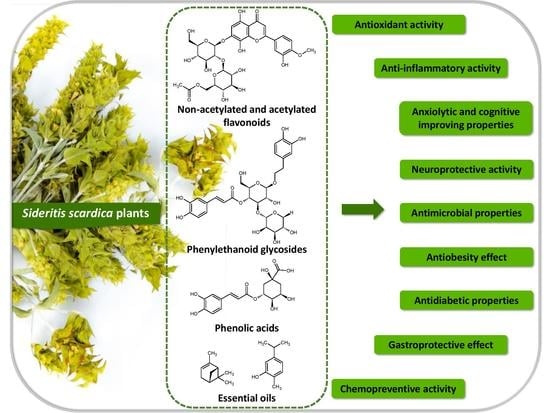
Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity
Abstract
:1. Introduction
2. Phytochemical Profile of Sideritis scardica and Other Sideritis Species
3. Beneficial Impact of Compounds Present in Sideritis Plants on Mental Health
4. Anti-Inflammatory and Antimicrobial Properties of Sideritis Plants
5. Effect of Sideritis scardica and Other Sideritis Plants on Blood and Liver Parameters
6. Interactions of Bioactive Compounds from Sideritis scardica with the Intestinal Microflora
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:21227-1 (accessed on 8 April 2020).
- Yaneva, I.; Balabanski, V. History of the uses of Pirin mountain tea (Sideritis scardica Griseb) in Bulgaria. Bulg. J. Public Health 2013, 5, 48–57. [Google Scholar]
- Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Aneva, I.; Evstatieva, L.N. Chemotaxonomic contribution to the Sideritis species dilemma on the Balkans. Biochem. Syst. Ecol. 2015, 61, 477–487. [Google Scholar] [CrossRef]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb., anendemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, I.; Petricevic, S.; Tadic, V.; Petrovic, D.; Tosic, J.; Stanojevic, Z.; Petronijevic, M.; Vidicevic, S.; Trajkovic, V.; Isakovic, A. Effects of Sideritis scardica extract on glucose tolerance, triglyceride levels and markers of oxidative stress in ovariectomized rats. Planta Med. 2019, 85, 465–472. [Google Scholar]
- Zhao, G.; Qin, G.W.; Wang, J.; Chu, W.J.; Guo, L.H. Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.). Britt. Neurochem. Int. 2010, 56, 168–176. [Google Scholar] [CrossRef]
- Knörle, R. Extracts of Sideritis scardica as triple monoamine reuptake inhibitors. J. Neural Transm. 2012, 119, 1477–1482. [Google Scholar] [CrossRef]
- Tadić, V.M.; Jeremic, I.; Dobric, S.; Isakovic, A.; Markovic, I.; Trajkovic, V.; Bojovic, D.; Arsic, I. Anti-inflammatory, gastroprotective, and cytotoxic effects of Sideritis scardica extracts. Planta Med. 2012, 78, 415–427. [Google Scholar] [CrossRef]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Petreska, J.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Stefova, M. Phenolic compounds of mountain tea from the Balkans: LC/DAD/ESI/MSn profile and content. Nat. Prod. Commun. 2011, 6, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Ibraliu, A.; Trendafilova, A.B.; Anđelković, B.D.; Qazimi, B.; Gođevac, D.M.; Shengjergji, D.; Bebeci, E.; Stefkov, G.; Zdunic, G.; Aneva, I.I.; et al. Comparative study of Balkan Sideritis species from Albania, Bulgaria and Macedonia. Eur. J. Med. Plants 2015, 5, 328–340. [Google Scholar] [CrossRef]
- Petreska, J.; Stefova, M.; Ferreres, F.; Moreno, D.A.; Tomás-Barberán, F.A.; Stefkov, G.; Kulevanova, S.; Gil-Izquierdo, A. Potential bioactive phenolics of Macedonian Sideritis species used for medicinal “Mountain Tea”. Food Chem. 2011, 125, 13–20. [Google Scholar] [CrossRef]
- Axiotis, E.; Petrakis, E.A.; Halabalaki, M.; Mitakou, S. Phytochemical profile and biological activity of endemic Sideritis sipylea Boiss. in North Aegean Greek islands. Molecules 2020, 25, 2022. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, A.; Houghton, P.J.; Duman, H.; Coskun, M.; Sahin, P. Antioxidant activity studies on selected Sideritis species native to Turkey. Pharm. Biol. 2005, 43, 173–177. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kirimer, N.; Tümen, G. Essential oil of Sideritis scardica Griseb subsp. scardica. J. Essent. Oil Res. 1997, 9, 205–207. [Google Scholar] [CrossRef]
- Kloukina, C.; Tomou, E.M.; Skaltsa, H. Essential oil composition of two Greek cultivated Sideritis spp. Nat. Volatiles Essent. Oils 2019, 6, 16–23. [Google Scholar]
- PubChem. National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 30 June 2020).
