Abstract
The two-step acidic hydrolysis of α-hydroxybenzylphosphonates and a few related derivatives was monitored in order to determine the kinetics and to map the reactivity of the differently substituted phosphonates in hydrolysis. Electron-withdrawing substituents increased the rate, while electron-releasing ones slowed down the reaction. Both hydrolysis steps were characterized by pseudo-first-order rate constants. The fission of the second P-O-C bond was found to be the rate-determining step.
1. Introduction
The hydrolysis of P-esters (e.g., phosphinates and phosphonates) resulting in the formation of the corresponding acids (phosphinic acids and phosphonic acids, respectively) is an important chemical transformation, and hence it is applied widely in syntheses. Most often, the hydrolyses were performed under acidic conditions [1,2,3,4], but the application of NaOH or KOH is also common [5,6,7,8]. An additional possibility is the fission of the P-O-C unit by the effect of Me3SiBr [9,10,11]. Usually, the acid- or base-catalyzed hydrolyses were carried out routinely, under “excessive” (unoptimized) conditions applying the acid or base catalysts in a larger quantity than required, and allowing longer reaction times. We undertook to explore the optimum conditions for the HCl-catalyzed hydrolysis of phosphinic and phosphonic esters. In the first round, the acid-catalyzed hydrolysis of cyclic phosphinates, such as 1-alkoxy-3-phospholene oxides, 1-alkoxyphospholane oxides, and an 1-alkoxy-1,2,3,4,5,6-hexahydrophosphinine oxide was investigated, optimized, and characterized by rate constants [12]. Then, the hydrolysis of a series of dialkyl arylphosphonates was studied. In this case, two-step conversions were monitored and quantified by k values [13]. α-Hydroxybenzylyphosphonates, obtained in the Pudovik reaction of substituted benzaldehydes and dialkyl phosphites, form a representative class of phosphonic acid derivatives [14]. α-Hydroxyphosphonates are versatile intermediates that may be transformed to α-aminophosphonates [15], can be phosphorylated [16], and may be rearranged to the corresponding phosphates [17]. The catalytic hydrogenation of α-dibenzyl hydroxyphosphonates afforded the respective α-hydroxyphosphonic acids [18]. Moreover, they may be of cytotoxic activity [18]. In this article, we describe our results on the HCl–promoted hydrolysis of α-hydroxyphosphonates and a few related analogues.
2. Results and Discussion
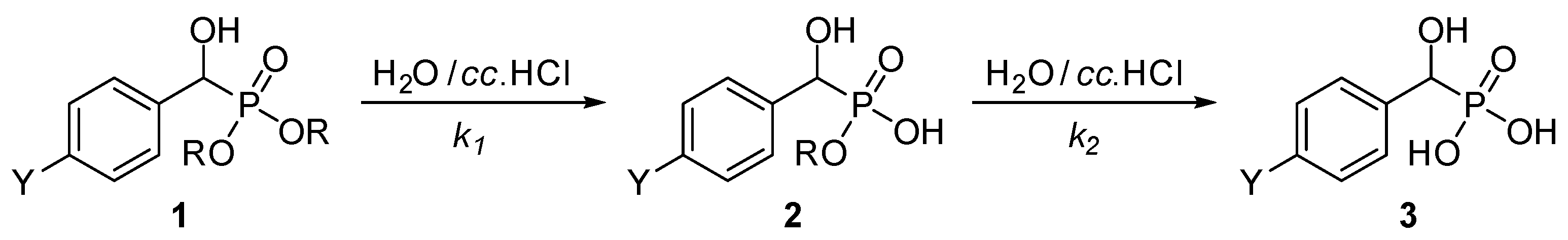
Substituted α-hydroxybenzylphosphonates (1a–j) prepared as described earlier [18] were subjected to acidic hydrolysis. The application of three equivalents (0.5 mL) of concentrated hydrochloric acid in 1 mL of water for ca. 2 mmol of the phosphonate (1) at reflux resulted in complete hydrolysis within 2.5–9.5 h depending on the substituents. The reactions followed a two-step protocol and took place via the corresponding ester–acid intermediate 2, and were monitored by 31P NMR spectroscopy (Scheme 1). Experimental data together with the calculated pseudo-first-order k1 and k2 rate constants are listed in Table 1, while the concentration–time diagrams exhibiting the relative proportions of components 1, 2, and 3 are shown in Figure 1 and Figure 2 and Figures S1–S8 in the Supplementary Materials.
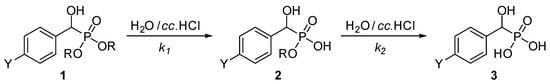
Scheme 1.
Two-step acidic hydrolysis of substituted α-hydroxybenzylphosphonates. For the Y substituents see Table 1.

Table 1.
Experimental and kinetic data on the two-step hydrolysis of α-hydroxybenzylphosphonates 1a–j.
