Metathesis Cyclopolymerization Triggered Self-Assembly of Azobenzene-Containing Nanostructure
Abstract
:1. Introduction
2. Results and Discussion
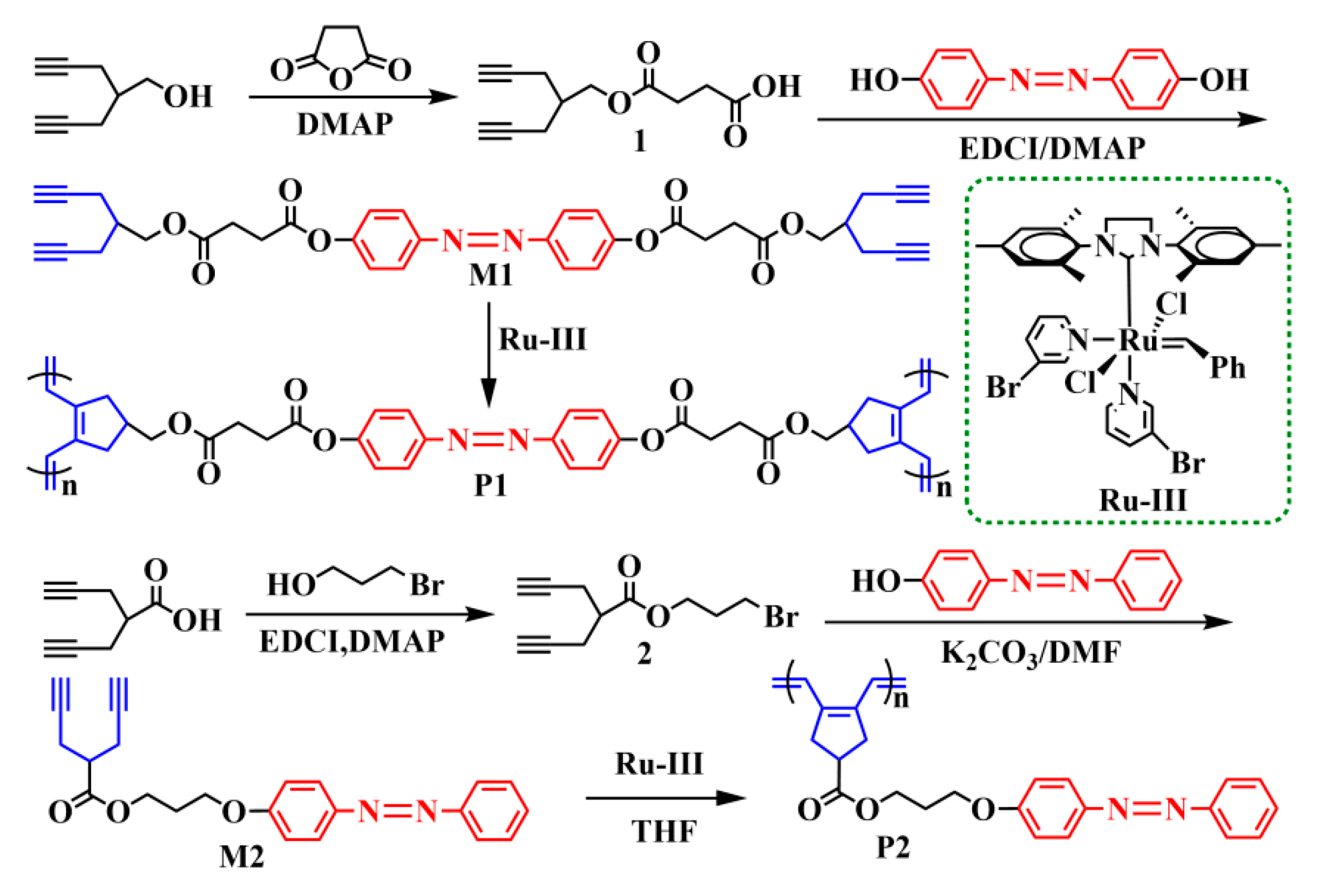
2.1. Synthesis and Characterization of AB-linked 1,6-Heptadiyne Monomers
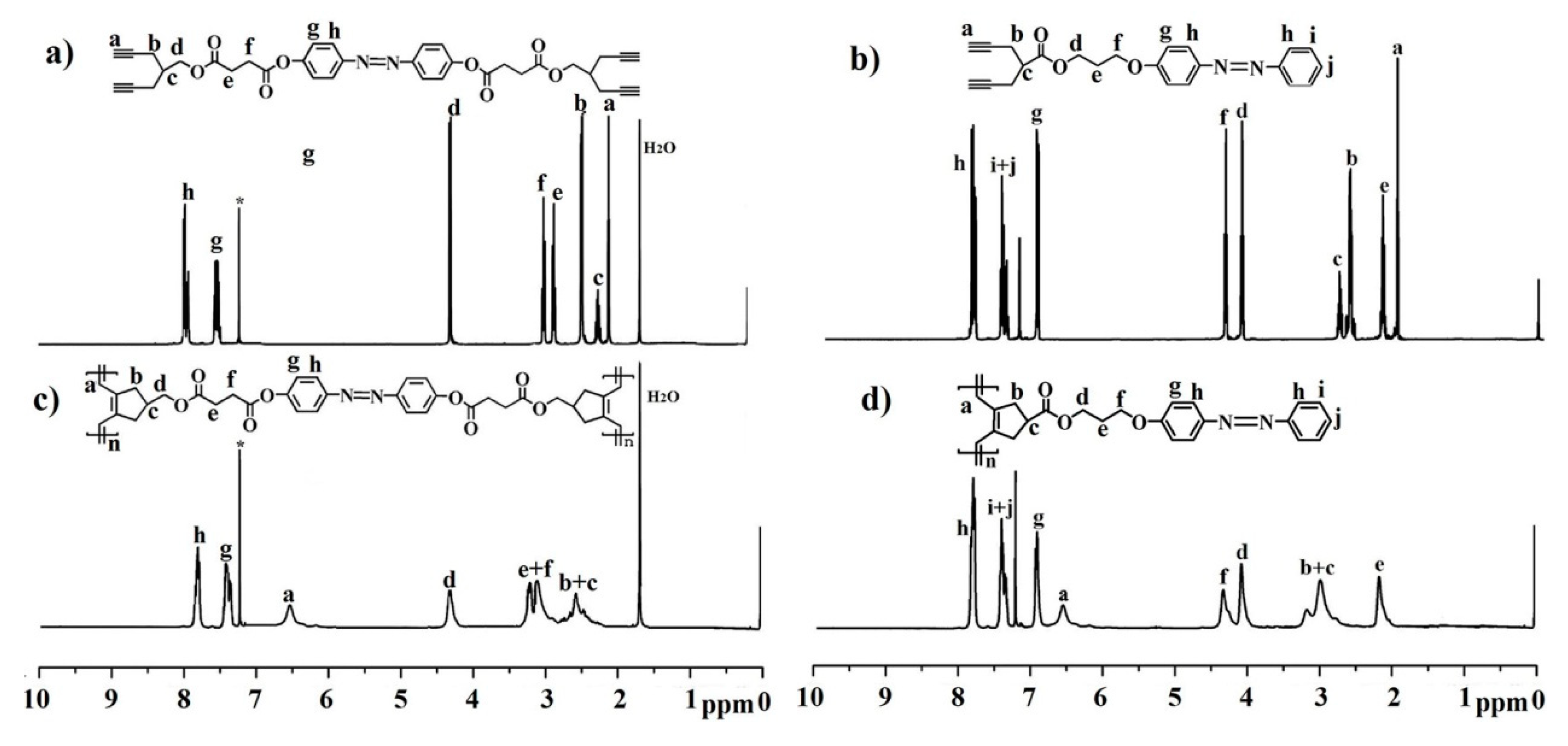
2.2. Synthesis and Characterization of AB-Linked Double- and Single-Stranded P1 and P2
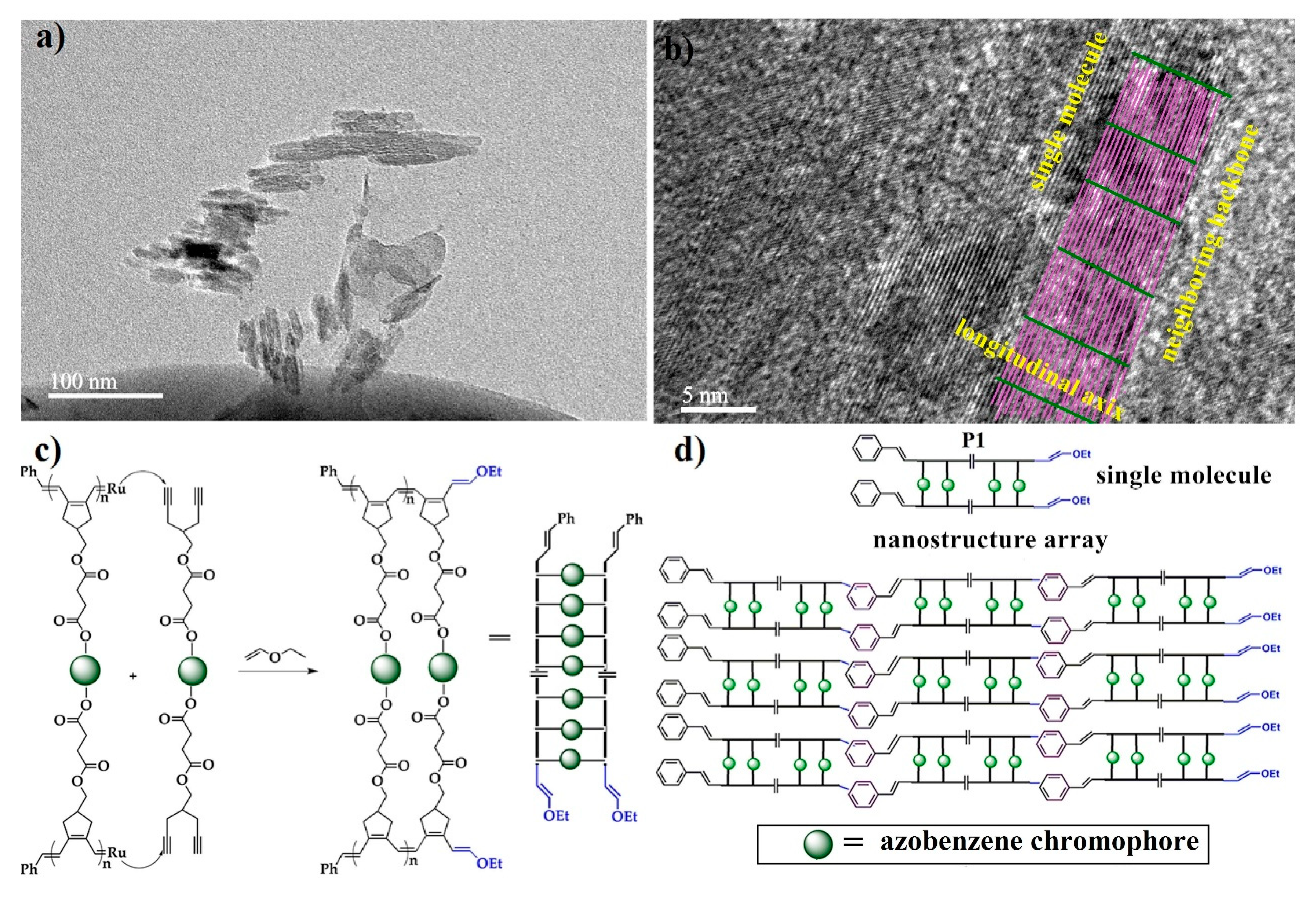
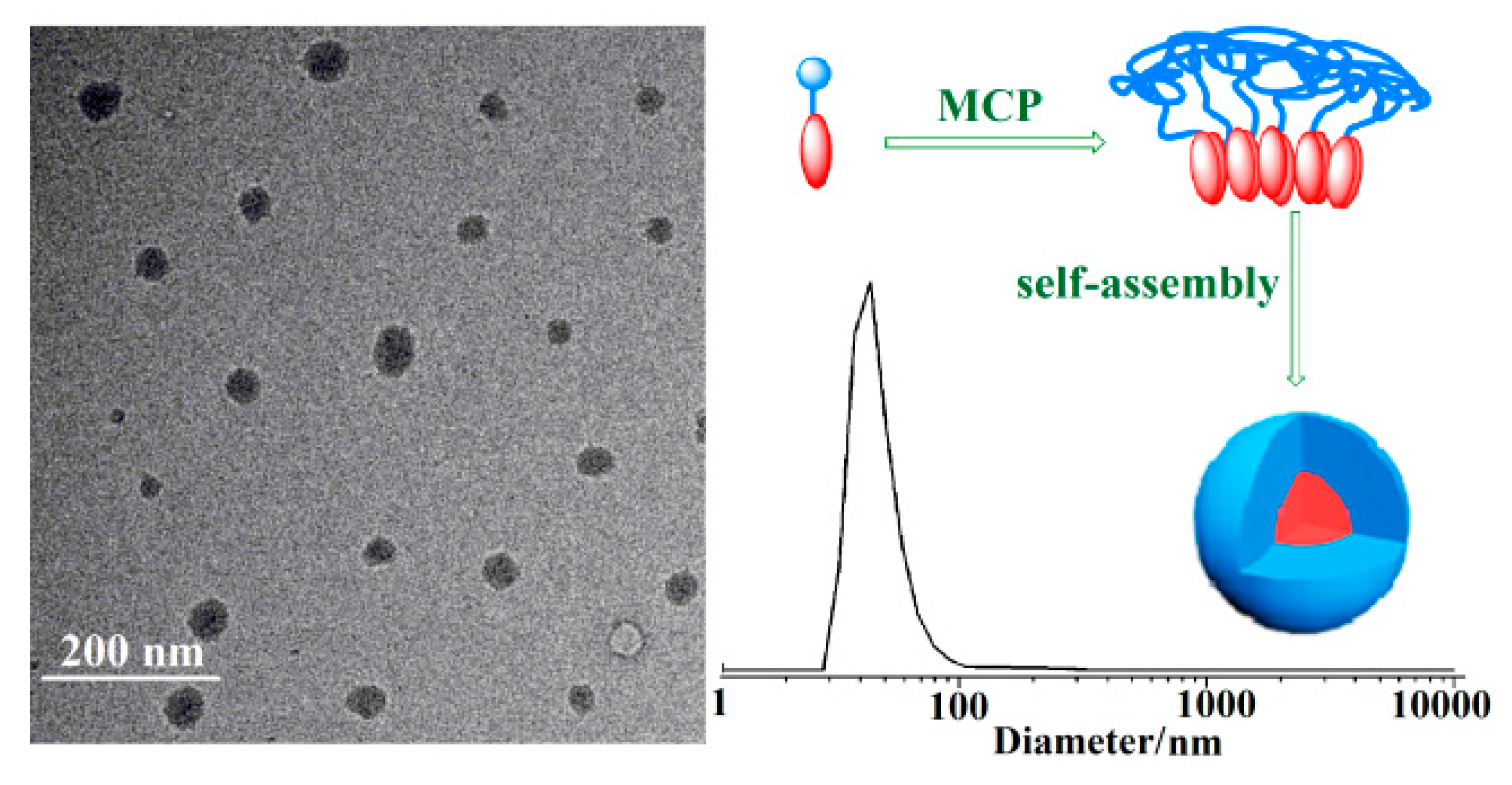
2.3. Morphology of Ordered Ladder Architecture
2.4. Photoisomerization Behaviors of P1 and P2
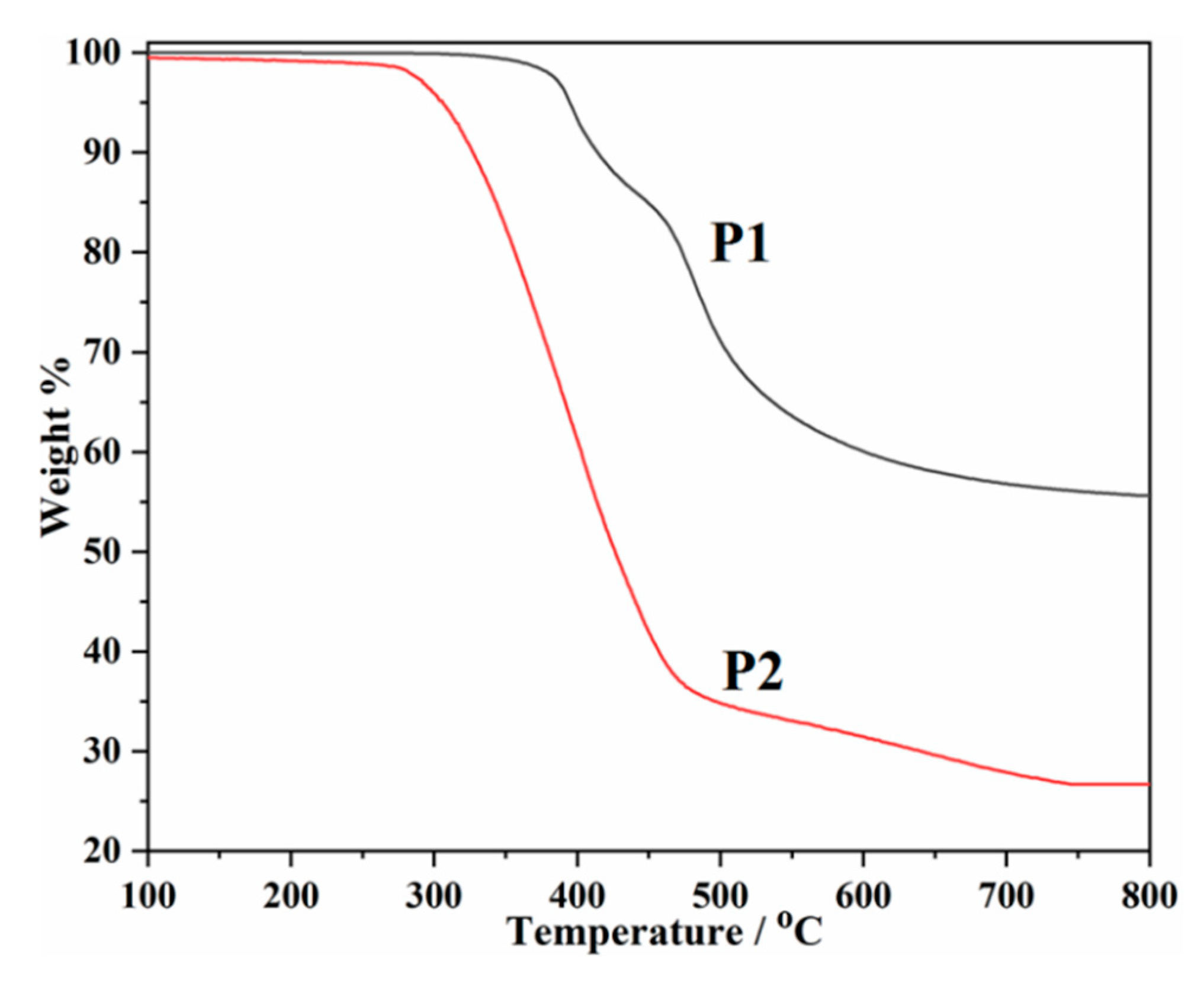
2.5. Thermal Stabilities of P1 and P2
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Compound 1
3.3. Synthesis of AB-Linked Bis(1,6-heptadiyne) Monomer M1
3.4. Synthesis of Compound 2
3.5. Synthesis of AB-Linked 1,6-Heptadiyne Monomer M2
3.6. General Procedure for Polymerization
3.6.1. Synthesis of AB-Linked Double-Stranded P1
3.6.2. Synthesis of AB-Linked Single-Stranded P2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warren, N.J.; Mykhaylyk, O.O.; Ryan, A.J.; Williams, M.; Doussineau, T.; Dugourd, P.; Antoine, R.; Portale, G.; Armes, S.P. Testing the Vesicular Morphology to Destruction: Birth and Death of Diblock Copolymer Vesicles Prepared via Polymerization-Induced Self-Assembly. J. Am. Chem. Soc. 2015, 137, 1929–1937. [Google Scholar] [CrossRef]
