Plants as Sources of Anti-Inflammatory Agents
Abstract
1. Introduction
2. Anti-Inflammatory Drugs
3. Plant Use and the Development of Drugs
4. Secondary Metabolite Biosynthesis
5. Anti-Inflammatory Molecules of Medicinal Plants and Mechanism of Action
Funding
Conflicts of Interest
References
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant. Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Useche, D.C.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.A.; Sloan, S.; Laurance, S.G.; Campbell, M.; Abernethy, K.; Álvarez, P.; et al. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.K.D.; Mendonça, A.C.A.M.; Silva, M.A.P. Ethnobotanical, phytochemical and pharmacological aspects Rubiaceae species in Brazil. Rev. Cubana Plant Med. 2013, 18, 140–156. [Google Scholar]
- Shazhni, J.A.; Renu, A.; Vijayaraghavan, P. Insights of antidiabetic, anti-inflammatory and hepatoprotective properties of antimicrobial secondary metabolites of corm extract from Caladium x hortulanum. Saudi J. Boil. Sci. 2018, 25, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Deng, Z. De Novo Assembly, Annotation, and Characterization of Root Transcriptomes of Three Caladium Cultivars with a Focus on Necrotrophic Pathogen Resistance/Defense-Related Genes. Int. J. Mol. Sci. 2017, 18, 712. [Google Scholar] [CrossRef]
- Locatelli, C.; Nardi, G.M.; Anuário, A.d.F.; Freire, C.G.; Megiolaro, F.; Schneider, K.; Perazzoli, M.R.A.; Nascimento, S.R.D.; Gon, A.C.; Mariano, L.N.B.; et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacogn. Res. 2016, 8, S42–S49. [Google Scholar] [CrossRef]
- Virshette, S.J.; Patil, M.K.; Somkuwar, A.P. A review on medicinal plants used as anti inflammatory agents. J. Pharmacogn. Phytochem. 2019, 8, 1641–1646. [Google Scholar]
- Liu, C.H.; Abrams, N.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; Prabhudas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat. Immunol. 2017, 18, 1175–1180. [Google Scholar] [CrossRef]
- Fialho, L.; Cunha-E-Silva, J.A.; Santa-Maria, A.F.; Madureira, F.A.; Iglesias, A.C. Comparative study of systemic early postoperative inflammatory response among elderly and non-elderly patients undergoing laparoscopic cholecystectomy. Rev. Col. Bras. Cir. 2018, 45, e1586. [Google Scholar] [CrossRef]
- Jang, C.H.; Kim, Y.Y.; Seong, J.Y.; Kang, S.H.; Jung, E.K.; Sung, C.M.; Kim, S.B.; Cho, Y.B.; Sung, J.Y. Clinical characteristics of pediatric external auditory canal cholesteatoma. Int. J. Pediatr. Otorhinolaryngol. 2016, 87, 5–10. [Google Scholar] [CrossRef]
- Kim, Y.; Bayona, P.W.; Kim, M.; Chang, J.; Hong, S.; Park, Y.; Budiman, A.; Kim, Y.-J.; Choi, C.Y.; Kim, W.S.; et al. Macrophage Lamin A/C Regulates Inflammation and the Development of Obesity-Induced Insulin Resistance. Front. Immunol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.; Sharma, A.; Zhi, W.; Bai, S.; Hopkins, D.; Steed, L.; Bode, B.; Anderson, S.W.; Reed, J.C.; Steed, R.D.; et al. Proteins of TNF-α and IL6 Pathways Are Elevated in Serum of Type-1 Diabetes Patients with Microalbuminuria. Front. Immunol. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Kroger, C.J.; Tisch, R.M. Type 1 Diabetes: A Chronic Anti-Self-Inflammatory Response. Front. Immunol. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Espigol-Frigole, G.; Planas-Rigol, E.; Lozano, E.; Corbera-Bellalta, M.; Terrades-García, N.; Prieto-González, S.; García-Martínez, A.; Hernández-Rodríguez, J.; Grau, J.M.; Cid, M.C. Expression and Function of IL12/23 Related Cytokine Subunits (p35, p40, and p19) in Giant-Cell Arteritis Lesions: Contribution of p40 to Th1- and Th17-Mediated Inflammatory Pathways. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Donninelli, G.; Del Cornò, M.; Pierdominici, M.; Scazzocchio, B.; Varì, R.; Varano, B.; Pacella, I.; Piconese, S.; Barnaba, V.; D’Archivio, M.; et al. Distinct Blood and Visceral Adipose Tissue Regulatory T Cell and Innate Lymphocyte Profiles Characterize Obesity and Colorectal Cancer. Front. Immunol. 2017, 8, 643. [Google Scholar] [CrossRef]
- Katare, P.B.; Bagul, P.K.; Dinda, A.K.; Banerjee, S.K. Toll-Like Receptor 4 Inhibition Improves Oxidative Stress and Mitochondrial Health in Isoproterenol-Induced Cardiac Hypertrophy in Rats. Front. Immunol. 2017, 8, 719. [Google Scholar] [CrossRef]
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front. Immunol. 2017, 8, 1058. [Google Scholar] [CrossRef]
- Qi, H.; Yang, S.; Zhang, L. Neutrophil Extracellular Traps and Endothelial Dysfunction in Atherosclerosis and Thrombosis. Front. Immunol. 2017, 8, 928. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant. Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Thumann, T.A.; Pferschy-Wenzig, E.-M.; Moissl-Eichinger, C.; Bauer, R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for gastrointestinal disorders. J. Ethnopharmacol. 2019, 245, 112153. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.S.; Alvim, H.G.O. Review on non-steroid antiinflammatory: Acetylsalicylic acid. Rev. Inic. Ciente. Ext. 2018, 1, 169–174. [Google Scholar]
