Synthesis, Crystal Structures and Anticancer Studies of Morpholinyldithiocarbamato Cu(II) and Zn(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
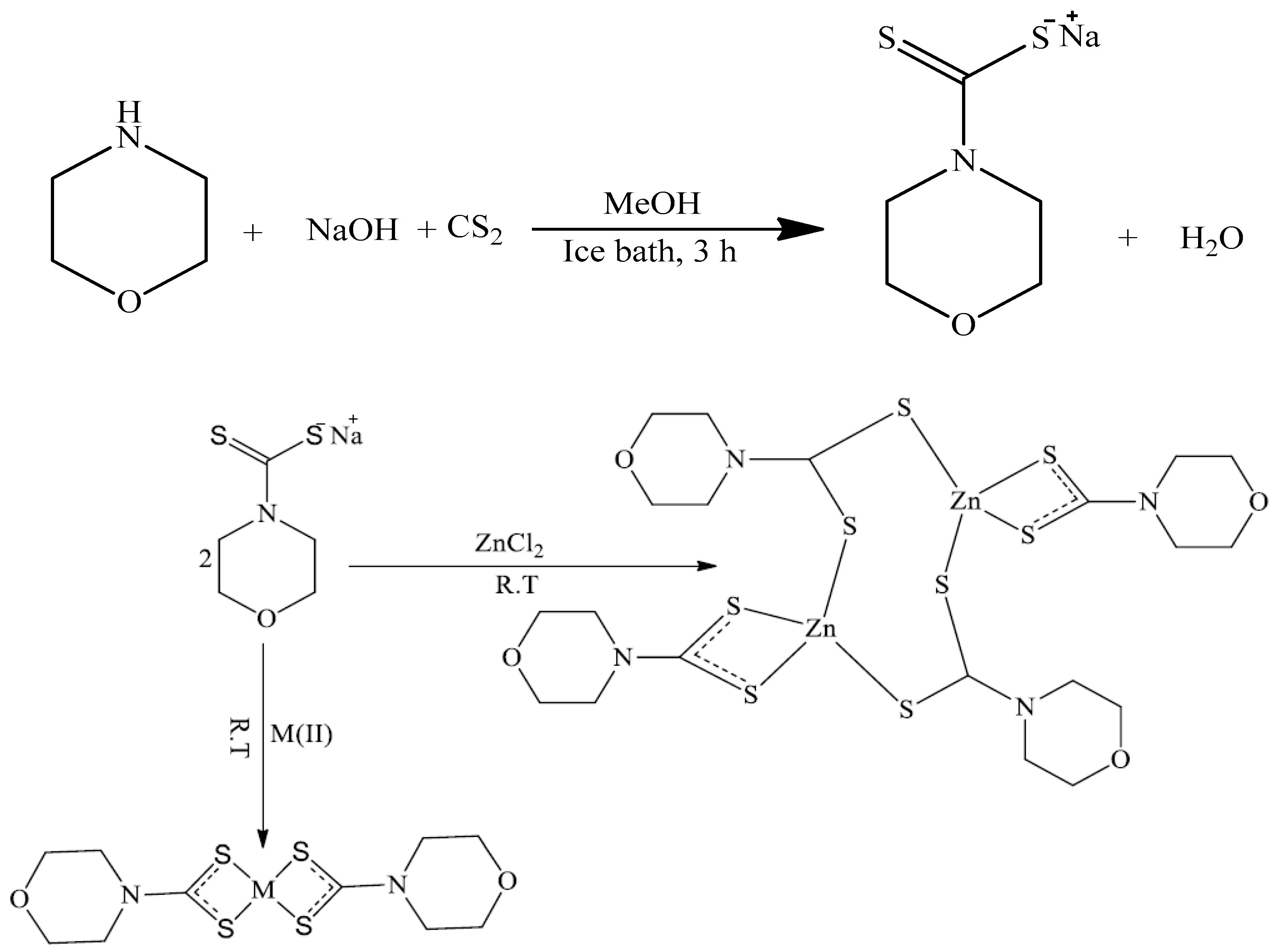
2.1. Syntheses
2.2. Spectral Studies of the Morpholine Dithiocarbamate and Its Cu(II) and Zn(II) Complexes
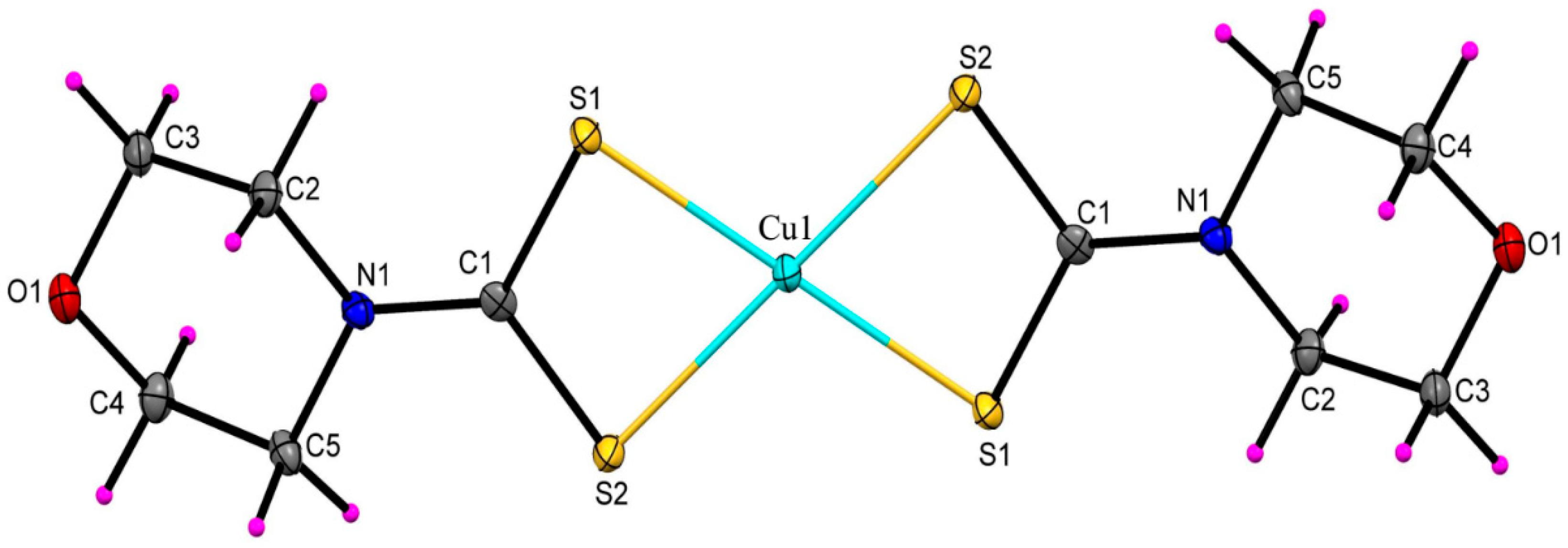
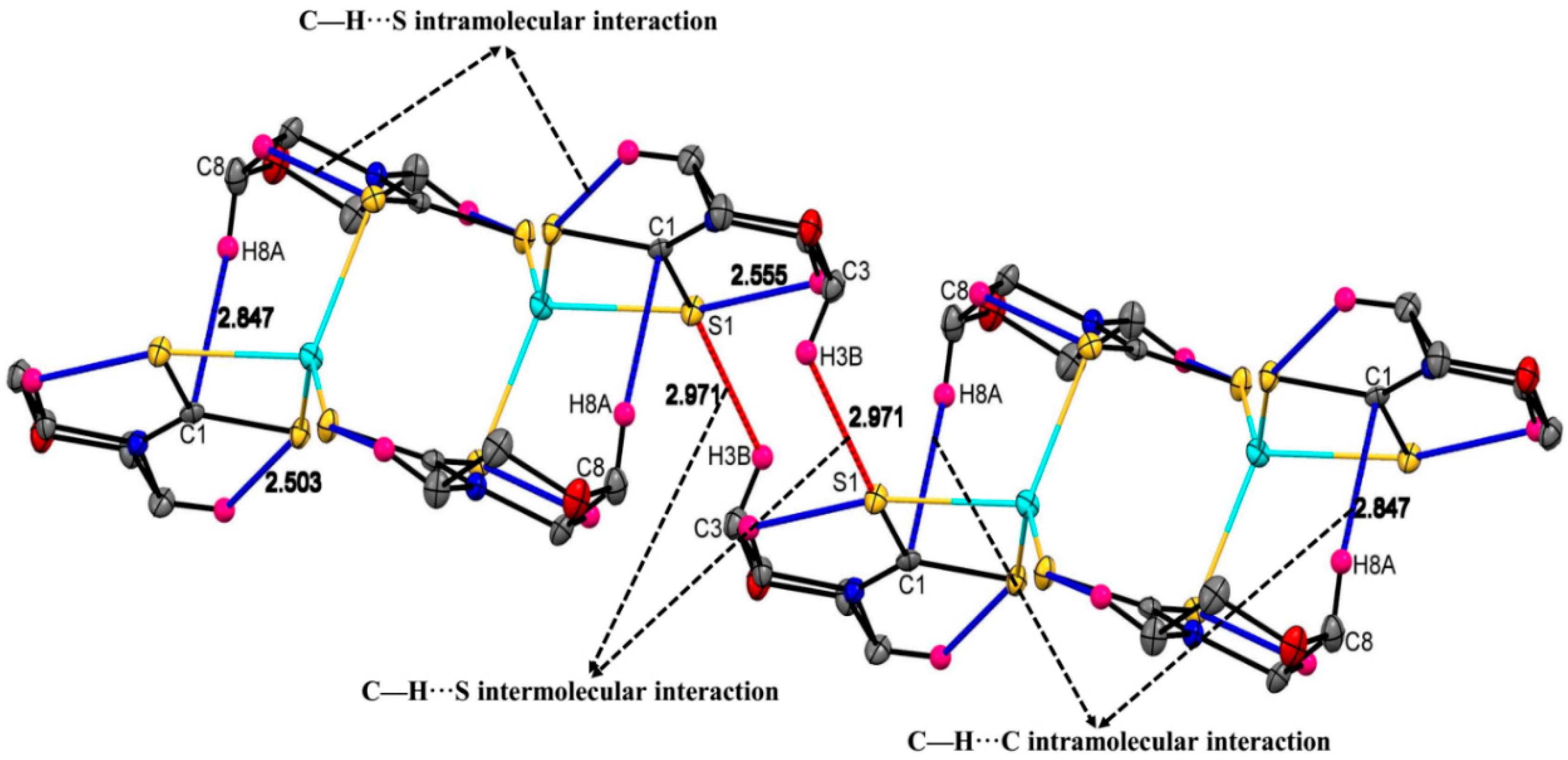
2.3. Molecular Structures of the Cu(II) and Zn(II) Morpholinyldithiocarbamate Complexes
2.4. Anticancer Studies
3. Materials and Methods
3.1. Apparatus, Materials and Analysis
3.2. Synthesis of Bis(Morpholinyldithiocarbamato) Cu(II) Complex [Cu(MphDTC)2]
3.3. Synthesis of Bis(Morpholinyldithiocarbamato) Zn(II) Complex [Zn(MphDTC)2]
3.4. Anticancer Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-S. Mechanisms of chemotherapeutic drug resistance in cancer therapy—A quick review. J. Obstet. Gynecol. 2009, 48, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connors, T. Anticancer drug development. Oncologist 1996, 1, 180–181. [Google Scholar] [CrossRef] [Green Version]
- Shazia, R.; Muhammad, I.; Anwar, N.; Haji, A.; Amin, A. Transition metal complexes as potential therapeutic agents. Biotechnol. Mol. Biol. Rev. 2010, 5, 38–45. [Google Scholar]
- Ajibade, P.A.; Kolawole, G.A. Synthesis, characterization and in vitro antiprotozoal studies of iron(III) complexes of some antimalarial drugs. J. Coord. Chem. 2008, 61, 3367–3374. [Google Scholar] [CrossRef]
- Ingo, O. On the medicinal chemistry of gold complexes as anticancer drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar]
- Adeniyi, A.A.; Ajibade, P.A. Development of ruthenium-based complexes as anticancer agents: Toward a rational design of alternative receptor targets. Rev. Inorg. Chem. 2016, 36, 53–75. [Google Scholar] [CrossRef]
- Olivier, R.; Waldo, O.; Manuel, A.; Ricardo, P.; Eddie, R.; Ken, P.; Tito, F. Oxaliplatin, Tetraplatin, cisplatin, and carboplatin: Spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s Anticancer Drug Screen Panel. Biochem. Pharmacol. 1996, 52, 1855–1865. [Google Scholar]