- Figg, C.A.; Carmean, R.N.; Bentz, K.C.; Mukherjee, S.; Savin, D.A.; Sumerlin, B.S. Tuning Hydrophobicity to Program Block Copolymer Assemblies from the Inside Out. Macromolecules 2017, 50, 935–943. [Google Scholar] [CrossRef]
- Chaduc, I.; Crepet, A.; Boyron, O.; Charleux, B.; D’Agosto, F.; Lansalot, M. Effect of the pH on the RAFT Polymerization of Acrylic Acid in Water. Application to the Synthesis of Poly(acrylic acid)—Stabilized Polystyrene Particles by RAFT Emulsion Polymerization. Macromolecules 2013, 46, 6013–6023. [Google Scholar] [CrossRef]
- Binauld, S.; Delafresnaye, L.; Charleux, B.; D’Agosto, F.; Lansalot, M. Emulsion Polymerization of Vinyl Acetate in the Presence of Different Hydrophilic Polymers Obtained by RAFT/MADIX. Macromolecules 2014, 47, 3461–3472. [Google Scholar] [CrossRef]
- Zhang, X.; Boisson, F.; Colombani, O.; Chassenieux, C.; Charleux, B. Synthesis of Amphiphilic Poly(acrylic acid)-b-poly(n-butyl acrylate-co-acrylic acid) Block Copolymers with Various Microstructures via RAFT Polymerization in Water/Ethanol Heterogeneous Media. Macromolecules 2013, 47, 51–60. [Google Scholar] [CrossRef]
- Wright, D.B.; Touve, M.A.; Adamiak, L.; Gianneschi, N.C. ROMPISA: Ring-Opening Metathesis Polymerization-Induced Self-Assembly. ACS Macro Lett. 2017, 6, 925–929. [Google Scholar] [CrossRef]