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, T.M. Essential oils of Lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Irakli, M.; Tsifodimou, K.; Sarrou, E.; Chatzopoulou, P. Optimization infusions conditions for improving phenolic content and antioxidant activity in Sideritis scardica tea using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2018, 8, 67–74. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Hsieh, M.T.; Tsai, F.S.; Wu, C.R.; Chiu, C.S.; Lee, M.M.; Xu, H.X.; Zhao, Z.Z.; Peng, W.H. Neuroprotective effect of luteolin on amyloid β protein (25-35)-induced toxicity in cultured rat cortical neurons. Phytother. Res. 2009, 24, 102–108. [Google Scholar] [CrossRef]
- Patil, S.P.; Jain, P.D.; Sancheti, J.S.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective and neurotrophic effects of apigenin and luteolin in MPTP induced parkinsonism in mice. Neuropharmacology 2014, 86, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savtschenko, A.; Dhein, S.; Rauwald, H.W. The antiarrhythmic effects of lavandulifolioside and ferulic acid from Leonurus cardiaca extracts on cardiac electrophysiology. Z. Phytother. 2013, 3, 25. [Google Scholar] [CrossRef]
- Jiménez, C.; Riguera, R. Phenylethanoid glycosides in plants: Structure and biological activity. Nat. Prod. Rep. 1994, 11, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Akcos, Y.; Ezer, N.; Çalis, I.; Demirdamar, R.; Tel, B.C. Polyphenolic compounds of Sideritis lycia and their anti-inflammatory activity. Pharm. Biol. 1999, 37, 118–122. [Google Scholar] [CrossRef]
- Korkina, L.G.; Mikhalchik, E.; Suprun, M.; Pastore, S.; Dal Toso, R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell Mol. Biol. 2007, 53, 78–83. [Google Scholar]
- Charami, M.T.; Lazari, D.; Karioti, A.; Skaltsa, H.; Hadjipavlou-Litina, D.; Souleles, C. Antioxidant and antiinflammatory activities of Sideritis perfoliata subsp. perfoliata (Lamiaceae). Phytother. Res. 2008, 22, 450–454. [Google Scholar] [CrossRef]
- Kostyuka, V.A.; Potapovich, A.I.; Suhan, T.O.; de Luca, C.; Korkina, L.G. Antioxidant and signal modulation properties of plant polyphenols in controlling vascular inflammation. Eur. J. Pharmacol. 2011, 658, 248–256. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; de Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404. [Google Scholar] [CrossRef]
- Mazzon, E.; Esposito, E.; di Paola, R.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Effects of verbascoside biotechnologically produced by Syringa vulgaris plant cell cultures in a rodent model of colitis. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 79–94. [Google Scholar] [CrossRef]
- Vertuani, S.; Erika Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.J.; Tan, N.H.; Zhu, H.Z.; Zeng, G.Z.; He, W.J.; Yu, B.S.; Chen, X. Anti-sports anaemia effects of verbascoside and martynoside in mice. J. Sports Med. 2010, 31, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharm. Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Wall, C.A.; Lappas, M. Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol. Hum. Reprod. 2013, 19, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmakar, S.; Davis, K.A.; Choudhury, S.R.; Deeconda, A.; Banik, N.L.; Ray, S.K. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2009, 388, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Ma, J.; Zhu, H.Y.; Zhang, X.H.; Du, Z.Y.; Xu, Y.J.; Yu, X.D. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol. Cancer 2011, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.G. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Viola, H.; Wasowski, C.; Levi de Stein, M.; Wolfman, C.; Silveira, R.; Dajas, F.; Medina, J.; Paladini, A. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995, 61, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhang, T.; Yang, H.; Lan, X.; Ying, J.; Du, G. The flavonoid apigenin protects brain neurovascular coupling against amyloid-β25-35-induced toxicity in mice. J. Alzheimers Dis. 2011, 24, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, F.; Chen, G. Neuroprotective effect of apigenin in rats after contusive spinal cord injury. Neurol. Sci. 2014, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.; Carlsen, H.; Nordström, O.; Blomhoff, R.; Moskaug, J.Ø. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Apigenin (4′,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007, 59, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.W. Protective effect of apigenin against oxidative stress-induced damage in osteoblastic cells. Int. J. Mol. Med. 2014, 33, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Papapetropoulos, A.; Mauromatis, A.; Economou, M.; Fotsis, T.; Roussos, C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 2001, 296, 181–187. [Google Scholar] [PubMed]
- Harris, G.K.; Qian, Y.; Leonard, S.S.; Sbarra, D.C.; Shi, X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. J. Nutr. 2006, 136, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Peng, W.H.; Tsai, K.D.; Hsu, S.L. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, J.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Gao, M.; Qiang, G.F.; Zhang, T.T.; Lan, X.; Ying, J.; Du, G.H. The anti-amnesic effects of luteolin against amyloid β25–35 peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience 2009, 162, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lan, X.; Ying, J.; Du, G.H. Protective Effects of luteolin against amyloid β25–35-induced toxicity on rat cerebral microvascular endothelial cells. Chin. J. Nat. Med. 2010, 8, 223–227. [Google Scholar]
- Farid, M.M.; Marzouk, M.M.; El-Shabrawy, M.; Salem, M.A.; Mounier, M.M.; Hussein, S.R. Isoscutellarein 8, 4′-dimethyl ether glycosides as cytotoxic agents and chemotaxonomic markers in Kickxia aegyptiaca. Biocatal. Agric. Biotechnol. 2019, 22, 101431. [Google Scholar] [CrossRef]
- Villar, A.; Gasco, M.A.; Alcaraz, M.J. Anti-inflammatory and anti-ulcer properties of hypolaetin-8-glucoside, a novel plant flavonoid. J. Pharm. Pharmacol. 1984, 36, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Tordera, M. Studies on the gastric anti-ulcer activity of hypolaetin-8-glucoside. Phytother. Res. 1988, 2, 85–88. [Google Scholar] [CrossRef]
- Raafat, K.M. Anti-inflammatory and anti-neuropathic effects of a novel quinic acid derivative from Acanthus syriacus. Avicenna J. Phytomed. 2019, 9, 221–236. [Google Scholar]
- Hur, J.Y.; Soh, Y.; Kim, B.H.; Suk, K.; Sohn, N.; Kim, H.C.; Kwon, H.C.; Lee, K.R.; Kim, S.Y. Neuroprotective and neurotrophic effects of quinic acids from Aster scaber in PC12 cells. Biol. Pharm. Bull. 2001, 24, 921–924. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Isoda, H. Neuroprotective effects of 3,5-di-o-caffeoylquinicacid in vitro and in vivo. BMC Proc. 2011, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007, 80, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, I.; Yeşiloğlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Jordão, N.; da Costa Pereira Soares, N.; de Mesquita, J.; Monteiro, M.; Teodoro, A. Pharmacokinetic, antiproliferative and apoptotic effects of phenolic acids in human colon adenocarcinoma cells using in vitro and in silico approaches. Molecules 2018, 23, 2569. [Google Scholar] [CrossRef] [Green Version]
- Guven, M.; Yuksel, Y.; Sehitoglu, M.H.; Tokmak, M.; Aras, A.B.; Akman, T.; Golge, U.H.; Goksel, F.; Karavelioglu, E.; Cosar, M. The Effect of coumaric acid on ischemia-reperfusion injury of sciatic nerve in rats. Inflammation 2015, 38, 2124–2132. [Google Scholar] [CrossRef]