- Pereira-Leite, C.; Nunes, C.; Jamal, S.K.; Cuccovia, I.M.; Reis, S. Nonsteroidal Anti-Inflammatory Therapy: A Journey Toward Safety. Med. Res. Rev. 2016, 37, 802–859. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, A.C.; Fernandes, D.R.; Silva, E.A.; Terra Júnior, A.T. The indiscriminated use of non-steroid anti-inflammatory (NSAID). Rev. Cient. FAEMA 2017, 8, 165–176. [Google Scholar] [CrossRef]
- Sostres, C.; Lanas, Á. Appropriate prescription, adherence and safety of non-steroidal anti-inflammatory drugs. Med. Clin. 2016, 146, 267–272. [Google Scholar] [CrossRef]
- Patel, D.P.; Schenk, J.M.; Darke, A.K.; Myers, J.B.; Brant, W.O.; Hotaling, J.M. Non-steroidal anti-inflammatory drug (NSAID) use is not associated with erectile dysfunction risk: Results from the Prostate Cancer Prevention Trial. BJU Int. 2015, 117, 500–506. [Google Scholar] [CrossRef]
- Inotai, A.; Hanko, B.; Meszaro, A. Trends in the non-steroidal anti-inflammatory drug market in six central-eastern european countries based on retail information. Pharmacoepidemiol. Drug Saf. 2010, 19, 183–190. [Google Scholar] [CrossRef]
- Golden, J.M.; Escobar, O.H.; Nguyen, M.V.L.; Mallicote, M.U.; Kavarian, P.; Frey, M.R.; Gayer, C.P. Ursodeoxycholic acid protects against intestinal barrier breakdown by promoting enterocyte migration via EGFR- and COX-2-dependent mechanisms. Am. J. Physiol. Liver Physiol. 2018, 315, G259–G271. [Google Scholar] [CrossRef]
- Suleyman, H.; Demircan, B.; Karagöz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–258. [Google Scholar]
- Oliveira, M.M.C.; Silva, M.M.; Moreira, T.L.M.; Couto, V.F.; Coelho, Y.N.; Nunes, C.P. The Chronic Use of Non-Steroid Anti-Inflammatory and Their Adverse Effects. Rev. Cad. Med. 2019, 2, 90–100. [Google Scholar]
- Ayyadevara, S.; Bharill, P.; Dandapat, A.; Hu, C.; Khaidakov, M.; Mitra, S.; Reis, R.J.S.; Mehta, J.L. Aspirin Inhibits Oxidant Stress, Reduces Age-Associated Functional Declines, and Extends Lifespan of Caenorhabditis elegans. Antioxid. Redox Signal 2013, 18, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-G.; Kim, Y.-S.; Choi, J.-P.; Choi, D.-S.; Yoon, C.M.; Jeon, S.G.; Gho, Y.S.; Kim, Y.-K. Aspirin attenuates the anti-inflammatory effects of theophylline via inhibition of cAMP production in mice with non-eosinophilic asthma. Exp. Mol. Med. 2009, 42, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Desborough, M.J.R.; Keeling, D.M. The aspirin story—from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2016, 164, 836. [Google Scholar] [CrossRef]
- Hayashi, S.; Sumi, Y.; Ueno, N.; Murase, A.; Takada, J. Discovery of a novel COX-2 inhibitor as an orally potent anti-pyretic and anti-inflammatory drug: Design, synthesis, and structure-activity relationship. Biochem. Pharmacol. 2011, 82, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, R.; Kazerouni, A.; Kazerouni, O. Evidence for Clinical Use of Honey in Wound Healing as an Anti-bacterial, Anti-inflammatory Anti-oxidant and Anti-viral Agent: A Review. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 100–104. [Google Scholar] [CrossRef]
- Lucas, G.N.C.; Leitão, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.D.F.; Júnior, G.B.D.S.; Da Silva, G.B. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. Braz. J. Nephrol. 2019, 41, 124–130. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.; Ghabbour, H.A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2-p53 interaction. Bioorg. Chem. 2019, 86, 598–608. [Google Scholar] [CrossRef]
- Abdellatif, K.R.A.; Fadaly, W.A.; Elshaier, Y.A.M.M.; Ali, W.A.; Kamel, G.M. Non-acidic 1,3,4-trisubstituted-pyrazole derivatives as lonazolac analogs with promising COX-2 selectivity, anti-inflammatory activity and gastric safety profile. Bioorg. Chem. 2018, 77, 568–578. [Google Scholar] [CrossRef]
- El-miligy, M.M.; Hazzaa, A.A.; El-messmary, H.; Nassra, R.A.; E-hawash, S.A. New hybrid molecules combining benzothiophene or benzofuran with rhodanine as dual COX-1/2 and 5-LOX inhibitors: Synthesis, biological evaluation and docking study. Bioorg. Chem. 2017, 72, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.M.M.; Ghabbour, H.A. Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv. 2018, 8, 14335–14346. [Google Scholar] [CrossRef]
- Owona, B.A.; Abia, W.A.; Moundipa, P.F. Natural compounds flavonoids as modulators of inflammasomes in chronic diseases. Int. Immunopharmacol. 2020, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.K. Lipids, inflammasomes, metabolism, and disease. Immunol. Rev. 2020, 1–15. [Google Scholar] [CrossRef]
- Newman, D.J. Developing natural product drugs: Supply problems and how they have been overcome. Pharmacol. Therapeut. 2015, 162, 1–9. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Arya, V.; Arya, M.L. A review on anti-inflammatory plant barks. Int. J. Pharm. Tech. Res. 2011, 3, 899–908. [Google Scholar]
