Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. var. “Camone” Tomato in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterisation of Camone Tomato Locular Gel and Serum
2.2. Animal Experiments: Effects of Four-Week Treatment with Vehicle (V), Gel/Serum (Gs), Tomatine (T) and Captopril (C)
2.2.1. Physiological Parameters
2.2.2. Systolic Blood Pressure (SBP) and Heart Rate (HR)
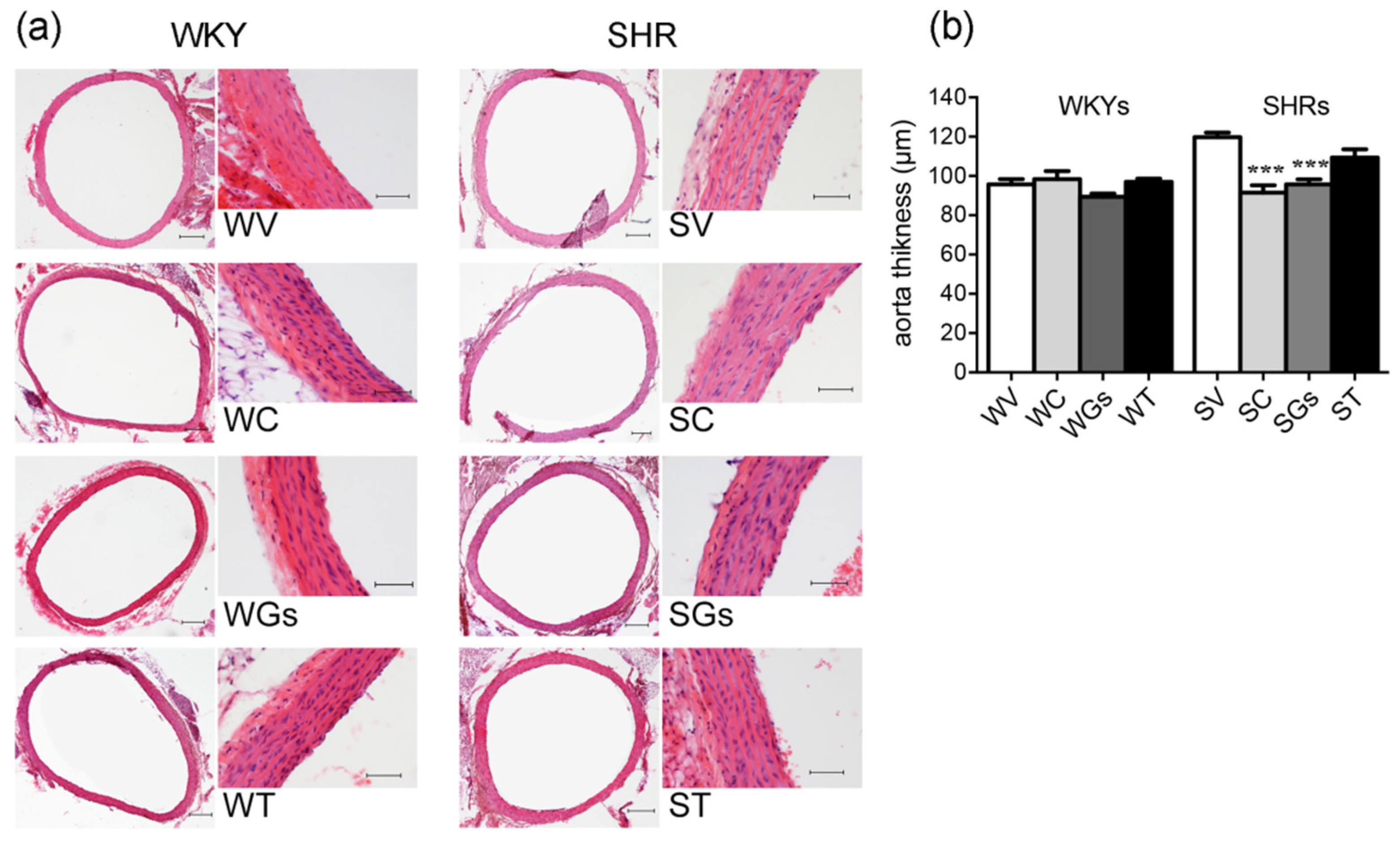
2.2.3. Histological Analysis
2.2.4. Serum Inflammatory Cytokine
2.2.5. Liver Cytochrome P450- and b5-content, NADPH-Cytochrome P450 Reductase Activity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Tomato Samples and Chemical Characterisation
4.3. Animals
4.4. Experimental Design
- V: Vehicle (physiologic solution, n = 7);
- C: Captopril (50 mg/kg, n = 7), a well-known antihypertensive drug; dose chosen according to the literature [24];
- Gs: lyophilised gel reconstituted in serum (400 mg lyophilised gel in 12 mL serum, i.e., 12.4 g/kg, n = 8);
- T: Tomatine (8 mg/kg, n = 8).