- Guven, M.; Aras, A.B.; Akman, T.; Sen, H.M.; Ozkan, A.; Salis, O.; Sehitoglu, I.; Kalkan, Y.; Silan, C.; Deniz, M.; et al. Neuroprotective effect of p-coumaric acid in rat model of embolic cerebral ischemia. Iran. J. Basic Med. Sci. 2015, 18, 356–363. [Google Scholar]
- Guven, M.; Sehitoglu, M.H.; Yuksel, Y.; Tokmak, M.; Aras, A.B.; Akman, T.; Golge, U.H.; Karavelioglu, E.; Bal, E.; Cosar, M. The neuroprotective effect of coumaric acid on spinal cord ischemia/reperfusion injury in rats. Inflammation 2015, 38, 1986–1995. [Google Scholar] [CrossRef]
- Shailasree, S.; Venkataramana, M.; Niranjana, S.R.; Prakash, H.S. Cytotoxic effect of p-coumaric acid on neuroblastoma, N2a cell via generation of reactive oxygen species leading to dysfunction of mitochondria inducing apoptosis and autophagy. Mol. Neurobiol. 2015, 51, 119–130. [Google Scholar] [CrossRef]
- El-Askary, H.; Handoussa, H.; Badria, F.; El-Khatib, A.H.; Alsayari, A.; Linscheid, M.W.; Motaal, A.A. Characterization of hepatoprotective metabolites from Artemisia annua and Cleome droserifolia using HPLC/PDA/ESI/MS–MS. Rev. Bras. Farmacogn. 2019, 29, 213–220. [Google Scholar] [CrossRef]
- Behrendt, I.; Schneider, I.; Schuchardt, J.P.; Bitterlich, N.; Hahn, A. Effect of an herbal extract of Sideritis scardica and B-vitamins on cognitive performance under stress: A pilot study. Int. J. Phytomed. 2016, 8, 95–103. [Google Scholar]
- Vasilopoulou, C.G.; Kontogianni, V.G.; Linardaki, Z.I.; Iatrou, G.; Lamari, F.N.; Nerantzaki, A.A.; Gerothanassis, I.P.; Tzakos, A.G.; Margarity, M. Phytochemical composition of “mountain tea” from Sideritis clandestina subsp. clandestina and evaluation of its behavioral and oxidant/antioxidant effects on adult mice. Eur. J. Nutr. 2013, 52, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Locatelli, M.; Mocan, A.; Zengin, G.; Kirkan, B. Phenolic Profile and Bioactivities of Sideritis perfoliata L.: The Plant, Its Most Active Extract, and Its Broad Biological Properties. Front. Pharmacol. 2020, 10, 1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The acute and chronic cognitive and cerebral blood flow effects of a Sideritis scardica (Greek mountain tea) extract: A double blind, randomized, placebo controlled, parallel groups study in healthy humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofrichter, J.; Krohn, M.; Schumacher, T.; Lange, C.; Feistel, B.; Walbroel, B.; Pahnke, J. Sideritis spp. extracts enhance memory and learning in Alzheimer’s β-amyloidosis mouse models and aged C57Bl/6 mice. J. Alzheimers Dis. 2016, 53, 967–980. [Google Scholar] [CrossRef] [Green Version]
- Tadić, V.M.; Djordjević, S.; Arsić, I.; Dobrić, S.; Milenković, M.; Antić-Stanković, J. Anti-inflammatory and antimicrobial activity of Sideritis scardica extracts. Planta Med. 2007, 73, 98. [Google Scholar] [CrossRef]
- Tadić, V.; Bojović, D.; Arsić, I.; Đorđević, S.; Aksentijevic, K.; Stamenić, M.; Janković, S. Chemical and Antimicrobial Evaluation of Supercritical and Conventional Sideritis scardica Griseb., Lamiaceae Extracts. Molecules 2012, 17, 2683–2703. [Google Scholar] [CrossRef] [Green Version]
- Sagdic, O.; Aksoy, A.; Ozkan, G.; Ekici, L.; Albayrak, S. Biological activities of the extracts of two endemic Sideritis species in Turkey. Innov. Food Sci. Emerg. Technol. 2008, 9, 80–84. [Google Scholar] [CrossRef]
- Uğur, A.; Varol, Ö.; Ceylan, Ö. Antibacterial Activity of Sideritis curvidens and Sideritis lanata from Turkey. Pharm. Biol. 2005, 43, 47–52. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Bazos, I.; Milenković, M.; Pavlović-Drobac, M.; Tzakou, O. Antimicrobial Activity and Essential Oil Composition of Five Sideritis taxa of Empedoclia and Hesiodia Sect. from Greece. Rec. Nat. Prod. 2013, 7, 6–14. [Google Scholar]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [Green Version]