- Shah, B.; Seth, A.; Maheshwari, K. A Review on Medicinal Plants as a Source of Anti-inflammatory Agents. Res. J. Med. Plant. 2011, 5, 101–115. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Hypoglycaemic and anti-diabetic activity of selected African medicinal plants. Int J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 224–237. [Google Scholar]
- Azab, A.N.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Sharopov, F.; Braun, M.S.; Gulmurodov, I.; Khalifaev, D.; Isupov, S.; Wink, M. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils of selected aromatic plants from Tajikistan. Foods 2015, 4, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Bauri, R.K.; Tigga, M.N.; Saleebkullu, S. A review on use of medicinal plants to control parasites. J. Nat. Prod. Resour. 2015, 6, 268–277. [Google Scholar]
- Cabral, B.; Siqueira, E.M.S.; Bitencourt, M.A.O.; Lima, M.C.J.S.; Lima, A.K.; Ortmannd, C.F.; Chaves, V.C.; Fernandes-Pedrosa, M.F.; Rochac, H.A.O.; Scortecci, K.C.; et al. Phytochemical study and anti-inflammatory and antioxidant potential of Spondias mombin leaves. Rev. Bras. Farmacog. 2016, 26, 304–311. [Google Scholar] [CrossRef]
- Barbosa, H.M.; Nascimento, J.N.D.; Araújo, T.A.; Duarte, F.S.; Albuquerque, U.P.; Vieira, J.R.; De Santana, E.R.; Yara, R.; Lima, C.S.; Lira, E.C.; et al. Acute Toxicity and Cytotoxicity Effect of Ethanolic Extract of Spondias tuberosa Arruda Bark: Hematological, Biochemical and Histopathological Evaluation. An. Acad. Bras. Ciênc. 2016, 88, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Rosas, E.; Correa, L.B.; Pádua, T.D.A.; Costa, T.E.M.M.; Mazzei, J.L.; Heringer, A.P.; Bizarro, C.A.; Kaplan, M.A.C.; Figueiredo, M.R.; Henriques, M.D.G.M.O. Anti-inflammatory effect of Schinus terebinthifolius Raddi hydroalcoholic extract on neutrophil migration in zymosan-induced arthritis. J. Ethnopharmacol. 2015, 175, 490–498. [Google Scholar] [CrossRef]
- Mostofora, R.; Ahmed, S.; Begum, M.M.; Rahman, M.S.; Begum, T.; Ahmed, S.U.; Tuhin, R.H.; Das, M.; Hossain, A.; Sharma, M.; et al. Evaluation of anti-inflammatory and gastric anti-ulcer activity of Phyllanthus niruri L. (Euphorbiaceae) leaves in experimental rats. BMC Complement Altern. Med. 2017, 17, 267. [Google Scholar]
- Zahidin, N.S.; Saidin, S.; Zulkifli, R.M.; Muhamad, I.I.; Ya’Akob, H.; Nur, H. A review of Acalypha indica L. (Euphorbiaceae) as traditional medicinal plant and its therapeutic potential. J. Ethnopharmacol. 2017, 207, 146–173. [Google Scholar] [CrossRef]
- Siraj, A.; Shilpi, J.A.; Hossain, G.; Uddin, S.J.; Islam, K.; Jahan, I.A.; Hossain, H. Anti-Inflammatory and Antioxidant Activity of Acalyphahispida Leaf and Analysis of its Major Bioactive Polyphenols by HPLC. Adv. Pharm. Bull. 2016, 6, 275–283. [Google Scholar] [CrossRef]
- Rios, R.; Silva, H.B.F.; Carneiro, N.V.Q.; Costa, R.S.; Carneiro, T.C.B.; Marques, C.R.; Machado, M.S.S.; Velozo, E.S.; Silva, T.M.; Silva, T.M.S.; et al. Anti-inflammatory Activity of Jurubeba (Solanum paniculatum L.) Through Reducing the T-bet and GATA3 Gene Expression, In Vitro. J. Allergy Clin. Immunol. 2017, 139, AB268. [Google Scholar] [CrossRef][Green Version]
- Ortiz, M.I.; Fernández-Martínez, E.; Soria-Jasso, L.E.; Lucas-Gómez, I.; Villagómez-Ibarra, R.; González-García, M.P.; Castañeda-Hernández, G.; Salinas-Caballero, M. Isolation, identification and molecular docking as cyclooxygenase (COX) inhibitors of the main constituents of Matricaria chamomilla L. extract and its synergistic interaction with diclofenac on nociception and gastric damage in rats. Biomed. Pharmacother. 2016, 78, 248–256. [Google Scholar] [CrossRef]
- Kyei, S.; Koffuor, G.; Ramkissoon, P.; Ameyaw, E.O.; Asiamah, E.A. Anti-inflammatory effect of Heliotropium indicum Linn on lipopolysaccharide-induced uveitis in New Zealand white rabbits. Int. J. Ophthalmol. 2016, 9, 528–535. [Google Scholar] [CrossRef]
- Cruz, M.P.; Andrade, C.M.F.; Silva, K.O.; De Souza, E.P.; Yatsuda, R.; Marques, L.M.; David, J.P.; David, J.M.; Napimoga, M.H.; Clemente-Napimoga, J.T. Antinoceptive and Anti-inflammatory Activities of the Ethanolic Extract, Fractions and Flavones Isolated from Mimosa tenuiflora (Willd.) Poir (Leguminosae). PLoS ONE 2016, 11, e0150839. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-Inflammatory Effects of the Essential Oils of Ginger (Zingiber officinale Roscoe) in Experimental Rheumatoid Arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2015, 30, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Rtibi, K.; Selmi, S.; Jabri, M.-A.; Mamadou, G.; Limas-Nzouzi, N.; Sebai, H.; El-Benna, J.; Marzouki, L.; Eto, B.; Amri, M. Effects of aqueous extracts from Ceratonia siliqua L. pods on small intestinal motility in rats and jejunal permeability in mice. RSC Adv. 2016, 6, 44345–44353. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Walker, J.; Reichelt, K.V.; Obst, K.; Widder, S.; Hans, J.; Krammer, G.E.; Ley, J.; Somoza, V. Identification of an anti-inflammatory potential of Eriodictyon angustifolium compounds in human gingival fibroblasts. Food Funct. 2016, 7, 3046–3055. [Google Scholar] [CrossRef]