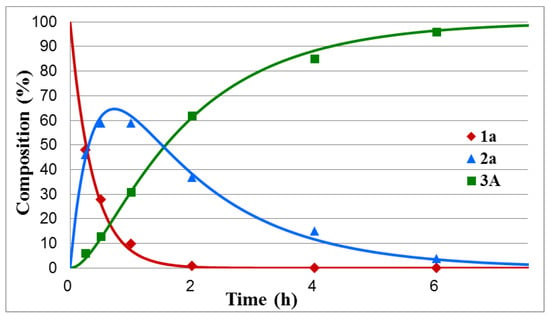
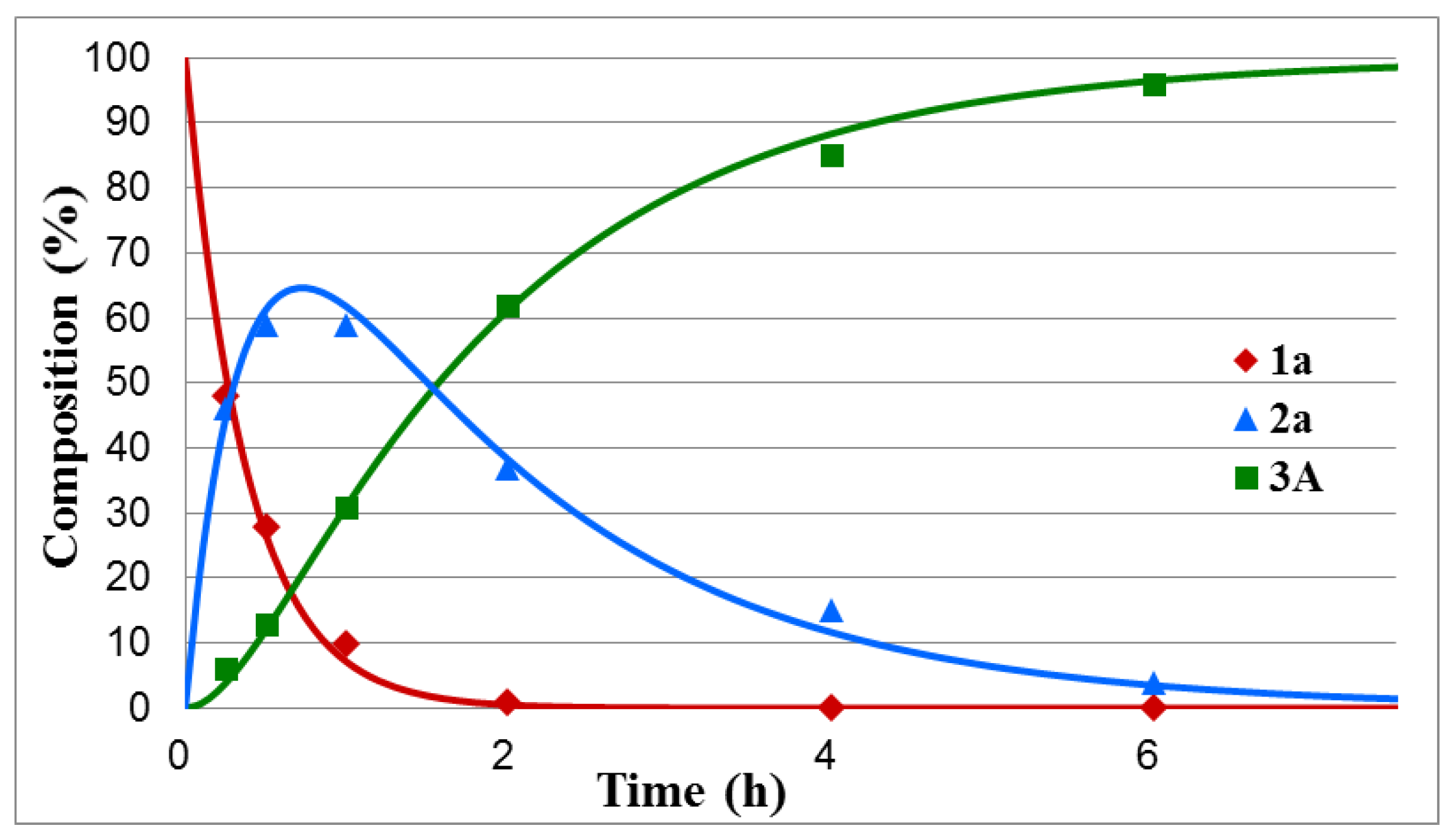
Figure 1.
Concentration profile for the components during the hydrolysis of dimethyl α-hydroxybenzylphosphonate (1a) under optimum conditions. The R2 measure of goodness of fit is 0.994.
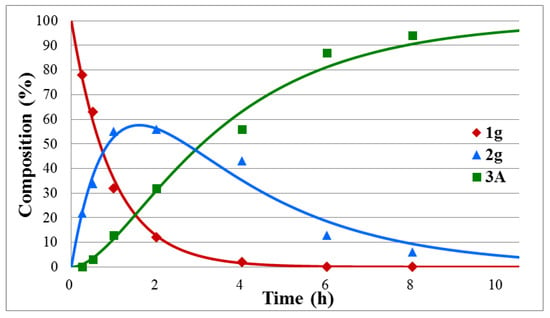
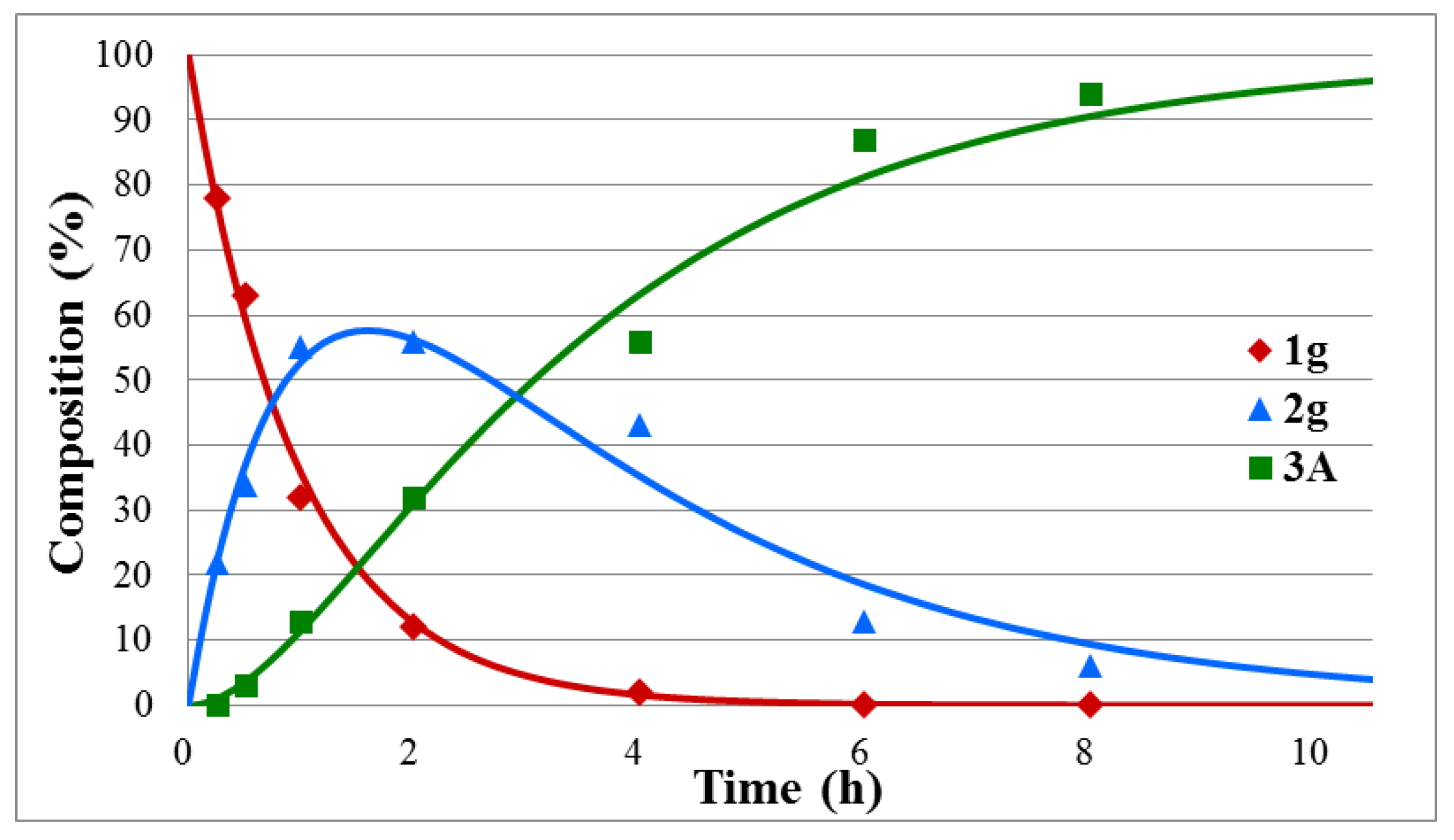
Figure 2.
