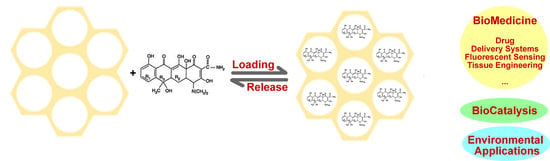
Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents
Abstract
:1. Introduction
2. Characteristics of Mesoporous Silica Materials
3. Mesoporous Silica Synthesis Methods
3.1. MCM-41 Synthesis
3.2. MCM-48 Synthesis
3.3. MCM-50 Synthesis
3.4. SBA-15 Synthesis
3.5. SBA-16 Synthesis
3.6. FDU-12 Synthesis
3.7. Design Principle
4. Surface Functionalization Strategies
Stimuli-Responsive Mesoporous Silica Systems
5. Application of Mesoporous Silica Nanoparticles
5.1. Biomedical Applications
5.2. Cosmetics
5.3. Fluorescent Sensing
5.4. Bio Catalysis Involvement and Natural Processes Behavior
5.5. Environmental Applications
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zdravkov, B.; Čermák, J.; Šefara, M.; Janku, J. Pore Classification in the Characterization of Porous Materials: A Perspective. Cent. Eur. J. Chem. 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.; Everett, D.; Haynes, J.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-Based Systems for Oral Delivery of Drugs, Macromolecules and Cells. Adv. Colloid Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Giorno, L.; Piacentini, E.; Bazzarelli, F. Macroporous, Mesoporous, and Microporous Membranes. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–2. [Google Scholar]
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of Structural and Compositional Characteristics of Ordered Microporous and Mesoporous Materials with Inorganic Hosts (Iupac Recommendations 2001). Pure Appl. Chem. 2001, 73, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regi, M.; Tamanoi, F. Chapter One—Overview of Studies Regarding Mesoporous Silica Nanomaterials and Their Biomedical Application. In The Enzymes; Tamanoi, F., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 43, pp. 1–10. [Google Scholar]
- Pattnaik, S.; Swain, K. 24—Mesoporous Nanomaterials as Carriers in Drug Delivery. In Applications of Nanocomposite Materials in Drug Delivery; Abdullah Asiri, I., Mohammad, A., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 589–604. [Google Scholar]
- Unger, K.K.; Ltidtke, S.; Thomas, A. Application of 0.5-Μm Porous Silanized Silica Beads in Electrochromatography. J. Chromatogr. 1997, 786, 229–235. [Google Scholar]
- Yamamoto, E.; Kuroda, K. Chapter One—Preparation and Controllability of Mesoporous Silica Nanoparticles. In The Enzymes; Tamanoi, F., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 44, pp. 1–10. [Google Scholar]
- Pednekar, P.P.; Godiyal, S.C.; Jadhav, K.R.; Kadam, V.J. Chapter 23—Mesoporous Silica Nanoparticles: A Promising Multifunctional Drug Delivery System. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–621. [Google Scholar]
- Yang, B.; Chen, Y.; Shi, J. Mesoporous Silica/Organosilica Nanoparticles: Synthesis, Biological Effect and Biomedical Application. Mater. Sci. Eng. R Rep. 2019, 137, 66–105. [Google Scholar] [CrossRef]
- Poonia, N.; Lather, V.; Pandita, D. Mesoporous Silica Nanoparticles: A Smart Nanosystem for Management of Breast Cancer. Drug Discov. Today 2018, 23, 315–332. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [Green Version]
- Şen Karaman, D.; Kettiger, H. Chapter 1—Silica-Based Nanoparticles as Drug Delivery Systems: Chances and Challenges. In Inorganic Frameworks as Smart Nanomedicines; Grumezescu, A.M., Ed.; William Andrew Publishing: Cambridge, UK, 2018; pp. 1–40. [Google Scholar]