- Bhargava, A.; Vaishampayan, U.N. Satraplatin: Leading the new generation of oral platinum agents. Expert Opin. Investig Drugs 2009, 18, 1787–1797. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.W.F.; Rocha, H.A.O.; Queiroz de Medeiros, W.M.T.; Silva, M.S. Application of Dithiocarbamates as Potential New Antitrypanosomatids-Drugs: Approach Chemistry, Functional and Biological. Molecules 2019, 24, 2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buac, D.; Schmitt, S.; Ventro, G.; Dou, Q.P. Dithiocarbamate-Based Coordination Compounds as Potent Proteasome Inhibitors in Human Cancer Cells. Mini Rev. Med. Chem. 2012, 12, 1193–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.A.; Moghadam, M.E.; Saeidifar, M.; Saboury, A.A. Biological effect and molecular docking of anticancer palladium and platinum complexes with morpholine dithiocarbamate on human serum albumin as a blood carrier protein. J. Physiol. Pharmacol. 2018, 96, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Andrew, F.P.; Ajibade, P.A. Metal complexes of alkyl-aryl dithiocarbamates: Structural studies, anticancer potentials and applications as precursors for semiconductor nanocrystals. J. Mol. Struct. 2018, 1155, 843–855. [Google Scholar] [CrossRef]
- Sankarganesh, M.; Rajesh, J.; Kumar, G.V.; Vadivel, M.; Mitu, L.; Kumar, R.S.; Raja, J.D. Synthesis, spectral characterization, theoretical, antimicrobial, DNA interaction and in vitro anticancer studies of Cu(II) and Zn(II) complexes with pyrimidine-morpholine based Schiff base ligand. J. Saudi Chem. Soc. 2018, 22, 416–426. [Google Scholar] [CrossRef]
- Amir, M.K.; Khan, S.Z.; Hayat, F.; Hassan, A.; Butler, I.S.; Rehman, Z. Review article: Anticancer activity, DNA-binding and DNA-denaturing aptitude of palladium(II) dithiocarbamates. Inorg. Chim. Acta 2016, 451, 31–40. [Google Scholar] [CrossRef]