Concentration profile for the components during the hydrolysis of diethyl α-hydroxybenzylphosphonate (1g) under optimum conditions. The R2 measure of goodness of fit is 0.986.
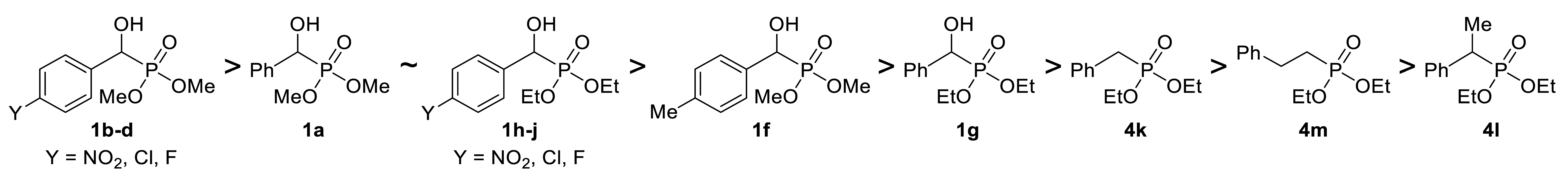
One can see that the hydrolysis of the unsubstituted dimethyl α-hydroxybenzylphosphonate was complete after tr = 6.5 h, and the maximum proportion of intermediate 2a could be observed at tmax = 44 min. In this case, k1 and k2 were found to be 2.64 h−1 and 0.60 h−1, respectively (Table 1/Entry 1). Electron-withdrawing substituents, such as 4-NO2, 4-Cl and 4-F in the phenyl ring facilitated the hydrolyses that were complete after 2.5 h, 5.5 h, and 6 h, respectively. The maximum concentration of intermediates 2b–d appeared in the range of 22–34 min. The k1 values fell in the range of 3.36–5.18 h−1, while the k2 constants were between 0.67 and 1.24 h−1 (Table 1/Entries 2–4). It seems that the 4-CF3Ph substituent acted overall as the 4-ClPh group, as marked by tr = 5.5 h. In this case (Y = CF3), k1 was 2.03, while k2 was 0.61 (Table 1/Entry 5). In the above series, the hydrolysis of the 4-Me-substituted benzylphosphonate (1f) was the slowest, as a complete hydrolysis required 8 h, and the rate constants were found to be 1.64 (k1) and 0.31 (k2) (Table 1/Entry 6). It is noteworthy that the fission of the second P–OMe unit is the rate-determining step. As can be seen from Table 1, the k1 values for the above cases are, in almost all cases, more than four times larger as compared to the k2 values.
Regarding the series of substituted diethyl α-hydroxybenzylphosphonates (1g–j), the hydrolysis of the unsubstituted model (1g) was significantly slower than that of the dimethyl analogue (1a) (compare the reaction times of 9.5 h (Table 1/Entry 7) and 6.5 h (Table 1/Entry 1)). The corresponding k1 and k2 rate constants for the hydrolysis of diethyl ester 1g were roughly the half the ones obtained for the methyl counterpart (1a) (compare rate constants 1.03/0.35 versus 2.64/0.60 (Table 1/Entry 7 versus Entry 1). Hydrolysis of the α-hydroxyphosphonates with electron-withdrawing 4-NO2, 4-Cl, and 4-F substituents in the phenyl ring (1h-j) required shorter reaction times of 5.5–9.0 h as compared with that (9.5 h) of the unsubstituted instance (1g) (Table 1/Entries 8–10). In these cases again, the k2 rate constants (0.61, 0.42, and 0.31, respectively) determined the overall reactivity.
