Immune Activity of Polysaccharide Fractions Isolated from Korean Red Ginseng
Abstract
:1. Introduction
2. Results and Discussion
2.1. Component Analysis
2.2. Weight Change of Immunity Organs
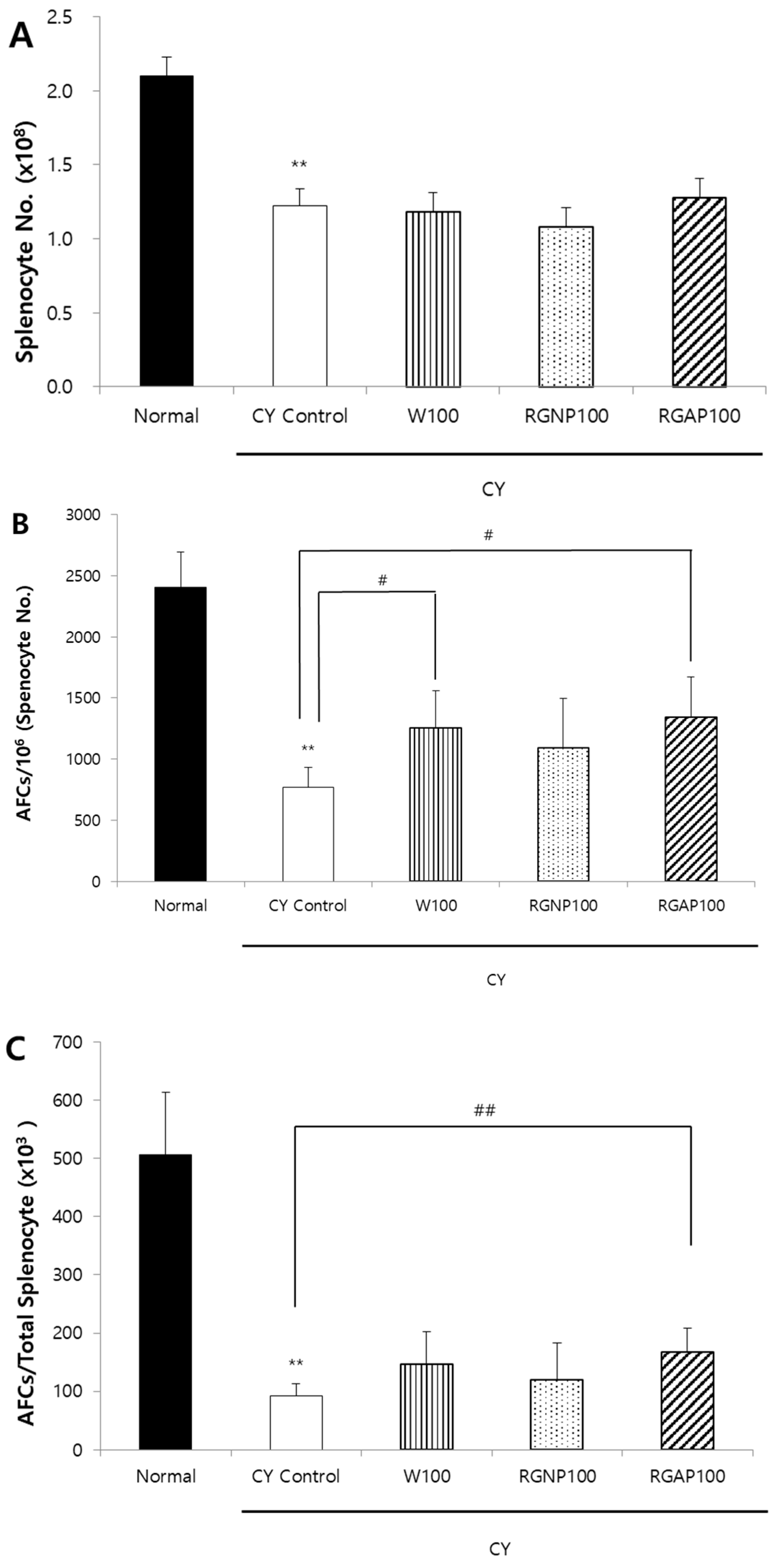
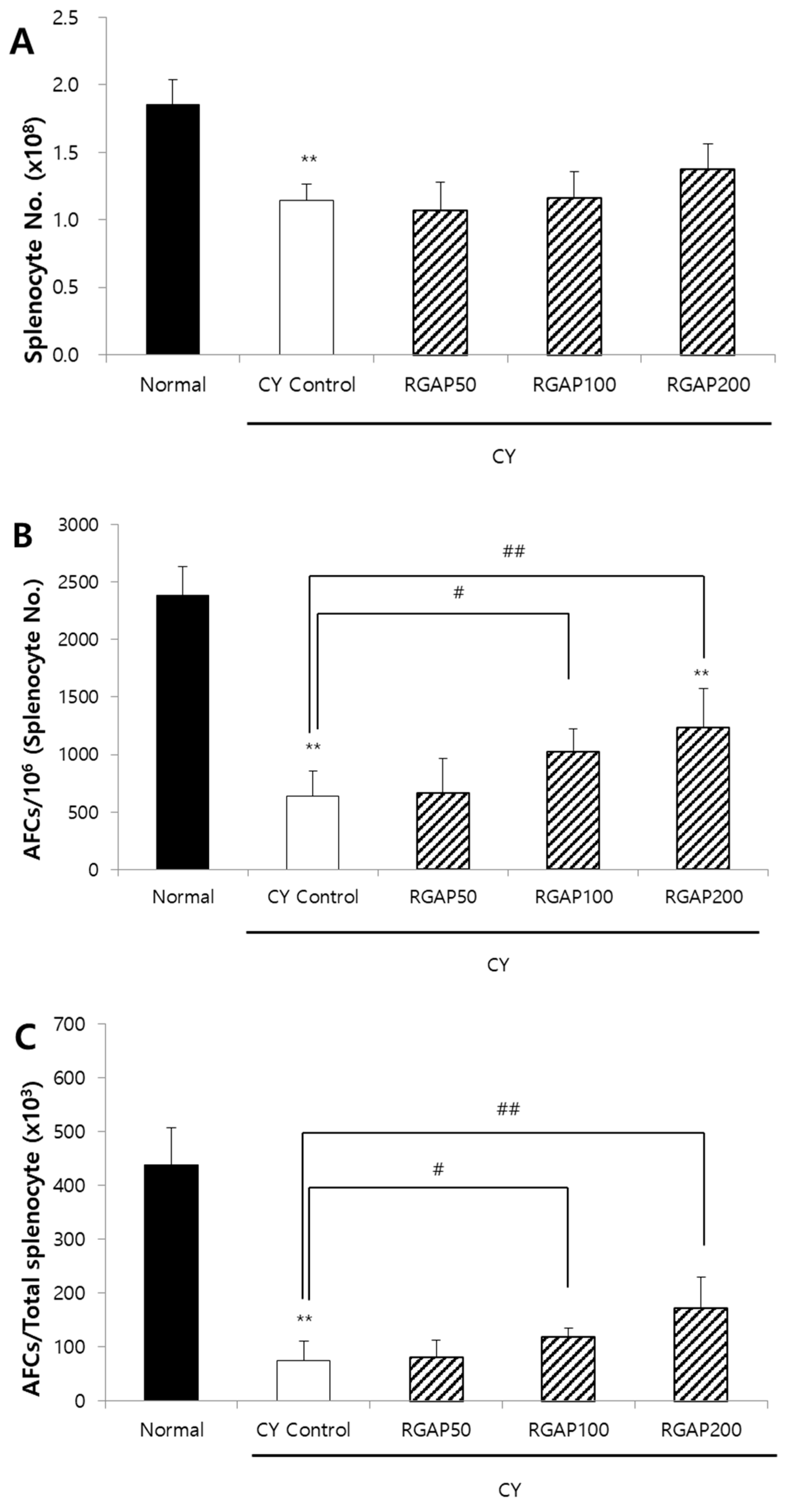
2.3. IgM Antibody-Forming Cells (AFCs) in Splenocyte against SRBCs
2.4. Phagocytosis Activity of Peritoneal Macrophage
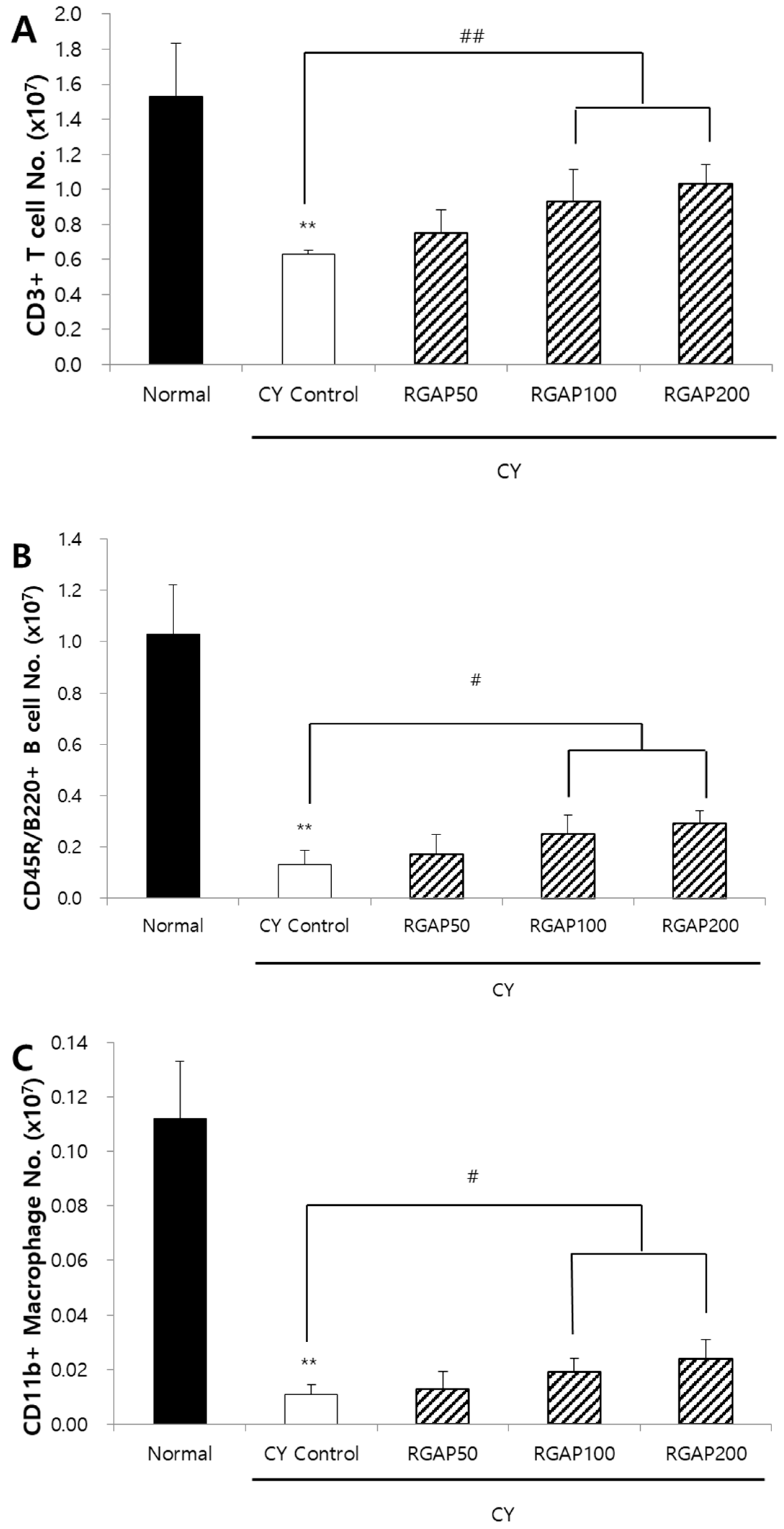
2.5. Distribution of Spleen Lymphocyte
2.6. Discussion
3. Methods
3.1. Manufacture of Korean Red Ginseng
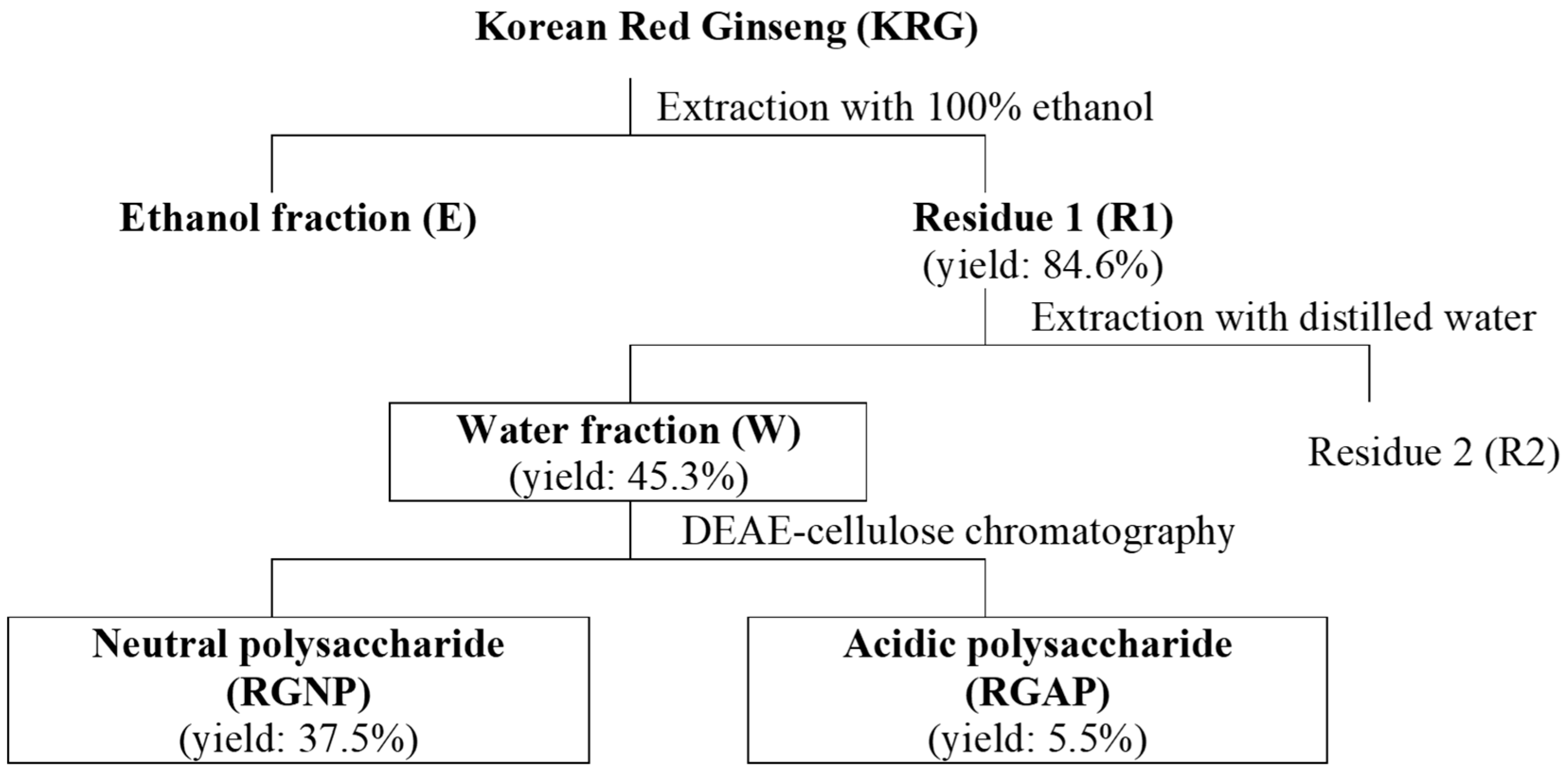
3.2. Production of Fractions
3.3. Component Analysis
3.4. Reagents and Analysis Devices
3.5. Animals
3.6. Sample Administration
3.7. Weight Measurement of Immune System Organs
3.8. Antibody-Forming Cells (AFCs) in Splenocytes against SRBCs
3.9. Phagocytosis Activity of Intraperitoneal Macrophage
3.10. Distribution of Spleen Lymphocyte
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- So, S.H.; Lee, J.W.; Kim, Y.S.; Hyun, S.H.; Han, C.K. Red ginseng monograph. J. Ginseng. Res. 2018, 42, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Kwak, Y.S.; Han, C.K.; Hyun, S.H.; Rhee, M.H. Adaptogenic effects of Panax ginseng on modulation of cardiovascular functions. J. Ginseng. Res. 2020, 44, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.K.; Han, C.K. Physiological and pharmacological features of the non-saponin components in Korean Red Ginseng. J. Ginseng. Res. 2020, 44, 527–537. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety of the Republic of Korea. Health Functional Food Code: Ministry of Food and Drug Safety Notification, 21 December 2016. Available online: https://www.khsa.or.kr/assets/extra/hfood/01.pdf (accessed on 6 August 2020).