- Kassi, E.; Dimas, C.; Dalamaga, M.; Panagiotou, A.; Papoutsi, Z.; Spilioti, E.; Moutsatsou, P. Sideritis euboea extract lowers total cholesterol but not LDL cholesterol in humans: A randomized controlled trial. Clin. Lipidol. 2013, 8, 627–634. [Google Scholar] [CrossRef]
- Singh, V.J.; Selvendiran, K.; Mumtaz Banu, S.; Padmavathi, R.; Sakthisekaran, D. Protective role of Apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine 2004, 11, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O. Ferulic acid modulates nitric oxide synthase expression in focal cerebral ischemia. Lab. Anim. Res. 2012, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Heiner, F.; Feistel, B.; Wink, M. Sideritis scardica extracts inhibit aggregation and toxicity of amyloid-β in Caenorhabditis elegans used as a model for Alzheimer’s disease. PeerJ 2018, 6, 4683. [Google Scholar] [CrossRef] [Green Version]
- Sarnowska, E.; Balcerak, A.; Olszyna-Serementa, M.; Kotlarek, D.; Sarnowski, T.J.; Siedlecki, J.A. Kinaza białkowa aktywowana przez AMP (AMPK) jako cel terapeutyczny. Postepy Hig. Med. Dosw. 2013, 67, 750–760. [Google Scholar] [CrossRef]
- Zhou, G.; Sebhat, I.K.; Zhang, B.B. AMPK activators—Potential therapeutics for metabolic and other diseases. Acta Physiol. 2009, 196, 175–190. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghoshal, S.; Porter, T.D. Phosphorylation of hepatic AMP-activated protein kinase and liver kinase B1 is increased after a single oral dose of green tea extract to mice. Nutr. Res. 2012, 32, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.W.; Hsu, A.; Tan, B.K. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Petreska Stanoeva, J.; Stefova, M. Assay of urinary excretion of polyphenols after ingestion of a cup of mountain tea (Sideritis scardica) measured by HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2013, 61, 10488–10497. [Google Scholar] [CrossRef]
- Simons, A.L.; Renouf, M.; Hendrich, S.; Murphy, P.A. Human gut microbial degradation of flavonoids: Structure-function relationships. J. Agric. Food Chem. 2005, 53, 4258–4263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Tand, X.; Shane, J.; Liu, Y.; Cai, B.; Di, L. Effect of chito-oligosaccharide on the intestinal absorptions of phenylethanoid glycosides in Fructus Forsythiae extract. Phytomedicine 2014, 21, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Huang, W.; Li, M.; Zhong, Y.; Wang, M.; Lu, B. Bioaccessibility and absorption mechanism of phenylethanoid glycosides using simulated digestion/Caco-2 intestinal cell models. J. Agric. Food Chem. 2018, 66, 4630–4637. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, X.; Luo, X.; Su, M.; Xu, R.; Chen, J.; Ding, Y.; Shi, Y. An integrated approach to characterize intestinal metabolites of four phenylethanoid glycosides and intestinal microbe-mediated antioxidant activity evaluation in vitro using UHPLC-Q-Exactive High-Resolution Mass Spectrometry and a 1,1-Diphenyl-2-picrylhydrazyl-based assay. Front. Pharmacol. 2019, 10, 826. [Google Scholar] [PubMed] [Green Version]
- de Moraes Barros, H.R.; García-Villalba, R.; Tomás-Barberán, F.A.; Inés Genovese, M.I. Evaluation of the distribution and metabolism of polyphenols derived from cupuassu (Theobroma grandiflorum) in mice gastrointestinal tract by UPLC-ESI-QTOF. J. Funct. Food 2016, 22, 477–489. [Google Scholar] [CrossRef]