- Asadi-Samani, M.; Kafash-Farkhad, N.; Azimi, N.; Fasihi, A.; Alinia-Ahandani, E.; Rafieian-Kopaei, M. Medicinal plants with hepatoprotective activity in Iranian folk medicine. Asian Pac. J. Trop. Biomed. 2015, 5, 146–157. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products; Wiley: Chichester, UK, 2009; p. 550. [Google Scholar]
- Płonka, J.; Górny, A.; Kokoszka, K.; Barchanska, H. Metabolic profiles in the course of the shikimic acid pathway of Raphanus sativus var. longipinnatus exposed to mesotrione and its degradation products. Chemosphere 2020, 245, 125616. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Thompson, C.B. A two-way street: Reciprocal regulation of metabolism and signalling. Nat. Rev. Mol. Cell Boil. 2012, 13, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Heinrich, M. Fundamentals of Pharmacognosy and Phytotherapy, 2nd ed.; Churchill Livingstone/Elsevier: Edinburgh, UK, 2012; p. 326. [Google Scholar]
- Kite, G.; Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach. Kew Bull. 1998, 53, 499. [Google Scholar] [CrossRef][Green Version]
- Bose, S.; Ramesh, V.; Locasale, J.W. Acetate Metabolism in Physiology, Cancer, and Beyond. Trends Cell Boil. 2019, 29, 695–703. [Google Scholar] [CrossRef]
- Karam, T.K.; Dalposso, L.M.; Casa, D.M.; De Freitas, G.B.L. Broom (Baccharis trimera): Therapeutic use and biosynthesis. Rev. Bras. Plantas Med. 2013, 15, 280–286. [Google Scholar] [CrossRef]
- Jesch, E.D.; Carr, T.P. Food Ingredients That Inhibit Cholesterol Absorption. Prev. Nutr. Food Sci. 2017, 22, 67–80. [Google Scholar]
- Le Pogam, P.; Boustie, J. Xanthones of Lichen Source: A 2016 Update. Molecules 2016, 21, 294. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Corey, E.J. Total Synthesis of Natural Products; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 295. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Bi, Y.; Gao, X.; Yan, X.; Zhang, Y.; Xue, P.; Bammert, C.E.; LeGalley, T.D.; Gibson, K.M.; Bi, L.; et al. Anti-inflammatory, analgesic and antioxidant activities of novel kyotorphin-nitroxide hybrid molecules. Bioorganic Med. Chem. Lett. 2016, 26, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for cancer prevention and therapy: Current progress and future perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. Herb. Med. 2019, 2, 11–30. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2014, 5, 129–151. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, J.A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.D.; Subramaniyan, A. Phytochemical Constituents of Gloriosa superba Seed, Tuber and Leaves. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 111–117. [Google Scholar]
- Mitra, I.; Saha, A.; Roy, K. Exploring quantitative structure-activity relationship studies of antioxidant phenolic compounds obtained from traditional Chinese medicinal plants. Mol. Simul. 2010, 36, 1067–1079. [Google Scholar] [CrossRef]
- Rex, J.R.S.; Muthukumar, N.M.S.A.; Selvakumar, P.M. Phytochemicals as a potential source for anti-microbial, anti-oxidant and wound healing—A review. MOJ Bioorg. Org. Chem. 2018, 2, 1. [Google Scholar] [CrossRef]
- Rajkapoor, B.; Burkan, Z.E.; Senthilkumar, R. Oxidants and human diseases: Role of antioxidant medicinal plants—A review. Pharmacologyonline 2010, 1, 1117–1131. [Google Scholar]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of Medicinal Plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Torres, D.A.; Pereira, E.C.V.; Sampaio, P.A.; Souza, N.A.C.; Ferraz, C.A.A.; Oliveira, A.P.; Moura, C.A.; Almeida, J.R.G.S.; Rolim-Neto, P.K.; Oliveira-Júniora, R.G.; et al. Influence of extraction process on flavonoid content from Cnidoscolus quercifoliuspohl (euhorbiaceae) and antioxidant activity. Quim. Nova 2018, 41, 743–747. [Google Scholar]
- Saxena, M.; Saxena, J.; Pradhan, A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 130–134. [Google Scholar]
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The Biological Efficacy of Natural Products against Acute and Chronic Inflammatory Diseases in the Oral Region. Medicines 2018, 5, 122. [Google Scholar] [CrossRef]
- Reginato, F.Z.; Da Silva, A.R.H.; Bauermann, L.F. Evaluation of the flavonoides use in the treatment of the inflammation. Rev. Cuba. Farm. 2015, 49, 569–582. [Google Scholar]
- Aravindaram, K.; Yang, N.-S. Anti-Inflammatory Plant Natural Products for Cancer Therapy. Planta Med. 2010, 76, 1103–1117. [Google Scholar] [CrossRef]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Teixeira, T.O.; Campos, K.M.; Cerqueira-Lima, A.T.; Carneiro, T.C.B.; Velozo, E.D.S.; De Melo, I.C.; Figueiredo, E.A.; Oliveira, E.; Silva, D.F.; Ivan, R.C.; et al. Potential therapeutic effect of Allium cepa L. and quercetin in a murine model of Blomia tropicalis induced asthma. DARU J. Pharm. Sci. 2015, 23, 18. [Google Scholar] [CrossRef]