- Kim, J.W.; Han, S.W.; Cho, J.Y.; Chung, I.J.; Kim, J.G.; Lee, K.H.; Park, K.U.; Baek, S.K.; Oh, S.C.; Lee, M.A.; et al. Korean red ginseng for cancer-related fatigue in colorectal cancer patients with chemotherapy: A randomised phase III trial. Eur. J. Cancer 2020, 130, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, S.K.; Hong, Y.; Yun, B.H.; Chon, S.J.; Jung, Y.S.; Park, J.H.; Cho, S.H.; Choi, Y.S.; Lee, B.S. Antioxidative effects of Korean red ginseng in postmenopausal women: a double-blind randomized controlled trial. J. Ethnopharmacol. 2014, 154, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.Y.; Kang, H.J.; Kim, O.Y.; Lee, J.H. Beneficial effects of Korean red ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: a randomized, double-blind, placebo-controlled trial. Nutr. J. 2012, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.W.; Kim, M.; Kim, M.; Chae, J.S.; Lee, J.H. Blood pressure-lowering effect of Korean red ginseng associated with decreased circulating Lp-PLA2 activity and lysophosphatidylcholines and increased dihydrobiopterin level in prehypertensive subjects. Hypertens. Res. 2016, 39, 449–456. [Google Scholar] [CrossRef]
- Bang, H.; Kwak, J.H.; Ahn, H.Y.; Shin, D.Y.; Lee, J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J. Med. Food 2014, 17, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Seo, S.K.; Choi, Y.M.; Jeon, Y.E.; Lim, K.J.; Cho, S.; Choi, Y.S.; Lee, B.S. Effects of red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women: A double-blind randomized controlled trial. Menopause 2012, 19, 461–466. [Google Scholar] [CrossRef]
