Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food
Abstract
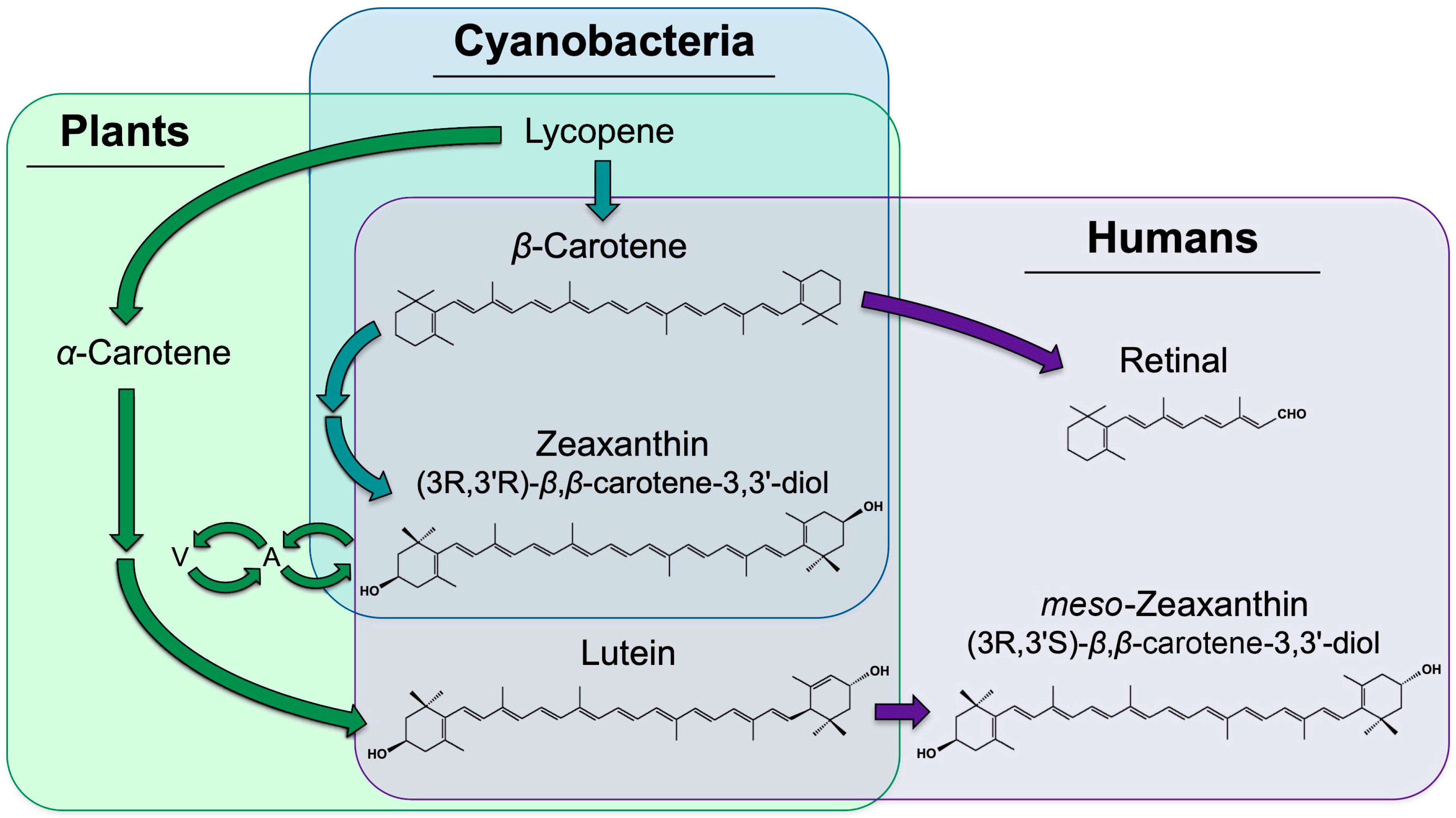
:1. Introduction
2. The Duality of Light
3. Zeaxanthin and Lutein in Plants
The Xanthophyll Cycle and Photoprotective Energy Dissipation as Harmless Heat
4. Zeaxanthin and Lutein in Humans
4.1. Transport and Storage of Carotenoids
4.2. Protection of the Eye against Intense Visible Light
4.3. Enhancement of Eye and Brain Function beyond Disease
4.4. Xanthophylls as Anti-Inflammatory Agents
5. Synergy between Xanthophylls and Other Antioxidation Systems
6. Dual Roles of Oxidants and Antioxidants
6.1. Enhancement of Eye and Brain Function beyond Disease
6.2. Positive and Negative Effects of Antioxidants
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, E.J.; Neuringer, M.; Russell, R.M.; Schalch, W.; Snodderly, D.M. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investig. Ophthalmol. Vis. Sci. 2005, 46, 692–702. [Google Scholar] [CrossRef]
- Chung, H.Y.; Rasmussen, H.M.; Johnson, E.J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. 2004, 134, 1887–1893. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, A.; Skibsted, L.H. Free radical transients in photobleaching of xanthophylls and carotenes. Free Radic. Res. 1997, 26, 549–563. [Google Scholar] [CrossRef]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M.; García-Plazaola, J.I. Beyond non-photochemical fluorescence quenching: The overlapping antioxidant functions of zeaxanthin and tocopherols. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603. [Google Scholar]
- Bhosale, P.; Bernstein, P.S. Synergistic effects of zeaxanthin and its binding protein in the prevention of lipid membrane oxidation. Biochim. Biophys. Acta 2005, 1740, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Sauer, L.; Li, B.; Bernstein, P.S. Ocular carotenoid status in health and disease. Annu. Rev. Nutr. 2019, 39, 95–120. [Google Scholar] [CrossRef]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and orientation of xanthophylls in a lipid bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef]
- Sujak, A.; Gabrielska, J.; Grudzinski, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef]
- Gabrielska, J.; Gruszecki, W.I. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: A 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta 1996, 1285, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 2005, 1740, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Asker, D.; Beppu, T.; Ueda, K. Unique diversity of carotenoid-producing bacteria isolated from Misasa, a radioactive site in Japan. Appl. Microbiol. Biotechnol. 2007, 77, 383–392. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B.; Lange, O.L. Carotenoid composition and metabolism in green and blue-green algal lichens in the field. Oecologia 1993, 94, 576–584. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Green, T.G.A.; Czygan, F.-C.; Lange, O.L. Differences in the susceptibility to light stress in two lichens forming a phycosymbiodeme, one partner possessing and one lacking the xanthophyll cycle. Oecologia 1990, 84, 451–456. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Hernández, A.; Becerril, J.M. Antioxidant and pigment composition during autumnal leaf senescence in woody deciduous species differing in their ecological traits. Plant Biol. 2003, 5, 557–566. [Google Scholar] [CrossRef]
- Jouni, Z.E.; Wells, M.A. Purification and partial characterization of a lutein-binding protein from the midgut of the silkworm Bombyx mori. J. Biol. Chem. 1996, 271, 14722–14726. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retinal Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [Green Version]