- Kaladhar, D.S.V.G.K.; Swathi, S.K.; Varahalarao, V.; Nagendra, S.Y. Evaluation of Anti-inflammatory and Anti-proliferative Activity of Abutilon indicum L. Plant Ethanolic Leaf Extract on Lung Cancer Cell Line A549 for System Network Studies. J. Cancer Sci. Ther. 2014, 6, 188–194. [Google Scholar] [CrossRef]
- Bose, S.; Laha, B.; Banerjee, S. Anti-inflammatory activity of isolated allicin from garlic with post-acoustic waves and microwave radiation. J. Adv. Pharm. Educ. Res. 2013, 3, 512–515. [Google Scholar]
- Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their Glycosides and Glycoconjugates. Synthesis and Biological Activity. Curr. Org. Chem. 2017, 21, 218–235. [Google Scholar] [CrossRef]
- Díaz-Tielas, C.; Reigosa, M.; Sánchez-Moreiras, A.; Graña, E. Biological activities and novel applications of chalcones. Planta Daninha 2016, 34, 607–616. [Google Scholar] [CrossRef]
- Roy, A. A review on the alkaloids an important therapeutic compound from plants. Int. J. Plant Biol. 2017, 3, 1–9. [Google Scholar]
- Carlos, L.D.A.; Amaral, K.A.D.S.; Vieira, I.J.C.; Mathias, L.; Braz-Filho, R.; Samarão, S.S.; Da Motta, O.V. Rauvolfia Grandiflora (Apocynaceae) Extract Interferes with Staphylococcal Density, Enterotoxin Production and Antimicrobial Activity. Braz. J. Microbiol. 2010, 41, 612–620. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar] [CrossRef]
- Sandini, T.M.; Udo, M.S.B.; Spinosa, H.D.S. Senecio brasiliensis e alcaloides pirrolizidínicos: Toxicidade em animais e na saúde humana. Biotemas 2013, 26, 83–92. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.S.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2016, 174, 1244–1262. [Google Scholar] [CrossRef]
- Xie, L.; Roto, A.V.; Bolling, B.W. Characterization of Ellagitannins, Gallotannins, and Bound Proanthocyanidins from California Almond (Prunus dulcis) Varieties. J. Agric. Food Chem. 2012, 60, 12151–12156. [Google Scholar] [CrossRef]
- He, M.; Tian, H.; Luo, X.; Qi, X.-H.; Chen, X. Molecular Progress in Research on Fruit Astringency. Molecules 2015, 20, 1434–1451. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Gupta, A.; Chaphalkar, S.R. Terpenoids from three medicinal plants and their potential anti-inflammatory and immunosuppressive activity on human whole blood and peripheral blood mononuclear cells. Asian J. Ethnopharmacol. Med. Foods 2016, 2, 13–17. [Google Scholar]
- Gu, J.; Luo, L.; Wang, Q.; Yan, S.; Lin, J.; Li, D.; Cao, B.; Mei, H.; Ying, B.; Bin, L.; et al. Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Lab. Investig. 2018, 98, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaf, M.I.; Alansari, W.S.; Ibrahim, E.A.; Elhalwagy, M.E. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. Sci. 2019, 31, 1358–1362. [Google Scholar] [CrossRef]
- Mensah, J.K.; Golomeke, D. Antioxidant and antimicrobial activities of the extracts of the Calyx of Hibiscus Sabdariffa Linn. Curr. Sci. Perspectiv. 2015, 1, 69–76. [Google Scholar]
- Zhang, Y.F.; Sun, C.C.; Duan, J.X.; Yang, H.H.; Zhang, C.Y.; Xiong, J.B.; Zhong, W.J.; Zu, C.; Guan, X.X.; Jiang, H.L.; et al. A COX-2/sEH dual inhibitor PTUPB ameliorates cecal ligation and punctureinduced sepsis in mice via anti-inflammation and anti-oxidative stress. Biomed. Pharmacother. 2020, 126, 109907. [Google Scholar] [CrossRef]
- Sun, L.-C.; Zhang, H.-B.; Gu, C.-D.; Guo, S.-D.; Li, G.; Lian, R.; Yao, Y.; Zhang, G.-Q. Protective effect of acacetin on sepsis-induced acute lung injury via its anti-inflammatory and antioxidative activity. Arch. Pharmacal. Res. 2017, 41, 1199–1210. [Google Scholar] [CrossRef]
- Hattori, Y.; Hattori, K.; Suzuki, T.; Matsuda, N. Recent advances in the pathophysiology and molecular basis of sepsis-associated organ dysfunction: Novel therapeutic implications and challenges. Pharmacol. Ther. 2017, 177, 56–66. [Google Scholar] [CrossRef]
- Wenzel, P.; Kossmann, S.; Münzel, T.; Daiber, A. Redox regulation of cardiovascular inflammation—Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic. Boil. Med. 2017, 109, 48–60. [Google Scholar] [CrossRef]
- Bernardes, N.R.; Pessanha, F.F.; Oliveira, D.B. Alimentos Funcionais: Uma breve revisão. Ciênc. Cult. 2010, 6, 2. (In Portuguese) [Google Scholar]
- Chen, X.; Wang, Y.; Xie, X.; Chen, H.; Zhu, Q.; Ge, Z.; Wei, H.; Deng, J.; Zhongyuan, X.; Lian, Q. Heme Oxygenase-1 Reduces Sepsis-Induced Endoplasmic Reticulum Stress and Acute Lung Injury. Mediat. Inflamm. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Ondua, M.; Shai, L.; Lebelo, S.L. Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants. J. Inflamm. Res. 2019, 12, 195–203. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H. Spiroindolone analogues bearing benzofuran moiety as a selective cyclooxygenase COX-1 with TNF-α and IL-6 inhibitors. Saudi J. Boil. Sci. 2020, 27, 1208–1216. [Google Scholar] [CrossRef]
