Encapsulation of Cinnamic Acid by Cucurbit[7]uril for Enhancing Photoisomerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Host–Guest Interactions
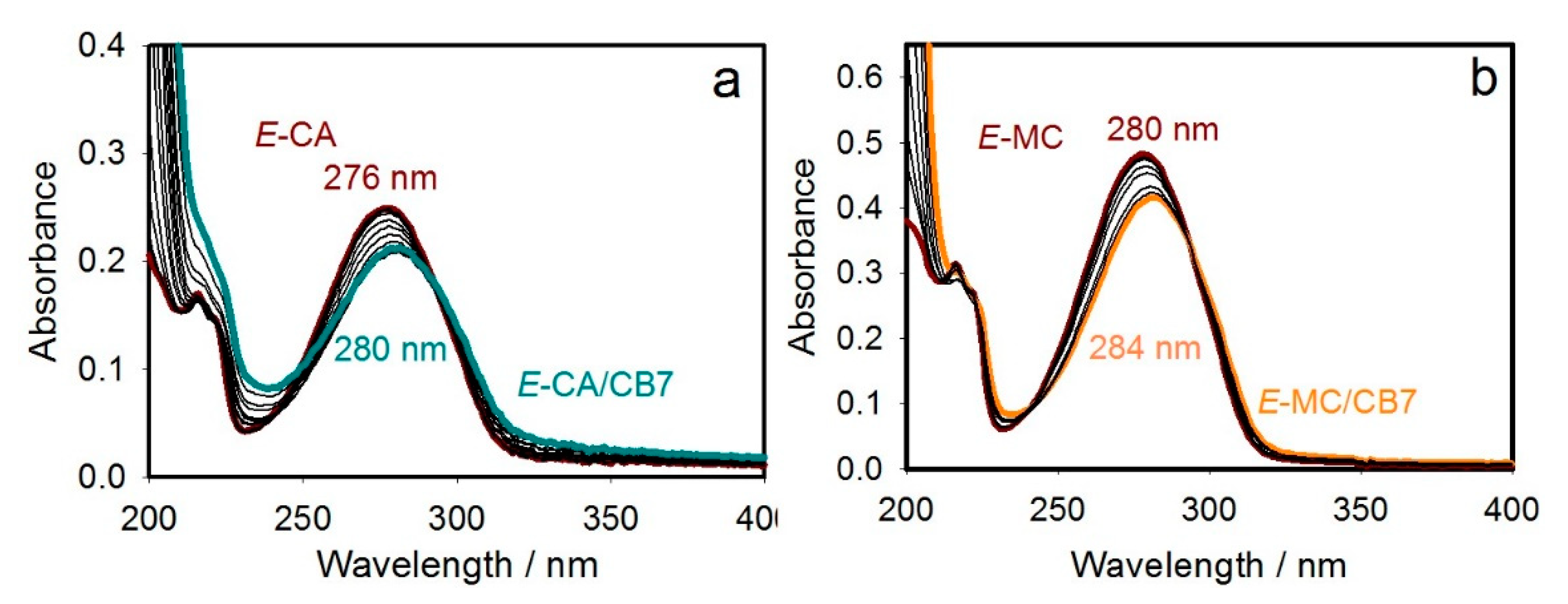
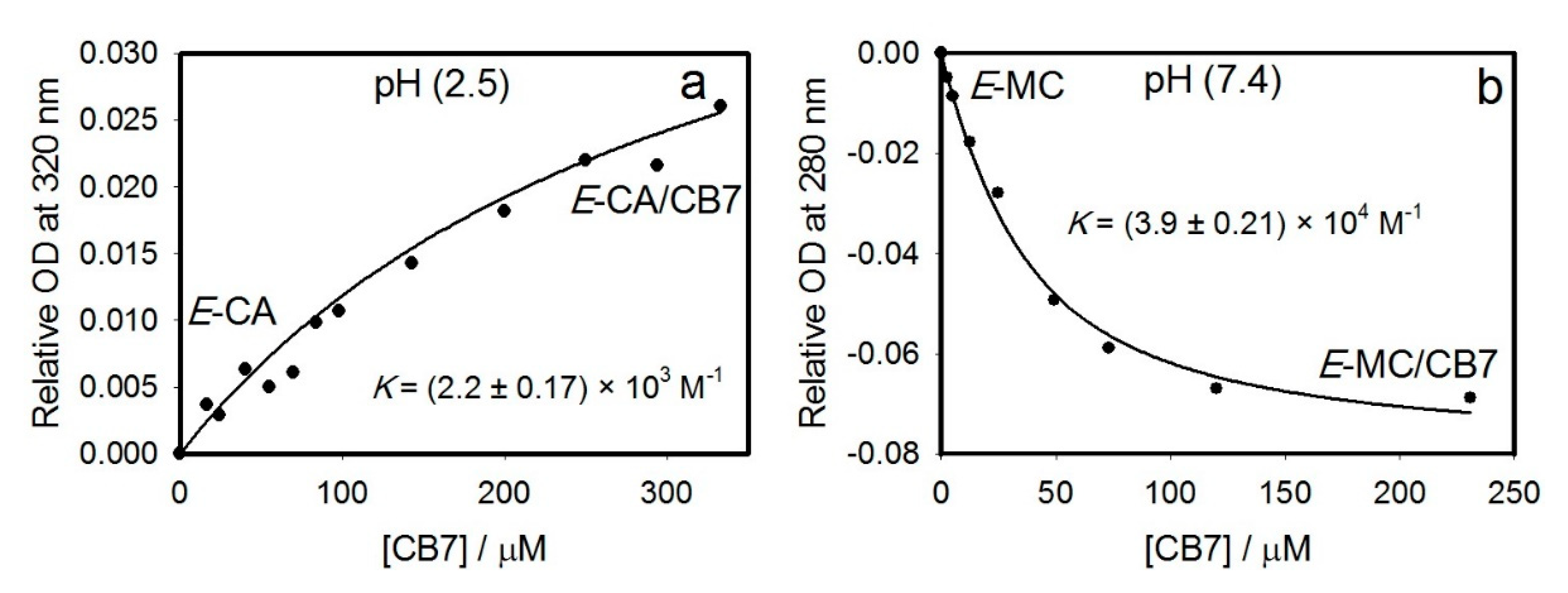
2.1.1. UV–Visible Absorption Titration (UV)
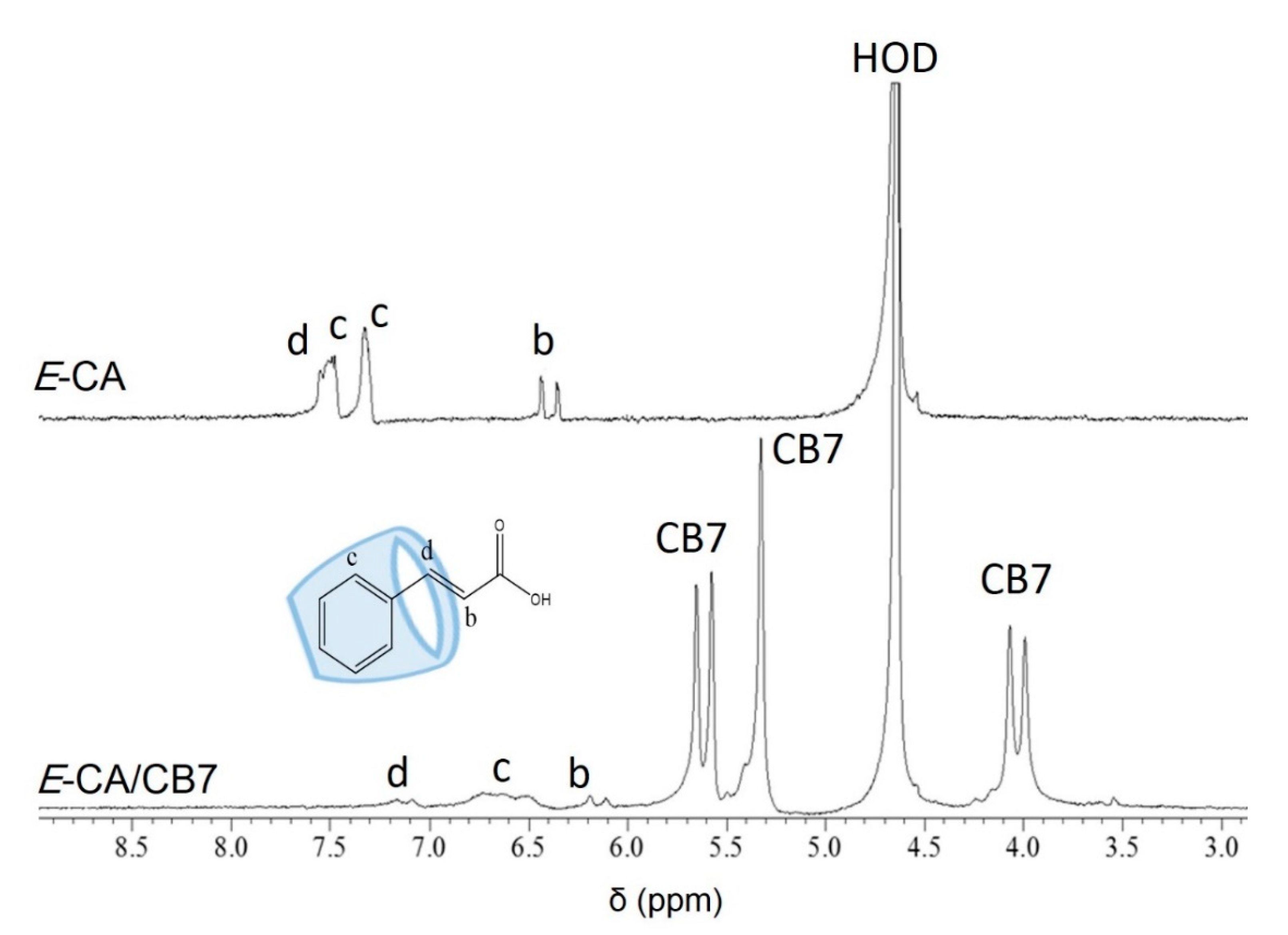
2.1.2. NMR Titration (NMR)
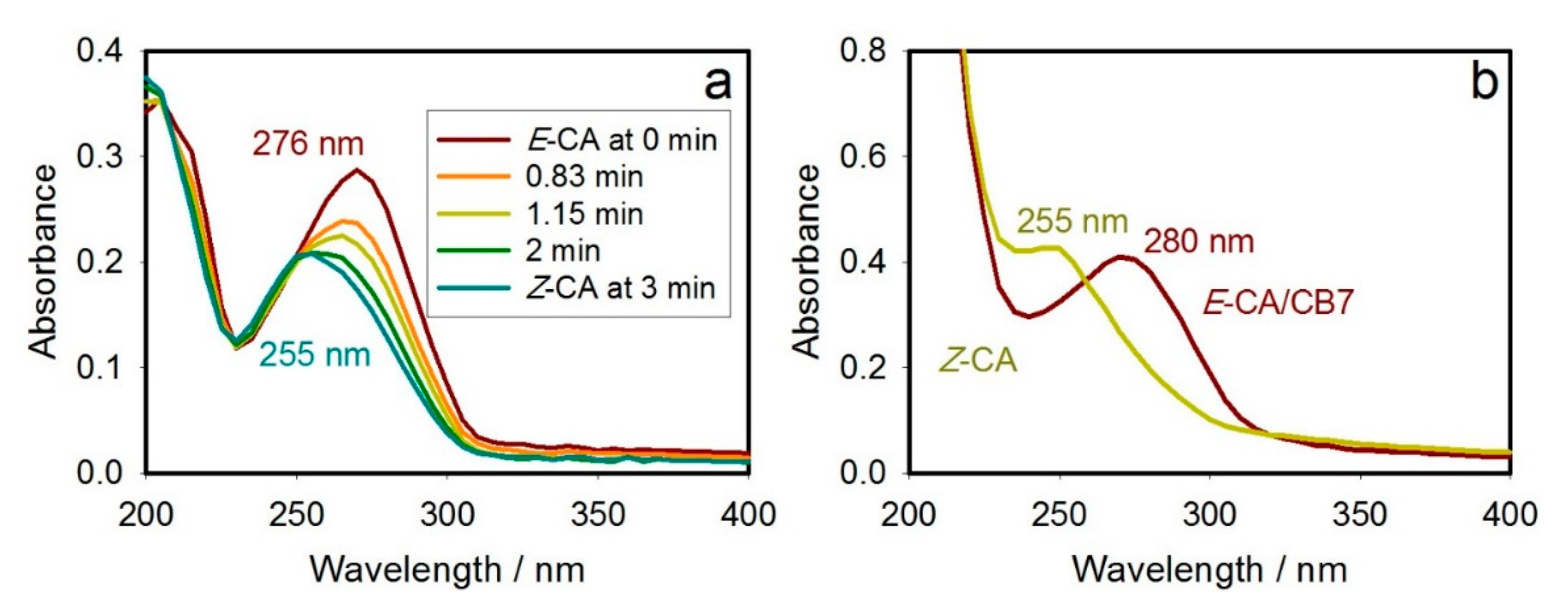
2.2. Stimuli-Responsiveness to Light through the Photoisomerization Process
2.3. Quantitation of Formed Photoisomerization Product Ratios
3. Experimental
3.1. Materials
3.2. 1H NMR Titration
3.3. Optical Measurements
3.4. Determination of Binding Affinities
3.5. Photoisomerization Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lunkenbein, S.; Bellido, M.; Aharoni, A.; Salentijn, E.M.J.; Kaldenhoff, R.; Coiner, H.A.; Muñoz-Blanco, J.; Schwab, W. Cinnamate metabolism in ripening fruit. Characterization of a UDP-glucose: Cinnamate glucosyltransferase from strawberry. Plant Physiol. 2006, 140, 1047–1058. [Google Scholar] [PubMed] [Green Version]
- Steenackers, W.; El Houari, I.; Baekelandt, A.; Witvrouw, K.; Dhondt, S.; Leroux, O.; Gonzalez, N.; Corneillie, S.; Cesarino, I.; Inzé, D.; et al. Cis-cinnamic acid is a natural plant growth-promoting compound. J. Exp. Bot. 2019, 70, 6293–6304. [Google Scholar] [CrossRef] [Green Version]