- Sung, H.; Kang, S.-M.; Lee, M.-S.; Kim, T.G.; Cho, Y.-K. Korean red ginseng slows depletion of CD4 T cells in human immunodeficiency virus type 1-infected patients. Clin. Diagn. Lab. Immunol. 2005, 12, 497–501. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Shim, J.S.; Lee, J.S.; Kim, M.-K.; Chung, M.-S.; Kim, K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr. Res. 2006, 341, 1154–1163. [Google Scholar] [CrossRef]
- Lee, S.M.; Bae, B.-S.; Park, H.-W.; Ahn, N.-G.; Cho, B.-G.; Cho, Y.-L.; Kwak, Y.-S. Characterization of Korean Red ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng. Res. 2015, 39, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, B.H.; Kim, H.S.; Chang, S.J. Effect of saponin and non-saponin of panax ginseng on the blood pressure in the renocascular hypertension rats. J. Ginseng Res. 1999, 23, 79–80. [Google Scholar]
- Kwak, Y.-S.; Kyung, J.-S.; Kim, J.S.; Cho, J.U.; Rhee, M.-H. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol. Pharm. Bull. 2010, 33, 468–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.C.; In, M.J.; Lee, J.Y.; Hwang, Y.K.; Lee, S.D. Antithrombin active polysaccharide isolated from the alkaline extract of red ginseng. J. Ginseng Res. 1999, 23, 217–221. [Google Scholar]
- Song, J.-Y.; Yi, S.-H.; Jung, I.-S.; Yun, Y.-S. Effect of polysaccharide extracted from Panax ginseng on murine hematopoiesis. J. Ginseng Res. 2001, 25, 63–67. [Google Scholar]
