A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Lavandula stoechas Essential Oil
2.2. Anti-Inflammatory Activity In Vitro
2.2.1. Irritation Test in Red Blood Cell System Cellular Model
2.2.2. Inhibition of Denaturation of Bovine Serum Albumin
2.3. In Vivo Anti-Inflammatory Activity Assay
2.3.1. Carrageenan-Induced Paw Edema
2.3.2. Xylene-Induced Ear Edema
2.3.3. Mouse Ear Tissue Morphology
2.4. Effects of LSEO on Cytotoxicity of Three Human Tumor Cell Lines
3. Materials and Methods
3.1. Material
3.1.1. Extraction of Lavandula stoechas Essential Oil
3.1.2. Solvents, Drugs and Chemicals
3.1.3. Animals
3.1.4. Cancer Cell Lines
3.2. Methods
3.2.1. Determination of the Chemical Composition of Essential Oil
3.2.2. In Vitro and In Vivo Anti-Inflammatory Activities
Anti-Inflammatory Test Using Erythrocyte System Cellular Model In Vitro
Anti-Inflammatory Activity Using the Inhibition of Denaturation of Albumin In Vitro
In Vivo Anti-Inflammatory Assay Using Carrageenan-Induced Paw Edema
In Vivo Anti-Inflammatory Activity Using Xylene-Induced Ear Edema
Morphological Analysis of Ear Tissue
3.2.3. Cytotoxic Activity Using MTT Test
3.2.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BSA | bovine serum albumin |
| DMSO | dimethyl sulfoxide |
| EO | essential oil |
| FBS | fetal bovine serum |
| GC-MS | gas chromatography-mass spectrometry |
| H&E | hematoxylin-eosin |
| HRBC | human red blood cells |
| IC50 | median inhibitory concentration |
| LE | left ear |
| LHP | left hind paw |
| LNCPP | Laboratoire National de Contrôle des Produits Pharmaceutiques |
| LSEO | Lavandula stoechas essential oil |
| MTT | 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-tetrazolium bromide |
| NIST | National Institute of Standards and Technology |
| NMRI | Naval Medical Research Institute |
| NSAID | non-steroidal anti-inflammatory drugs |
| PBS | phosphate-buffered saline |
| p.o. | per os |
| PARP | poly(ADP-ribose) polymerase |
| PMN | polymorphonuclear cells |
| R&D | Research and Development Center |
| RE | right ear |
| RHP | right hind paw |
| RI | retention index |
| rpm | rotation per minute |
| RPMI | Roswell Park Memorial Institute |
| RT | retention times |
References
- Monteleone, G.; Pallone, F.; Stolfi, C. The dual role of inflammation in colon carcinogenesis. Int. J. Mol. Sci. 2012, 13, 11071–11084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE 2014, 9, e0092122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Karin, M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e0114767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkan, A.; Erdoğan, A. Membrane and DNA damaging/protective effects of eugenol, eucalyptol, terpinen-4-ol, and camphor at various concentrations on parental and drug-resistant H1299 cells. Turk. J. Biol. 2013, 37, 405–413. [Google Scholar] [CrossRef]
- Leighton, X.; Bera, A.; Eidelman, O.; Eklund, M.; Puthillathu, N.; Pollard, H.B.; Srivastava, M. High ANXA7 Potentiates eucalyptol toxicity in hormone-refractory prostate cancer. Anticancer Res. 2018, 38, 3831–3842. [Google Scholar] [CrossRef] [Green Version]