- Lewis, F.D.; Oxman, J.D.; Gibson, L.L.; Hampsch, H.L.; Quillen, S.L. Lewis acid catalysis of photochemical reactions. 4. Selective isomerization of cinnamic esters. J. Am. Chem. Soc. 1986, 108, 3005–3015. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Ramamurthy, V. Templating photodimerization of trans-cinnamic acid esters with a water-soluble pd nanocage. J. Org. Chem. 2007, 72, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Samanta, S.R.; Ramamurthy, V. Photodimerization of hydrophobic guests within a water–soluble nanocapsule. Res. Chem. Intermed. 2013, 39, 73–87. [Google Scholar] [CrossRef]
- Kim, Y.; Ko, Y.H.; Jung, M.; Selvapalam, N.; Kim, K. A new photo-switchable “on-off” host–guest system. Photochem. Photobiol. Sci. 2011, 10, 1415–1419. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.F.; Al-Sadeh, K.S.; Al-Bitar, M.-B. Physicochemical study on microencapsulation of hydroxypropyl-β-cyclodextrin in dermal preparations. Drug Dev. Ind. Pharm. 2010, 36, 688–697. [Google Scholar] [CrossRef]
- Das, D.; Assaf, K.I.; Nau, W.M. Applications of cucurbiturils in medicinal chemistry and chemical biology. Front. Chem. 2019, 7, 619. [Google Scholar] [CrossRef] [Green Version]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Ciobanu, A.; Landy, D.; Fourmentin, S. Complexation efficiency of cyclodextrins for volatile flavor compounds. Food Res. Int. 2013, 53, 110–114. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Promising applications of cyclodextrins in food: Improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 2015, 131, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Tech. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhen, Z.; Jiang, H.; Li, X.-G.; Liu, J.-A. Encapsulation of the ethylene inhibitor 1-methylcyclopropene by cucurbit[6]uril. J. Agric. Food Chem. 2011, 59, 10539–10545. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.; Scherman, O.A.; Mazzei, P.; Piccolo, A. pH-controlled release of auxin plant hormones from cucurbit[7]uril macrocycle. Chem. Biol. Tech. Agric. 2014, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-based molecular recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, L. Stimuli responsive systems constructed using cucurbit[n]uril-type molecular containers. Acc. Chem. Res. 2014, 47, 2052–2062. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.A.; Basílio, N.; Moro, A.J.; Domingues, M.; González-Delgado, J.A.; Arteaga, J.F.; Pischel, U. Photocaged competitor guests: A general approach toward light-activated cargo release from cucurbiturils. Chem. Eur. J. 2017, 23, 13105–13111. [Google Scholar] [CrossRef]
- Saleh, N.; Suwaid, A.R.B.; Alhalabi, A.; Abuibaid, A.Z.A.; Maltsev, O.V.; Hintermann, L.; Naumov, P. Bioinspired molecular lantern: Tuning the firefly oxyluciferin emission with host–guest chemistry. J. Phys. Chem. B 2016, 120, 7671–7680. [Google Scholar] [CrossRef]
- Ghosh, S.; Isaacs, L. Biological catalysis regulated by cucurbit[7]uril molecular containers. J. Am. Chem. Soc. 2010, 132, 4445–4454. [Google Scholar] [CrossRef]
- Pal, K.; Chandra, F.; Mallick, S.; Koner, A.L. pH Dependent supramolecular recognition of dapoxyl sodium sulfonate with 2-Hydroxypropyl-β-Cyclodextrin: An application towards food-additive formulation. New J. Chem. 2016, 40, 6093–6100. [Google Scholar] [CrossRef]