- Lee, C.K.; Choi, J.W.; Kim, S.H.; Kim, H.K.; Han, Y.N. Biological activities of acidic polysaccharide of Korean red ginseng I. Effects on alcohol detoxification system in the liver of alcohol-intoxicated rats. J. Ginseng Res. 1998, 22, 260–266. [Google Scholar]
- Lee, C.K.; Choi, J.W.; Kim, S.H.; Kim, H.K.; Han, Y.N. Biological activities of acidic polysaccharide of Korean red ginseng. III: Effects on metabolizing activities in acetaminophen-treated rats. J. Ginseng Res. 1998, 22, 267–273. [Google Scholar]
- Hwang, Y.K.; Lee, S.D. Inhibitory activity of acidic polysaccharides of Korean red ginseng on lipolytic action of Toxohormone-L from cancerous ascites fluid. Korean J. Food Nutr. 1992, 5, 7–12. [Google Scholar]
- Suh, S.O.; Kroh, M.; Kim, N.R.; Joh, Y.G.; Cho, M.Y. Effects of red ginseng upon postoperative immunity and survival in patients with stage III gastric cancer. Am. J. Chin. Med. 2002, 30, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, K.M.; Shin, H.J.; Song, K.S.; Nam, K.Y.; Park, J.D. Anticancer activities of red ginseng acidic polysaccharide by activation of macrophages and natural killer cells. Yakhak Hoeji 2002, 46, 113–119. [Google Scholar]
- Kwak, Y.-S.; Shin, H.-J.; Song, Y.-B.; Kyung, J.-S.; Wee, J.-J.; Park, J.-D. Effect of oral administration of red ginseng acidic polysaccharide (RGAP) on the tumor growth inhibition. J. Ginseng Res. 2005, 29, 176–181. [Google Scholar]
- Kwak, Y.S.; Kim, Y.S.; Shin, H.J.; Song, Y.B.; Park, J.D. Anticancer activities by combined treatment of red ginseng acidic polysaccharide (RGAP) and anticancer agents. J. Ginseng Res. 2003, 27, 47–51. [Google Scholar]