4.5. Physiological Parameters
4.6. Blood Pressure and Heart Rate
4.7. Histological Analysis
4.8. Inflammatory Cytokines
4.9. Liver Cytochrome P450- and b5-content, NADPH-Cytochrome P450 Reductase Activity
4.10. Alkoxyresorufin Assay for Determination of Rat Liver Microsome CYP-Dependent-Activities
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Jackson, T.; et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czegle, I.; Csala, M.; Mandl, J.; Benedetti, A.; Karádi, I.; Bánhegyi, G. G6PT-H6PDH-11βHSD1 triad in the liver and its implication in the pathomechanism of the metabolic syndrome. World J. Hepatol. 2012, 4, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.P.; Stevens, S.; Stevens, R.; Martin, U.; Mant, J.; Hobbs, F.D.R.; McManus, R.J. Benefits and harms of antihypertensive treatment in low-risk patients with mild hypertension. JAMA Intern. Med. 2018, 178, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Functional Foods and Nutraceuticals in the Primary Prevention of Cardiovascular Diseases. J. Nutr. Metab. 2012. [Google Scholar] [CrossRef] [Green Version]
- Valeri, A.; Fiorenzani, P.; Rossi, R.; Aloisi, A.M.; Valoti, M.; Pessina, F. The soy phytoestrogens genistein and daidzein as neuroprotective agents against anoxia-glucopenia and reperfusion damage in rat urinary bladder. Pharmacol. Res. 2012, 66, 309–316. [Google Scholar] [CrossRef]
- Tamasi, G.; Baratto, M.C.; Bonechi, C.; Byelyakova, A.; Pardini, A.; Donati, A.; Leone, G.; Consumi, M.; Lamponi, S.; Magnani, A.; et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci. Nut. 2019, 7, 2907–2920. [Google Scholar] [CrossRef] [Green Version]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Leone, G.; Consumi, M.; Magnani, A.; Rossi, C. Solution dynamics of the natural bioactive molecule, Capsaicin: A relaxation study. Spectrosc. Lett. 2019, 52, 74–79. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Franzi, C.; Tamasi, G.; Lamponi, S.; Donati, A.; Magnani, A.; Rossi, C.; Bonechi, C. Development of liposomal formulations to potentiate natural lovastatin inhibitory activity towards 3- hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase. J. Drug Del. Sci. Technol. 2018, 43, 107–112. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Seybold, C.; Fröhlich, K.; Bitsch, R.; Otto, K.; Böhm, V. Changes in Contents of Carotenoids and Vitamin E during Tomato Processing. J. Agric. Food Chem. 2004, 52, 7005–7010. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Verheul, M.J. Content of chalconaringenin and chlorogenic acid in cherry tomatoes is strongly reduced during postharvest ripening. J. Agric. Food Chem. 2005, 53, 7251–7256. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Analytical determination of antioxidants in tomato: Typical components of the Mediterranean diet. J. Sep. Sci. 2007, 30, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Tamasi, G.; Pardini, A.; Bonechi, C.; Donati, A.; Pessina, F.; Marcolongo, P.; Gamberucci, A.; Leone, G.; Consumi, M.; Magnani, A.; et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 2019, 245, 907–918. [Google Scholar] [CrossRef]