- Adams, W.W., III; Demmig-Adams, B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 1992, 186, 390–398. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B.; Logan, B.A.; Barker, D.H.; Osmond, C.B. Rapid changes in xanthophyll cycle-dependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilax australis, growing in the understory of an open Eucalyptus forest. Plant Cell Environ. 1999, 22, 125–136. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W., III. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef]
- Cohu, C.M.; Lombardi, E.; Adams, W.W., III; Demmig-Adams, B. Increased nutritional quality of plants for long-term spaceflight missions through choice of plant variety and manipulation of growth conditions. Acta Astronaut. 2014, 94, 799–806. [Google Scholar] [CrossRef]
- Park, S.; Fischer, A.L.; Steen, C.J.; Iwai, M.; Morris, J.M.; Walla, P.J.; Niyogi, K.K.; Fleming, G.R. Chlorophyll–carotenoid excitation energy transfer in high-light-exposed thylakoid membranes investigated by snapshot transient absorption spectroscopy. J. Am. Chem. Soc. 2018, 140, 11965–11973. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Steen, C.J.; Lyska, D.; Fischer, A.L.; Endelman, B.; Iwai, M.; Niyogi, K.K.; Fleming, G.R. Chlorophyll–carotenoid excitation energy transfer and charge transfer in Nannochloropsis oceanica for the regulation of photosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 3385–3390. [Google Scholar] [CrossRef] [Green Version]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Dall’Osto, L.; Holt, N.E.; Kaligotia, S.; Fuciman, M.; Cazzaniga, S.; Carbonera, D.; Frank, H.A.; Alric, J.; Bassi, R. Zeaxanthin protects plant photosynthesis by modulating chlorophyll triplet yield in specific light-harvesting antenna subunits. J. Biol. Chem. 2012, 287, 41820–41834. [Google Scholar] [CrossRef] [Green Version]
- Connor, W.E.; Duell, P.B.; Kean, R.; Wang, Y. The prime role of HDL to transport lutein into the retina: Evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4226–4231. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef]
- González, S.; Astner, S.; An, W.; Pathak, M.A.; Goukassian, D. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J. Investig. Dermatol. 2003, 121, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Khachik, F.; de Moura, F.F.; Zhao, D.Y.; Aebischer, C.P.; Bernstein, P.S. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3383–3392. [Google Scholar]
- Stahl, W.; Nicolai, S.; Briviba, K.; Hanusch, M.; Broszeit, G.; Peters, M.; Martin, H.D.; Sies, H. Biological activities of natural and synthetic carotenoids: Induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis 1997, 18, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Thomson, L.R.; Toyoda, Y.; Langner, A.; Delori, F.C.; Garnett, K.M.; Craft, N.; Nichols, C.R.; Cheng, K.M.; Dorey, C.K. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3538–3549. [Google Scholar]
- Toyoda, Y.; Thomson, L.R.; Langner, A.; Craft, N.E.; Garnett, K.M.; Nichols, C.R.; Cheng, K.M.; Dorey, C.K. Effect of dietary zeaxanthin on tissue distribution of zeaxanthin and lutein in quail. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1210–1221. [Google Scholar]
- Demmig-Adams, B.; Adams, R.B. Eye nutrition in context: Mechanisms, implementation, and future directions. Nutrients 2013, 5, 2483–2501. [Google Scholar] [CrossRef] [PubMed]
