Proton Conductive Zr-Phosphonate UPG-1—Aminoacid Insertion as Proton Carrier Stabilizer
Abstract
:1. Introduction
2. Results
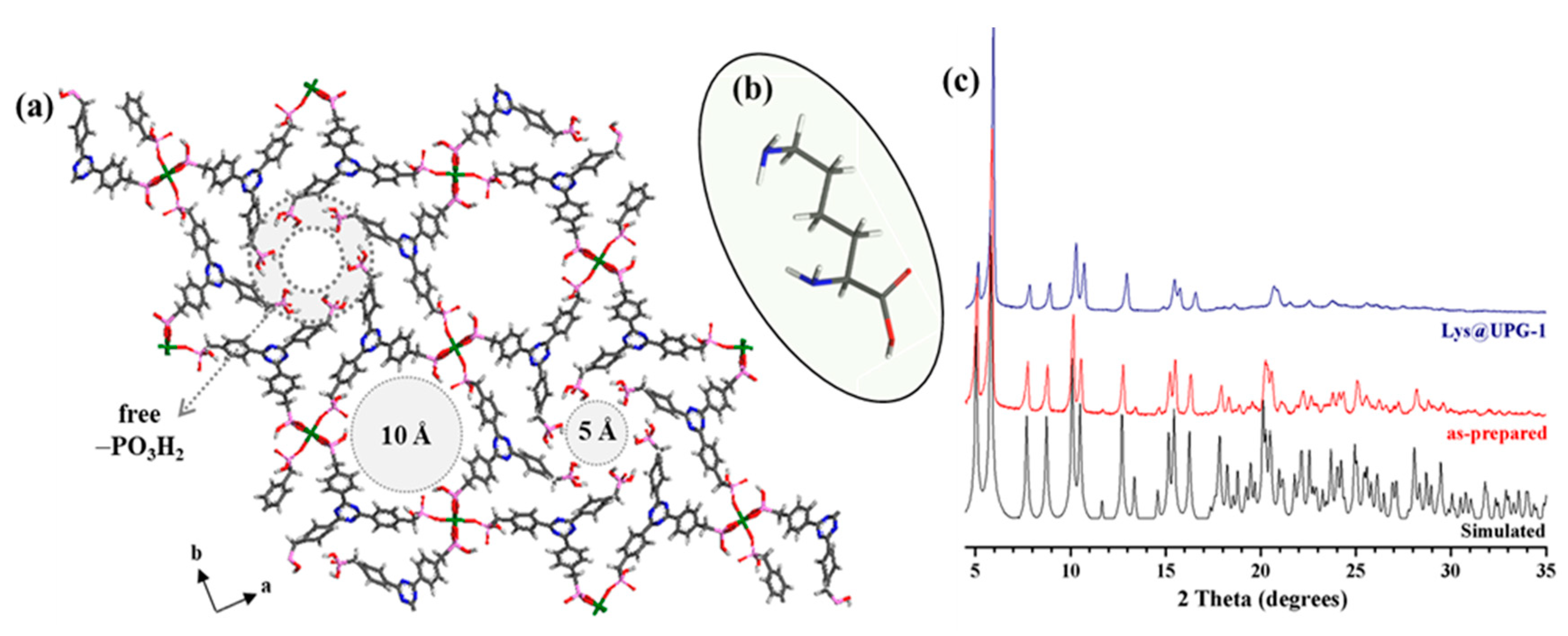
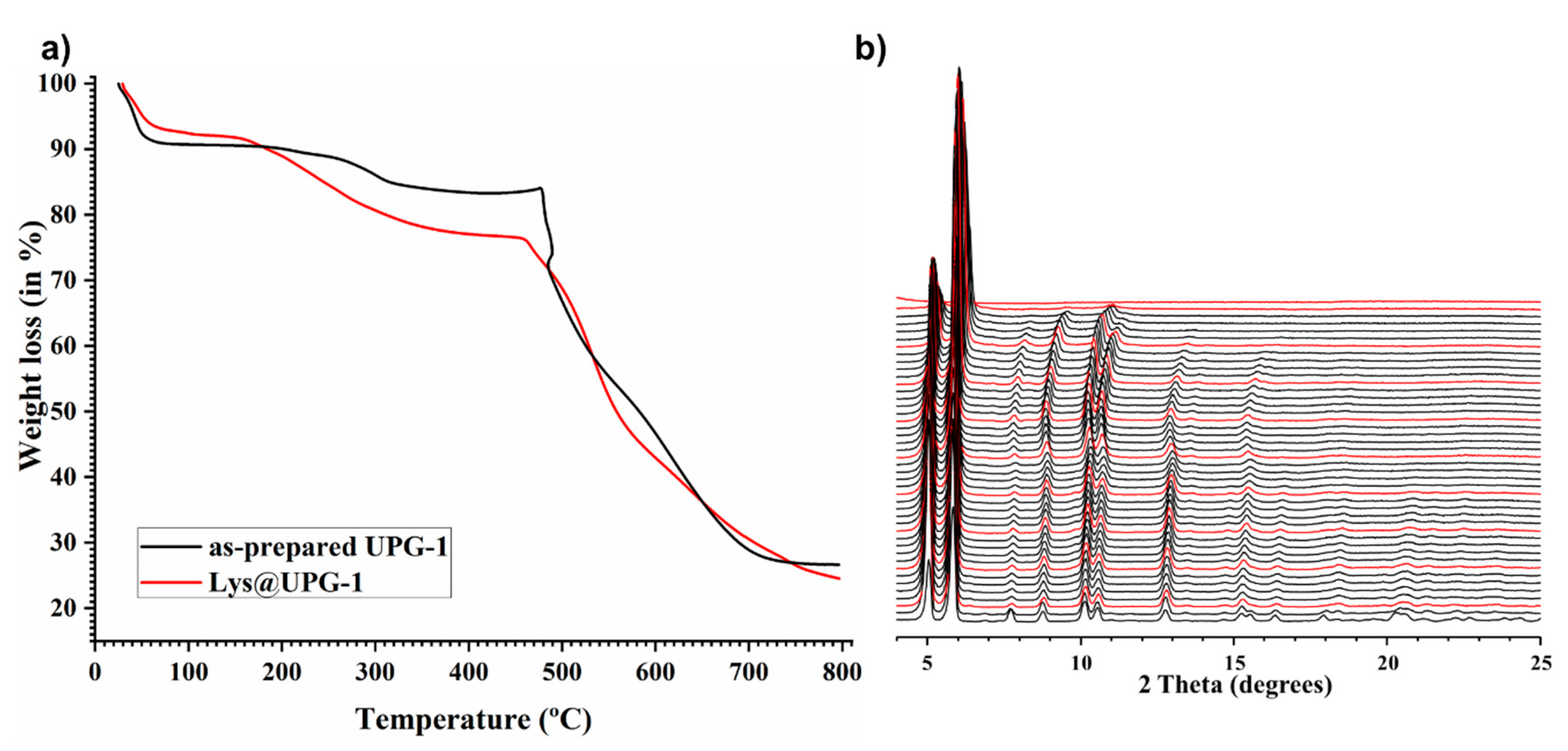
2.1. Synthesis and Characterization of UPG-1 and Lys@UPG-1
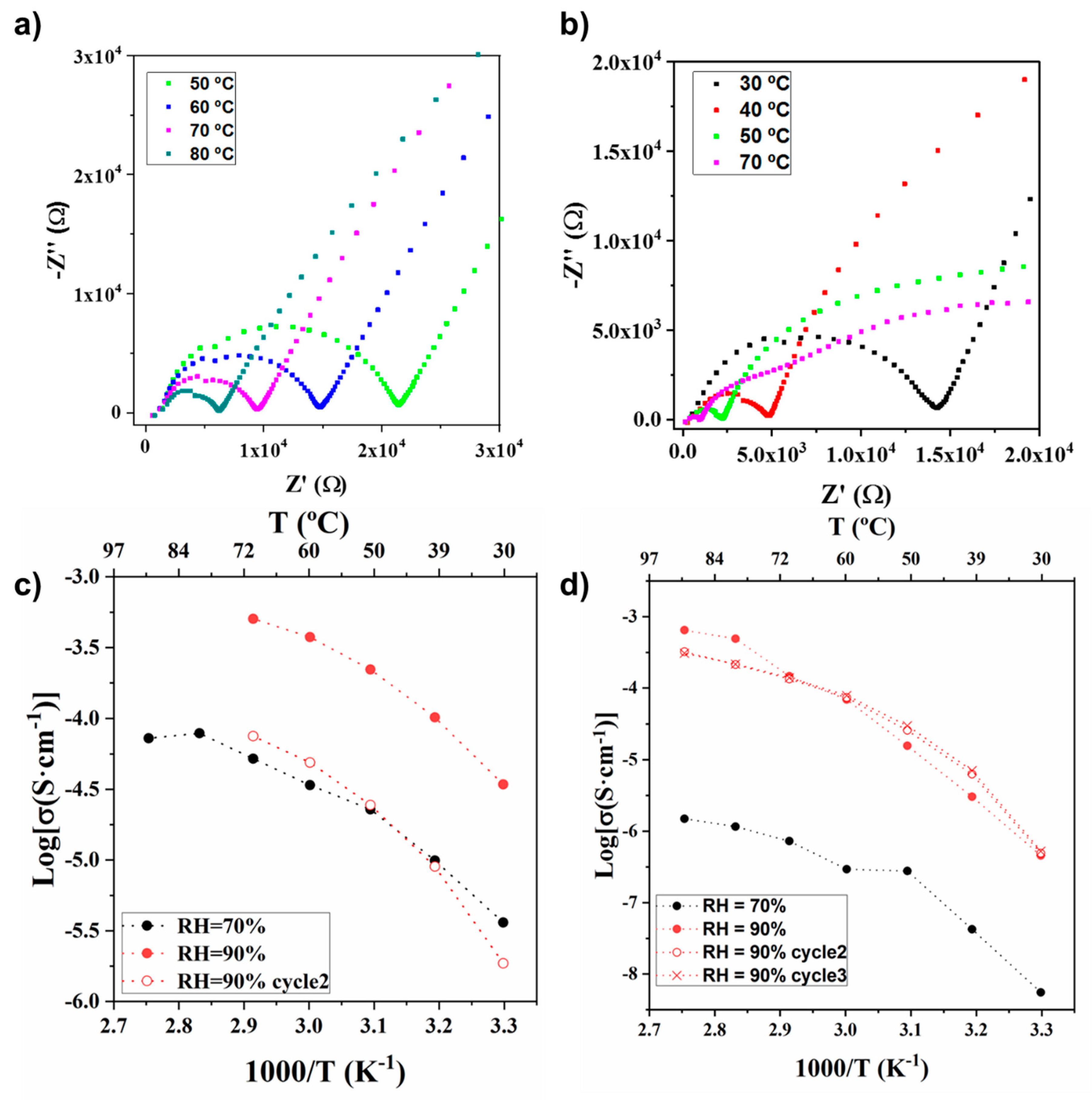
2.2. Evaluation of UPG-1 and Lys@UPG-1 Proton Conductivity
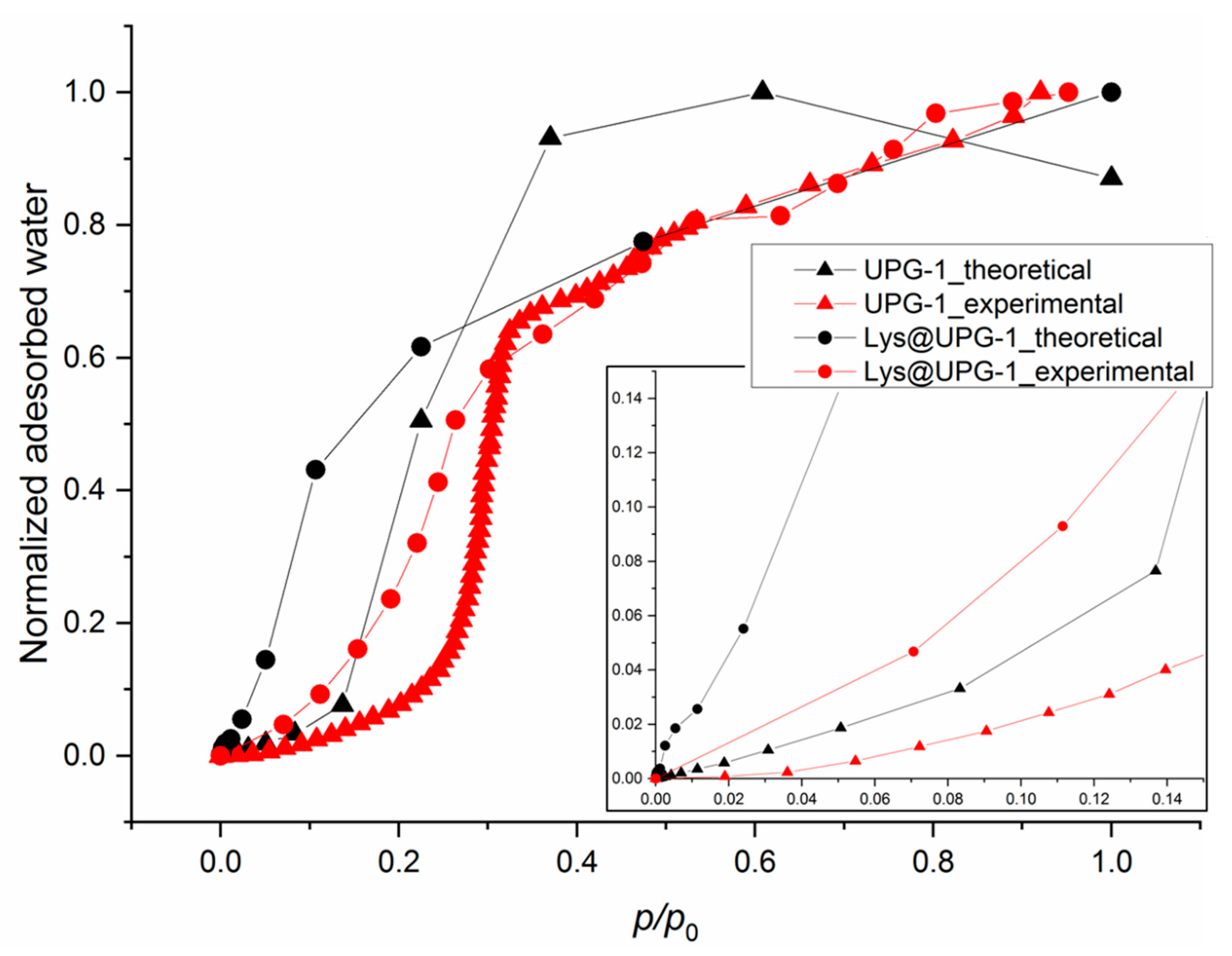
2.3. Water Adsorption Properties of UPG-1 and Lys@UPG-1
3. Discussion
4. Materials and Methods
4.1. Reagents and Solvents
4.2. Synthesis of [Zr(H4ttbmp)2]·10H2O (UPG-1)
4.3. Preparation of Lys@UPG-1
4.4. General Instrumentation
4.5. Proton Conductivity
4.6. Water Adsorption Measurements
4.7. Theoretical Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Yonoff, R.E.; Ochoa, G.V.; Cardenas-Escorcia, Y.; Silva-Ortega, J.I.; Meriño-Stand, L. Research trends in proton exchange membrane fuel cells during 2008–2018: A bibliometric analysis. Heliyon 2019, 5, e01724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollet, B.G.; Staffell, I.; Shang, J.L.; Molkov, V. Fuel-cell (hydrogen) electric hybrid vehicles. In Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 685–735. ISBN 9780857095220. [Google Scholar]
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes in fuel cells. J. Membr. Sci. 2014, 469, 379–396. [Google Scholar] [CrossRef] [Green Version]
- Zatoń, M.; Rozière, J.; Jones, D.J. Current understanding of chemical degradation mechanisms of perfluorosulfonic acid membranes and their mitigation strategies: A review. Sustain. Energy Fuels 2017, 1, 409–438. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Liu, H.; Gong, C.; Wen, S.; Wang, X.; Tu, Z. Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: A review. Fuel Process. Technol. 