- Khosroshahi, M.; Tehrani, I.M.; Nouri, A. Fabrication and Characterization of Multilayer MSiO2@Fe3O4@Au Mesoporous Nanocomposite for near-Infrared Biomedical Applications. Adv. Nano Bio Mater. Devices 2018, 2, 230–246. [Google Scholar]
- Mitran, R.-A.; Deaconu, M.; Matei, C.; Berger, D. Chapter 11—Mesoporous Silica as Carrier for Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 351–374. [Google Scholar]
- Kumar, V.; Kaur, G.; Pickrell, G.R. Chapter 19—Silica Nanospheres. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Kaur, G., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 521–544. [Google Scholar]
- Ghosh, S. Chapter 9—Mesoporous Silica-Based Nano Drug-Delivery System Synthesis, Characterization, and Applications. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–317. [Google Scholar]
- Doadrio, A.L.; Salinas, A.J.; Sánchez-Montero, J.M.; Vallet-Regi, M. Drug Release from Ordered Mesoporous Silicas. Curr. Pharm. Des. 2015, 21, 6213–6819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parida, K.; Rath, D.; Rana, S. Cheminform Abstract: Organic Amine-Functionalized Silica-Based Mesoporous Materials: An Update of Syntheses and Catalytic Applications. RSC Adv. 2014, 4, 57111–57124. [Google Scholar]
- Thanabodeekij, N.; Sadthayanon, S.; Gulari, E.; Wongkasemjit, S. Extremely High Surface Area of Ordered Mesoporous Mcm-41 by Atrane Route. Mater. Chem. Phys. 2006, 98, 131–137. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sakamoto, Y.; Terasaki, O.; Ryoo, R.; Ko, C.H. Determination of Pore Size and Pore Wall Structure of Mcm-41 by Using Nitrogen Adsorption, Transmission Electron Microscopy, and X-Ray Diffraction. J. Phys. Chem. B 2000, 104, 292–301. [Google Scholar] [CrossRef]
- Widenmeyer, M.; Anwander, R. Pore Size Control of Highly Ordered Mesoporous Silica Mcm-48. Chem. Mater. 2002, 14, 1827–1831. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874. [Google Scholar] [CrossRef] [Green Version]
- Ukmar, T.; Planinsek, O. Ordered Mesoporous Silicates as Matrices for Controlled Release of Drugs. Acta Pharm. (ZagrebCroat.) 2010, 60, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Romo, P.; de Lourdes Guzmán-Castillo, M.; Armendáriz-Herrera, H.; Navarrete, J.; Acosta, D.R.; Montoya, J.A. Synthesis of Fsm-16 Mesoporous Materials: Effect of the Anion (F−, Cl−, SO42−) in the Ion Exchange Process on the Thermal Stability. Langmuir 2003, 19, 3446–3452. [Google Scholar] [CrossRef]
- Inagaki, S. Fsm-16 and Mesoporous Organosilicas. In Studies in Surface Science and Catalysis; Terasaki, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 148, pp. 109–132. [Google Scholar]
- Zimowska, M.; Michalik-Zym, A.; Kryſciak-Czerwenka, J.; Dula, R.; Socha, R.P.; Pamin, K.; Bazarnik, M.; Bahranowski, K.; Olejniczak, Z.; Lityſska-Dobrzyſska, L.; et al. A Comparative Study of Direct Versus Post-Synthesis Alumination of Mesoporous Fsm-16 Silica. Mater. Res. Bull. 2016, 83, 623–631. [Google Scholar] [CrossRef]
- Tang, L.; Luo, G.; Zhu, M.; Kang, L.; Dai, B. Preparation, Characterization and Catalytic Performance of Hpw-Tud-1 Catalyst on Oxidative Desulfurization. J. Ind. Eng. Chem. 2013, 19, 620–626. [Google Scholar] [CrossRef]
- Telalović, S.; Ramanathan, A.; Mul, G.; Hanefeld, U. Tud-1: Synthesis and Application of a Versatile Catalyst, Carrier, Material…. New J. Chem. 2010, 20, 642–658. [Google Scholar] [CrossRef] [Green Version]