- Polutchko, S.K.; Stewart, J.J.; Demmig-Adams, B. Integrative view of the nutrition of the eye. In Nutraceuticals and Functional Foods in Human Health and Disease Prevention; Bagchi, D., Preuss, H.G., Swaroop, A., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015; pp. 407–417. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Niedzwiedzki, D.M.; Enriquez, M.M.; LaFountain, A.M.; Frank, H.A. Ultrafast time-resolved absorption spectroscopy of geometric isomers of xanthophylls. Chem. Phys. 2010, 373, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R.; Wooten, B.R.; Engles, M.; Wong, J.C. The influence of filtering by the macular carotenoids on contrast sensitivity measured under simulated blue haze conditions. Vis. Res. 2012, 63, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Stringham, J.M.; Hammond, B.R. Macular pigment and visual performance under glare conditions. Optom. Vis. Sci. 2008, 85, 82–88. [Google Scholar] [CrossRef]
- Ceravolo, S.A.; Hammond, B.R.; Oliver, W.; Clementz, B.; Miller, L.S.; Renzi-Hammond, L.M. Dietary carotenoids lutein and zeaxanthin change brain activation in older adult participants: A randomized, double-masked, placebo-controlled trial. Mol. Nutr. Food Res. 2019, 63, 1801051. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.C.; Kaplan, H.S.; Hammond, B.R. Lutein and zeaxanthin status and auditory thresholds in a sample of young healthy adults. Nutr. Neurosci. 2017, 20, 1–7. [Google Scholar] [CrossRef]
- Mewborn, C.M.; Terry, D.P.; Renzi-Hammond, L.M.; Hammond, B.R.; Miller, L.S. Relation of retinal and serum lutein and zeaxanthin to white matter integrity in older adults: A diffusion tensor imaging study. Arch. Clin. Neuropsychol. 2018, 33, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Min, K.B. Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 2014, 37, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional intervention to prevent Alzheimer’s disease: Potential benefits of xanthophyll carotenoids and omega-3 fatty acids combined. J. Alzheimers Dis. 2018, 367–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavich, G.M. Understanding inflammation, its regulation, and relevance for health: A top scientific and public priority. Brain Behav. Immun. 2015, 45, 13–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf-ganjouei, A.; Moradi, K.; Bagheri, S.; Aarabi, M.H. The association between systemic inflammation and cognitive performance in healthy adults. J. Neuroimmunol. 2020, 345, 577272. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell. Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef] [Green Version]
- Yong, L.C.; Petersen, M.R.; Sigurdson, A.J.; Sampson, L.A.; Ward, E.M. High dietary antioxidant intakes are associated with decreased chromosome translocation frequency in airline pilots. Am. J. Clin. Nutr. 2009, 90, 1402–1410. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Ding, X.; Qin, L.; Mao, Y.; Chen, L.; Li, W.; Ying, C. Zeaxanthin improves diabetes-induced cognitive deficit in rats through activiting PI3K/AKT signaling pathway. Brain Res. Bull. 2017, 132, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Stringham, J.M.; Johnson, E.J.; Hammond, B.R. Lutein across the lifespan: From childhood cognitive performance to the aging eye and brain. Curr. Dev. Nutr. 2019, 3, nzz066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renzi-Hammond, L.M.; Bovier, E.R.; Fletcher, L.M.; Miller, L.S.; Mewborn, C.M.; Lindbergh, C.A.; Baxter, J.H.; Hammond, B.R. Effects of a lutein and zeaxanthin intervention on cognitive function: A randomized, double-masked, placebo-controlled trial of younger healthy adults. Nutrients 2017, 9, 