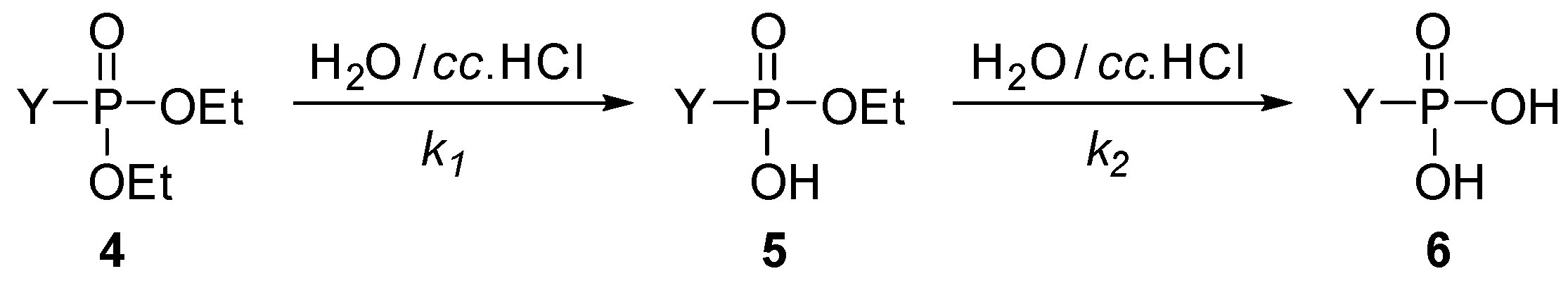
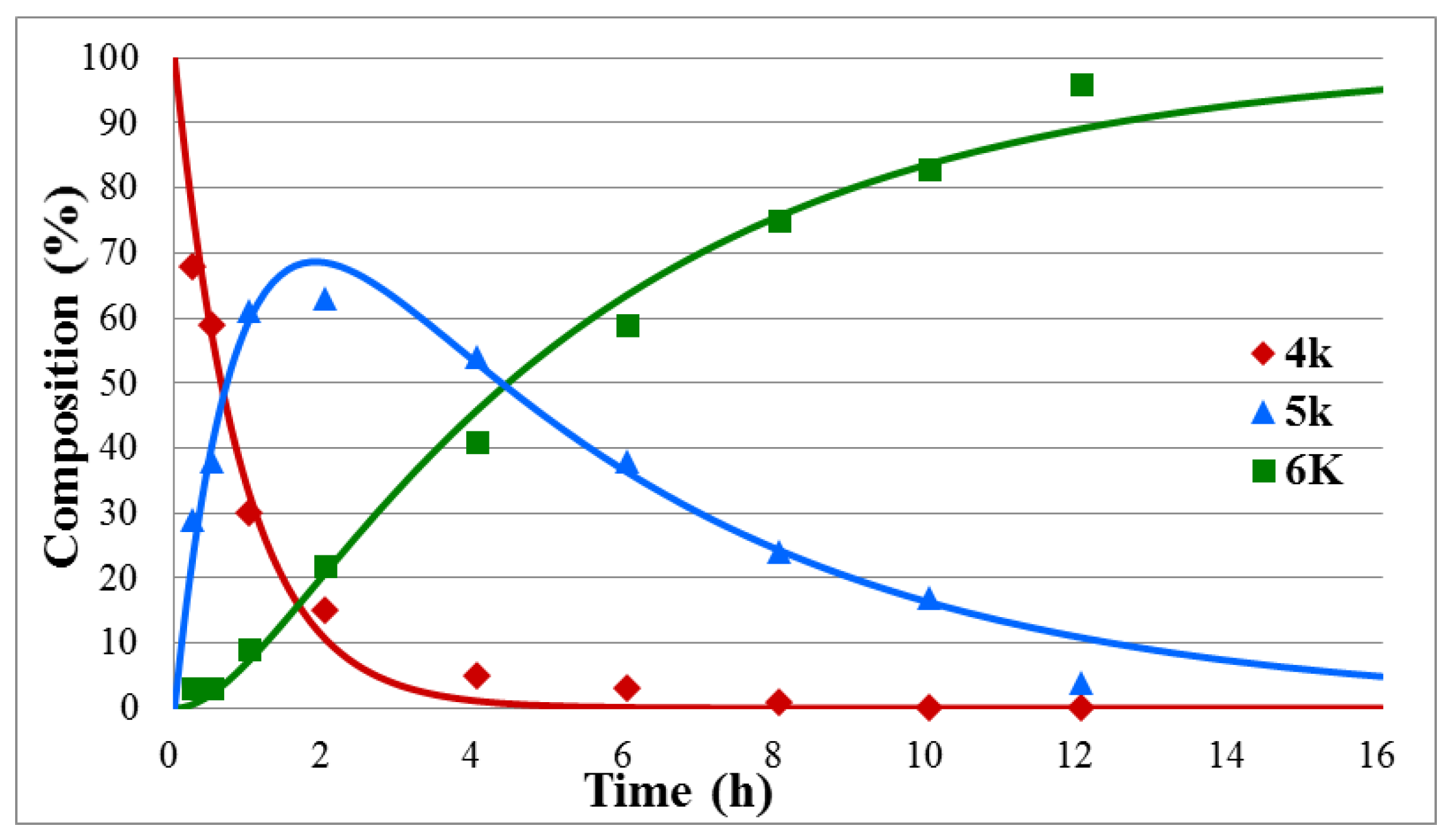
To study the effect of substituents on the rate of the hydrolysis further, three additional model compounds, diethyl benzylphosphonate (4k), diethyl α-phenylethylphosphonate (4l), and diethyl β-phenylethylphosphonate (4m) were also subjected to hydrolysis, under the conditions applied for the α-hydroxybenzylphosphonates (1a–j) above (Scheme 2, Table 2, Figure 3 and Figures S9 and S10 in Supplementary Materials). It was found that the hydrolysis of the benzylphosphonate (4k) took longer than that of the α-hydroxy derivative 1g (15 h versus 9.5 h, Table 2/Entry 1 and Table 1/Entry 7). The k1 constant was somewhat larger for the hydrolysis of species 4k than that for 1g, but the decisive k2 value become lower, as demonstrated by 1.12 h−1 and 0.20 h−1 versus 1.03 h−1 and 0.35 h−1 data pairs, respectively. Placing a Me group instead of the OH function on the α C atom, i.e., starting from α-phenylethylphosphonate 4l, the hydrolysis became even slower, and it was complete only after 25 h. The smallest k values (k1 = 0.51 h−1, k2 = 0.11 h−1) were obtained in this case (Table 2/Entry 2). It is obvious that the lack of the electron-withdrawing OH group in position α, or the appearance of an Me group instead of the HO function decreases the electrophilicity of the P atom of the P=O-function. The hydrolysis of β-phenylethylphosphonate (4m) with a reaction time of 20 h and k values of 0.70 h−1 and 0.15 h−1 (Table 2/Entry 3) occupied an intermediate position.
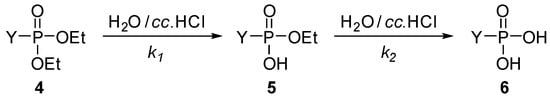
Scheme 2.
The two-step hydrolysis of other phosphonate derivatives. For the Y substituents see Table 2.

Table 2.
Experimental and kinetic data on the two-step hydrolysis of phosphonates 4k–m.