- Putschögl, M.; Zirak, P.; Penzkofer, A. Absorption and emission behaviour of trans-p-coumaric acid in aqueous solutions and some organic solvents. Chem. Phys. 2008, 343, 107–120. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. Cucurbiturils: From synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1786. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Morales, J.; Rial-Otero, R.; Simal-Gándara, J. Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Res Int. 2010, 43, 1212–1218. [Google Scholar] [CrossRef]
- Kokkinou, A.; Makedonopoulou, S.; Mentzafos, D. The crystal structure of the 1:1 complex of β-cyclodextrin with trans-cinnamic acid. Carbohydr. Res. 2000, 2, 135–140. [Google Scholar] [CrossRef]
- Hursthouse, M.B.; Thornton-Pett, M.; Utley, J.H.P. The X-ray crystal structure of an ethyl cinnamate-β-cyclodextrin guest-host complex. J. Chem. Soc. Chem. Commun. 1982, 15, 881–882. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
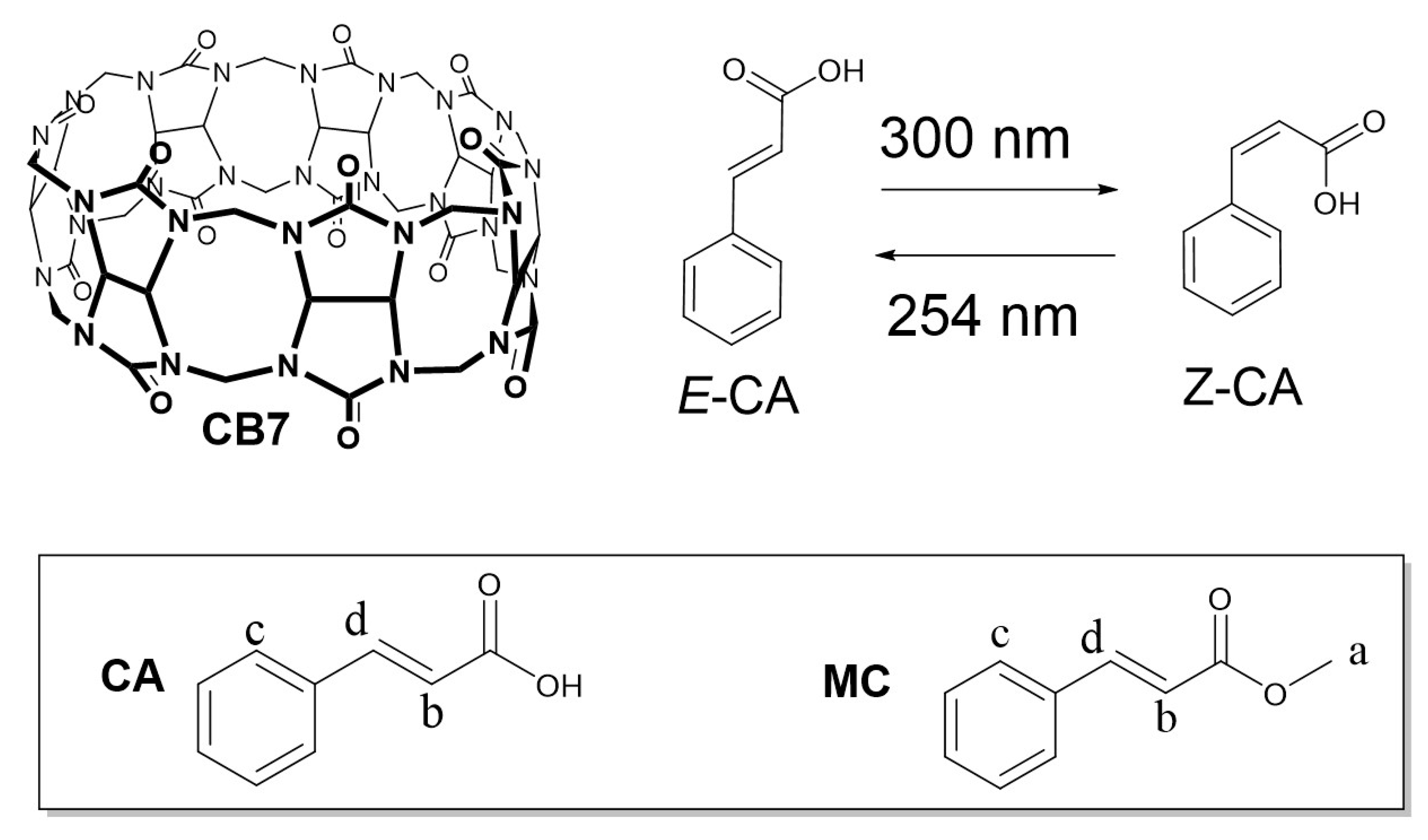

| Substrates | CB7 | CB8 | α-CD | β-CD | γ-CD |
|---|---|---|---|---|---|
| E-CA | (2.2 ± 0.2) × 103 M−1 (UV) (2.6 ± 1.2) × 103 M−1 (NMR) | ND a | ND a | ND a | ND a |
| E-MC | (3.9 ± 0.2) × 104 M−1 (UV) | NF b | (1.9 ± 0.1) × 103 M−1 (UV) (3.5 ± 0.2) × 102 M−1 (NMR) | (5.9 ± 0.2) × 102 M−1 (UV) (1.4 ± 0.6) × 102 M−1 (NMR) | (1.7 ± 0.2) × 102 M−1 (NMR) |
| E-C c | NF b | ND a | ND a | ND a | ND a |