- Al-Jaroudi, S.S.; Altaf, M.; Seliman, A.A.; Yadav, S.; Arjmand, F.; Alhoshani, A.; Korashy, H.M.; Ahmad, S.; Isab, A.A. Synthesis, characterization, in vitro cytotoxicity and DNA interaction study of phosphanegold(I) complexes with dithiocarbamate ligands. Inorg. Chiim. Acta 2017, 464, 37–48. [Google Scholar] [CrossRef]
- Hayat, F.; Rehman, Z.; Khan, M.H. Two new heteroleptic ruthenium(II) dithiocarbamates: Synthesis, characterization, DFT calculation and DNA binding. J. Coord. Chem. 2017, 70, 279–295. [Google Scholar] [CrossRef]
- Altaf, M.; Monim-ul-Mehboob, M.; Kawde, A.N.; Corona, G.; Larcher, R.; Ogasawara, M.; Casagrande, N.; Celegato, M.; Borghese, C.; Siddik, Z.H.; et al. New bipyridine gold(III) dithiocarbamate-containing complexes exerted a potent anticancer activity against cisplatin-resistant cancer cells independent of p53 status. Oncotarget 2017, 8, 490–505. [Google Scholar] [CrossRef] [Green Version]
- Ohui, K.; Afanasenko, E.; Bacher, F.; Ting, R.L.X.; Zafar, A.; Blanco-Cabra, N.; Torrents, E.; Domotor, O.; May, N.V.; Darvasiova, D.; et al. New water-soluble copper(II) complexes with morpholine–thiosemicarbazone hybrids: Insights into the anticancer and antibacterial mode of action. J. Med. Chem. 2019, 62, 512–530. [Google Scholar]
- Dhahagani, K.; Kumar, S.M.; Chakkaravarthi, G.; Anitha, K.; Rajesh, J.; Ramu, A.; Rajagopal, G. Synthesis and spectral characterization of Schiff base complexes of Cu(II), Co(II), Zn(II) and VO(IV) containing 4-(4-aminophenyl) morpholine derivatives: Antimicrobial evaluation and anticancer studies. Spectrochim. Acta Part A 2014, 117, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Gwaram, N.S.; Hassandarvish, P. Synthesis, characterization, and anticancer studies of some morpholine derived Schiff bases and their metal complexes. J. Appl. Pharm. Sci. 2014, 4, 075–080. [Google Scholar] [CrossRef]