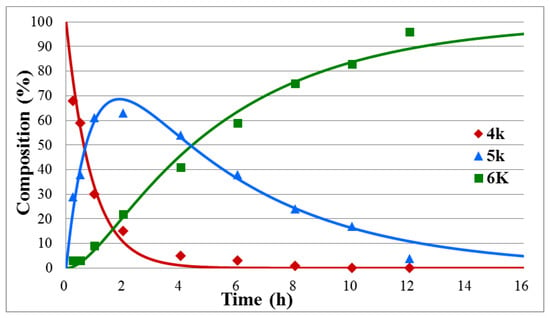
Figure 3.
Concentration profile for the components during the hydrolysis of diethyl benzylphosphonate (4k) under optimum conditions. The R2 measure of goodness of fit is 0.983.
It is noted that the hydrolyses of the phosphonate function take place via the SN2 mechanism, i.e., by the nucleophilic attack of the water molecule on the P=O function. In the consecutive series, the fission of the second P-O-C bond is the rate-determining step.
The overall order of reactivity of the phosphonates (1a–j and 4k–m) observed under acidic conditions was summarized in Table 3.

Table 3.
Reactivity order of the phosphonates 1a–j and 4k–m in acidic hydrolyses characterized by tr, as well as k1 and k2.
3. Materials and Methods
3.1. General Information
The 31P, 13C, and 1H NMR spectra were taken on a Bruker DRX-500 spectrometer operating at 202.4, 125.7, and 500 MHz, respectively. The couplings are given in Hz. LC-MS measurements were performed with an Agilent 1200 liquid chromatography system coupled with a 6130 quadrupole mass spectrometer equipped with an ESI ion source (Agilent Technologies, Palo Alto, CA, USA). High-resolution mass spectrometric measurements were performed using a Thermo Velos Pro Orbitrap Elite hybrid mass spectrometer in positive electrospray mode.
3.2. Use of the 31P NMR Spectra in Quantitative Analysis
The composition of the reaction mixture was determined by the integration of the areas under the corresponding peaks of the starting material, intermediate, and product in the 31P NMR spectra.
3.3. Curve Fitting on the Time–Relative Quantity Data Pairs
The acidic hydrolysis was modeled assuming pseudo-first-order kinetics. The concentration of water and hydrochloric acid was constant during the reaction, and their initial concentration is incorporated in the pseudo-first-order rate constants k1 and k2. The corresponding differential equations used in the model are the following:
where [diester], [ester-acid], and [acid] are the time-dependent molarities of the dialkyl α-hydroxyphosphonate, the phosphonic ester–acid intermediate, and the phosphonic acid, respectively, and k1 and k2 are the pseudo-first-order rate constants of the first and the second step of the hydrolysis.
The solution of the differential equations is the following (k1 ≠ k2):
where c0 is the initial molarity of the dialkyl α-hydroxyphosphonate.
The relative quantity of the components is their molarity divided by the sum of the three molarities (which is c0). The calculated time–composition curves are described by the following equations, by leaving the k1 and k2 rate constants as parameters:
During the calculation of the rate constants, we first gave arbitrary initial values to k1 and k2, then, we optimized their values such that the sum of the squares of the differences between the experimental and calculated compositions became minimal:
where n is the number of experimental time–composition data points measured at reaction times t1, t2, …, tn.
The resulting k1 and k2 rate constants and the associated time–composition curves were considered as the best fits. The best fits were found iteratively, using the nonlinear generalized reduced gradient method [19] of Microsoft Excel Solver.
The R2 measure of goodness of fit is calculated as , where SSres is described above and
The reaction time (tmax) corresponding to the maximal ratio of the phosphonic ester–acid intermediate in the reaction mixture was found as follows:
3.4. General Procedure for the Hydrolysis of Phosphonates (1a–j, 4k–m)
A mixture of 3.8 mmol of phosphonate (1a: 0.82 g, 1b: 0.99 g, 1c: 0.95 g, 1d: 0.89 g, 1e: 1.1 g, 1f: 0.87 g, 1g: 0.93 g, 1h: 1.1 g, 1i: 1.1 g, 1j: 1.0 g, 4k: 0.87 g, 4l: 0.92 g, 4m: 0.92 g), 1.0 mL (6.0 mmol) of cc. hydrochloric acid, and 2.0 mL of water was stirred at reflux for 2.5–25 h. The concentration of an aliquot part of the reaction mixture, or the whole mixture, afforded an oil that was analyzed by 31P NMR spectroscopy and LC-MS. Identification of the starting materials (1a–j, 4k–m), intermediates (2a–j, 5k–m), and products (3A–F, 6K–M) can be found in Table 4. The 13C and 1H NMR spectral data of the new intermediates (2a–f, h–j) were obtained from the spectra of the corresponding mixtures containing also the phosphonic acids (3A–F).

Table 4.
Identification of the starting phosphonates (1a–j, 4k–m), ester–acid intermediates (2a–j, 5k–m), and phosphonic acids (3A–F, 6K–M).
13C and 1H NMR characterization of the new ester acids:
Methyl hydrogen 1-(4-nitrophenyl)-1-hydroxymethylphosphonate (2b). 13C NMR (DMSO-d6) δ: 52.6 (d, 2J = 6.5, OCH3), 69.1 (d, 1J = 157.5, PCH), 122.7 (d, 4J = 2.4, C3), 128.1 (d, 3J = 5.0, C2), 146.5 (d, 5J = 3.3, C4), 147.6 (C1); 1H NMR (DMSO d6) δ: 3.58 (d, 3J = 10.4, 3H, OMe), 5.05 (d, 2J = 15.5, 1H, PCH), 7.64–7.73 (m, 2H, H2), 8.17–8.23 (m, 2H, H3).
Methyl hydrogen 1-(4-chlorophenyl)-1-hydroxymethylphosphonate (2c). 13C NMR (DMSO-d6) δ: 53.0 (d, 2J = 6.5, OCH3), 69.4 (d, 1J = 160.3, PCH), 128.1 (d, 4J = 2.2, C3), 129.5 (d, 3J = 5.3, C2), 132.1 (d, 5J = 3.6, C4), 139.0 (C1); 1H NMR (DMSO-d6) δ: 3.55 (d, 3J = 10.3, 3H, OMe), 4.85 (d, 2J = 13.8, 1H, PCH), 7.32–7.47 (m, 4H, Ar).