- Williams, M.; Penfold, N.J.W.; Lovett, J.R.; Warren, N.J.; Douglas, C.W.I.; Doroshenko, N.; Verstraete, P.; Smets, J.; Armes, S.P. Bespoke cationic nano-objects via RAFT aqueous dispersion polymerisation. Polym. Chem. 2016, 7, 3864–3873. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Sun, H.; Yu, M.; Sumerlin, B.S.; Zhang, L. Photo-PISA: Shedding Light on Polymerization-Induced Self-Assembly. ACS Macro Lett. 2015, 4, 1249–1253. [Google Scholar] [CrossRef]
- Blanazs, A.; Ryan, A.J.; Armes, S.P. Predictive Phase Diagrams for RAFT Aqueous Dispersion Polymerization: Effect of Block Copolymer Composition, Molecular Weight, and Copolymer Concentration. Macromolecules 2012, 45, 5099–5107. [Google Scholar] [CrossRef]
- Song, W.; Han, H.; Wu, J.; Xie, M. Ladder-like polyacetylene with excellent optoelectronic properties and regular architecture. Chem. Commun. 2014, 50, 12899–12902. [Google Scholar] [CrossRef]
- Kang, E.H.; Lee, I.S.; Choi, T.L. Ultrafast Cyclopolymerization for Polyene Synthesis: Living Polymerization to Dendronized Polymers. J. Am. Chem. Soc. 2011, 133, 11904–11907. [Google Scholar] [CrossRef]
- Kang, E.H.; Lee, I.H.; Choi, T.L. Brush Polymers Containing Semiconducting Polyene Backbones: Graft-Through Synthesis via Cyclopolymerization and Conformational Analysis on the Coil-to-Rod Transition. ACS Macro Lett. 2012, 1, 1098–1102. [Google Scholar] [CrossRef]
- Lee, I.S.; Kang, E.H.; Park, H.; Choi, T.-L. Controlled cyclopolymerisation of 1,7-octadiyne derivatives using Grubbs catalyst. Chem. Sci. 2012, 3, 761–765. [Google Scholar] [CrossRef]