2018, 179, 203–228. [Google Scholar] [CrossRef]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton Conductivity of Composite Polyelectrolyte Membranes with Metal-Organic Frameworks for Fuel Cell Applications. Adv. Mater. Interfaces 2019, 6, 1801146. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Wong, N.E.; Shimizu, G.K.H. MOFs as proton conductors – challenges and opportunities. Chem. Soc. Rev. 2014, 43, 5913–5932. [Google Scholar] [CrossRef]
- Zakil, F.A.; Kamarudin, S.K.; Basri, S. Modified Nafion membranes for direct alcohol fuel cells: An overview. Renew. Sustain. Energy Rev. 2016, 65, 841–852. [Google Scholar] [CrossRef]
- Han, W.; Kwan, S.M.; Yeung, K.L. Zeolite applications in fuel cells: Water management and proton conductivity. Chem. Eng. J. 2012, 187, 367–371. [Google Scholar] [CrossRef]
- Pornprasertsuk, R.; Kosasang, O.; Somroop, K.; Horprathum, M.; Limnonthakul, P.; Chindaudom, P.; Jinawath, S. Proton conductivity of Y-doped BaZrO3: Pellets and thin films. Solid State Sci. 2011, 13, 1429–1437. [Google Scholar] [CrossRef]
- Abouzari-lotf, E.; Jacob, M.V.; Ghassemi, H.; Ahmad, A.; Nasef, M.M.; Zakeri, M.; Mehdipour-Ataei, S. Enhancement of fuel cell performance with less-water dependent composite membranes having polyoxometalate anchored nanofibrous interlayer. J. Power Sources 2016, 326, 482–489. [Google Scholar] [CrossRef]
- Montoro, C.; Rodríguez-San-Miguel, D.; Polo, E.; Escudero-Cid, R.; Ruiz-González, M.L.; Navarro, J.A.R.; Ocón, P.; Zamora, F. Ionic Conductivity and Potential Application for Fuel Cell of a Modified Imine-Based Covalent Organic Framework. J. Am. Chem. Soc. 2017, 139, 10079–10086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, M.R.; Hatakeyama, K.; Koinuma, M.; Hayami, S. Proton conductors produced by chemical modifications of carbon allotropes, perovskites and metal organic frameworks. J. Mater. Chem. A 2017, 5, 7243–7256. [Google Scholar] [CrossRef]