Methyl hydrogen 1-(4-fluorophenyl)-1-hydroxymethylphosphonate (2d). 13C NMR (DMSO-d6) δ: 53.0 (d, 2JP,C = 6.5, OCH3), 69.4 (d, 1JP,C = 161.3, PCH), 114.9 (dd, 2JF,C = 21.2, 4JP,C = 1.9, C3), 129.7 (dd, 3JF,C = 8.0, 3JP,C = 5.6, C2), 136.1 (d, 4JF,C = 2.6), 161.9 (dd, 1JF,C = 242.5, 5JP,C = 3.0, C4); 1H NMR (DMSO-d6) δ: 3.54 (d, 3J = 10.2, 3H, OMe), 4.84 (d, 2J = 13.2, 1H, PCH), 7.09–7.19 (m, 2H, H3), 7.38–7.49 (m, 2H, H2).
Methyl hydrogen 1-(4-trifluoromethylphenyl)-1-hydroxymethylphosphonate (2e). 13C NMR (DMSO-d6) δ: 53.1 (d, 2JP,C = 6.5, OCH3), 69.7 (d, 1JP,C = 157.9, PCH), 124.7–125.0 (m, C3), 124.9 (q, 1JF,C = 271.1, CF3), 127.3–128.7 (m, C2, C4), 145.0 (C1); 1H NMR (DMSO-d6) δ: 3.56 (d, 3J = 10.3, 3H, OMe), 4.96 (d, 2J = 14.6, 1H, PCH), 7.57–7.72 (m, 4H, Ar).
Methyl hydrogen 1-(4-methylphenyl)-1-hydroxymethylphosphonate (2f). 13C NMR (DMSO-d6) δ: 21.2 (Ar-CH3), 52.8 (d, 2JP,C = 6.6, OCH3), 70.0 (d, 1JP,C = 160.2, PCH), 127.7 (d, 3J = 5.6, C2), 128.7 (d, 4J = 2.1, C3), 136.5 (d, 5J = 3.1, C4), 136.9 (C1); 1H NMR (DMSO-d6) δ: 2.28 (s, 3H, Ar-CH3), 3.52 (d, 3J = 10.2, 3H, OMe), 4.76 (d, 2J = 13.0, 1H, PCH), 7.06–7.38 (m, 4H, Ar).
Ethyl hydrogen 1-(4-nitrophenyl)-1-hydroxymethylphosphonate (2h) 13C NMR (DMSO-d6) δ: 16.9 (d, 3J = 5.6, CH3), 62.0 (d, 2J = 6.5, OCH2), 69.1 (d, 1J = 157.5, PCH), 122.7 (d, 4J = 2.4, C3), 128.2 (d, 3J = 4.9, C2), 146.5 (d, 5J = 3.6, C4), 147.6 (C1); 1H NMR (DMSO-d6) δ: 1.17 (t, 3JH,H = 7.0, 3H, CH3), 3.91 (dq, 3JP,H = 8.1, 3JH,H = 7.0, 2H, OCH2), 5.03 (d, 2J = 15.7, 1H, PCH), 7.65–7.72 (m, 2H, H2), 8.16–8.23 (m, 2H, H3).
Ethyl hydrogen 1-(4-chlorophenyl)-1-hydroxymethylphosphonate (2i). 13C NMR (DMSO-d6) δ: 16.6 (d, 3J = 5.5, CH3), 61.7 (d, 2J = 6.5, OCH2), 69.3 (d, 1J = 160.7, PCH), 127.7 (d, 4J = 1.9, C3), 129.2 (d, 3J = 5.3, C2), 131.8 (d, 5J = 3.7, C4), 138.6 (C1); 1H NMR (DMSO-d6) δ: 1.15 (t, 3JH,H = 7.1, 3H, CH3), 3.91 (dq, 3JP,H = 7.1, 3JH,H = 7.1, 2H, OCH2), 4.84 (d, 2J = 13.8, 1H, PCH), 7.33–7.47 (m, 4H, Ar).
Ethyl hydrogen 1-(4-fluorophenyl)-1-hydroxymethylphosphonate (2j). 13C NMR (DMSO-d6) δ: 16.9 (d, 3J = 5.6, CH3), 61.9 (d, 2J = 6.5, OCH2), 69.5 (d, 1JP,C = 161.7, PCH), 114.8 (dd, 2JF,C = 21.1, 4JP,C = 2.0, C3), 129.7 (dd, 3JF,C = 8.2, 3JP,C = 5.5, C2), 136.1 (d, 4JF,C = 2.8), 161.9 (dd, 1JF,C = 242.4, 5JP,C = 3.2, C4); 1H NMR (DMSO-d6) δ: 1.15 (t, 3JH,H = 7.0, 3H, CH3), 3.87–3.95 (m, 2H, OCH2), 4.82 (d, 2J = 13.3, 1H, PCH), 7.11–7.17 (m, 2H, H3), 7.42–7.47 (m, 2H, H2).
1-(4-Trifluoromethylphenyl)-1-hydroxymethylphosphonic acid (3e). 13C NMR (DMSO-d6) δ: 70.5 (d, 1JP,C = 157.7, PCH), 124.7–125.0 (m, C3), 124.9 (q, 1JF,C = 272.3, CF3), 127.2–128.6 (m, C4), 128.4 (d, 3JP,C = 5.0, C2), 145.6 (C1); 1H NMR (DMSO-d6) δ: 4.80 (d, 2J = 15.0, 1H, PCH), 7.55–7.72 (m, 4H, Ar).
4. Conclusions
Kinetic study of the two-step acidic hydrolysis of a series of dialkyl α-hydroxybenzylphosphonates and a few related model compounds allowed the mapping of the reactivity of the different substrates. The two-step hydrolyses were characterized by k1 and k2 pseudo-first-order rate constants belonging to the formation of the corresponding monoester monoacids and the phosphonic acids, respectively. Electron-withdrawing substituents increased the rate, while electron-releasing ones slowed down the hydrolyses starting with the nucleophilic attack of the water molecule. It turned out that the fission of the second P-O-C unit is the rate-determining step. The intermediate ester–acid species were identified and characterized.