- Ali, F.; Rahul, F.; Naz, S.; Jyoti, V.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. Biomed. Res. Int. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Shimoi, K.; Okada, H.; Furugori, M.; Goda, T.; Takase, S.; Suzuki, M.; Hara, Y.; Yamamoto, H.; Kinae, N. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998, 438, 220–224. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Appl. Environ. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Q.; Pan, Y.; Zhang, W.; Zhang, Y.; Ren, S.; Wang, D.; Wang, Z.; Liu, X.; Xiao, W. Metabolites of dietary acteoside: Profiles, isolation, identification, and hepatoprotective capacities. J. Agric. Food Chem. 2018, 66, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gan, L.; Li, G.Q.; Deng, L.; Zhang, X.; Deng, Y. Pharmacokinetics of plantamajoside and acteoside from Plantago asiatica in rats by liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2014, 89, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, G.; Peng, Y.; Tu, P.; Li, X. Screening and identification of three typical phenylethanoid glycosides metabolites from Cistanches Herba by human intestinal bacteria using UPLC/Q-TOF-MS. J. Pharm. Biomed. Anal. 2016, 118, 167–176. [Google Scholar] [CrossRef]
- Wu, Y.T.; Lin, L.C.; Sung, J.S.; Tsai, T.H. Determination of acteoside in Cistanche deserticola and Boschniakia rossica and its pharmacokinetics in freely-moving rats using LC–MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 844, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Xiong, A.; Li, P.; Yang, Q.; Yang, L.; Wang, Z. Identification of acteoside and its major metabolites in rat urine by Ultra-Performance Liquid Chromatography combined with Electrospray Ionization Quadrupole Time-Of-Flight Tandem Mass Spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 940, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Herranz-López, M.; Funes, L.; Borrás-Linares, I.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia Citriodora in rats. Phytomedicine 2013, 20, 1112–1118. [Google Scholar] [CrossRef]
- Del Rio, D.; Stalmach, A.; Calani, L.; Crozier, A. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients 2010, 2, 820–833. [Google Scholar] [CrossRef]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Casalini, C.; Lodovici, M.; Briani, C.; Paganelli, G.; Remy, S.; Cheynier, V.; Dolara, P. Effect of complex polyphenols and tannins from red wine (WCPT) on chemically induced oxidative DNA damage in the rat. Eur. J. Nutr. 1999, 38, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Casalini, C.; De Filippo, C.; Copeland, E.; Xu, X.; Clifford, M.; Dolara, P. Inhibition of 1,2-dimethylhydrazine-induced oxidative DNA damage in rat colon mucosa by black tea complex polyphenols. Food Chem. Toxicol. 2000, 38, 1085–1088. [Google Scholar] [CrossRef]
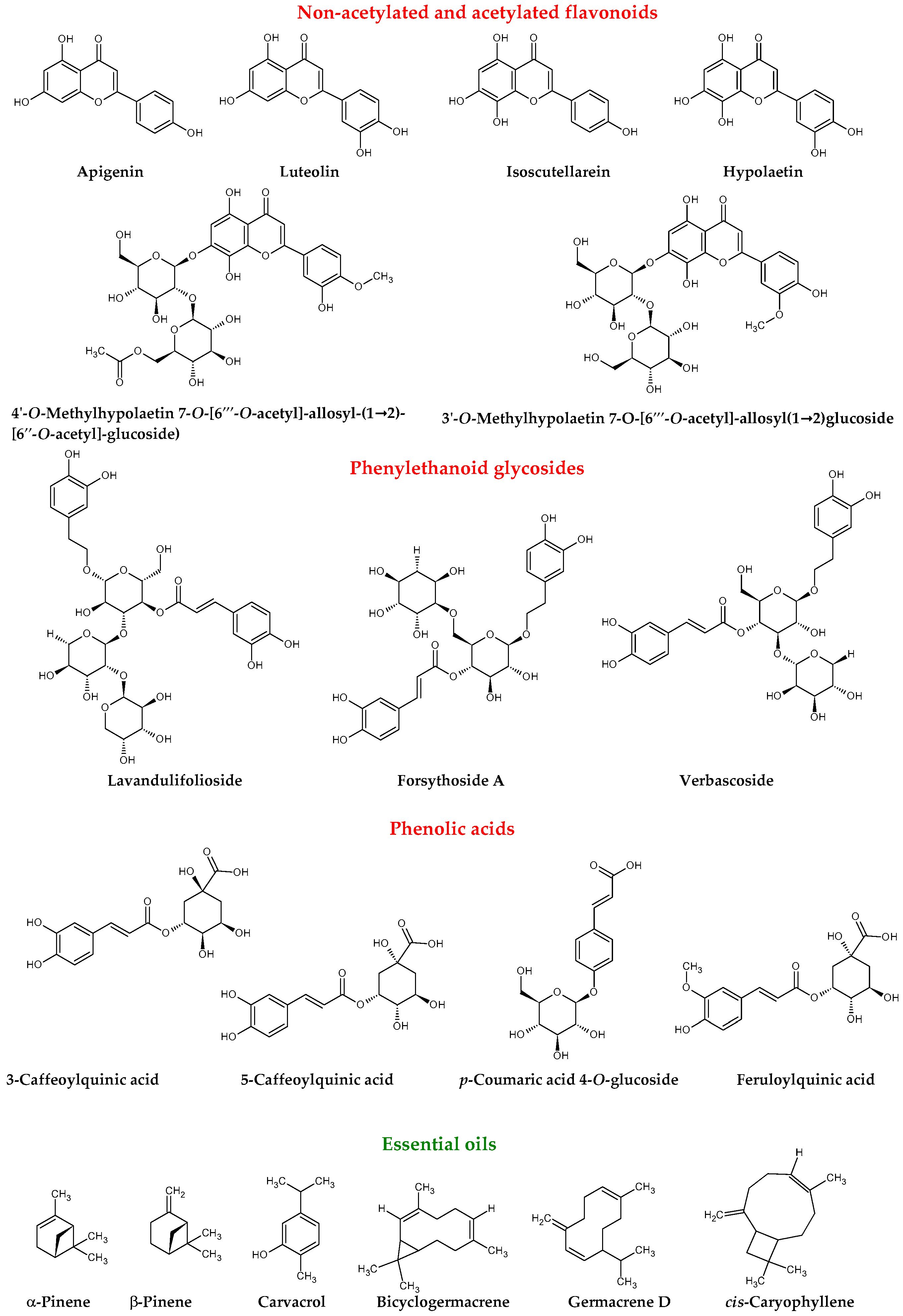
| Compound | Ref. |
|---|---|
| Phenylethanoid glycosides | |
| Lavandulifolioside | [3,11,12] |
| Verbascoside | [3,11,12,13,14] |
| Forsythoside A | [3,11,12,13] |
| Echinacoside | [3,11,12,13,14] |
| Isoverbascoside | [3,11,12,14] |
| Samioside | [3,11,12,13,14] |
| Leucoseptoside A | [3,11,12,13,14] |
| Allysonoside | [3,11,13,14] |
| Martynoside | [14] |
| Flavonoid glycosides | |
| 3′-O-Methylhypolaetin 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | [3,11,12,13] |
| 4′-O-Methylhypolaetin 7-O-[6‴-O-acetyl]-allosyl-(1→2)- [6″-O-acetyl]-glucoside | [3,11,12,13,14] |
| 4′-O-Methylisoscutellarein 7-O-[6″-O-acetyl]-allosyl-(1→2)glucoside | [11,12,13] |
| 4′-O-Methylisoscutellarein 7-O-allosyl(1→2)glucoside | [3,11,12,13] |
| 3′-O-Methylhypolaetin 7-O-allosyl(1→2)glucoside | [3,12,13] |
| 4′-O-Methylisoscutellarein 7-O-[6‴-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glucoside | [3,11,13] |
| Isoscutellarein 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | [3,11,13] |
| Isoscutellarein 7-O-[6‴-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glucoside | [13] |
| Isoscutellarein 7-O-allosyl(1→2)glucoside | [3,11,12,13] |
| Hypolaetin 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | [11,12,13,14] |
| Hypolaetin 7-O-allosyl-(1→2)-[6″-O-acetyl]glucoside | [3,11,12,14] |
| Apigenin 7-(4″-p-coumaroylglucoside) | [3,11,14] |