- Li, A.; Gao, Q.; Xu, J.; Bu, X. Proton-conductive metal-organic frameworks : Recent advances and perspectives. Coord. Chem. Rev. 2017, 344, 54–82. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-organic frameworks for energy applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef] [Green Version]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Hydrated Proton-Conductive Metal–Organic Frameworks. ChemPlusChem 2016, 81, 691–701. [Google Scholar] [CrossRef]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion conductivity and transport by porous coordination polymers and metal-organic frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974–986. [Google Scholar] [CrossRef] [Green Version]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigematsu, A.; Yamada, T.; Kitagawa, H. Wide Control of Proton Conductivity in Porous Coordination Polymers. J. Am. Chem. Soc. 2011, 133, 2034–2036. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, P.; Matsuda, R.; Kosaka, W.; Akiyama, G.; Jeon, H.J.; Kitagawa, S. Highly proton conductive nanoporous coordination polymers with sulfonic acid groups on the pore surface. Chem. Commun. 2014, 50, 1144–1146. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, G.; Dou, Y.; Wang, B.; Zhang, H.; Wu, H.; Zhou, W.; Li, J.-R.; Chen, B. A flexible metal–organic framework with a high density of sulfonic acid sites for proton conduction. Nat. Energy 2017, 877–883. [Google Scholar] [CrossRef]
- Phang, W.J.; Jo, H.; Lee, W.R.; Song, J.H.; Yoo, K.; Kim, B.; Hong, C.S. Superprotonic conductivity of a uio-66 framework functionalized with sulfonic acid groups by facile postsynthetic oxidation. Angew. Chem. Int. Ed. 2015, 54, 5142–5146. [Google Scholar] [CrossRef]