- Serratì, S.; Porcelli, L.; Guida, S.; Ferretta, A.; Iacobazzi, R.M.; Cocco, T.; Maida, I.; Tamasi, G.; Rossi, C.; Manganelli, M.; et al. Tomatine Displays Antitumor Potential in In Vitro Models of Metastatic Melanoma. Int. J. Mol. Sci. 2020, 21, 5243. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Engelhard, Y.N.; Gazer, B.; Paran, E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am. Heart J. 2006, 151. [Google Scholar] [CrossRef]
- Pessina, F.; Kalfin, R.; Esposito, L.; Fusi, F.; Valoti, M.; Ponticelli, F.; Sgaragli, G. Neuroprotection afforded by some hindered phenols and alpha-tocopherol in guinea-pig detrusor strips subjected to anoxia-glucopenia and reperfusion-like conditions. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 462–471. [Google Scholar] [CrossRef]
- Friedman, M. Tomato Glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef]
- Biswas, D.; Uddin, M.; Dizdarevic, L.L.; Jørgensen, A.; Duttaroy, A.K. Inhibition of angiotensin-converting enzyme by aqueous extract of tomato. Eur. J. Nutr. 2014, 53, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Fitch, T.E.; Levin, C.E.; Yokoyama, W.H. Feeding Tomatoes to Hamsters Reduces their Plasma Low-density Lipoprotein Cholesterol and Triglycerides. J. Food Sci. 2000, 65, 897–900. [Google Scholar] [CrossRef]
- López-Carreras, N.; Fernández-Vallinasa, S.; Hernández, R.; Miguel, M.; Aleixandre, A. Short-term effect of an aqueous Fraxinus excelsior L. seed extract in spontaneously hypertensive rats. Food Res. Int. 2013, 53, 81–87. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Fitch, T.E.; Yokoyama, W.E. Lowering of plasma LDL cholesterol in hamsters by the tomato glycoalkaloid tomatine. Food Chem. Toxicol. 2000, 38, 549–553. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of Angiotensin Converting Enzyme (ACE) by Flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. 2007, 21, 32–36. [Google Scholar] [CrossRef]
- Vazquez, A.; Sanchez-Rodriguez, E.; Vargas, F.; Montoro-Molina, S.; Romero, M.; Espejo-Calvo, J.A.; Vilchez, P.; Jaramillo, S.; Olmo-García, L.; Carrasco-Pancorbo, A.; et al. Cardioprotective Effect of a Virgin Olive Oil Enriched with Bioactive Compounds in Spontaneously Hypertensive Rats. Nutrients 2019, 11, 1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randriamboavonjy, J.I.; Loirand, G.; Vaillant, N.; Lauzier, B.; Derbré, S.; Michalet, S.; Pacaud, P.; Tesse, A. Cardiac Protective Effects of Moringa oleifera Seeds in Spontaneous Hypertensive Rats. Am. J. Hypertens. 2016, 29, 873–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Zhang, C.; Hou, Y.; Laudon, M.; She, M.; Yang, S.; Ding, L.; Wang, H.; Wang, Z.; He, P.; et al. Blood pressure reducing effects of piromelatine and melatonin in spontaneously hypertensive rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2449–2456. [Google Scholar] [PubMed]