| Apigenin 7-(6″-p-coumaroylglucoside) | [3,11,14] |
| Apigenin 7-O-allosyl(1→2)glucoside | [11,12,14] |
| Apigenin 7-O-[6″-O-acetyl]-allosyl(1→2)glucoside | [3,11,12,13,14] |
| Luteolin 7-O-allosyl(1→2)glucoside | [13] |
| Luteolin 7-O-allosyl-(1→2)-[6″-O-acetyl]-glucoside | [11,12,14] |
| Luteolin 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | [11,12] |
| Luteolin 7-O-[6‴-O-acetyl]-allosyl-(1→2)-[6″-O-acetyl]-glucoside | [11,12,14] |
| Hypolaetin 7-O-allosyl(1→2)glucoside | [3,11,13] |
| Apigenin 7-O-glucoside | [3,11,14] |
| Chryseriol 7-O-[6‴-O-acetyl]-allosyl(1→2)glucoside | [3,11] |
| Phenolic acids | |
| 3-Caffeoylquinic acid | [13] |
| 5-Caffeoylquinic acid | [3,11,12,13,14] |
| 6-O-Caffeoyl-glucose | [14] |
| p-Coumaric acid 4-O-glucoside | [3,11] |
| Feruloylquinic acid | [3,11,12,13,14] |
| Polyphenols | Biological Activity | Ref. |
|---|---|---|
| Phenylethanoid glycosides | ||
| Lavandulifolioside | antiarrhythmic effect peroxylipid formation inhibitor anti-inflammatory activity | [25,26,27] |
| Verbascoside | antioxidant activity anti-inflammatory activity prevention red blood cell from free radical damage tyrosinase and/or melanin production inhibition activity | [14,28,29,30,31,32,33,34,35] |
| Forsythoside | anti-inflammatory activity antibacterial activity inhibitory of cAMP-phosphodiesterase in vitro 5-HETE formation inhibitor | [26,27] |
| antioxidant activity anti-inflammatory activity tyrosinase and/or melanin production inhibition activity | [14,27,29,35] | |
| Flavonoids | ||
| Apigenin | antioxidant activity anti-inflammatory effect cytotoxicity to cancer cells promoting apoptosis of cancer cells anxiolytic effect memory improvement neuroprotective effect, protective effect against amyloid-β-neurotoxicity | [9,36,37,38,39,40,41,42,43,44,45,46,47,48,49] [23,24,44,45,49] |
| Luteolin | antioxidant and anti-inflammatory activities cytotoxicity to cancer cells promoting apoptosis of cancer cells neuroprotective effect | [9,22,23,50,51,52,53,54,55] |
| Isoscutellarein | moderate to weak cytotoxicity to cancer cells | [56] |
| Hypolaetin | anti-inflammatory activity gastric protection (increase in mucus production) anti-ulcer activity | [57,58] |
| Phenolic acids | ||
| Caffeoylquinic acid | antioxidant activity reduced blood pressure neuroprotective effect | [59,60,61,62,63,64] |
| p-Coumaric acid 4-O-glucoside | antioxidant activity cytotoxicity to cancer cells promoting apoptosis of cancer cells neuroprotective effect cytotoxicity to cancer cells | [64,65,66,67,68,69,70] |
| Feruloylquinic acid | antioxidant activity hepatoprotective activity anti-proliferative activity | [35,71] |
| Biological Function | Extract | Ref. |
|---|---|---|
| Antioxidant activity | S. perfoliata air-dried aerial parts extract, S. clandestina aqueous extract | [29,73,74] |
| Anxiolytic, cognitive improving and neuroprotective properties | S. scardica extract (aqueous or ethanolic), S. euboea extract, S. clandestina infusion | [72,73,75,76] |
| Inhibition of lipid peroxidation | herbal tea from Sideritis | [73] |
| Anti-inflammatory activity | S. scardica ethanol, diethyl ether, ethyl acetate, and N-butanol extracts | [77,78] |
| Antimicrobial properties | S. scardica extract, S. ozturkii and S. caesarea methanolic extracts, essential oils from S. curvidens, S. lanata. S. clandestina, S. euboea, and S. romana | [77,78,79,80,81] |
| Gastroprotective effect | S. scardica ethanol, diethyl ether, ethyl acetate, and N-butanol extracts | [9] |
| Anti-obesity and antidiabetic properties | S. scardica extract, S. euboea aqueous extract | [6,82,83] |
| Chemopreventive activity | S. scardica diethyl ether extract | [41,42] |
| Polyphenol Class | Compounds | Phase I and II Possible Metabolites | Potential Microorganisms Involved | Microbial Biotransformation | Possible Gut Microbial Metabolites | Ref. |
