Synthesis, Structure and Supramolecular Properties of a Novel C3 Cryptand with Pyridine Units in the Bridges
Abstract
1. Introduction
2. Results and Discussion
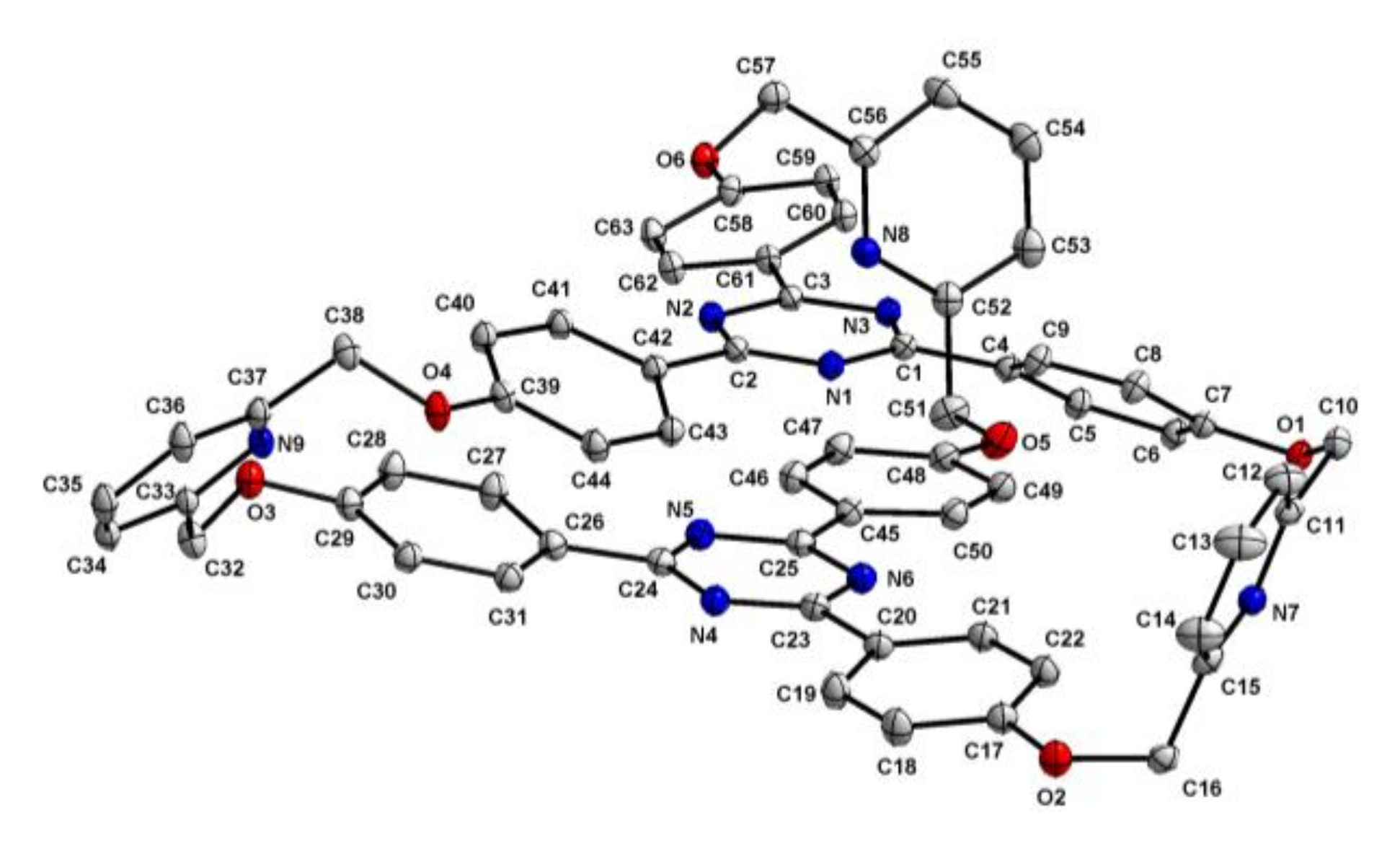
2.1. Synthesis and Structure of Cryptand 4
2.2. Complexation Abilities of Cryptand 4
3. Materials and Methods
3.1. General Data
3.2. Procedure for the Synthesis of Cryptand 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; Wiley & Sons: New York, USA, 2009; pp. 23–24. [Google Scholar]
- Crisan, C.; Terec, A.; Hădade, N.D.; Grosu, I. Cryptands with 2,4,6-tris(p-phenylene)-1,3,5-triazine central units and oligoethyleneoxide bridges: Synthesis, structure and complexation abilities. Tetrahedron 2015, 71, 6888–6893. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, M.; Zhao, Y.; Yang, Z.; Jiang, J.; Wang, L.; Pan, Y. Redox-switchable host–guest systems based on a bisthiotetrathiafulvalene-bridged cryptand. Chem. Commun. 2014, 50, 15585–15588. [Google Scholar] [CrossRef]
- Delecluse, M.; Colomban, C.; Chatelet, B.; Chevallier-Michaud, S.; Moraleda, D.; Dutasta, J.-P.; Martinez, A. Highly Selective Fluoride Recognition by a Small Tris-Urea Covalent Cage. J. Org. Chem. 2020, 85, 4706–4711. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, W.-J.; Liu, Y.A.; Zhao, X.-L.; Li, J.-S.; Jiang, B.; Wen, K. A Shape-Persistent Cryptand for Capturing Polycyclic Aromatic Hydrocarbons. J. Org. Chem. 2016, 81, 5649–5654. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, X.; Huang, F.; Niu, Z.; Gibson, H.W. Stimuli-Responsive Host–Guest Systems Based on the Recognition of Cryptands by Organic Guests. Acc. Chem. Res. 2014, 47, 1995–2005. [Google Scholar] [CrossRef]
- Lar, C.; Woiczechowski-Pop, A.; Bende, A.; Grosu, I.G.; Miklášová, N.; Bogdan, E.; Hădade, N.D.; Terec, A.; Grosu, I. A three-armed cryptand with triazine and pyridine units: Synthesis, structure and complexation with polycyclic aromatic compounds. Beilstein J. Org. Chem. 2018, 14, 1370–1377. [Google Scholar] [CrossRef]
- Woiczechowski-Pop, A.; Gligor, D.; Bende, A.; Varodi, C.; Bogdan, E.; Terec, A.; Grosu, I. Synthesis, structure, electrochemical behaviour and electrochemical investigations on the assembling with pyrene of a novel C 3 cryptand. Supramol. Chem. 2015, 27, 52–58. [Google Scholar] [CrossRef]
- Dale, E.J.; Vermeulen, N.A.; Thomas, A.A.; Barnes, J.C.; Jurίček, M.; Blackburn, A.K.; Strutt, N.L.; Sarjeant, A.A.; Stern, C.L.; Denmark, S.E.; et al. ExCage. J. Am. Chem. Soc. 2014, 136, 10669–10682. [Google Scholar] [CrossRef]
- Mastalerz, M. Shape-Persistent Organic Cage Compounds by Dynamic Covalent Bond Formation. Angew. Chem. Int. Ed. 2010, 49, 5042–5053. [Google Scholar] [CrossRef]