- Wilson, J.C.; Anderson, L.A.; Murray, L.J.; Hughes, C.M. Non-steroidal anti-inflammatory drug and aspirin use and the risk of head and neck cancer: A systematic review. Cancer Causes Control 2011, 22, 803–810. [Google Scholar] [CrossRef]
- Halliwell, B. The wanderings of a free radical. Free Radic. Boil. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef]
- Ali, S.S.; Kasoju, N.; Luthra, A.; Singh, A.; Sharanabasava, H.; Sahu, A.; Bora, U. Indian medicinal herbs as sources of antioxidants. Food Res. Int. 2008, 41, 1–15. [Google Scholar] [CrossRef]
- González-Costa, M.; Padrón González, A.A. Inflammation from an immunologic perspective: A challenge to medicine in the 21st century. Rev. Haban. Cienc. Méd. 2018, 18, 30–44. [Google Scholar]
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301. [Google Scholar] [CrossRef]
- Padrón-González, A.; Dorta-Contreras. Vía de las lectinas, una ruta del complemento en construcción. Arch. Alerg. Inmunol. Clin. 2018, 49, 0005–0012. (In Spanish) [Google Scholar]
- Isailovic, N.; Daigo, K.; Mantovani, A.; Selmi, C. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 2015, 60, 1–11. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Świrski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of their Molecular Mechanisms and Experimental Models. Phytother. Res. 2014, 29, 323–331. [Google Scholar] [CrossRef]
- Bassiouni, W.; Daabees, T.; Louedec, L.; Norel, X.; Senbel, A.M. Evaluation of some prostaglandins modulators on rat corpus cavernosum in-vitro: Is relaxation negatively affected by COX-inhibitors? Biomed. Pharmacother. 2019, 111, 1458–1466. [Google Scholar] [CrossRef]
- Zeliha, P.K.; Dilek, O.; Ezgi, O.; Halil, K.; Cihan, U.; Gul, O. Association between ABCB1, ABCG2 carrier protein and COX-2 enzyme gene polymorphisms and breast cancer risk in a Turkish population. Saudi Pharm. J. 2020, 28, 215–219. [Google Scholar] [CrossRef]
- Ucan, B.; Özbek, M.; Sahin, M.; Kızılgül, M.; Çakal, E. Cyclooxygenase-2 (COX-2) gene polymorphism in patients with differentiated thyroid carcinomas in the Turkish population. Turk. J. Med. Sci. 2017, 47, 1848–1853. [Google Scholar] [CrossRef]
- Lelubre, C.; Vincent, J.-L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol. 2018, 14, 417–427. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. Br. Med. J. 2016, 353, i1585. [Google Scholar] [CrossRef]
- Van Der Poll, T.; Van De Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef]
- Davis, C.M.; Liu, X.; Alkayed, N.J. Cytochrome P450 eicosanoids in cerebrovascular function and disease. Pharmacol. Ther. 2017, 179, 31–46. [Google Scholar] [CrossRef]
- Hanke, T.; Merk, D.; Steinhilber, D.; Geisslinger, G.; Schubert-Zsilavecz, M. Small molecules with anti-inflammatory properties in clinical development. Pharmacol. Ther. 2016, 157, 163–187. [Google Scholar] [CrossRef]
- Wu, J.; Liu, B.; Mao, W.; Feng, S.; Yao, Y.; Bai, F.; Shen, Y.; Guleng, A.; Jirigala, B.; Cao, J. Prostaglandin E2 Regulates Activation of Mouse Peritoneal Macrophages by Staphylococcus aureus through Toll-Like Receptor 2, Toll-Like Receptor 4, and NLRP3 Inflammasome Signaling. J. Innate Immun. 2019, 12, 154–169. [Google Scholar] [CrossRef]
- Gartung, A.; Yang, J.; Sukhatme, V.P.; Bielenberg, D.R.; Fernandes, D.; Chang, J.; Schmidt, B.A.; Hwang, S.H.; Zurakowski, D.; Huang, S.; et al. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc. Natl. Acad. Sci. USA 2019, 116, 1698–1703. [Google Scholar] [CrossRef]
- Darwesh, A.M.; Jamieson, K.L.; Wang, C.; Samokhvalov, V.; Seubert, J.M. Cardioprotective effects of CYP-derived epoxy metabolites of docosahexaenoic acid involve limiting NLRP3 inflammasome activation. Can. J. Physiol. Pharmacol. 2019, 97, 544–556. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Chen, P. Sulfur dioxide attenuates sepsis-induced cardiac dysfunction via inhibition of NLRP3 inflammasome activation in rats. Nitric Oxide 2018, 81, 11–20. [Google Scholar] [CrossRef]
- Schunck, W.-H.; Konkel, A.; Fischer, R.; Weylandt, K.-H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef]
- Rahim, I.; Djerdjouri, B.; Sayed, R.K.A.; Ortiz, F.; Fernandez-Gil, B.I.; Hidalgo-Gutiérrez, A.; Lopez, L.C.; Escames, G.; Reiter, R.J.; Acuña-Castroviejo, D. Melatonin administration to wild-type mice and nontreated NLRP3 mutant mice share similar inhibition of the inflammatory response during sepsis. J. Pineal Res. 2017, 63, e12410. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, A.; Lan, T.; Cepinskas, G.; Kao, R.; Martin, C.M.; Rui, T. Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res. Cardiol. 2017, 112, 16. [Google Scholar] [CrossRef]