- Oliveira, S.A.; Okoshi, K.; Lima-Leopoldo, A.P.; Leopoldo, A.S.; Campos, D.H.S.; Martinez, P.F.; Okoshi, M.P.; Padovani, C.R.; Dal Pai-Silva, M.; Cicogna, A.C. Nutritional and Cardiovascular Profiles of Normotensive and Hypertensive Rats kept on a High Fat Diet. Arq. Bras. Cardiol. 2009, 93, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kellems, R.E.; Xia, Y. Inflammation, Autoimmunity, and Hypertension: The Essential Role of Tissue Transglutaminase. Am. J. Hypertens. 2017, 30, 756–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Zhou, B.; Bao, L.; Yang, Y.; Guo, K. Alpha-Tomatine Exhibits Anti-inflammatory Activity in Lipopolysaccharide-Activated Macrophages. Inflammation 2015, 38, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kiyota, N.; Tsurushima, K.; Yoshitomi, M.; Horlad, H.; Ikeda, T.; Nohara, T.; Takeya, M.; Nagai, R. Tomatidine, a tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in apoE-deficient mice by inhibiting acyl-CoA:cholesterol acyl-transferase (ACAT). J. Agric. Food Chem. 2012, 60, 2472–2479. [Google Scholar] [CrossRef]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Serpe, L.; Canaparo, R.; Dell’Osso, B.; Galentino, R.; De Michele, S.; Zanaboni Dina, C.; Porta, M.; et al. Food Bioactive Compounds and Their Interference in Drug Pharmacokinetic/Pharmacodynamic Profiles. Pharmaceutics 2018, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Fragoso, L.; Martínez-Arismendi, J.L.; Orozco-Bustos, D.; Reyes-Esparza, J.; Torres, E.; Burchiel, S.W. Potential risks resulting from fruit/vegetable-drug interactions: Effects on drug-metabolizing enzymes and drug transporters. J. Food Sci. 2011, 76, R112–R124. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Agawa, C.; Ueda, S.; Yamane, T.; Kitayama, H.; Terao, A.; Fukuda, T.; Minegaki, T.; Nishiguchi, K. Inhibitory Effects of Juices Prepared from Individual Vegetables on CYP3A4 Activity in Recombinant CYP3A4 and LS180 Cells. Biol. Pharm. Bull. 2017, 40, 1561–1565. [Google Scholar] [CrossRef]
- Liu, A.G.; Volker, S.E.; Jeffery, E.H.; Erdman, J.W., Jr. Feeding tomato and broccoli powders enriched with bioactives improves bioactivity markers in rats. J. Agric. Food Chem. 2009, 57, 7304–7310. [Google Scholar] [CrossRef]
- Pessina, F.; Frosini, M.; Marcolongo, P.; Fusi, F.; Saponara, S.; Gamberucci, A.; Valoti, M.; Giustarini, D.; Fiorenzani, P.; Gorelli, B.; et al. Antihypertensive, cardio- and neuro-protective effects of Tenebrio molitor (Coleoptera: Tenebrionidae) defatted larvae in spontaneously hypertensive rats. PLoS ONE 2020, 15, e0233788. [Google Scholar] [CrossRef]