- Hosseini-Kharat, M.; Rahimi, R.; Zargarian, D.; Lighvan, Z.M.; Momtazi-Borojeni, A.A.; Sharifi, T.; Abdollahi, E.; Tavakol, H.; Mohammadi, T. Antiproliferative activity of morpholine-based compounds on MCF-7 breast cancer, colon carcinoma C26, and normal fibroblast NIH-3T3 cell lines and study of their binding affinity to calf thymus-DNA and bovine serum albumin. J. Biomol. Struct. Dyn. 2019, 37, 3788–3802. [Google Scholar] [CrossRef] [PubMed]
- Rupak, K.; Vulichi, S.R.; Suman, K. Emphasizing morpholine and its derivatives (MAID): A typical candidate of pharmaceutical importance. Int. J. Chem. Sci. 2016, 14, 1777–1788. [Google Scholar]
- War, J.A.; Srivastava, S.K.; Srivastava, S.D. Design, synthesis and DNA-binding study of some novel morpholine linked thiazolidinone derivatives. Spectrochim. Acta Part A 2017, 173, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Bissantz, C.; Kuhn, B.; Stahl, M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem 2010, 53, 5061–5084. [Google Scholar]
- Morgenthaler, M.; Schweizer, E.; Hoffmann-Roder, A.; Benini, F.; Martin, R.E.; Jaeschke, G.; Zimmerli, D. Predicting and tuning physicochemical properties in lead optimization: Amine basicities. Chem. Med. Chem. 2007, 2, 1100–1115. [Google Scholar] [CrossRef]
- Ndungu, J.M.; Krumm, S.A.; Yan, D.; Arrendale, R.F.; Reddy, G.P.; Evers, T.; Liotta, D.C. Non-nucleoside inhibitors of the measles virus RNA-dependent RNA polymerase: Synthesis, structure–activity relationships, and pharmacokinetics. J. Med. Chem. 2012, 55, 4220–4230. [Google Scholar]
- Stasevich, M.V.; Zvarich, V.I.; Novikov, V.P.; Zagorodnyaya, S.D.; Povnitsa, O.Y.; Chaika, M.A.; Nesterkina, M.V.; Kravchenko, I.A.; Druzhilovskii, D.S.; Poroikov, V.V. 9,10-Anthraquinone dithiocarbamates as potential pharmaceutical substances with pleiotropic actions: Computerized prediction of biological activity and experimental validation. Pharm. Chem. J. 2020, 53, 905–913. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Duraisamy, S.; Kasi, M.; Kandasamy, S.; Sarkar, R.; Kumarasamy, A. Syntheses, physicochemical characterization, antibacterial studies on potassium morpholine dithiocarbamate nickel(II), copper(II) metal complexes and their ligands. Heliyon 2019, 5, e01687. [Google Scholar] [CrossRef] [Green Version]