- Dong, L.; Zhou, Y.; Zhu, Z.-Q.; Liu, T.; Duan, J.-X.; Zhang, J.; Li, P.; Hammcok, B.D.; Guan, C.-X. Soluble Epoxide Hydrolase Inhibitor Suppresses the Expression of Triggering Receptor Expressed on Myeloid Cells-1 by Inhibiting NF-kB Activation in Murine Macrophage. Inflammation 2016, 40, 13–20. [Google Scholar] [CrossRef]
- Hye Khan, M.A.; Hwang, S.H.; Sharma, A.; Corbett, J.A.; Hammock, B.D.; Imig, J.D. A dual COX-2/sEH inhibitor improves the metabolic profile and reduces kidney injury in Zucker diabetic fatty rat. Prostag. Oth. Lipid Mediat. 2016, 125, 40–47. [Google Scholar] [CrossRef]
- Warner, T.D.; Mitchell, J.A. Cyclooxygenase-3 (COX-3): Filling in the gaps toward a COX continuum? Proc. Natl. Acad. Sci. USA 2002, 99, 13371–13373. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y.-M. Recent advances in the biology of IL-1 family cytokines and their potential roles in development of sepsis. Cytokine Growth Factor Rev. 2019, 45, 24–34. [Google Scholar] [CrossRef]
- Mckenna, S.; Eckman, M.; Parker, A.; Bok, R.; Hurt, K.J.; Wright, C.J. Perinatal endotoxemia induces sustained hepatic COX-2 expression through an NFkappaB-Dependent mechanism. J. Innate Immun. 2016, 8, 386–399. [Google Scholar] [CrossRef]
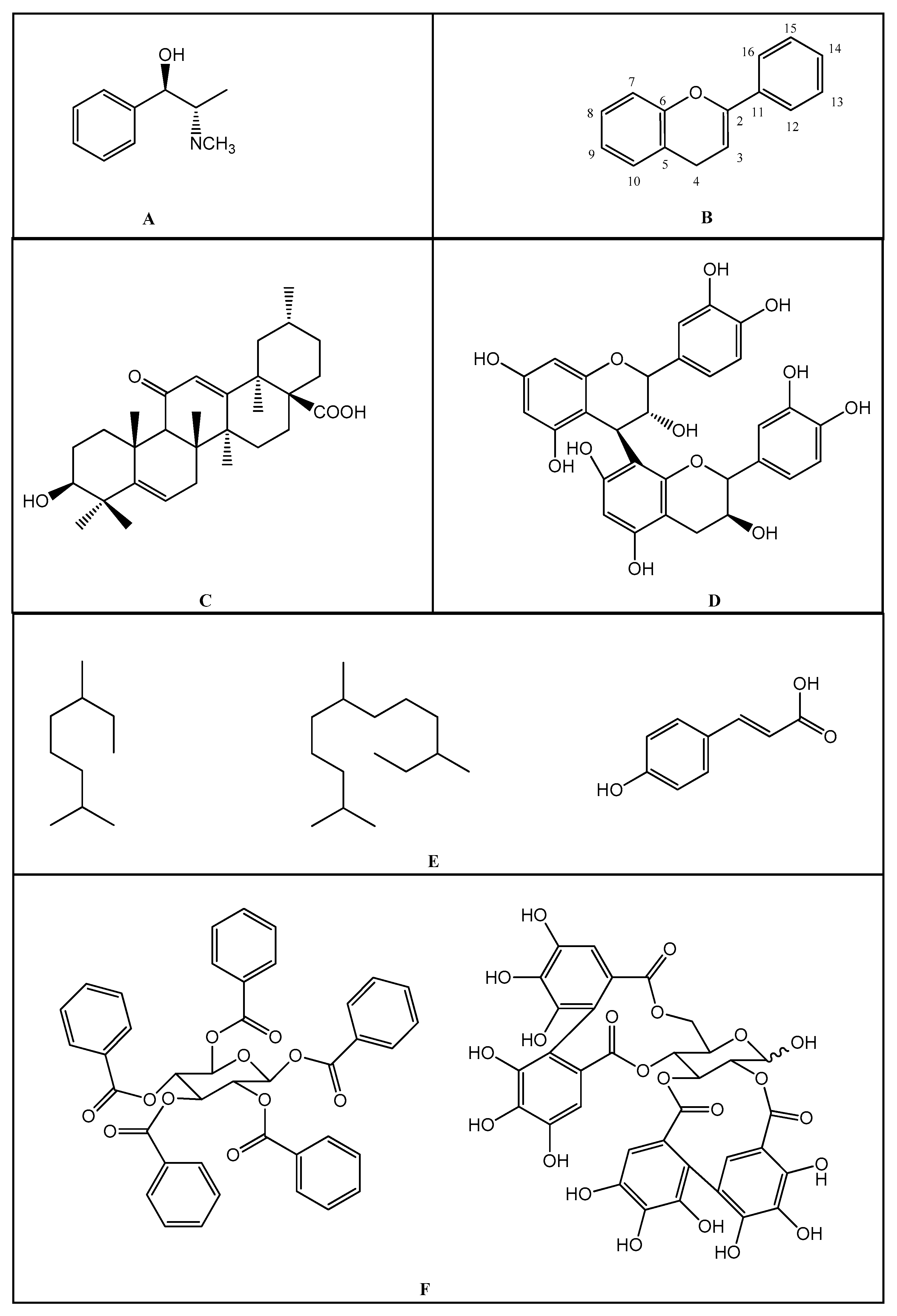
| Salicylates | Indoleacetic Acid Derivatives | Aryl Acetic Derivatives | Enolic Acids | |
|---|---|---|---|---|
| Acetylsalicylic acid Lysine clonixinate Benorilate Diflunisal Salicylamide Etersalate Salsalate or salicylic acid | Acemethacin Glucamethacin Indomethacin Proglumethacin Oxamethacin Sulindac Tolmetin Difenpiramide | Aceclofenac Diclofenac Etodolac Fentiazac Ketorolac Bufexamac Lonazolac Alclofenac Zomepirac | Oxicans: Droxicam Meloxicam Piroxicam Tenoxicam Oxaprozin Lornoxicam | Pyrazolones: Phenylbutazone Mofebutazone Oxyphenbutazone Kebuzone Metamizole (Dipyrone) Feprazone Nifenazone Suxibuzone Aminophenazone |
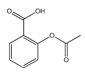 |  |  | 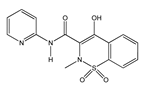 | 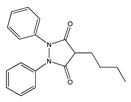 |
| Aspirin | Sulindac | Etodolac | Piroxicam | Phenylbutazone |
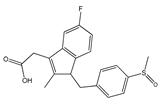
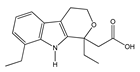
| Arylpropionic Derivatives | Phenemates | Others | ||
| Butibufen Phenoprofen Phenobufen Flurbiprofen Benoxaprofen Suprofen Ibuprofen Ibuproxam | Ketoprofen Dexetoprofen Pyprophene Indoprofen Naproxen Oxaprozin Tiaprofen Dexibuprofen Phenoprofen Flunoxaprofen Alminoprofen | Meclofenamic acid Mefenamic acid Flufenamic acid Tolipanic acidNiflumic acid Etofenamate | Nabumetone Glucosamine Diacerhein Nimesulide Proquazone Azapropazone Benzidamine Orgotein Feprazone Morniflumato Tenidap Glucosaminoglycan | Coxibs: Celecoxib Rofecoxib Parecoxib Valdecoxib Etoricoxib 4-Aminophenol Paracetamol (Acetaminophen) |
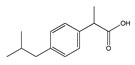 | 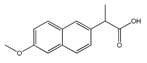 | 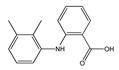 | 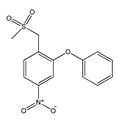 |  |
| (S)-Ibuprofen | Naproxen | Mefenamic acid | Nimesulide | Valdecoxib |
| Number | Botanical Name | Plant/Family | Parts Used | Constituent Compounds |
|---|---|---|---|---|
| 01 | Acacia catechu | Mimosaceae | Bark, wood, flowering tops, gum. | Tannin, gum, catechuic acid |
| 02 | Azadirachta indica | Meliaceae | Leaf, root, oil, seed, gum, fruit, flower. | Margosine, bitter oil, azadirachtin. |
| 03 | Caesalpinia crista | Caesalpiniaceae | Seeds, root, leaf, root bark. | Oleic, linoleic, palmitic, stearic acid, phytosterols. |
| 04 | Cassia angustifolia | Caeasalpinaceae | Pods, dried leaves. | Emodin, eatharitin, mucilage, senna-picrin, opleanic acid. |
| 05 | Coriandrum sativum | Umbelliferaeapiaceae | Leaf, bark, flower | Tannin, cathartin, malic acid, cathartin, albuminoids. |
| 06 | Cuscuta reflexa | Convolvulaceae | Plant, seed, fruit, stem. | Cuscutine, flavonoid, glucoside, bergenin, coumarin. |
| 07 | Enicostema littorale | Gentianaceae | Whole plant. | Alkaloids, gentiocrucine |
| 08 | Erythrina variegate | Papilionaceae | Leaves, bark, roots, flower. | 2-Hydroxygenistein, genistein. |
| 09 | Euphorbia hirta | Euphorbiaceae | Plant, roots, leaves | Ascorbic acid, β-amyrin, choline, inositol, linoleic acid, β-sitosterol. |
| 10 | Euphorbia tirucalli | Euphorbiaceae | Root, plant (milk, juice). | β-sitosterol, ellagic acid, citric acid, malic acid, eupholglucose. |
| 11 | Fagonia cretica | Zygophyllaceae | Leaves, twigs, bark. | Betulin |
| 12 | Ficus benghalensis | Moraceae | Aerial roots, bark, seeds, leaves, buds, fruits, latex. | Skin, fruits contain 10% tannin. |
| 13 | Ficus carica | Moraceae | Fruit, root. | Alkaloids, ascorbic acid, caffeic acid, niacin, linoleic acid, lutein, β-carotene, pantothenic acid, β-amyrin. |
| 14 | Ficus religiosa | Moraceae | Bark, leaves, fruits, tender shoots, seeds. | The bark contains tannins, rubber, wax. |
| 15 | Foeniculum vulgare | Apiaceae | Fruit, root, seeds, leaves. | Ascorbic acid, estragole, coumaric acid, caffeic acid, α-terpinene, scoparone, scopoletin, cynarin, D-limonene, α-phellandrene. |