- Løvdal, T.; Van Droogenbroeck, B.; Eroglu, E.C.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of Tomato Surplus and Waste Fractions: A Case Study Using Norway, Belgium, Poland, and Turkey as Examples. Food 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995, 28, 23–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Gómez-Roso, M.; Montero, M.J.; Carrón, R.; Sevilla, M.A. Cardiovascular changes in spontaneously hypertensive rats are improved by chronic treatment with zofenopril. Br. J. Pharmacol. 2009, 158, 1911–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayen, M.N. Effect of dietary tomatine on cholesterol metabolism in the rat. J. Lipid Res. 1971, 12, 482–490. [Google Scholar]
- Gangwar, A.; Kumar, P.; Rawat, A.; Tiwari, S. Noninvasive measurement of systolic blood pressure in rats: A novel technique. Indian J. Pharmacol. 2014, 46, 351–352. [Google Scholar] [CrossRef]
- Bánhegyi, G.; Marcolongo, P.; Fulceri, R.; Hinds, C.; Burchell, A.; Benedetti, A. Demonstration of a metabolically active glucose-6-phosphate pool in the lumen of liver microsomal vesicles. J. Biol. Chem. 1997, 272, 13584–13590. [Google Scholar] [CrossRef] [Green Version]
- Schagger, H.; Cramer, W.A.; Vonjagow, G. Analysis of Molecular Masses and Oligomeric States of Protein Complexes by Blue Native Electrophoresis and Isolation of Membrane Protein Complexes by Two-Dimensional Native Electrophoresis. Anal. Biochem. 1994, 217, 220–230. [Google Scholar] [CrossRef]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar]
- Masters, B.S.S.; William, C.H.; Kamin, H. The preparation and properties of microsomal NADPH-cytochrome c reductase from pig liver. In Methods in Enzymology; Estabrook, R.W., Pulman, E.E., Eds.; Academy Press: New York, NY, USA, 1967; Volume 10, pp. 565–573. [Google Scholar]
- Dragoni, S.; Materozzi, G.; Pessina, F.; Frosini, M.; Marco, J.L.; Unzeta, M.; Sgaragli, G.; Valoti, M. CYP-dependent metabolism of PF9601N, a new monoamine oxidase-B inhibitor, by C57BL/6 mouse and human liver microsomes. J. Pharm. Pharm. Sci. 2007, 10, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Shiraga, T.; Yamasaki, S.; Ishibashi, K.; Ohno, Y.; Kagayama, A. In vitro activation of 7-benzyloxyresorufin O-debenzylation and nifedipine oxidation in human liver microsomes. Xenobiotica 2003, 33, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Lechón, M.J.; Donato, M.T.; Castell, J.V.; Jover, R. Human hepatocytes in primary culture: The choice to investigate drug metabolism in man. Curr. Drug Metab. 2004, 5, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Abass, K.; Reponen, P.; Jalonen, J.; Pelkonen, O. In vitro metabolism and interactions of the fungicide metalaxyl in human liver preparations. Environ. Toxicol. Pharmacol. 2007, 23, 39–47. [Google Scholar] [CrossRef]