- Ajibade, P.A.; Fatokun, A.A.; Andrew, F.P. Synthesis, characterization and anti-cancer studies of Mn(II), Cu(II), Zn(II) and Pt(II) dithiocarbamate complexes—Crystal structures of the Cu(II) and Pt(II) complexes. Inorg. Chim. Acta 2020, 504, 119431. [Google Scholar] [CrossRef]
- Kartina, D.; Wahab, A.W.; Ahmad, A.; Irfandi, R.; Raya, I. In vitro antibacterial and anticancer activity of Zn (II) Valinedithiocarbamate complexes. J. Phys. Conf. Ser. 2019, 1341, 032042. [Google Scholar] [CrossRef]
- Andrew, F.P.; Ajibade, P.A. Synthesis, characterization and anticancer studies of bis-(N-methyl-1-phenyldithiocarbamato) Cu(II), Zn(II), and Pt(II) complexes: Single crystal X-ray structure of the copper complex. J. Coord. Chem. 2018, 71, 2776–2786. [Google Scholar] [CrossRef]
- Mbese, J.Z.; Ajibade, P.A. Synthesis, structural and optical properties of ZnS, CdS and HgS nanoparticles from dithiocarbamato single molecule precursors. J. Sulfur Chem. 2014, 35, 438–449. [Google Scholar] [CrossRef]
- Ronconi, L.; Giovagnini, L.; Marzano, C.; Bettìo, F.; Graziani, R.; Pilloni, G.; Fregona, D. Gold dithiocarbamate derivatives as potential antineoplastic agents: Design, spectroscopic properties, and in vitro antitumor activity. Inorg. Chem. 2005, 44, 1867–1881. [Google Scholar] [CrossRef]
- Oluwalana, A.E.; Ajibade, P.A. Synthesis and crystal structures of Pb(II) dithiocarbamates complexes: Precursors for PbS nanophotocatalyst. J. Sulfur Chem. 2020, 41, 182–199. [Google Scholar] [CrossRef]
- Gurumoorthy, G.; Thirumaran, S.; Ciattini, S. Synthesis and characterization of copper(II) dithiocarbamate complexes involving pyrrole and ferrocenyl moieties and their utility for sensing anions and preparation of copper sulfide and copper–iron sulfide nanoparticles. Appl. Organomet. Chem. 2018, 32, e4363. [Google Scholar] [CrossRef]