|---|---|---|---|---|---|---|
| Flavonoids | Hypolaetin | Hypolaetin sulfate, glucuronide, diglucuronide, and glucuronide-sulfate | Human and mice fecal flora | Hydrolysis by gut microflora into their aglycones | Hypolaetin, isoscutellarein, and methylhypolaetin | [92,97] |
| Methylhypolaetin | Methylhypolaetin sulfate, glucuronide, and glucuronide + pentose | |||||
| Isoscutellarein | Isoscutellarein sulfate, glucuronide, and glucuronide-sulfate | |||||
| Methylisoscutellarein | Isoscutellarein disulfate, glucuronide, and diglucuronide | |||||
| Apigenin | Apigenin disulfate, glucuronide, diglucuronide, and glucuronide-sulfate | Human and rats fecal flora | Hydrolysis by gut microflora to simple phenolic acids | 3-(4-Hydroxyphenyl)propionic acid, 3-(3-hydroxyphenyl)propionic acid, 3-(3,4-dihydroxyphenyl)propionic acid, phenylacetic acid, 4-hydroxycinnamic acid, phloretin | [92,96,98,99,100] | |
| Luteolin | Luteolin glucuronide and sulfate, o-methyl luteolin (diosmetin or chrysoeryol) | Human and rats fecal flora | Hydrolysis by gut microflora to simple phenolic acids | 3-(3,4-dihydroxyphenyl)propionic acid, 3-(4-hydroxyphenyl)propionic acid and 4-hydroxycinnamic acid, phloretin, eriodictyol, and phloroglucinol | [92,99,101,102,103] | |
| Phenylethanoid glycosides | Verbascoside (acteoside) | Methyl acteoside, dimethyl acteoside, methyl acteosideglucuronide, dimethyl acteosideglucuronide, caffeic acid sulfate and glucuronide, methyl caffeic acid sulfate, hydroxytyrosolsulfate and glucuronide, homovanillic alcohol sulfate and glucuronide, homovanillin glucuronide, homovanillic acid, homovanillic acid sulfate and glucuronide, ferulic acid, ferulic acid glucuronide, and homoprotocatechuic acid | Human and rats fecal flora | Deglycosylation, de-rhamnose, de-HT, de-caffeoyl, deacetylation, reduction, acetylation, and sulfate conjugation | Caffeic acid,3-hydroxyphenylpropionic acid and hydroxytyrosol | [104,105,106,107,108,109] |
| Phenolic acids | Caffeoylquinic acid | Caffeoylquinic acid sulfate, disulfate, and glucuronide, caffeic acid glucuronide-sulfate, dimethylcaffeic acid glucuronide, quinic acid trisulfate, glucuronide, and glucuronide-sulfate | Human fecal flora | Deesterification, reduction of a double bond, dihydroxylation and futher β-oxidation by gut microflora to simple phenolic acids | Dihydrocaffeic acid, dihydro-isoferulic acid, 3-hydroxyphenylpropionic acid and benzoic acid | [92,105,106,107,108,110] |
| Feruloylquinic acid | Ferulic acid sulfate, glucuronide, and glucuronide-sulfate, feruloylquinic acid disulfate, glucuronide, and dimethylferuloylquinic acid glucuronide | Feruloylglycine, dihydroferulic acid, and 3-(4-hydroxyphenyl)-propionic acid, benzoic acid, 3-(4-hydroxyphenyl)propionic acid, vanillin | [92,110,111,112] | |||
| p-Coumaric acid 4-O-glucoside | Coumaric acid glucuronide | 3-Hydroxyphenylpropionic acid, benzoic acid, 3-(4-hydroxyphenyl)propionic acid, vanillin | [92,112,113] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules 2020, 25, 3763. https://doi.org/10.3390/molecules25163763
Żyżelewicz D, Kulbat-Warycha K, Oracz J, Żyżelewicz K. Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules. 2020; 25(16):3763. https://doi.org/10.3390/molecules25163763
Chicago/Turabian StyleŻyżelewicz, Dorota, Kamila Kulbat-Warycha, Joanna Oracz, and Kacper Żyżelewicz. 2020. "Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity" Molecules 25, no. 16: 3763. https://doi.org/10.3390/molecules25163763
APA StyleŻyżelewicz, D., Kulbat-Warycha, K., Oracz, J., & Żyżelewicz, K. (2020). Polyphenols and Other Bioactive Compounds of Sideritis Plants and Their Potential Biological Activity. Molecules, 25(16), 3763. https://doi.org/10.3390/molecules25163763