- Kim, H.J.; Kim, M.H.; Byon, Y.Y.; Park, J.W.; Jee, Y.; Joo, H.G. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J. Vet. Sci. 2007, 8, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Reyes, A.W.; Simborio, H.L.; Hop, H.T.; Arayan, L.T.; Min, W.G.; Lee, H.J.; Rhee, M.H.; Chang, H.H.; Kim, S. Inhibitory effect of red ginseng acidic polysaccharide from Korean red ginseng on phagocytic activity and intracellular replication of Brucella abortus in RAW 264.7 cells. J. Vet. Sci. 2016, 17, 315–321. [Google Scholar] [CrossRef]
- Bing, S.J.; Kim, M.J.; Ahn, G.; Im, J.; Kim, D.S.; Ha, D.; Cho, J.; Kim, A.; Jee, Y. Acidic polysaccharide of Panax ginseng regulates the mitochondria/caspase-dependent apoptotic pathway in radiation-induced damage to the jejunum in mice. Acta Histochem. 2014, 116, 514–521. [Google Scholar] [CrossRef]
- Park, K.M.; Jeong, T.C.; Kim, Y.S.; Shin, H.J.; Nam, K.Y.; Park, J.D. Immunomodulatory effect of acidic polysaccharide fraction from Korean red ginseng (Panax ginseng). Nat. Prod. Sci. 2000, 6, 31–35. [Google Scholar]
- Park, K.M.; Kim, Y.S.; Jeong, T.C.; Joe, C.O.; Shin, H.J.; Lee, Y.H.; Nam, K.Y.; Park, J.D. Nitric oxide is involved in the immunomodulating activities of acidic polysaccharide from Panax ginseng. Planta. Med. 2001, 67, 122–126. [Google Scholar] [CrossRef]
- Kang, S.W.; Min, H.Y. Ginseng, the ’Immunity Boost’: The effects of Panax ginseng on immune system. J. Ginseng. Res. 2012, 36, 354–368. [Google Scholar] [CrossRef] [Green Version]
- Goldsby, R.A.; Kindt, T.J.; Osbome, B.A.; Kudy, J. Immunology, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2003; pp. 5–11. [Google Scholar]