- Boukhatem, M.N.; Ferhat, M.A.; Benassel, N.; Kameli, A. Lavande papillon (Lavandula stoechas L.): Une plante à parfum aux multiples vertus. Phytothérapie 2019, in press. [Google Scholar] [CrossRef]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolifer. 2004, 37, 221–229. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Bousta, D.; Farah, A. A Phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 9. [Google Scholar] [CrossRef]
- Rahmati, B.; Kiasalari, Z.; Roghani, M.; Khalili, M.; Ansari, F. Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharmac. Biol. 2017, 55, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, M.; Kiani, Z.; Moezi, S.A.; Rad, G.H.M. The effects of lavender, valerian, and oxazepam on anxiety among hospitalized patients with coronary artery disease. Mod. Care J. 2018, 15. [Google Scholar] [CrossRef]
- Vokou, D.; Chalkos, D.; Karamanlidou, G.; Yiangou, M. Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J. Chem. Ecol. 2002, 28, 755–768. [Google Scholar] [CrossRef]
- Amara, N.; Boukhatem, M.N.; Ferhat, M.A.; Kaibouche, N.; Laissaoui, O.; Boufridi, A. Applications potentielles de l’huile essentielle de lavande papillon (Lavandula stoechas L.) comme conservateur alimentaire naturel. Phytothérapie 2017, 1–9. [Google Scholar] [CrossRef]
- Wells, R.; Truong, F.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. Lavandula Essential oils: A current review of applications in medicinal, food, and cosmetic industries of Lavender. Nat. Prod. Comm. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Kameli, A. Essential oils from wild populations of Algerian Lavandula stoechas L.: Composition, chemical variability, and in vitro biological properties. Chem. Biodiv. 2011, 8, 937–953. [Google Scholar] [CrossRef]
- Ristorcelli, D.; Tomi, F.; Casanova, J. 13C-NMR as a tool for identification and enantiomeric differentiation of major terpenes exemplified by the essential oil of Lavandula stoechas L. ssp. stoechas. Flav. Fragr. J. 1998, 13, 154–158. [Google Scholar] [CrossRef]
- Parvin, M.S.; Das, N.; Jahan, N.; Akhter, M.A.; Nahar, L.; Islam, M.E. Evaluation of in vitro anti-inflammatory and antibacterial potential of Crescentia cujete leaves and stem bark. BMC Res. Notes 2015, 8, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, H.; Eswaraiah, M.C.; Dutta, A.M. In-vitro anti-inflammatory and anti-arthritic activity of Oryza Sativa Var. joha rice (an aromatic indigenous rice of Assam). Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 115–121. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; El Hilaly, J.; Ennassir, J.; Benlyas, M.; Alem, C.; Amarouch, M.Y.; Filali-Zegzouti, Y. Anti-inflammatory properties and phenolic profile of six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. King Saud Univ.-Sci. 2018, 30, 519–526. [Google Scholar] [CrossRef]
- Karthik, E.V.; Vishnu, V.; Gayathri Priya, R. Anti-inflammatory activity of lavender oil using HRBC membrane stabilising method. Int. J. Pharm. Sci. Rev. Res. 2016, 2016. 40, 254–258. [Google Scholar]
- Williams, L.A.D.; O’Connar, A.; Latore, L.; Dennis, O.; Ringer, S.; Whittaker, J.A.; Kraus, W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med. J. 2008, 57, 327–331. [Google Scholar]