- Sambasivan, S.; Kim, S.-G.; Choi, S.M.; Rhee, Y.M.; Ahn, K.H. C3-Symmetric Cage-like Receptors: Chiral Discrimination of α-Chiral Amines in a Confined Space. Org. Lett. 2010, 12, 4228–4231. [Google Scholar] [CrossRef]
- Gomez-Lor, B.; Hennrich, G.; Alonso, B.; Monge, A.; Gutierrez-Puebla, E.; Echavarren, A.M. A Redox-Active C3-Symmetric Triindole-Based Triazacyclophane. Angew. Chem. Int. Ed. 2006, 45, 4491–4494. [Google Scholar] [CrossRef]
- Greenaway, R.L.; Santolini, V.; Szczypiński, F.T.; Bennison, M.J.; Little, M.A.; Marsh, A.; Jelfs, K.E.; Cooper, A.I. Organic Cage Dumbbells. Chem. Eur. J. 2020, 26, 3718–3722. [Google Scholar] [CrossRef]
- Chakraborty, S.; Saha, S.; Lima, L.M.P.; Warzok, U.; Sarkar, S.; Akhuli, B.; Nandi, M.; Bej, S.; Adarsh, N.N.; Schalley, C.A.; et al. Polyamide–Polyamine Cryptand as Dicarboxylate Receptor: Dianion Binding Studies in the Solid State, in Solution, and in the Gas Phase. J. Org. Chem. 2017, 82, 10007–10014. [Google Scholar] [CrossRef]
- Naseer, M.M.; Wang, D.-X.; Zhao, L.; Huang, Z.-T.; Wang, M.-X. Synthesis and Functionalization of Heteroatom-Bridged Bicyclocalixaromatics, Large Molecular Triangular Prisms with Electron-Rich and -Deficient Aromatic Interiors. J. Org. Chem. 2011, 76, 1804–1813. [Google Scholar] [CrossRef]
- Morales-Sanfrutos, J.; Ortega-Muñoz, M.; Lopez-Jaramillo, J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F.; Lopez-Jaramillo, F.J. Synthesis of Molecular Nanocages by Click Chemistry. J. Org. Chem. 2008, 73, 7772–7774. [Google Scholar] [CrossRef]
- Pop, F.; Socaci, C.; Terec, A.; Condamine, E.; Varga, R.A.; Raţ, C.I.; Roncali, J.; Grosu, I. Cryptands and bismacrocycles with cyanuric and isocyanuric units: Synthesis and structural investigations. Tetrahedron 2012, 68, 8581–8588. [Google Scholar] [CrossRef]
- Ballester, P. Anion binding in covalent and self-assembled molecular capsules. Chem. Soc. Rev. 2010, 39, 3810–3830. [Google Scholar] [CrossRef]
- Kang, S.O.; Llinares, J.M.; Day, V.W.; Bowman-James, K. Cryptand-like anion receptors. Chem. Soc. Rev. 2010, 39, 3980–4003. [Google Scholar] [CrossRef]
- Stollenz, M.; Barbasiewicz, M.; Nawara-Hultzsch, A.J.; Fiedler, T.; Laddusaw, R.M.; Bhuvanesh, N.; Gladysz, J.A. Dibridge head Diphosphines that Turn Themselves Inside Out. Angew. Chem. Int. Ed. 2011, 50, 6647–6651. [Google Scholar] [CrossRef]
- Bauer, I.; Habicher, W.D. In, out-Isomerism of Phosphorus Bridgehead Cage Compounds. A Review. Collect. Czech. Chem. Commun. 2004, 69, 1195–1230. [Google Scholar] [CrossRef]