Supplementary Materials
The following are available online: Figure S1: Concentration profile for the components during the hydrolysis of dimethyl α-hydroxy-4-nitrobenzylphosphonate (1b) under optimum conditions. The R2 measure of goodness of fit is 0.989. Figure S2: Concentration profile for the components during the hydrolysis of dimethyl α-hydroxy-4-chlorobenzylphosphonate (1c) under optimum conditions. The R2 measure of goodness of fit is 0.987. Figure S3: Concentration profile for the components during the hydrolysis of dimethyl α-hydroxy-4-fluorobenzylphosphonate (1d) under optimum conditions. The R2 measure of goodness of fit is 0.965. Figure S4: Concentration profile for the components during the hydrolysis of dimethyl α-hydroxy-4-trifluoromethylbenzylphosphonate (1e) under optimum conditions. The R2 measure of goodness of fit is 0.988. Figure S5: Concentration profile for the components during the hydrolysis of dimethyl α-hydroxy-4-methylbenzylphosphonate (1f) under optimum conditions. The R2 measure of goodness of fit is 0.962. Figure S6: Concentration profile for the components during the hydrolysis of diethyl α-hydroxy-4-nitrobenzylphosphonate (1h) under optimum conditions. The R2 measure of goodness of fit is 0.992. Figure S7: Concentration profile for the components during the hydrolysis of diethyl α-hydroxy-4-chlorobenzylphosphonate (1i) under optimum conditions. The R2 measure of goodness of fit is 0.992. Figure S8: Concentration profile for the components during the hydrolysis of diethyl α-hydroxy-4-fluorobenzylphosphonate (1j) under optimum conditions. The R2 measure of goodness of fit is 0.970. Figure S9: Concentration profile for the components during the hydrolysis of diethyl α-phenylethylphosphonate (4l) under optimum conditions. The R2 measure of goodness of fit is 0.940. Figure S10: Concentration profile for the components during the hydrolysis of diethyl β-phenylethylphosphonate (4m) under optimum conditions. The R2 measure of goodness of fit is 0.949.
Author Contributions
Conceptualization, G.K. and Z.R.; methodology, N.H.; software, Á.S.; formal analysis, N.H. and J.K.; investigation, N.H.; resources, G.K.; data curation, Á.S./N.H.; writing—original draft preparation, G.K. and N.H.; writing—review and editing, G.K.; supervision, G.K.; project administration, G.K.; funding acquisition, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the National Research, Development and Innovation Office (K119202 and K134318).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Desai, J.; Wang, Y.; Wang, K.; Malwal, S.R.; Oldfield, E. Isoprenoid biosynthesis inhibitors targeting bacterial cell growth. Chem. Med. Chem. 2016, 11, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Tcarkova, K.V.; Artyushin, O.I.; Bondarenko, N.A. Synthetic routes to bis(3-aminophenyl) phosphinic acid. Phosphorus Sulfur Silicon 2016, 191, 1520–1522. [Google Scholar] [CrossRef]
- Keglevich, G.; Grün, A.; Bölcskei, A.; Drahos, L.; Kraszni, M.; Balogh, G.T. Synthesis and proton dissociation properties of arylphosphonates; A microwave-assisted catalytic Arbuzov reaction with aryl bromides. Heteroat. Chem. 2012, 23, 574–582. [Google Scholar] [CrossRef]
- Gavande, N.; Yamamoto, I.; Salam, N.K.; Ai, T.-H.; Burden, P.M.; Johnston, G.A.R.; Hanrahan, J.R.; Chebib, M. Novel cyclic phosphinic acids as GABAC ρ receptor antagonists: Design, synthesis, and pharmacology. ACS Med. Chem. Lett. 2011, 2, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rahil, J.; Haake, P. Rates and mechanism of the alkaline-hydrolysis of a sterically hindered phosphinate ester—Partial reaction by nucleophilic-attack at carbon. J. Org. Chem. 1981, 46, 3048–3052. [Google Scholar] [CrossRef]
- Cook, R.D.; Farah, S.; Ghawi, L.; Itani, A.; Rahil, J. The influence of the changing of P=O to P=S and P-O-R to P-S-R on the reactivity of phosphinate esters under alkaline-hydrolysis conditions. Can. J. Chem. 1986, 64, 1630–1637. [Google Scholar] [CrossRef]
- Wróblewski, A.E.; Verkade, J.G. 1-oxo-2-oxa-1-phosphabicyclo [2.2.2]octane: A new mechanistic probe for the basic hydrolysis of phosphate esters. J. Am. Chem. Soc. 1996, 118, 10168–10174. [Google Scholar] [CrossRef]
- Cevasco, G.; Thea, S. The quest for carbanion-promoted dissociative pathways in the hydrolysis of aryl phosphinates. J. Chem. Soc. Perkin Trans. 2 1993, 1103–1106. [Google Scholar] [CrossRef]
- Salomon, C.J.; Breuer, E. Efficient and selective dealkylation of phosphonate diisopropyl esters using ME3SiBr. Tetrahedron Lett. 1995, 36, 6759–6760. [Google Scholar] [CrossRef]
- Tulsi, N.S.; Downey, A.M.; Cairo, C.W. A protected L-bromophosphonomethylphenylalanine amino acid derivative (BrPmp) for synthesis of irreversible protein tyrosine phosphatase inhibitors. Bioorg. Med. Chem. 2010, 18, 8679–8686. [Google Scholar] [CrossRef]
- Jansa, P.; Hradil, O.; Baszczyňski, O.; Dračínský, M.; Janeba, Z. An efficient microwave-assisted synthesis and biological properties of polysubstituted pyrimidinyl- and 1,3,5-triazinylphosphonic acids. Tetrahedron 2012, 68, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Keglevich, G.; Rádai, Z.; Harsági, N.; Szigetvári, Á.; Kiss, N.Z. A study on the acidic hydrolysis of cyclic phosphinates: 1-Alkoxy-3-phospholene 1-oxides, 1-ethoxy-3-methylphospholane 1-oxide, and 1-ethoxy-3-methyl-1,2,3,4,5,6-hexahydrophosphinine 1-oxide. Heteroat. Chem. 2017, 28, e21394. [Google Scholar] [CrossRef]