- Yang, H.; Li, L.; Song, Y.; Hou, H.; Fan, Y. Syntheses and characterization of ferrocenylthiocarboxylate-containing coordination compounds for nonlinear optics. J. Organomet. Chem. 2008, 693, 2624–2630. [Google Scholar] [CrossRef]
- Khan, S.; Ahmad, W.; Munawar, K.; Kanwal, S. Synthesis, Spectroscopic Characterization and Biological Evaluation of Ni(II), Cu(II) and Zn(II) complexes of diphenyldithiocarbamate. Indian J. Pharm. Sci. 2018, 80, 480–488. [Google Scholar] [CrossRef]
- Hogarth, G.; Faulkner, S. The crystal structure of [Cu (S2CNC4H8O)2]: An interesting structural motif constructed by intermolecular S … S and C–H … Cu interactions. Inorg. Chim. Acta 2013, 408, 222–224. [Google Scholar] [CrossRef]
- Sathiyaraj, E.; Perumal, M.V.; Nagarajan, E.R.; Ramalingan, C. Functionalized zinc(II) dithiocarbamate complexes: Synthesis, spectral and molecular structures of bis (N-cyclopropyl-N-4-methoxybenzyldithiocarbamato-S, S′) zinc(II) and (2, 2′-bipyridine) bis (N-cyclopropyl-N-4-methoxybenzyldithiocarbamato-S, S′) zinc(II). J. Saudi Chem. Soc. 2018, 22, 527–537. [Google Scholar]
- Radha, A.; Seshasayee, M.; Aravamudan, G. Structures of bis (piperidine-1-dithiocarbamato) nickel (II) and bis (piperidine-1-dithiocarbamato) copper (II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 1378–1381. [Google Scholar] [CrossRef]
- Pan, S.; Wang, Y. Crystal structure and spectroscopic studies of Bis (morpholine dithiocarbamate) Nickel (II) complex, Ni (C4H8ONCS2)2. Chin. J. Chem. 2001, 19, 856–859. [Google Scholar] [CrossRef]
- Andrew, F.P.; Ajibade, P.A. Synthesis, characterization and anticancer studies of bis (1-phenylpiperazine dithiocarbamato) Cu(II), Zn(II) and Pt(II) complexes: Crystal structures of 1-phenylpiperazine dithiocarbamato-S, S′ zinc(II) and Pt(II). J. Mol. Struct. 2018, 1170, 24–29. [Google Scholar] [CrossRef]
- Almeida Paz, F.A.; Neves, M.C.; Trindade, T.; Klinowski, J. The first dinuclear zinc (II) dithiocarbamate complex with butyl substituent groups. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m1067–m1069. [Google Scholar] [CrossRef] [Green Version]
