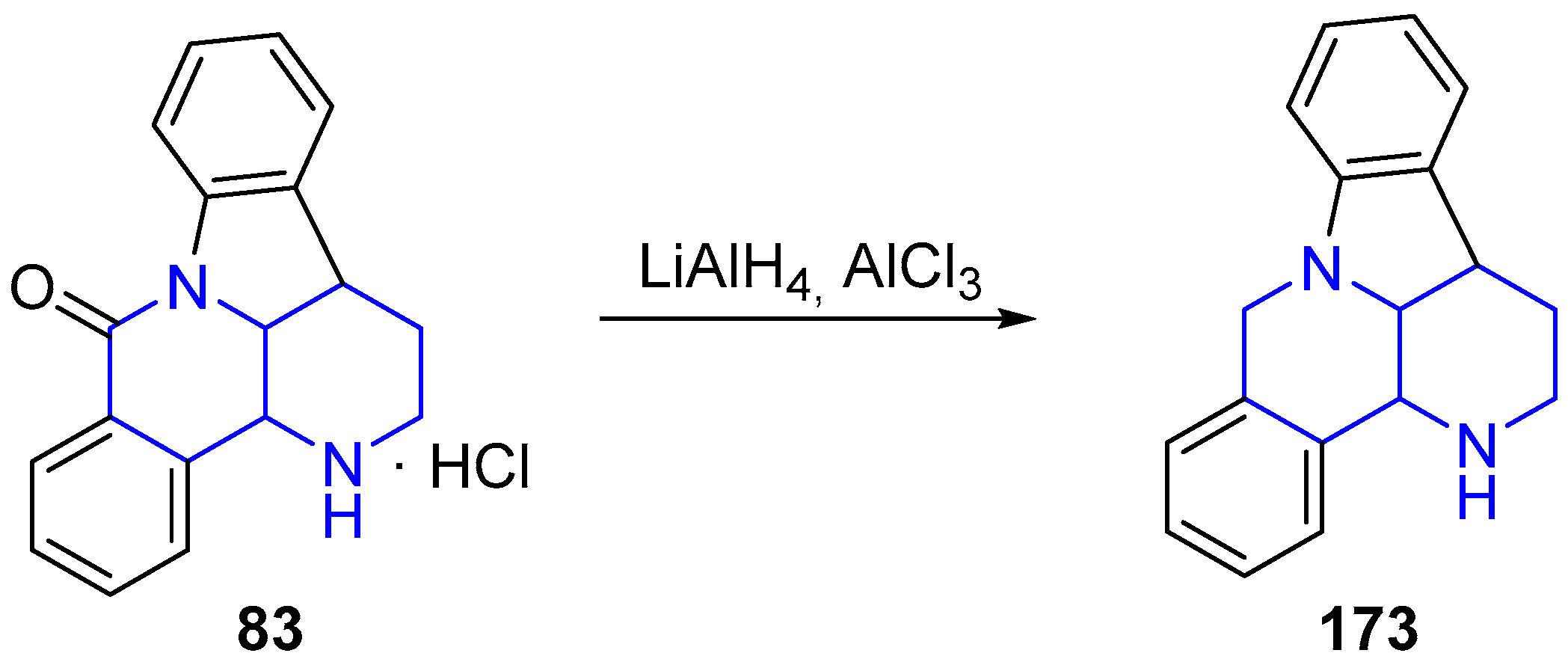
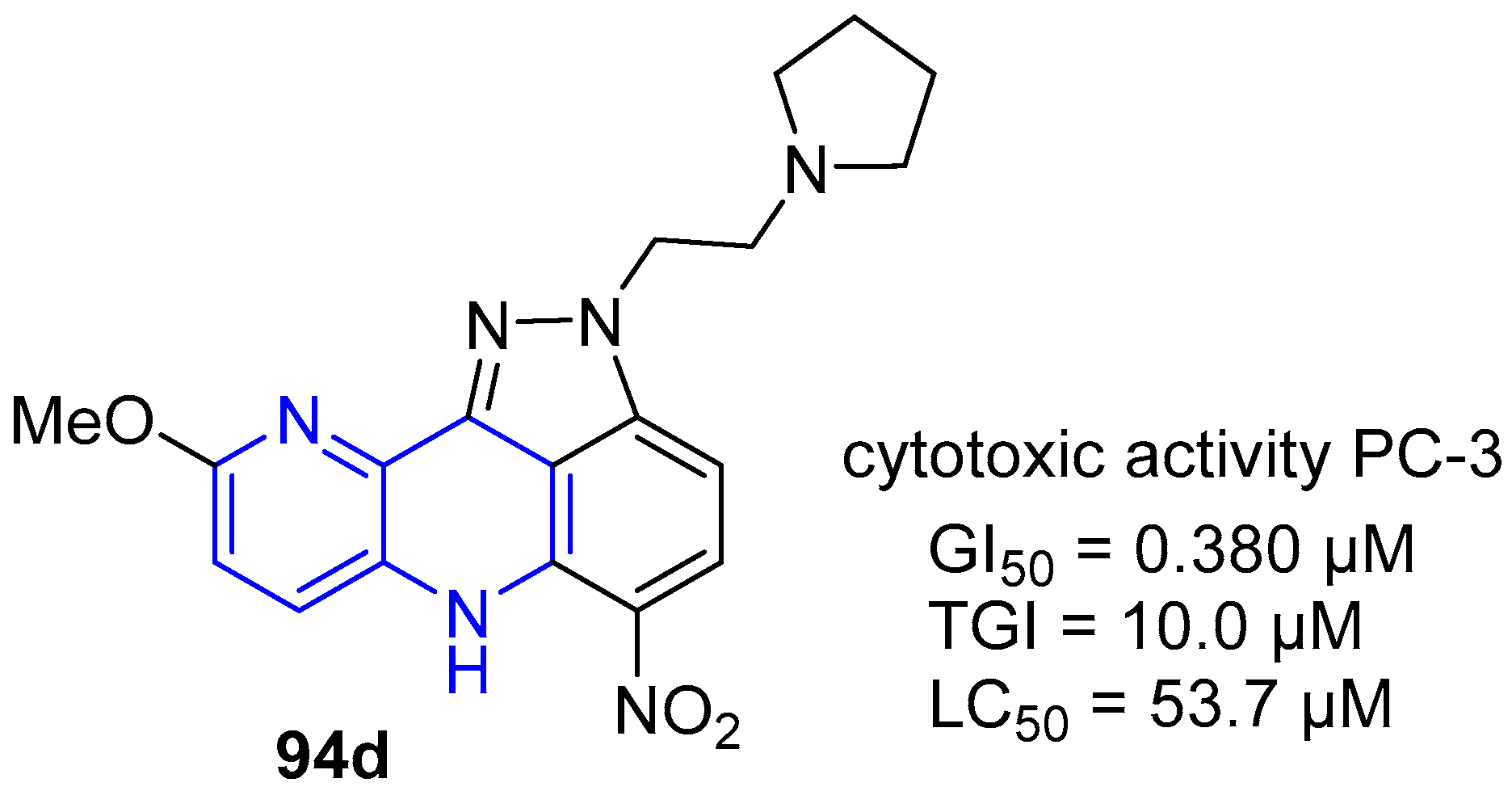



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masdeu, C.; Fuertes, M.; Martin-Encinas, E.; Selas, A.; Rubiales, G.; Palacios, F.; Alonso, C. Fused 1,5-Naphthyridines: Synthetic Tools and Applications. Molecules 2020, 25, 3508. https://doi.org/10.3390/molecules25153508
Masdeu C, Fuertes M, Martin-Encinas E, Selas A, Rubiales G, Palacios F, Alonso C. Fused 1,5-Naphthyridines: Synthetic Tools and Applications. Molecules. 2020; 25(15):3508. https://doi.org/10.3390/molecules25153508
Chicago/Turabian StyleMasdeu, Carme, Maria Fuertes, Endika Martin-Encinas, Asier Selas, Gloria Rubiales, Francisco Palacios, and Concepcion Alonso. 2020. "Fused 1,5-Naphthyridines: Synthetic Tools and Applications" Molecules 25, no. 15: 3508. https://doi.org/10.3390/molecules25153508
APA StyleMasdeu, C., Fuertes, M., Martin-Encinas, E., Selas, A., Rubiales, G., Palacios, F., & Alonso, C. (2020). Fused 1,5-Naphthyridines: Synthetic Tools and Applications. Molecules, 25(15), 3508. https://doi.org/10.3390/molecules25153508