- Kim, J.; Kang, E.H.; Choi, T.L. Cyclopolymerization To Synthesize Conjugated Polymers Containing Meldrum’s Acid as a Precursor for Ketene Functionality. ACS Macro Lett. 2012, 1, 1090–1093. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Liao, X.; Han, H.; Li, Y.; Xie, M. Tandem Metathesis Polymerization-Induced Self-Assembly to Nanostructured Block Copolymer and the Controlled Triazolinedione Modification for Enhancing Dielectric Properties. Macromolecules 2018, 51, 10202–10213. [Google Scholar] [CrossRef]
- Guo, M.; Sun, R.; Han, H.; Wu, J.; Xie, M.; Liao, X. Metathesis Cyclopolymerization of 1.6-Heptadiyne Derivative toward Triphenylamine-Functionalized Polyacetylene with Excellent Optoelectronic Properties and Nanocylinder Morphology. Macromolecules 2015, 48, 2378–2387. [Google Scholar] [CrossRef]
- Ling, W.; Cheng, X.; Miao, T.; Zhang, S.; Zhang, W.; Zhu, X. Synthesis and Photocontrolled Supramolecular Self-Assembly of Azobenzene-Functionalized Perylene Bisimide Derivatives. Polymers 2019, 11, 1143. [Google Scholar] [CrossRef] [Green Version]
- Lambeth, R.H.; Moore, J.S. Light-Induced Shape Changes in Azobenzene Functionalized Polymers Prepared by Ring-Opening Metathesis Polymerization. Macromolecules 2007, 40, 1838–1842. [Google Scholar] [CrossRef]
- Haque, H.A.; Kakehi, S.; Hara, M.; Nagano, S.; Seki, T. High-Density Liquid-Crystalline Azobenzene Polymer Brush Attained by Surface-Initiated Ring-Opening Metathesis Polymerization. Langmuir 2013, 29, 7571–7575. [Google Scholar] [CrossRef]