- Santos, F.A.; Rao, V.S.N. Anti-inflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1,8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef]
- Al-Reza, S.M.; Yoon, J.I.; Kim, H.J.; Kim, J.S.; Kang, S.C. Anti-inflammatory activity of seed essential oil from Zizyphus jujuba. Food Chem. Toxicol. 2010, 48, 639–643. [Google Scholar] [CrossRef]
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.; Donadio, M.V.; Mello, R.O. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Ciênc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef] [Green Version]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Cuman, R.K.N. Effect of lavender (Lavandula angustifolia) essential oil on acute inflammatory response. Evid.-Based Complem. Altern. Med. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukhatem, M.N.; Kameli, A.; Ferhat, M.A.; Saidi, F.; Mekarnia, M. Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J. Med. 2013, 8, 22520. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Koda, K. Antitumor effect of 1,8-cineole against colon cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.D.; Kim, Y.H.; Kim, J.Y. Essential oil and 1,8-cineole from Artemisia lavandulae folia induces apoptosis in KB cells via mitochondrial stress and caspase activation. Food Sci. Biotechnol. 2010, 19, 185–191. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Amiri, A.; Karimi, G.; Emami, S.A.; Asili, J.; Mousavi, S.H. Comparative studies of cytotoxic and apoptotic properties of different extracts and the essential oil of Lavandula angustifolia on malignant and normal cells. Nutr. Cancer 2014, 66, 424–434. [Google Scholar] [CrossRef]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants used against cancer—An extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Gezici, S. Promising anticancer activity of lavender (Lavandula angustifolia Mill.) essential oil through induction of both apoptosis and necrosis. Ann. Phytomed. 2018, 7, 38–45. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Lavandula stoechas Essential Oil are available from the authors. |
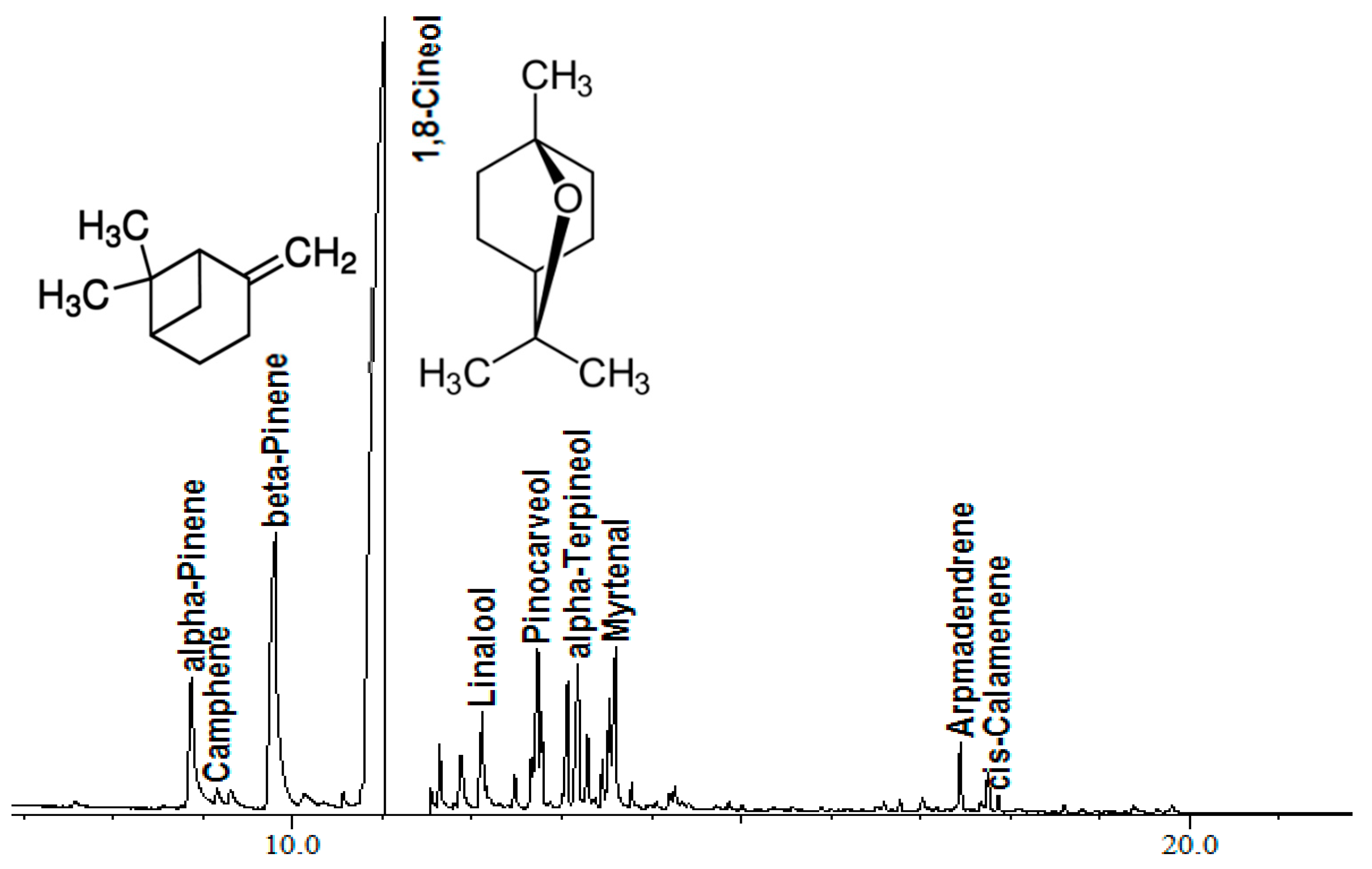



| Retention Time (min) | Name | % |
|---|---|---|
| 8.874 | α-Pinene | 4.75 |
| 9.155 | Camphene | 0.35 |
| 9.808 | β-Pinene | 13.83 |
| 11.031 | 1,8-Cineole | 61.36 |
| 11.640 | cis-Linalool oxide | 0.92 |
| 11.887 | trans-Linalool oxide | 1.39 |
| 12.114 | Linalool | 1.63 |
| 12.475 | α-Campholenal | 0.44 |
| 12.734 | Pinocarveol | 2.12 |
| 12.778 | Camphor | 0.63 |
| 13.067 | Pinocarvone | 2.04 |
| 13.181 | α-Terpineol | 3.15 |
| 13.288 | Terpineol-4 | 0.96 |
| 13.453 | Cryptone | 0.59 |
| 13.532 | α-Terpineol | 1.14 |
| 13.598 | Myrtenal | 2.36 |
| 13.781 | Verbenone | 0.29 |
| 14.261 | Carvone | 0.21 |
| 17.442 | Aromadendrene | 0.98 |
| 17.749 | δ-Elemene | 0.66 |
| 17.863 | cis-Calamenene | 0.20 |
| Oxygenated Monoterpenes | 79.23 | |
| Monoterene Hydrocarbons | 18.93 | |
| Sesquiterpene Hydrocarbons | 1.84 |
| Treatment | Concentration | Absorbance (560 nm) | % Inhibition of Hemolysis | IC50 # |
|---|---|---|---|---|
| Control (PBS) | 0.568 | - | - | |