- Hyun, S.H.; Kim, E.S.; Lee, S.M.; Kyung, J.S.; Lee, S.M.; Lee, J.W.; Kim, M.R.; Hong, J.T.; Kim, Y.S. Comparative Study on immuno-enhancing effects of red ginseng fractions. J. Food Sci. Nutr. 2014, 43, 1665–1673. [Google Scholar]
- Byeon, S.E.; Lee, J.H.; Kim, J.H.; Yang, W.S.; Kwak, Y.S.; Kim, S.Y.; Choung, E.S.; Rhee, M.H.; Cho, J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012, 2012, 732860. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.S.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Cardiovascular Diseases and Panax ginseng: A review on molecular mechanisms and medical applications. J. Ginseng. Res. 2012, 36, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J. Ginseng. Res. 2012, 36, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.H.; Shin, H.J.; Jang, K.H.; Park, C.K.; Koo, B.S.; Shin, H.J.; Yuk, S.H.; Lee, K.Y. Anti-acne properties of hydrophobic fraction of red ginseng (Panax ginseng C.A. Meyer) and its active components. Phytother. Res. 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A. Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.Y.; Song, J.Y.; Yun, Y.S.; Yang, H.O.; Rhee, D.K.; Pyo, S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol. Immunotoxicol. 2002, 24, 469–482. [Google Scholar] [CrossRef]
- Xiao, F.D.; Jiang, C.Z.; We, C.F.; Won, E.K.; Choung, S.Y. Synergistic immunostimulatory effect of pidotimod and red ginseng acidic polysaccharide on humoral immunity of immunosuppressed mice. Pharmazie 2008, 63, 904–908. [Google Scholar]