- Fu, L.; Yang, J.; Dong, L.; Yu, H.; Yan, Q.; Zhao, F.; Zhai, F.; Xu, Y.; Dang, Y.; Hu, W.; et al. Solar Thermal Storage and Room-Temperature Fast Release Using a Uniform Flexible Azobenzene-Grafted Polynorborene Film Enhanced by Stretching. Macromolecules 2019, 52, 4222–4231. [Google Scholar] [CrossRef]
- Wang, D.; Ye, G.; Zhu, Y.; Wang, X. Photoinduced Mass-Migration Behavior of Two Amphiphilic Side-Chain Azo Diblock Copolymers with Different Length Flexible Spacers. Macromolecules 2009, 42, 2651–2657. [Google Scholar] [CrossRef]
- Zhou, X.; Du, Y.; Wang, X. Azo Polymer Janus Particles and Their Photoinduced, Symmetry-Breaking Deformation. ACS Macro Lett. 2016, 5, 234–237. [Google Scholar] [CrossRef]
- Xu, B.; Qian, H.; Lin, S. Self-Assembly and Photoinduced Spindle-Toroid Morphology Transition of Macromolecular Double-Brushes with Azobenzene Pendants. ACS Macro Lett. 2020, 9, 404–409. [Google Scholar] [CrossRef]
- Guan, S.; Deng, Z.; Huang, T.; Wen, W.; Zhao, Y.; Chen, A. Light-Triggered Reversible Slimming of Azobenzene-Containing Wormlike Nanoparticles Synthesized by Polymerization-Induced Self-Assembly for Nanofiltration Switches. ACS Macro Lett. 2019, 8, 460–465. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Jin, H.; Jiang, B.; Zhu, X.; Zhou, Y.; Lu, Z.; Yan, D. A Supramolecular Janus Hyperbranched Polymer and Its Photoresponsive Self-Assembly of Vesicles with Narrow Size Distribution. J. Am. Chem. Soc. 2013, 135, 4765–4770. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Crespi, S.; Simeth, N.A.; König, B. Heteroaryl azo dyes as molecular photoswitches. Nat. Rev. Chem. 2019, 3, 133–146. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, H.; Li, Z.; Zhang, Y.; Zhang, Y.; Zhang, H. Synthesis of Reactive Azobenzene Main-Chain Liquid Crystalline Polymers via Michael Addition Polymerization and Photomechanical Effects of Their Supramolecular Hydrogen-Bonded Fibers. Macromolecules 2013, 46, 7650–7660. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, L.; Han, H.; Huang, W.; Song, C.; Xie, M.; Zhang, Y. Hyperbranched Azo-Polymers Synthesized by Acyclic Diene Metathesis Polymerization of an AB2Monomer. Macromolecules 2009, 42, 5036–5042. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.; Cang, H.; Li, J.; Wang, C.; Song, W. Controlled synthesis of azobenzene-containing block copolymers both in the main- and side-chain from SET-LRP polymers via ADMET polymerization. Polymers 2020, 190, 122229–122238. [Google Scholar] [CrossRef]
- Ding, L.; Li, J.; Jiang, R.; Song, W. Photoresponsive Polymeric Reversible Nanoparticles via Self-Assembly of Reactive ABA Triblock Copolymers and Their Transformation to Permanent Nanostructures. Materials 2016, 9, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luh, T.-Y. Ladderphanes: A New Type of Duplex Polymers. Acc. Chem. Res. 2012, 46, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.T.; Lin, S.Y.; Lee, S.L.; Chen, C.H.; Hsu, C.H.; Hwang, L.P.; Xie, Z.Y.; Chen, C.H.; Huang, S.L.; Luh, T.Y. From Polynorbornene to the Complementary Polynorbornene by Replication. Angew. Chem. Int. Ed. 2007, 46, 4481–4485. [Google Scholar] [CrossRef]