- Heikkilä, T.; Salonen, J.; Tuura, J.; Hamdy, M.; Mul, G.; Kumar, N.; Salmi, T.; Murzin, D.Y.; Laitinen, L.; Kaukonen, A.M.; et al. Mesoporous Silica Material Tud-1 as a Drug Delivery System. Int. J. Pharm. 2007, 331, 133–138. [Google Scholar] [CrossRef] [PubMed]
- de Clippel, F.; Dusselier, M.; Van de Vyver, S.; Peng, L.; Pierre, J.; Sels, B. Cheminform Abstract: Tailoring Nanohybrids and Nanocomposites for Catalytic Applications. Green Chem. 2013, 15, 1398–1430. [Google Scholar] [CrossRef]
- Henning, L.M.; Cubas, D.D.; Colmenares, M.G.; Schmidt, J.; Bekheet, M.F.; Pauw, B.R.; Gurlo, A.; Simon, U. High Specific Surface Area Ordered Mesoporous Silica Cok-12 with Tailored Pore Size. Microporous Mesoporous Mater. 2019, 280, 133–143. [Google Scholar] [CrossRef]
- Shen, S.; Li, Y.; Zhang, Z.; Fan, J.; Tu, B.; Zhou, W.; Zhao, D. A Novel Ordered Cubic Mesoporous Silica Templated with Tri-Head Group Quaternary Ammonium Surfactant. Chem. Commun. 2002, 2212–2213. [Google Scholar] [CrossRef]
- Shen, S.; Garcia-Bennett, A.E.; Liu, Z.; Lu, Q.; Shi, Y.; Yan, S.; Yu, C.; Liu, W.; Cai, Y.; Terasaki, O.; et al. Three-Dimensional Low Symmetry Mesoporous Silica Structures Templated from Tetra-Headgroup Rigid Bolaform Quaternary Ammonium Surfactant. J. Am. Chem. Soc. 2005, 127, 6780–6787. [Google Scholar] [CrossRef]
- Huang, L.; Yan, X.; Kruk, M. Synthesis of Ultralarge-Pore Fdu-12 Silica with Face-Centered Cubic Structure. Langmuir 2010, 26, 14871–14878. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; González-Álvarez, I.; González-Álvarez, M.; Martínez-Máñez, R.; Marcos, M.D.; Bernardos, A.; Aznar, E. Surfactant-Triggered Molecular Gate Tested on Different Mesoporous Silica Supports for Gastrointestinal Controlled Delivery. Nanomaterials 2020, 10, 1290. [Google Scholar] [CrossRef]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous Silica Nanoparticles: Synthesis, Pharmaceutical Applications, Biodistribution, and Biosafety Assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Cao, V.D.; Nguyen, T.N.Q.; Hoang, D.T.; Ngo, V.C.; Nguyen, D.H. Functionalized Mesoporous Silica Nanoparticles and Biomedical Applications. Mater. Sci. Eng. C 2019, 99, 631–656. [Google Scholar] [CrossRef]
- Zielińska, A.; Pereira, I.; Antunes, S.; Veiga, F.J.; Santos, A.C.; Nowak, I.; Silva, A.M.; Souto, E.B. Chapter 10—Mesoporous Silica Nanoparticles as Drug Delivery Systems against Melanoma. In Design of Nanostructures for Theranostics Applications; Grumezescu, A.M., Ed.; William Andrew Publishing: Cambridge, UK, 2018; pp. 437–466. [Google Scholar]
- Han, Y.; Lu, Z.; Teng, Z.; Liang, J.; Guo, Z.; Wang, D.; Han, M.-Y.; Yang, W. Unraveling the Growth Mechanism of Silica Particles in the Stöber Method: In Situ Seeded Growth Model. Langmuir 2017, 33, 5879–5890. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yang, G.; Huang, D.; Cao, W.; Ge, L.; Li, L. Synthesis and Characterization of Spherical Silica Nanoparticles by Modified Stöber Process Assisted by Slow-Hydrolysis Catalyst. Colloid Polym. Sci. 2018, 296, 379–384. [Google Scholar] [CrossRef]
- Kim, T.G.; An, G.S.; Han, J.S.; Hur, J.U.; Park, B.G.; Choi, S.-C. Synthesis of Size Controlled Spherical Silica Nanoparticles Via Sol-Gel Process within Hydrophilic Solvent. J. Korean Ceram. Soc. 2017, 54, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Comite, A. Chapter 1—Preparation of Silica Membranes by Sol-Gel Method. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Ghasemzadeh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–23. [Google Scholar]