| Chemical Form | Max λabs/nm for Given Form (ε/M−1cm−1) |
|---|---|
| E-CA | 276 (13,835) |
| Z-CA | 255 (10,370) |
| E-CA/CB7 | 280 (10,635) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, N.; Bufaroosha, M.S.; Moussa, Z.; Bojesomo, R.; Al-Amodi, H.; Al-Ahdal, A. Encapsulation of Cinnamic Acid by Cucurbit[7]uril for Enhancing Photoisomerization. Molecules 2020, 25, 3702. https://doi.org/10.3390/molecules25163702
Saleh N, Bufaroosha MS, Moussa Z, Bojesomo R, Al-Amodi H, Al-Ahdal A. Encapsulation of Cinnamic Acid by Cucurbit[7]uril for Enhancing Photoisomerization. Molecules. 2020; 25(16):3702. https://doi.org/10.3390/molecules25163702
Chicago/Turabian StyleSaleh, Na’il, Muna S. Bufaroosha, Ziad Moussa, Rukayat Bojesomo, Hebah Al-Amodi, and Asia Al-Ahdal. 2020. "Encapsulation of Cinnamic Acid by Cucurbit[7]uril for Enhancing Photoisomerization" Molecules 25, no. 16: 3702. https://doi.org/10.3390/molecules25163702
APA StyleSaleh, N., Bufaroosha, M. S., Moussa, Z., Bojesomo, R., Al-Amodi, H., & Al-Ahdal, A. (2020). Encapsulation of Cinnamic Acid by Cucurbit[7]uril for Enhancing Photoisomerization. Molecules, 25(16), 3702. https://doi.org/10.3390/molecules25163702