- Lin, C.L.; Yang, H.C.; Lin, N.T.; Hsu, I.J.; Wang, Y.; Luh, T.Y. Electrochemical oxidation of double-stranded polybisnorbornenes containing linearly aligned ferrocene linkers. Chem. Commun. 2008, 37, 4484. [Google Scholar] [CrossRef]
- Chou, C.M.; Lee, S.L.; Chen, C.H.; Biju, A.T.; Wang, H.W.; Wu, Y.L.; Zhang, G.F.; Yang, K.W.; Lim, T.S.; Huang, M.J.; et al. Polymeric Ladderphanes. J. Am. Chem. Soc. 2009, 131, 12579–12585. [Google Scholar] [CrossRef]
- Yang, K.W.; Xu, J.; Chen, C.H.; Huang, H.H.; Yu, T.J.Y.; Lim, T.S.; Chen, C.H.; Luh, T.Y. Triple-Stranded Polymeric Ladderphanes. Macromolecules 2010, 43, 5188–5194. [Google Scholar] [CrossRef]
- Zhu, L.; Flook, M.M.; Lee, S.L.; Chan, L.W.; Huang, S.L.; Chiu, C.W.; Chen, C.H.; Schrock, R.R.; Luh, T.Y. Cis, Isotactic Selective ROMP of Norbornenes Fused with N-Arylpyrrolidines. Double Stranded Polynorbornene-Based Ladderphanes with Z-Double Bonds. Macromolecules 2012, 45, 8166–8171. [Google Scholar] [CrossRef]
- Carney, J.M.; Donoghue, P.J.; Wuest, W.M.; Wiest, O.; Helquist, P. Intramolecular Hydroamination of Aminoalkynes with Silver−Phenanthroline Catalysts. Org. Lett. 2008, 10, 3903–3906. [Google Scholar] [CrossRef]