| 16 | Gentiana kuroo | Gentianaceae | Rhizomes (roots) | Gentiopicrine, gentianic acid |
| 17 | Gloriosa superba | Liliaceae | Rhizome, tuber, leaves, flower | Choline, colchicine, stigmasterol, salicylic acid, 2-methylcolchicine. |
| 18 | Glycyrrhiza glabra | Papilionaceae | Roots, leaves. | Genistein, eugenol, bergapten, glycyrrhizin, acetophenone, estragole, camphor, ascorbic acid, apigenin, anethole. |
| 19 | Gmelina arbórea Roxb | Verbenaceae | Whole plant. | Betulin |
| 20 | Grewia asiatica | Tiliaceae | Leaves, roots, fruits, bark. | Betulin |
| 21 | Hibiscus rosa-Sinensis | Malvaceae | Buds, roots, leaves, flower | Quercetin, ascorbic acid. |
| 22 | Hygrophila auriculata | Acanthaceae | Roots, leaves, seeds. | Oleic and linoleic acids in seed oil, palmitic acid, stearic acid. |
| 23 | Manihot esculenta | Euphorbiaceae | Tuberous roots. | Ascorbic acid, palmitic acid, lauric acid, stearic acid, oleic acid. |
| 24 | Martynia annua | Pedaliaceae | Fruits, leaves. | Pelargonidin-3,5-diglucoside, cyanidin-3-galactoside, semi-drying oil. |
| 25 | Momordica charantia | Cucurbitaceae | Whole plant | 5-Hydroxytryptamine, alkaloids, ascorbic acid, β-carotene, cholesterol, lutein, diosgenin, lanosterol, lycopene, momordicin, charantin niacin, momordicoside. |
| 26 | Moringa oleifera | Moringaceae | Roots, bark, leaves, seeds. | Choline, moringinine, myristic, ascorbic acid, β-carotene, niacin, oleic acid, spirochin, stearic acid, tocopherol, vanillin. |
| 27 | Nelumbo nucifera | Nymphaeaceae | Whole plant. | Anonaine, ascorbic acid, β-carotene, copper, erucic acid, glutathione, hyperoside, myristic acid, nuciferine, oxoushinsunine, rutin, stearic acid, trigonelline, kaempferol, D-catechin. |
| 28 | Nicotiana tobacum | Solanaceae | Leaves. | 1,8-Cineole, 4-vinylguaiacol, acetaldehyde, acetophenone, alkaloids, anabasine, nicotinic acid, nicotine, scopoletin, quercitrin, sorbitol, tocopherol stigmasterol, trigonelline. |
| 29 | Nigella sativa | Ranunculaceae | Seeds. | α-spinasterol, ascorbic acid, β-sitosterol, carvone, D-limonene, linoleic acid, myristic acid, methionine, nigellone, stearic acid, stigmasterol, tannin, thymoquinone, hederagenin. |
| 30 | Ocimum basilicum | Laminaceae | Whole plant | Acetic acid, ascorbic acid, aspartic acid, apigenin, arginine. |
| 31 | Plumbago zeylanica | Plumbaginaceae | Root, leaves, root, bark. | Plumbagin, droserone, 3-chloroplumbagin, chitranone, zeylinone, elliptione, isozeylinone. |
| 32 | Portulaca oleraceae | Portulaceae | Stem, leaves, seeds. | Oleracins I and II, acylated betacyanins, carbohydrate, galacturonic acid, mucilage. |
| 33 | Pterocarpus marsupium | Fabaceae | leaves, flower, gum Heartwood, | Alkaloids, gum, essential oil, semi-drying fixed oil. |
| 34 | Solanum melongena | Solanaceae | Roots, leaves, tender fruits. | Ascorbic acid, alanine, arginine, caffeic acid. |
| 35 | Solanum nigrum | Solanaceae | Whole plant. | Solenin, solasodine. |
| 36 | Stereopermum suaveolens | Bignoniaceae | Roots, flower | Mucilage, albumin, sugar, wax, lapachol, dehydrotectol, β-sitosterol, n-triacontanol. |
| 37 | Tephrosia purpurea | Fabaceae | Whole plant | Tephrosin, betulinic acid, lupeol, rutin. |
| 38 | Terminalia chebula | Combretaceae | Mature, immature fruits. | Ascorbic acid, gallic acid, ellagic acid, chebulic acid. |
| 39 | Thespesia populnea | Malvaceae | Whole plant | Gossypol, herbacetin, kaempferol. |
| 40 | Thespesia populneoides | Malvaceae | Whole plant | Populneol, gossypol, kaempferol, quercetin-5-glucoside, calycopterin, kaempferol-5-glucoside, kaempferol-3-gluoside. |
| 41 | Tinospora cordifolia | Menispemaceae | Stem | Alkaloids, starch. |
| 42 | Vernonia cinerea | Asteraceae | Whole plant | Linoleic acid, lupeol, vernolic acid. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, C.d.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. https://doi.org/10.3390/molecules25163726
Nunes CdR, Barreto Arantes M, Menezes de Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de Moraes L, Vieira IJC, Barros de Oliveira D. Plants as Sources of Anti-Inflammatory Agents. Molecules. 2020; 25(16):3726. https://doi.org/10.3390/molecules25163726
Chicago/Turabian StyleNunes, Clara dos Reis, Mariana Barreto Arantes, Silvia Menezes de Faria Pereira, Larissa Leandro da Cruz, Michel de Souza Passos, Luana Pereira de Moraes, Ivo José Curcino Vieira, and Daniela Barros de Oliveira. 2020. "Plants as Sources of Anti-Inflammatory Agents" Molecules 25, no. 16: 3726. https://doi.org/10.3390/molecules25163726
APA StyleNunes, C. d. R., Barreto Arantes, M., Menezes de Faria Pereira, S., Leandro da Cruz, L., de Souza Passos, M., Pereira de Moraes, L., Vieira, I. J. C., & Barros de Oliveira, D. (2020). Plants as Sources of Anti-Inflammatory Agents. Molecules, 25(16), 3726. https://doi.org/10.3390/molecules25163726