- Chovan, J.P.; Ring, S.C.; Yu, E.; Baldino, J.P. Cytochrome P450 probe substrate metabolism kinetics in Sprague Dawley rats. Xenobiotica 2007, 37, 459–473. [Google Scholar] [CrossRef]
- Donato, M.T.; Gómez-Lechón, M.J.; Castell, J.V. A microassay for measuring cytochrome P450IA1 and P450IIB1 activities in intact human and rat hepatocytes cultured on 96-well plates. Anal. Biochem. 1993, 213, 29–33. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds used in this study are available from the authors. |





| Locular Gel | ||||
| Week 1 | Week 2 | Week 3 | Week 4 | |
| Caffeic acid | 5.13 ± 0.04 a | 9.85 ± 0.17 b | 6.12 ± 0.49 c | 8.11 ± 0.24 d |
| Chlorogenic acid | 68.2 ± 1.7 a | 90.7 ± 3.5 b | 53.4 ± 2.3 c | 29.5 ± 0.6 d |
| p-Coumaric acid | 0.200 ± 0.001 a | 0.513 ± 0.007 b | 0.233 ± 0.004 c | 0.368 ± 0.009 d |
| Rutin | 0.717 ± 0.021 a | 0.321 ± 0.007 b | 0.210 ± 0.008 c | trace |
| α-Tomatine | 61.7 ± 0.9 a | 9.05 ± 0.37 b | 13.7 ± 0.3 c | 4.41 ± 0.32 d |
| Dehydrotomatine | 7.30 ± 0.25 a | 2.04 ± 0.09 b | 2.80 ± 0.19 c | 0.78 ± 0.09 d |
| Serum | ||||
| Week 1 | Week 2 | Week 3 | Week 4 | |
| Caffeic acid | 2.31 ± 0.04 a | 6.36 ± 0.52 b | 3.94 ± 0.17 c | 3.82 ± 0.04 c |
| Chlorogenic acid | 83.6 ± 3.0 a | 92.3 ± 4.7 b | 51.5 ± 1.0 c | 26.1 ± 0.8 d |
| p-Coumaric acid | nd | 0.140 ± 0.017 a | trace | trace |
| Rutin | 0.281 ± 0.016 a | 0.163 ± 0.005 b | 0.112 ± 0.001 c | trace |
| α-Tomatine | 12.5 ± 0.5 a | 1.51 ± 0.04 b | 1.90 ± 0.09 c | 0.65 ± 0.04 d |
| Dehydrotomatine | 1.86 ± 0.05 a | 0.305 ± 0.048 b | 0.362 ± 0.001 c | 0.118 ± 0.001 d |
| Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|
| Chlorogenic acid | 1276 ± 37 a | 1470 ± 58 b | 832 ± 15 c | 431 ± 10 d |
| Caffeic acid | 48.2 ± 0.5 a | 116 ± 6 b | 72 ± 3 c | 78 ± 1 c |
| α-Tomatine | 396 ± 7 a | 54 ± 2 b | 78 ± 2c | 25 ± 1 d |
| Strain | Diet | Body Weight (g) | Heart Weight/Body Weight (× 10−3) | |
|---|---|---|---|---|
| Time 0 | Week 4 | Week 4 | ||
| WKYs | Vehicle | 262.4 ± 3.8 | 303.4 ± 3.7 | 3.83 ± 0.03 |
| Captopril | 267.6 ± 4.8 | 304.2 ± 4.4 | 3.87 ± 0.43 | |
| Gel/serum | 266.0 ± 4.2 | 305.5 ± 8.4 | 3.60 ± 0.27 | |
| Tomatine | 262.2 ± 4.4 | 301.7 ± 4.4 | 3.68 ± 0.08 | |
| SHRs | Vehicle | 239.2 ± 4.3 | 282.2 ± 8.1 | 4.20 ± 0.13 |
| Captopril | 254.4 ± 2.9 | 292.4 ± 5.3 | 3.88 ± 0.10 | |
| Gel/serum | 246.5 ± 4.6 | 289.2 ± 5.9 | 3.98 ± 0.15 | |
| Tomatine | 249.0 ± 7.7 | 290.0 ± 7.7 | 3.93 ± 0.14 | |
| WKY V | WKY C | WKY Gs | WKY T | |
| Glucose (mg/dL) | 86.80 ± 5.48 | 88.00 ± 6.31 | 83.17 ± 6.26 | 78.83 ± 10.17 |
| Triglycerides (mg/dL) | 129.16 ± 9.48 | 119.60 ± 4.53 | 126.83 ± 9.85 | 125.33 ± 3.18 |
| Urine volume (mL) | 8.00 ± 0.10 | 13.50 ± 3.58 | 10.00 ± 0.82 | 9.00 ± 0.82 |
| SHR V | SHR C | SHR GS | SHR T | |
| Glucose (mg/dL) | 75.80 ± 5.56 | 75.80 ± 5.67 | 73.67 ± 5.94 | 72.50 ± 2.75 |