- Brotin, T.; Dutasta, J.-P. Cryptophanes and Their Complexes—Present and Future. Chem. Rev. 2009, 109, 88–130. [Google Scholar] [CrossRef] [PubMed]
- Holman, K.T.; Drake, S.D.; Steed, J.W.; Orr, G.W.; Atwood, J.L. Anion binding, aryl-extended cyclotriguaiacylenes and an aryl-bridged cryptophane that provides snapshots of a molecular gating mechanism. Supramol. Chem. 2010, 22, 870–890. [Google Scholar] [CrossRef]
- Li, M.-J.; Lai, C.-C.; Liu, Y.-H.; Peng, S.-M.; Chiu, S.-H. Two guest complexation modes in a cyclotriveratrylene-based molecular container. Chem. Commun. 2009, 39, 5814–5816. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Wang, H.; Jie, K.; Huang, F. Taco complex-templated highly regio- and stereo-selective photodimerization of a coumarin-containing crown ether. Chem. Commun. 2017, 53, 1688–1691. [Google Scholar] [CrossRef]
- Cheng, M.; Yao, C.; Cao, Y.; Wang, Q.; Pan, Y.; Jiang, J.; Wang, L. 4-Methylcoumarin-bridged fluorescent responsive cryptand: From [2+2] photodimerization to supramolecular polymer. Chem. Commun. 2016, 52, 8715–8718. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, X.; Liang, J.; Zhang, S.; Zhou, S.; Chen, M.; Tang, M.; Jiang, L. Efficient Syntheses of Novel Cryptands Based on Bis(m-phenylene)-26-crown-8 and Their Complexation with Paraquat. Eur. J. Org. Chem. 2010, 1904–1911. [Google Scholar] [CrossRef]
- Pascanu, V.; Cîrcu, M.; Socaci, C.; Terec, A.; Soran, A.; Grosu, I. Synthesis of cryptands with di-yne units via acetylenic homocoupling reactions of C3 tripodands. Tetrahedron Lett. 2013, 54, 6133–6136. [Google Scholar] [CrossRef]
- Otte, M.; Lutz, M.; Gebbink, R.J.M.K. Selective Synthesis of Hetero-Sequenced Aza-Cyclophanes. Eur. J. Org. Chem. 2017, 1657–1661. [Google Scholar] [CrossRef]
- Pederson, A.M.-P.; Price, T.L.; Slebodnick, C.; Schoonover, D.V.; Gibson, H.W. The Long and the Short of It: Regiospecific Syntheses of Isomers of Dicarbomethoxydibenzo-27-crown-9 and Binding Abilities of Their Pyridyl Cryptands. J. Org. Chem. 2017, 82, 8489–8496. [Google Scholar] [CrossRef]
- Price, T.L.; Wessels, H.R.; Slebodnick, C.; Gibson, H.W. High-Yielding Syntheses of Crown Ether-Based Pyridyl Cryptands. J. Org. Chem. 2017, 82, 8117–8122. [Google Scholar] [CrossRef]
- Kocher, L.; Durot, S.; Heitz, V. Control of the cavity size of flexible covalent cages by silver coordination to the peripheral binding sites. Chem. Commun. 2015, 51, 13181–13184. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.-T.; Zhao, X.; Jiang, X.-K.; Li, Z.-T. Hydrogen Bonding-Directed Quantitative Self-Assembly of Cyclotriveratrylene Capsules and Their Encapsulation of C60 and C70. J. Org. Chem. 2011, 76, 3531–3535. [Google Scholar] [CrossRef] [PubMed]