- Pardo, E.; Train, C.; Gontard, G.; Boubekeur, K.; Fabelo, O.; Liu, H.; Dkhil, B.; Lloret, F.; Nakagawa, K.; Tokoro, H.; et al. High proton conduction in a chiral ferromagnetic metal-organic quartz-like framework. J. Am. Chem. Soc. 2011, 133, 15328–15331. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Yamada, T.; Kitagawa, H. Rational Designs for Highly Proton-Conductive Metal—Organic Frameworks. J. Am. Chem. Soc. 2009, 131, 9906–9907. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Z.; Ye, Y.; Liu, C.; Lin, Q.; Chen, S.; Xiang, S.; Zhang, Z. Enhancement of Intrinsic Proton Conductivity and Aniline Sensitivity by Introducing Dye Molecules into the MOF Channel. ACS Appl. Mater. Interfaces 2019, 11, 16490–16495. [Google Scholar] [CrossRef]
- Luo, H.B.; Ren, Q.; Wang, P.; Zhang, J.; Wang, L.; Ren, X.M. High Proton Conductivity Achieved by Encapsulation of Imidazole Molecules into Proton-Conducting MOF-808. ACS Appl. Mater. Interfaces 2019, 11, 9164–9171. [Google Scholar] [CrossRef]
- Khatua, S.; Kumar Bar, A.; Konar, S. Tuning Proton Conductivity by Interstitial Guest Change in Size-Adjustable Nanopores of a CuI-MOF: A Potential Platform for Versatile Proton Carriers. Chem. Eur. J. 2016, 22, 16277–16285. [Google Scholar] [CrossRef]
- Bureekaew, S.; Horike, S.; Higuchi, M.; Mizuno, M.; Kawamura, T.; Tanaka, D.; Yanai, N.; Kitagawa, S. One-dimensional imidazole aggregate in aluminium porous coordination polymers with high proton conductivity. Nat. Mater. 2009, 8, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Ponomareva, V.G.; Kovalenko, K.A.; Chupakhin, A.P.; Dybtsev, D.N.; Shutova, E.S.; Fedin, V.P. Imparting high proton conductivity to a metal-organic framework material by controlled acid impregnation. J. Am. Chem. Soc. 2012, 134, 15640–15643. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Suh, K.; Natarajan, S.; Kim, K. Proton conduction in metal-organic frameworks and related modularly built porous solids. Angew. Chem. Int. Ed. 2013, 52, 2688–2700. [Google Scholar] [CrossRef] [PubMed]