| Triglycerides (mg/dL) | 115.80 ± 4.81 | 126.80 ± 11.43 | 110.83 ± 7.95 | 132.83 ± 6.16 |
| Urine volume (mL) | 10.00 ± 3.79 | 9.00 ± 0.10 | 11.00 ± 1.78 | 11.00 ± 1.47 |
| Strain | Treatment | Cytochrome P450 | Cytochrome b5 | Cytochrome P450-NADPH-Reductase |
|---|---|---|---|---|
| nmol/mg | nmol/mg | nmol/min/mg | ||
| WKYs | Vehicle | 0.80 ± 0.13 | 0.29 ± 0.07 | 19.01 ± 4.06 |
| Captopril | 0.76 ± 0.10 | 0.27 ± 0.04 | 13.90 ± 3.67 | |
| Gel/serum | 0.60 ± 0.06 | 0.32 ± 0.03 | 13.24 ± 2.27 | |
| Tomatine | 0.69 ± 0.13 | 0.32 ± 0.04 | 17.75 ± 1.44 | |
| SHRs | Vehicle | 0.50 ± 0.17 | 0.25 ± 0.07 | 30.17 ± 7.08 |
| Captopril | 0.68 ± 0.19 | 0.28 ± 0.05 | 40.27 ± 18.30 | |
| Gel/serum | 0.46 ± 0.11 | 0.23 ± 0.03 | 33.13 ± 7.27 | |
| Tomatine | 0.68 ± 0.13 | 0.32 ± 0.04 | 37.70 ± 14.34 |
| SUBSTRATE | ||||
|---|---|---|---|---|
| Strain | Treatment | ETR | PTR | BZR |
| pmol/min/mg | pmol/min/mg | pmol/min/mg | ||
| WKYs | Vehicle | 9.09 ± 2.39 | 2.40 ± 0.21 | 2.14 ± 0.46 |
| Captopril | 7.68 ± 1.77 | 1.88 ± 0.25 | 2.58 ± 0.43 | |
| Gel/serum | 9.23 ± 1.61 | 2.27 ± 0.41 | 2.98 ± 0.74 | |
| Tomatine | 9.76 ± 1.46 | 2.70 ± 0.64 | 2.55 ± 0.22 | |
| SHRs | Vehicle | 7.57 ± 1.87 | 2.64 ± 0.65 | 4.25 ± 0.26 |
| Captopril | 7.27 ± 1.27 | 2.39 ± 0.28 | 4.83 ± 0.52 | |
| Gel/serum | 7.81 ± 0.84 | 2.93 ± 0.46 | 4.55 ± 0.46 | |
| Tomatine | 8.60 ± 0.53 | 2.45 ± 0.29 | 5.01 ± 1.08 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Bonechi, C.; Rossi, C.; Giunti, R.; Barone, V.; Borghini, A.; Fiorenzani, P.; et al. Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. var. “Camone” Tomato in Spontaneously Hypertensive Rats. Molecules 2020, 25, 3758. https://doi.org/10.3390/molecules25163758
Marcolongo P, Gamberucci A, Tamasi G, Pardini A, Bonechi C, Rossi C, Giunti R, Barone V, Borghini A, Fiorenzani P, et al. Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. var. “Camone” Tomato in Spontaneously Hypertensive Rats. Molecules. 2020; 25(16):3758. https://doi.org/10.3390/molecules25163758
Chicago/Turabian StyleMarcolongo, Paola, Alessandra Gamberucci, Gabriella Tamasi, Alessio Pardini, Claudia Bonechi, Claudio Rossi, Roberta Giunti, Virginia Barone, Annalisa Borghini, Paolo Fiorenzani, and et al. 2020. "Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. var. “Camone” Tomato in Spontaneously Hypertensive Rats" Molecules 25, no. 16: 3758. https://doi.org/10.3390/molecules25163758
APA StyleMarcolongo, P., Gamberucci, A., Tamasi, G., Pardini, A., Bonechi, C., Rossi, C., Giunti, R., Barone, V., Borghini, A., Fiorenzani, P., Frosini, M., Valoti, M., & Pessina, F. (2020). Chemical Characterisation and Antihypertensive Effects of Locular Gel and Serum of Lycopersicum esculentum L. var. “Camone” Tomato in Spontaneously Hypertensive Rats. Molecules, 25(16), 3758. https://doi.org/10.3390/molecules25163758