- Bharti, A.; Bharati, P.; Chaudhari, U.; Singh, A.; Kushawaha, S.; Singh, N.; Bharty, M. Syntheses, crystal structures and photoluminescent properties of new homoleptic and heteroleptic zinc (II) dithiocarbamato complexes. Polyhedron 2015, 85, 712–719. [Google Scholar] [CrossRef]
- Shahid, M.; Rüffer, T.; Lang, H.; Awan, S.A.; Ahmad, S. Synthesis and crystal structure of a dinuclear zinc(II)-dithiocarbamate complex, bis{[(μ2-pyrrolidinedithiocarbamato-S, S′) (pyrrolidinedithiocarbamato-S, S′) zinc(II)]}. J. Coord. Chem. 2009, 62, 440–445. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Solomane, N. Synthesis and crystal structure of bis (thiomorpholinyldithiocarbamato) Zn (II): Structural, optical, and photocatalytic studies of ZnS nanoparticles from the complex. J. Coord. Chem. 2020, 73, 1292–1305. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds; morpholinyl dithiocarbamate, and the corresponding Cu(II) and Zn(II) complexes are available from the authors. |


| Compound | [Cu(MphDTC)2] | [Zn(μ-MphDTC)2(MphDTC)2] |
|---|---|---|
| Formula | C10H16CuN2O2S4 | C20H32N4O4S8Zn2 |
| Dcalc./g cm−3 | 1.806 | 1.734 |
| μ/mm−1 | 2.113 | 2.208 |
| Formula Weight | 388.03 | 779.76 |
| Size/mm3 | 0.34 × 0.22 × 0.14 | 0.33 × 0.22 × 0.14 |
| T/K | 100(2) | 100(2) |
| Crystal System | monoclinic | triclinic |
| Space Group | P21/n | P-1 |
| a/Å | 4.1987(5) | 7.9581(6) |
| b/Å | 20.646(3) | 8.7769(6) |
| c/Å | 8.3714(10) | 11.8945(9) |
| α/° | 90 | 103.680(2) |
| β/° | 100.564(8) | 91.204(1) |
| γ/° | 90 | 111.329(3) |
| V/Å3 | 713.39(16) | 746.67(10) |
| Z | 2 | 1 |
| Z’ | 0.5 | 0.5 |
| Θmin/° | 1.973 | 1.778 |
| Θmax/° | 26.497 | 27.412 |
| Measured Refl. | 6298 | 12596 |
| Independent Refl. | 1473 | 3370 |
| Reflections Used | 1222 | 3208 |
| Rint | 0.0672 | 0.0345 |
| Parameters | 88 | 172 |
| Largest Peak | 0.496 | 0.849 |
| Deepest Hole | −0.468 | −0.359 |
| GooF | 1.088 | 1.078 |
| wR2 (all data) | 0.0930 | 0.0725 |
| wR2 | 0.0873 | 0.0718 |