- Harsági, N.; Rádai, Z.; Kiss, N.Z.; Szigetvári, Á.; Keglevich, G. Two-step acidic hydrolysis of dialkyl arylhosphonates. Mendeleev Commun. 2020, 30, 38–39. [Google Scholar] [CrossRef]
- Rádai, Z.; Keglevich, G. Synthesis and reactions of α-hydroxyphosphonates. Molecules 2018, 23, 1493. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.Z.; Rádai, Z.; Mucsi, Z.; Keglevich, G. Synthesis of aminophosphonates from α-hydroxyphosphonates; a theoretical study. Heteroat. Chem. 2016, 27, 260–268. [Google Scholar] [CrossRef]
- Rádai, Z.; Hodula, V.; Kiss, N.Z.; Kóti, J.; Keglevich, G. Phosphorylation of (1-aryl-1-hydroxymethyl)phosphonates. Mendeleev Commun. 2019, 29, 153–154. [Google Scholar] [CrossRef]
- Rádai, Z.; Szabó, R.; Szigetvári, Á.; Kiss, N.Z.; Mucsi, Z.; Keglevich, G. A study on the rearrangement of dialkyl 1-aryl-1-hydroxymethylphosphonates to benzyl phosphates. Curr. Org. Chem. 2020, 24, 465–471. [Google Scholar] [CrossRef]
- Rádai, Z.; Szeles, P.; Kiss, N.Z.; Hegedűs, L.; Windt, T.; Nagy, V.; Keglevich, G. Green synthesis and cytotoxic activity of dibenzyl α-hydroxyphosphonates and α-hydroxyphosphonic acids. Heteroat. Chem. 2018, 29, e21436. [Google Scholar] [CrossRef]
- Lasdon, L.S.; Waren, A.D.; Jain, A.; Ratner, M. Design and testing of a generalized reduced gradient code for nonlinear programming. ACM T Math. Softw. 1978, 4, 34–50. [Google Scholar] [CrossRef]
- Keglevich, G.; Tóth, V.R.; Drahos, L. Microwave-assisted synthesis of α-hydroxy-benzylphosphonates and -benzylphosphine oxides. Heteroat. Chem. 2011, 22, 15–17. [Google Scholar] [CrossRef]
- Seven, O.; Polat-Cakir, S.; Hossain, M.S.; Emrullahoglu, M. Reactions of acyl phosphonates with organoaluminum reagents: A new method for the synthesis of secondary and tertiary alpha-hydroxy phosphonates. Tetrahedron 2011, 67, 3464–3469. [Google Scholar] [CrossRef]
- Cai, Z.-H.; Du, G.-F.; He, L.; Gu, C.-Z.; Dai, B. N-Heterocyclic carbene catalyzed hydrophosphonylation of aldehydes. Synthesis 2011, 2073–2078. [Google Scholar] [CrossRef]
- de Noronha, R.G.; Costa, P.J.; Romano, C.C.; Calhorda, M.J.; Fernandes, A.C. MoO2Cl2 as a novel catalyst for C–P bond formation and for hydrophosphonylation of aldehydes. Organometallics 2009, 28, 6206–6212. [Google Scholar] [CrossRef]
- Kedrowski, S.M.A.; Dougherty, D.A. Room-temperature alternative to the Arbuzov reaction: The reductive deoxygenation of acyl phosphonates. Org. Lett. 2010, 12, 3990–3993. [Google Scholar] [CrossRef]
- Huang, T.; Chen, T.; Han, L.-B. Oxidative dephosphorylation of benzylic phosphonates with dioxygen generating symmetrical trans-stilbenes. J. Org. Chem. 2018, 83, 2959–2965. [Google Scholar] [CrossRef]
- St. Maurice, M.; Bearne, S.L. Reaction intermediate analogues for mandelate racemase: Interaction between Asn 197 and the α-hydroxyl of the substrate promotes catalysis. Biochemistry 2000, 39, 13324–13335. [Google Scholar] [CrossRef]
- Hamerschmidt, F.; Hanninger, A. Enantioselective deprotonation of benzyl phosphates by homochiral lithium amide bases—Configurational stability of benzyl carbanions with a dialkoxyphosphoryloxy substituent and their rearrangement to optically-active α-hydroxy phosphonates. Chem. Ber. 1995, 128, 823–830. [Google Scholar] [CrossRef]
- Mortier, J.; Gridnev, I.D.; Guénot, P. Reactions of phosphonates with organohaloboranes: New route to molecular borophosphonates. Organometallics 2000, 19, 4266–4275. [Google Scholar] [CrossRef]
- Eom, D.; Jeong, Y.; Kim, Y.R.; Lee, E.; Choi, W.; Lee, P.H. Palladium-catalyzed C(sp2 and sp3)-H activation/C-O bond formation: Synthesis of benzoxaphosphole 1- and 2-oxides. Org. Lett. 2013, 15, 5210–5213. [Google Scholar] [CrossRef]
- Galardy, R.E.; Kontoyiannidou-Ostrem, V.; Kortylewicz, Z.P. Inhibition of angiotensin converting enzyme by phosphonic amides and phosphonic-acids. Biochemistry 1983, 22, 1990–1995. [Google Scholar] [CrossRef]
- Prishchenko, A.A.; Livantsov, M.V.; Novikova, O.P.; Livantsova, L.I.; Maryashkin, A.V. Reaction of trimethylsilyl phosphites with functionalyzed aromatic aldehydes. Russ. J. Gen. Chem. 2005, 75, 1965–1967. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Zhang, Y.-J.; Wang, W.-M.; Yang, K.-W. Synthesis and inhibitory activity of acetamidophosphonic acids against metallo-β-lactamases. Phosphorus Sulfur Silicon 2017, 192, 14–18. [Google Scholar] [CrossRef]
- Safonova, T.Y.; Gulyukina, N.S.; Novakovskaya, Y.V.; Astaf’ev, E.A.; Bondarenko, G.N.; Petrii, O.A.; Tsirlina, G.A.; Beletskaya, I.P. Electrochemical hydrogenation of substituted alpha-phenylvinylphosphonic acids: General characteristics of the reaction layer and prediction of preparative electrolysis conditions. Russ. J. Electrochem. 2002, 38, 457–466. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Vonder Embse, R.A. The invention of radical reactions. Part 39. The reaction of white phosphorus with carbon-centered radicals. An improved procedure for the synthesis of phosphonic acids and further mechanistic insights. Tetrahedron 1998, 54, 12475–12496. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).