1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrona, M.; Korytowksi, W.; Różanowska, M.; Sarna, T.; Truscott, T.G. Cooperation of antioxidants in protection against photosensitized oxidation. Free Rad. Biol. Med. 2003, 35, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Różanowska, M.; Sarna, T. Zeaxanthin in combination with ascorbic acid or α-tocopherol protects APRE-19 cells against photosensitized peroxidation of lipids. Free Rad. Biol. Med. 2004, 36, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Cohu, C.M.; Amiard, V.; van Zadelhoff, G.; Veldink, G.A.; Muller, O.; Adams, W.W., III. Emerging trade-offs – impact of photoprotectants (PsbS, xanthophylls, and vitamin E) on oxylipins as regulators of development and defense. New Phytol. 2013, 197, 720–729. [Google Scholar] [CrossRef]
- Dangles, O. Antioxidant activity of plant phenols: Chemical mechanisms and biological significance. Curr. Org. Chem. 2012, 16, 692. [Google Scholar] [CrossRef]
- Skibsted, L.H. Anthocyanidins regenerating xanthophylls: A quantum mechanical approach to eye health. Curr. Opin. Food Sci. 2018, 20, 24–29. [Google Scholar] [CrossRef]
- Mukai, K.; Nagai, K.; Ouchi, A.; Suzuki, T.; Izumisawa, K.; Nagaoka, S.-I. Finding of remarkable synergistic effect on the aroxyl-radical-scavenging rates under the coexistence of α-tocopherol and catechins. Int. J. Chem. Kinet. 2019, 51, 643–656. [Google Scholar] [CrossRef]
- Artiach, G.; Sarajlic, P.; Bäck, M. Inflammation and its resolution in coronary artery disease: A tightrope walk between omega-6 and omega-3 polyunsaturated fatty acids. Kardiol. Pol. 2020, 78, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Przybylska, S. Lycopene—A bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Kühn, H.; Borchert, A. Regulation of enzymatic lipid peroxidation: The interplay of peroxidizing and peroxide reducing enzymes. Free Rad. Biol. Med. 2002, 33, 154–172. [Google Scholar] [CrossRef]
- Calder, P. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and beneficial role of ROS. Oxid. Med. Cell. Longev. 2016, 2016, 7909186. [Google Scholar] [CrossRef] [Green Version]
- Fichman, Y.; Mittler, R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020, 102, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.B.; Egbo, K.N.; Demmig-Adams, B. High-dose vitamin C supplements diminish the benefits of exercise in athletic training and disease prevention. Nutr. Food Sci. 2014, 44, 95–101. [Google Scholar] [CrossRef]
- Bessa, A.L.; Oliveira, V.N.; Agostini, G.G.; Oliveira, R.J.S.; Oliveira, A.C.S.; White, G.E.; Wells, G.D.; Teixeira, D.N.S.; Espindola, F.S. Exercise intensity and recovery: Biomarkers of injury, inflammation, and oxidative stress. J. Strength Cond. Res. 2016, 30, 311–319. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Adams, W.W., III. Acclimation of photosynthesis to the environment. In Concepts in Photobiology: Photosynthesis and Photomorphogenesis; Singhal, G.S., Renger, G., Sopory, S.K., Irrgang, K.-D., Govindjee, Eds.; Narosa Publishing House: New Delhi, India, 1999; pp. 477–512. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; Adams, W.W., III. Chloroplast photoprotection and the trade-off between abiotic and biotic defense. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 631–643. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Kunkowska, A.B.; Schippers, J.H.M. Role of reactive oxygen species during cell expansion in leaves. Plant Physiol. 2016, 172, 2098–2106. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demmig-Adams, B.; Stewart, J.J.; Adams, W.W., III. Less photoprotection can be good in some genetic and environmental contexts. Biochem. J. 2019, 476, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Külheim, C.; Ågren, J.; Jansson, S. Rapid regulation of light harvesting and plant fitness in the field. Science 2002, 297, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Maharijaya, A.; Vosman, B.; Verstappen, F.; Steenhuis-Broers, G.; Mumm, R.; Purwito, A.; Visser, R.G.; Voorrips, R.E. Resistance factors in pepper inhibit larval development of thrips (Frankliniella occidentalis). Entomol. Exp. Appl. 2012, 145, 62–71. [Google Scholar] [CrossRef]
- Glowacka, K.; Kromdijk, J.; Kucera, K.; Xie, J.; Cavanagh, A.P.; Leonelli, L.; Leakey, A.D.B.; Ort, D.R.; Niyogi, K.K.; Long, S.P. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 2018, 9, 868. [Google Scholar] [CrossRef] [Green Version]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- Frenkel, M.; Külheim, C.; Jänkänpää, H.J.; Skogström, O.; Dall’Osto, L.; Ågren, J.; Bassi, R.; Moritz, T.; Moen, J.; Jansson, S. Improper excess light energy dissipation in Arabidopsis results in a metabolic reprogramming. BMC Plant Biol. 2009, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Zulfugarov, I.S.; Tovuu, A.; Kim, C.-Y.; Vo, K.T.X.; Ko, S.Y.; Hall, M.; Seok, H.-Y.; Kim, Y.-K.; Skogstrom, O.; Moon, Y.-H.; et al. Enhanced resistance of PsbS-deficient rice (Oryza sativa L.) to fungal and bacterial pathogens. J. Plant Biol. 2016, 59, 616–626. [Google Scholar] [CrossRef]
- Kromdijk, J.; Glowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.J.; Polutchko, S.K.; Adams, W.W., III; Cohu, C.M.; Wenzl, C.A.; Demmig-Adams, B. Light, temperature, and tocopherol status influence foliar vascular anatomy and leaf function in Arabidopsis thaliana. Physiol. Plant. 2017, 160, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Adams, W.W., III; Cohu, C.M.; Demmig-Adams, B. Tocopherols modulate leaf vein arrangement and composition without impacting photosynthesis. Photosynthetica 2018, 56, 382–391. [Google Scholar] [CrossRef]
- Stewart, J.J.; Baker, C.R.; Sharpes, C.S.; Wong-Michalak, S.T.; Polutchko, S.K.; Adams, W.W., III; Demmig-Adams, B. Effects of foliar redox status on leaf vascular organization suggest avenues for cooptimization of photosynthesis and heat tolerance. Int. J. Mol. Sci. 2018, 19, 2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patwardhan, R.S.; Singh, B.; Pal, D.; Checker, R.; Bandekar, M.; Sharma, D.; Sandur, S.K. Redox regulation of regulatory T-cell differentiation and functions. Free Radic. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; London, E.; de Moura, F.F.; Johnson, M.; Steidl, S.; Detolla, L.; Shipley, S.; Sanchez, R.; Chen, X.Q.; Flaws, J.; et al. Chronic ingestion of (3R,3′R,6′R)-lutein and (3R,3′R)-zeaxanthin in the female rhesus macaque. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5476–5486. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Tran, E.; Demmig-Adams, B. Vitamins and minerals: Powerful medicine or potent toxins? Nutr. Food Sci. 2007, 37, 50–60. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food. Molecules 2020, 25, 3607. https://doi.org/10.3390/molecules25163607
Demmig-Adams B, López-Pozo M, Stewart JJ, Adams WW III. Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food. Molecules. 2020; 25(16):3607. https://doi.org/10.3390/molecules25163607
Chicago/Turabian StyleDemmig-Adams, Barbara, Marina López-Pozo, Jared J. Stewart, and William W. Adams, III. 2020. "Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food" Molecules 25, no. 16: 3607. https://doi.org/10.3390/molecules25163607