- Yoo, D.G.; Kim, M.C.; Park, M.K.; Park, K.M.; Quan, F.S.; Song, J.M.; Wee, J.J.; Wang, B.Z.; Cho, Y.K.; Compas, R.W.; et al. Protective effect of ginseng polysaccharides on influenza viral infection. PLoS ONE 2012, 7, e33678. [Google Scholar] [CrossRef]
- Sadiman, S.; Islamiyati, I.; Poddar, S. The differences in hemoglobin levels before and after consuming ambon bananas in students. Enferm. Clin. 2020, 30, 115–118. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Kim, S.W.; Youn, S.H.; Hyun, S.H.; Kyung, J.S.; In, G.; Park, C.K.; Jung, H.R.; Moon, S.J.; Kang, M.J.; et al. Biological effects of Korean red ginseng polysaccharides in aged rat using global proteomic approach. Molecules 2020, 25, 3019. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Bi, H.; Li, X.; Ni, W.; Han, H.; Li, N.; Wang, B.; Zhou, Y.; Tai, G. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. Carbohydr. Polym 2009, 77, 544–552. [Google Scholar] [CrossRef]
- Joo, K.M.; Park, C.W.; Jeong, H.J.; Lee, S.J.; Chang, I.S. Simultaneous determination of two Amadori compounds in Korean red ginseng (Panax ginseng) extracts and rat plasma by high-performance anion-exchange chromatography with pulsed amperometric detection. J. Chromatogr. B 2008, 865, 159–166. [Google Scholar] [CrossRef] [PubMed]
- In, G.; Ahn, N.-G.; Bae, B.-S.; Han, S.-T.; Noh, K.-B.; Kim, C.S. New method for simultaneous quantification of 12 ginsenosides in red ginseng powder and extract: In-house method validation. J. Ginseng. Res. 2012, 36, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, Y.S.; Kim, E.M. The physicochemical properties of crude polysaccharide fraction isolated from Korean ginseng (Panax ginseng C. A. Meyer). Korean J. Food Sci. Technol. 1996, 127, 389–392. [Google Scholar]