- Filipová, L.; Kohagen, M.; Štacko, P.; Muchová, E.; Slavíček, P.; Klán, P. Photoswitching of Azobenzene-Based Reverse Micelles above and at Subzero Temperatures As Studied by NMR and Molecular Dynamics Simulations. Langmuir 2017, 33, 2306–2317. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Run | Polymer | (M)/(I) | Mn a (kDa) | PDI a | DP b | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | P1 | 10:1 | 6.7 | 1.0 | 10 | 100 |
| 2 | P1 | 20:1 | 12.3 | 11.2 | 20 | 100 |
| 3 | P1 | 30:1 | 18.1 | 11.3 | 29 | 92 |
| 4 | P1 | 40:1 | 23.7 | 11.4 | 38 | 84 |
| 5 | P1 c | 50:1 | 26.1 | 11.4 | 42 | 79 |
| 6 | P2 | 25:1 | 9.4 | 11.2 | 26 | 100 |
| 7 | P2 | 50:1 | 18.7 | 11.3 | 52 | 98 |
| 8 | P2 | 75:1 | 28.1 | 11.4 | 60 | 80 |
| 9 | P2 | 100:1 | 37.4 | 11.4 | 78 | 65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Shen, J.; Li, X.; Huang, J.; Ding, L.; Wu, J. Metathesis Cyclopolymerization Triggered Self-Assembly of Azobenzene-Containing Nanostructure. Molecules 2020, 25, 3767. https://doi.org/10.3390/molecules25173767
Song W, Shen J, Li X, Huang J, Ding L, Wu J. Metathesis Cyclopolymerization Triggered Self-Assembly of Azobenzene-Containing Nanostructure. Molecules. 2020; 25(17):3767. https://doi.org/10.3390/molecules25173767
Chicago/Turabian StyleSong, Wei, Jiamin Shen, Xiang Li, Jinhui Huang, Liang Ding, and Jianhua Wu. 2020. "Metathesis Cyclopolymerization Triggered Self-Assembly of Azobenzene-Containing Nanostructure" Molecules 25, no. 17: 3767. https://doi.org/10.3390/molecules25173767