- Dybtsev, D.N.; Ponomareva, V.G.; Aliev, S.B.; Chupakhin, A.P.; Gallyamov, M.R.; Moroz, N.K.; Kolesov, B.A.; Kovalenko, K.A.; Shutova, E.S.; Fedin, V.P. High proton conductivity and spectroscopic investigations of metal-organic framework materials impregnated by strong acids. ACS Appl. Mater. Interfaces 2014, 6, 5161–5167. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M.; Costantino, F.; Marmottini, F.; Comotti, A.; Sozzani, P.; Vivani, R. The first route to highly stable crystalline microporous zirconium phosphonate metal–organic frameworks. Chem. Commun. 2014, 50, 14831–14834. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wahiduzzaman, M.; Davis, L.; Tissot, A.; Shepard, W.; Marrot, J.; Martineau-Corcos, C.; Hamdane, D.; Maurin, G.; Devautour-Vinot, S.; et al. A robust zirconium amino acid metal-organic framework for proton conduction. Nat. Commun. 2018, 9, 4937. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, K.; Li, J.; Zeng, H.; Zhang, Q.; Lin, Z. Exploration of new water stable proton-conducting materials in an amino acid-templated metal phosphate system. Dalt. Trans. 2018, 47, 654–658. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, L.; Xiu, Z.; Yang, Y.; Wang, X.; Yin, Y.; Bi, Y.; Zheng, Z. High proton conductivity behavior in a 2D metal sulfite constructed from a histidine ligand. RSC Adv. 2019, 9, 16130–16135. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.; Vieira, F.; Gomes, A.C.; Ananias, D.; Fernandes, J.A.; Bruno, S.M.; Soares, R.; Valente, A.A.; Rocha, J.; Paz, F.A.A. Thermal Transformation of a Layered Multifunctional Network into a Metal-Organic Framework Based on a Polymeric Organic Linker. J. Am. Chem. Soc. 2011, 133, 15120–15138. [Google Scholar] [CrossRef]
- Taddei, M.; Shearan, S.J.I.; Donnadio, A.; Casciola, M.; Vivani, R.; Costantino, F. Investigating the effect of positional isomerism on the assembly of zirconium phosphonates based on tritopic linkers. Dalt. Trans. 2020, 49, 3662–3666. [Google Scholar] [CrossRef]