Sample Availability: Not available. |

| Group | W 1 | RGNP 2 | RGAP 3 |
|---|---|---|---|
| Glucose | 2.40 | 1.14 | N/D 4 |
| Fructose | 1.70 | 1.70 | N/D |
| Sucrose | 1.53 | 1.70 | N/D |
| Maltose | 5.10 | 5.80 | N/D |
| Acidic polysaccharides | 7.46 | N/D 5 | 57.25 |
| Ginsenosides 5 | 6.11 | 5.51 | N/D |
| Experimental Group (mg/kg) | Absolute Organ Weight | Relative Organ Weight (g/100 g of Body Weight) | ||
|---|---|---|---|---|
| Spleen | Thymus | Spleen | Thymus | |
| Normal | 112.8 ± 0.016 | 55.9 ± 0.008 | 0.47 ± 0.0007 | 0.23 ± 0.0003 |
| CY Control | 84.4 ± 0.013 ** | 42.7 ± 0.009 ** | 0.37 ± 0.0005 ** | 0.17 ± 0.0004 ** |
| CY + W100 | 88.3 ± 0.013 | 43.1 ± 0.007 | 0.37 ± 0.0007 | 0.18 ± 0.0003 |
| CY + RGNP100 | 92.6 ± 0.015 | 44.4 ± 0.004 | 0.38 ± 0.0006 | 0.19 ± 0.0002 |
| CY + RGAP100 | 94.3 ± 0.009 | 45.8 ± 0.005 | 0.4 ± 0.0004 | 0.19 ± 0.0002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, S.H.; Lee, S.M.; Han, C.-K.; In, G.; Park, C.-K.; Hyun, S.H. Immune Activity of Polysaccharide Fractions Isolated from Korean Red Ginseng. Molecules 2020, 25, 3569. https://doi.org/10.3390/molecules25163569
Youn SH, Lee SM, Han C-K, In G, Park C-K, Hyun SH. Immune Activity of Polysaccharide Fractions Isolated from Korean Red Ginseng. Molecules. 2020; 25(16):3569. https://doi.org/10.3390/molecules25163569
Chicago/Turabian StyleYoun, Soo Hyun, Sang Min Lee, Chang-Kyun Han, Gyo In, Chae-Kyu Park, and Sun Hee Hyun. 2020. "Immune Activity of Polysaccharide Fractions Isolated from Korean Red Ginseng" Molecules 25, no. 16: 3569. https://doi.org/10.3390/molecules25163569