| LSEO (µL/mL) | 6 | 0.370 | 34.917 ± 1.939 D | 6.214 ± 0.776 B |
| 3 | 0.145 | 74.471 ± 0.465 C | ||
| 1.5 | 0.064 | 88.791 ± 0.101 B | ||
| 0.8 | 0.042 | 92.605 ± 0.000 A | ||
| 0.4 | 0.042 | 92.605 ± 0.465 A | ||
| Sodium diclofenac (mg/mL) | 30 | 0.46 | 19.014 ± 12.707 F | 1.198 ± 0.735 A |
| 3 | 0.411 | 27.552 ± 3.354 E | ||
| 0.3 | 0.045 | 92.165 ± 0.419 A | ||
| 0.03 | 0.041 | 92.693 ± 0.227 A | ||
| 0.003 | 0.04 | 92.913 ± 0.221 A |
| Treatment | Concentration | Absorbance (560 nm) | % Inhibition of BSA | IC50 # |
|---|---|---|---|---|
| Control (PBS) | 1.288 | - | - | |
| LSEO (µL/mL) | 6 | 0.067 | 62.569 ± 0.967 E | 2.447 ± 0.873 A |
| 3 | 0.061 | 65.735 ± 0.853 D | ||
| 1.5 | 0.054 | 69.832 ± 0.558 C | ||
| 0.8 | 0.048 | 73.184 ± 0.558 B | ||
| 0.4 | 0.049 | 72.625 ± 2.560 B | ||
| Sodium diclofenac (mg/mL) | 10 | 0.165 | 7.960 ± 7.741 F | 8.260 ± 0.943 B |
| 1 | 0.04 | 77.932 ± 0.721 A | ||
| 0.1 | 0.044 | 75.279 ± 2.555 A | ||
| 0.01 | 0.043 | 76.117 ± 0.534 A | ||
| 0.001 | 0.045 | 74.720 ± 0.279 AB |
| Treatment (Dose μg/kg) | Weight (mean, mg) ± SD | % Inhibition of Edema | ||
|---|---|---|---|---|
| Left Hind Paw | Right Hind Paw | Edema Weight # | ||
| LSEO (200) | 126.10 ± 8.00 | 110.12 ± 7.12 | 15.975 ± 7.31 A | 47.0588 |
| LSEO (20) | 138.48 ± 10.1 | 121.00 ± 6.72 | 17.480 ± 8.71 A | 42.0712 |
| LSEO (2) | 146.08 ± 8.61 | 122.35 ± 6.00 | 23.733 ± 9.22 A,B | 21.3476 |
| Positive control (Indomethacin) | 161.25 ± 6.12 | 144.50 ± 6.18 | 16.750 ± 7.50 A | 44.4904 |
| Negative control | 154.30 ± 7.65 | 124.12 ± 10.0 | 30.175 ± 13.41 B | / |
| Treatment (Dose mg/kg) | Weight (mean, mg) ± SD | % Inhibition of Edema | ||
|---|---|---|---|---|
| Left Ear | Right Ear | Edema Weight # | ||
| LSEO (820) | 22.97 ± 4.13 | 16.52 ± 0.73 | 6.45 ± 2.80 B | 25.8620 |
| LSEO (410) | 16.37 ± 1.92 | 14.65 ± 2.22 | 1.72 ± 1.20 A | 80.1724 |
| LSEO (82) | 22.72 ± 5.16 | 18.50 ± 1.99 | 4.22 ± 2.88 B | 51.4367 |
| Betasone® 0.5% | 18.08 ± 2.79 | 15.50 ± 1.50 | 2.58 ± 1.49 A,B | 70.3448 |
| Voltarene Emulgel® 1% | 20.70 ± 4.94 | 13.32 ± 1.35 | 7.38 ± 3.17 B | 15.1724 |
| Negative control (Vehicle) | 28.10 ± 6.12 | 19.40 ± 4.13 | 8.70 ± 3.55 C | / |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. https://doi.org/10.3390/molecules25163671
Boukhatem MN, Sudha T, Darwish NHE, Chader H, Belkadi A, Rajabi M, Houche A, Benkebailli F, Oudjida F, Mousa SA. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules. 2020; 25(16):3671. https://doi.org/10.3390/molecules25163671
Chicago/Turabian StyleBoukhatem, Mohamed Nadjib, Thangirala Sudha, Noureldien H.E. Darwish, Henni Chader, Asma Belkadi, Mehdi Rajabi, Aicha Houche, Fatma Benkebailli, Faiza Oudjida, and Shaker A. Mousa. 2020. "A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer" Molecules 25, no. 16: 3671. https://doi.org/10.3390/molecules25163671
APA StyleBoukhatem, M. N., Sudha, T., Darwish, N. H. E., Chader, H., Belkadi, A., Rajabi, M., Houche, A., Benkebailli, F., Oudjida, F., & Mousa, S. A. (2020). A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules, 25(16), 3671. https://doi.org/10.3390/molecules25163671