- Cîrcu, M.; Soran, A.; Hadade, N.D.; Rednic, M.; Terec, A.; Grosu, I. Cryptands with 1,3,5-Tris(1′,3′-dioxan-2′-yl)-benzene Units: Synthesis and Structural Investigations. J. Org. Chem. 2013, 78, 8722–8729. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Natarajan, R. Cofacial Organic Click Cage to Intercalate Polycyclic Aromatic Hydrocarbons. Org. Lett. 2016, 18, 3394–3397. [Google Scholar] [CrossRef] [PubMed]
- Prokhorov, A.M.; Kozhevnikov, D.N. Triazines, Tetrazines, and Fused Ring Polyaza Systems. Prog. Heterocycl. Chem. 2012, 24, 421–441. [Google Scholar]
- Soler-Illia, G.J.A.A.; Azzaroni, O. Multifunctional hybrids by combining ordered mesoporous materials and macromolecular building blocks. Chem. Soc. Rev. 2011, 40, 1107–1150. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Maguire, J.A.; Hosmane, N.S. Facile Synthetic Routes to Phenylene and Triazine Core Based Dendritic Cobaltabisdicarbollides. Organometallics 2010, 29, 5230–5235. [Google Scholar] [CrossRef]
- Woiczechowski-Pop, A.; Dobra, I.L.; Roiban, G.D.; Terec, A.; Grosu, I. Synthesis and Structural Analysis of Some Podands with C3 Symmetry. Synth. Commun. 2012, 42, 3579–3588. [Google Scholar] [CrossRef]
- Cismaş, C.; Grosu, I.; Piron, F.; Oprea, C.; Terec, A.; Roncali, J. Synthesis of Podands with Cyanurate or Isocyanurate Cores and Terminal Triple Bonds. Synthesis 2010, 2010, 1639–1644. [Google Scholar] [CrossRef]
- Bogdan, E.; Hădade, N.D.; Terec, A.; Grosu, I. The 1,3-Dioxane Motif—A Useful Tool in Monitoring Molecular and Supramolecular Architectures. Tetrahedron Lett. 2016, 57, 2683–2691. [Google Scholar] [CrossRef]
- Medruţ, I.; Turdean, R.; Gropeanu, R.; Pop, F.; Toupet, L.; Hădade, N.D.; Bogdan, E.; Grosu, I. Macrocycles with a phenothiazine core: Synthesis, structural analysis, and electronic properties. Tetrahedron Lett. 2013, 54, 1107–1111. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999; Volume 9, pp. 1–480. ISBN 0-19-850252-4. [Google Scholar]
- Hunter, C.A.; Sanders, J.K.M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Uchimaru, T.; Mikami, M. Intermolecular Interaction between Hexafluorobenzene and Benzene: Ab Initio Calculations Including CCSD(T) Level Electron Correlation Correction. J. Phys. Chem. A 2006, 110, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Bruker 2012: APEX3; Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Boil. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
- DIAMOND—Crystal and Molecular Structure Visualization; Crystal Impact; Kreuzherrenstr: Bonn, Germany, 2018.