| R1 (all data) | 0.0448 | 0.0271 |
| R1 | 0.0359 | 0.0260 |
| [Cu(MphDTC)2] | [Zn(μ-MphDTC)2(MphDTC)2] | ||
|---|---|---|---|
| Bond | Length(Å) | Bonds | Length(Å) |
| Cu1–S1 | 2.3019(7) | S1–Zn1 | 2.4373(5) |
| Cu1–S2 | 2.3119(8) | S2–Zn1 | 2.3347(5) |
| S2–C1 | 1.717(3) | S3–Zn1 | 2.342(5) |
| O1–C3 | 1.436(3) | S4–Zn1 | 2.3169(5) |
| O1–C4 | 1.423(3) | C1–S1 | 1.7290(19) |
| N1–C1 | 1.332(3) | C3–O1 | 1.426(2) |
| C8–O2 | 1.428(2) | ||
| C1–N1 | 1.323(2) | ||
| Bond | Angle(°) | Bonds | Angle(°) |
| S11–Cu1–S1 | 180.0 | S2–Zn1–S1 | 76.408(16) |
| S11–Cu1–S2 | 77.22(2) | S2–Zn1–S3 | 115.480(18) |
| S1–Cu1–S2 | 102.78(2) | S1–Zn1–S3 | 112.705(18) |
| S11–Cu–S21 | 102.78(2) | S1–Zn1–S4 | 107.689(18) |
| S1–Cu1–S21 | 77.22(2) | S2–Zn1–S4 | 130.37(2) |
| S2–Cu1–S21 | 180.0 | S3–Zn1–S4 | 108.203(19) |
| C11–S1–Cu1 | 84.58(9) | C1–Zn1–S1 | 81.75(7) |
| C1–S2–Cu1 | 84.37(9) | S2–Zn1–S1 | 76.408(16) |
| Compounds/Cancer Cells | Renal (TK-10) IC50, µM | Melanoma (UACC-62) IC50, µM | Breast (MCF-7) IC50, µM |
|---|---|---|---|
| Mphdtc | 1.51 | 12.73 | 2.65 |
| [Cu(Mphdtc)2] | 4.64 | 4.47 | 4.37 |
| [Zn(μ-MphDTC)2(MphDTC)2] | 8.70 | 16.54 | 3.17 |
| Parthenolide | 4.64 | 11.37 | 3.52 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajibade, P.A.; Andrew, F.P.; Botha, N.L.; Solomane, N. Synthesis, Crystal Structures and Anticancer Studies of Morpholinyldithiocarbamato Cu(II) and Zn(II) Complexes. Molecules 2020, 25, 3584. https://doi.org/10.3390/molecules25163584
Ajibade PA, Andrew FP, Botha NL, Solomane N. Synthesis, Crystal Structures and Anticancer Studies of Morpholinyldithiocarbamato Cu(II) and Zn(II) Complexes. Molecules. 2020; 25(16):3584. https://doi.org/10.3390/molecules25163584
Chicago/Turabian StyleAjibade, Peter A., Fartisincha P. Andrew, Nandipha L. Botha, and Nolwazi Solomane. 2020. "Synthesis, Crystal Structures and Anticancer Studies of Morpholinyldithiocarbamato Cu(II) and Zn(II) Complexes" Molecules 25, no. 16: 3584. https://doi.org/10.3390/molecules25163584