- Fulcher, G.S. Analysis of Recent Measurements of the Viscosity of Glasses. J. Am. Ceram. Soc. 1992, 75, 1043–1055. [Google Scholar] [CrossRef]
- Ogawa, T.; Aonuma, T.; Tamaki, T.; Ohashi, H.; Ushiyama, H.; Yamashita, K.; Yamaguchi, T. The proton conduction mechanism in a material consisting of packed acids. Chem. Sci. 2014, 5, 4878–4887. [Google Scholar] [CrossRef]
- Gao, Y.; Broersen, R.; Hageman, W.; Yan, N.; Mittelmeijer-Hazeleger, M.C.; Rothenberg, G.; Tanase, S. High proton conductivity in cyanide-bridged metal-organic frameworks: Understanding the role of water. J. Mater. Chem. A 2015, 3, 22347–22352. [Google Scholar] [CrossRef] [Green Version]
- de Grotthuss, C.J.T. Memoir on the decomposition of water and of the bodies that it holds in solution by means of galvanic electricity. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 871–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allcock, H.R.; Phelps, M.V.B.; Barrett, E.W.; Pishko, M.V.; Koh, W.G. Ultraviolet photolithographic development of polyphosphazene hydrogel microstructures for potential use in microarray biosensors. Chem. Mater. 2006, 18, 609–613. [Google Scholar] [CrossRef]
- Salles, F.; Bourrelly, S.; Jobic, H.; Devic, T.; Guillerm, V.; Llewellyn, P.; Serre, C.; Ferey, G.; Maurin, G. Molecular Insight into the Adsorption and Diffusion of Water in the Versatile Hydrophilic/Hydrophobic Flexible MIL-53(Cr) MOF. J. Phys. Chem. C 2011, 115, 10764–10776. [Google Scholar] [CrossRef]
- Tominaka, S.; Cheetham, A.K. Intrinsic and extrinsic proton conductivity in metal-organic frameworks. RSC Adv. 2014, 4, 54382–54387. [Google Scholar] [CrossRef]
- Boudjema, L.; Long, J.; Salles, F.; Larionova, J.; Guari, Y.; Trens, P. A Switch in the Hydrophobic/Hydrophilic Gas-Adsorption Character of Prussian Blue Analogues: An Affinity Control for Smart Gas Sorption. Chem. A Eur. J. 2018, 25, 479–484. [Google Scholar] [CrossRef]