- Chai, J.-D.; Head-Gordon, M. Systematic optimization of long-range corrected hybrid density functionals. J. Chem. Phys. 2008, 128, 84106. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |













  | ||||
|---|---|---|---|---|
| C−H····X | d1 (Å) | γ (°) | D (Å) | |
| C10−H10A····O5a | 2.83(1) | 160.7(1) | 3.78(1) | |
| C38−H38A····O3b | 2.45(1) | 154.0(1) | 3.36(1) | |
| C47−H47····O6c | 2.80(1) | 133,0(1) | 3,51(1) | |
| C6−H6····N7d | 2.45(1) | 155,2(1) | 3.33(1) | |
| C35−H35····N3e | 2.68(1) | 165.2(1) | 3.60(1) | |
| C−H····π interactions | ||||
| C−H····plane | lateral shift, d0 (Å) | Dpln (Å) | α (°) | βpln(°) |
| C16−H16B····C(33-37)N9f | 0.90 | 2.58 | 18.3(1) | - |
| C41−H41····C(33-37)N9b | 0.25 | 2.88 | 41.2(1) | 69.3(1) |
| C49−H49····C(11-15)N7d | 0.37 | 2.99 | 28.5(1) | 72.9(1) |
| π····π interactions | ||||
| plane····plane | - | d (Å) | - | βpln(°) |
| C(1-3)N(1-3)····C(58-63)g | 3.60(1) | 16.8(1) | ||
| Compound | 4 |
|---|---|
| Empirical formula | C63H45N9O6 |
| Formula weight | 1024.08 |
| Crystal size (mm) | 0.147 × 0.136 × 0.126 |
| Crystal habit | colorless-yellow block |
| Wavelength (Å) | 0.71073 |
| Temperature (K) | 103.(2) |
| Crystal system | Triclinic |
| Space group | P-1 |
| a (Å) | 11.4981(6) |
| b (Å) | 15.6040(9) |
| c (Å) | 17.0964(9) |
| α (°) | 108.841(2) |
| β (°) | 105.346(2) |
| γ (°) | 91.215(2) |
| Volume(Å3) | 2780.3(3) |
| Z | 2 |
| Density (calculated) (g cm−1) | 1.223 |
| Absorption coefficient(mm−1) | 0.081 |
| F(000) | 1068 |
| θ range for data collection (°) | 2.18–28.26 |
| Tmax/Tmin | 0.99/0.97 |
| Reflections collected | 188,523 |
| Independent reflections, Rint | 13,743; 0.0292 |
| Completeness to θ = 28.26° | 99.8 % |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 13,743/0/706 |
| Goodness-of-fit on F2 | 1.053 |
| Final R indices [I > 2σ(I)] | R1 = 0.0351 |
| wR2= 0.0822 | |
| R indices (all data) | R1 = 0.0382 |
| wR2 = 0.0845 | |
| Largest diff. peak and hole, eA−3 | 0.326, −0.189 |
| CCDC No. | 2019839 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crişan, C.V.; Soran, A.; Bende, A.; Hӑdade, N.D.; Terec, A.; Grosu, I. Synthesis, Structure and Supramolecular Properties of a Novel C3 Cryptand with Pyridine Units in the Bridges. Molecules 2020, 25, 3789. https://doi.org/10.3390/molecules25173789
Crişan CV, Soran A, Bende A, Hӑdade ND, Terec A, Grosu I. Synthesis, Structure and Supramolecular Properties of a Novel C3 Cryptand with Pyridine Units in the Bridges. Molecules. 2020; 25(17):3789. https://doi.org/10.3390/molecules25173789
Chicago/Turabian StyleCrişan, Cosmin Vasile, Albert Soran, Attila Bende, Niculina Daniela Hӑdade, Anamaria Terec, and Ion Grosu. 2020. "Synthesis, Structure and Supramolecular Properties of a Novel C3 Cryptand with Pyridine Units in the Bridges" Molecules 25, no. 17: 3789. https://doi.org/10.3390/molecules25173789
APA StyleCrişan, C. V., Soran, A., Bende, A., Hӑdade, N. D., Terec, A., & Grosu, I. (2020). Synthesis, Structure and Supramolecular Properties of a Novel C3 Cryptand with Pyridine Units in the Bridges. Molecules, 25(17), 3789. https://doi.org/10.3390/molecules25173789