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef]
- Zview 2 for Windows (Version 2.0); Scribner Assoc. Inc.: Charlottesville, VA, USA, 2000.
- Tanchoux, N.; Trens, P.; Maldonado, D.; Di Renzo, F.; Fajula, F. The adsorption of hexane over MCM-41 type materials. Colloids Surfaces A Physicochem. Eng. Asp. 2004, 246, 1–8. [Google Scholar] [CrossRef]
- Trens, P.; Belarbi, H.; Shepherd, C.; Gonzalez, P.; Ramsahye, N.A.; Lee, U.H.; Seo, Y.K.; Chang, J.S. Coadsorption of n -hexane and benzene vapors onto the chromium terephthalate-based porous material MIL-101(Cr) an experimental and computational study. J. Phys. Chem. C 2012, 116, 25824–25831. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Gelb, L.D.; Gubbins, K.E. Pore Size Distributions in Porous Glasses: A Computer Simulation Study. Langmuir 1999, 15, 305–308. [Google Scholar] [CrossRef]
- Abascal, J.L.F.; Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, S.M.F.; Salcedo-Abraira, P.; Gómez-Peña, A.; Trens, P.; Várez, A.; Salles, F.; Horcajada, P. Proton Conductive Zr-Phosphonate UPG-1—Aminoacid Insertion as Proton Carrier Stabilizer. Molecules 2020, 25, 3519. https://doi.org/10.3390/molecules25153519
Vilela SMF, Salcedo-Abraira P, Gómez-Peña A, Trens P, Várez A, Salles F, Horcajada P. Proton Conductive Zr-Phosphonate UPG-1—Aminoacid Insertion as Proton Carrier Stabilizer. Molecules. 2020; 25(15):3519. https://doi.org/10.3390/molecules25153519
Chicago/Turabian StyleVilela, Sérgio M. F., Pablo Salcedo-Abraira, Alejandro Gómez-Peña, Philippe Trens, Alejandro Várez, Fabrice Salles, and Patricia Horcajada. 2020. "Proton Conductive Zr-Phosphonate UPG-1—Aminoacid Insertion as Proton Carrier Stabilizer" Molecules 25, no. 15: 3519. https://doi.org/10.3390/molecules25153519