- Zheng, K.; Boccaccini, A.R. Sol-Gel Processing of Bioactive Glass Nanoparticles: A Review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef]
- Ng, E.-P.; Bahaman, N.; Mukti, R.R.; Ling, T.-C.; Ng, Y.H.; Adam, F. Detailed Kinetic Observation Revealing the Formation Mechanism of Chiral Mesoporous Silica (Cms) Synthesized by Cooperative Self-Assembly of Anionic Chiral Surfactant. Mater. Res. Bull. 2015, 62, 192–199. [Google Scholar] [CrossRef]
- Shio, S.; Kimura, A.; Yamaguchi, M.; Yoshida, K.; Kuroda, K. Morphological Control of Ordered Mesoporous Silica Formation of Fine and Rod-Like Mesoporous Powders from Completely Dissolved Aqueous Solutions of Sodium Metasilicate and Cationic Surfactants. Chem. Commun. 1998, 2461–2462. [Google Scholar] [CrossRef]
- Tolbert, S.H.; Landry, C.C.; Stucky, G.D.; Chmelka, B.F.; Norby, P.; Hanson, J.C.; Monnier, A. Phase Transitions in Mesostructured Silica Surfactant Composites Surfactant Packing and the Role of Charge Density Matching. Chem. Mater. 2001, 13, 2247–2256. [Google Scholar] [CrossRef]
- Brankovic, M.; Zarubica, A.; Andjelkovic, T.; Andjelkovic, D. Mesoporous Silica (Mcm-41): Synthesis/Modification, Characterization and Removal of Selected Organic Micro-Pollutants from Water. Adv. Technol. 2017, 6, 50–57. [Google Scholar] [CrossRef]
- Martínez-Edo, G.; Balmori, A.; Pontón, I.; Martí del Rio, A.; Sánchez-García, D. Functionalized Ordered Mesoporous Silicas (Mcm-41): Synthesis and Applications in Catalysis. Catalysts 2018, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Deekamwong, K.; Kaiyasuan, C.; Jitcharoen, J.; Wittayakun, J. Influence of Gel Composition and Microwave-Assisted Hydrothermal Time in Mcm-41 Synthesis. Mater. Chem. Phys. 2017, 201, 384–390. [Google Scholar] [CrossRef]
- Sarı Yılmaz, M.; Dere Özdemir, O.; Pişkin, S. Synthesis and Characterization of Mcm-41 with Different Methods and Adsorption of Sr2+ on Mcm-41. Res. Chem. Intermed. 2015, 41, 199–211. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Jafarzadeh, A.; Pourahmad, A. Synthesis and Characterization of Mcm-41 Ropes. Mater. Lett. 2018, 212, 16–19. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Adam, F. Imx-Mcm-41 (X = Cl, Br and I): Active Catalysts for the Solvent Free Synthesis of Phenyl Glycidyl Carbonate. Surfaces Interfaces 2019, 14, 305–313. [Google Scholar] [CrossRef]
- Shah, B.A.; Patel, A.V.; Bagia, M.I.; Shah, A.V. Green Approach Towards the Synthesis of Mcm-41 from Siliceous Sugar Industry Waste. Int. J. Appl. Chem. 2017, 13, 497–514. [Google Scholar]
- Wei, F.-Y.; Liu, Z.-W.; Lu, J.; Liu, Z.-T. Synthesis of Mesoporous Mcm-48 Using Fumed Silica and Mixed Surfactants. Microporous Mesoporous Mater. 2010, 131, 224–229. [Google Scholar] [CrossRef]
- Boote, B.; Subramanian, H.; Ranjit, K.T. Rapid and Facile Synthesis of Siliceous Mcm-48 Mesoporous Materials. Chem. Commun. 2007, 4543–4545. [Google Scholar] [CrossRef]
- Seo, J.W.; Lee, W.-J.; Nam, S.; Ryoo, H.; Kim, J.-N.; Ko, C.H. Mesoporous Structure Control of Silica in Room-Temperature Synthesis under Basic Conditions. J. Nanomater. 2015, 2015, 7. [Google Scholar] [CrossRef]
- Malhis, A.A.; Arar, S.H.; Fayyad, M.K.; Hodali, H.A. Amino- and Thiol-Modified Microporous Silicalite-1 and Mesoporous Mcm-48 Materials as Potential Effective Adsorbents for Pb (Ii) in Polluted Aquatic Systems. Adsorpt. Sci. Technol. 2018, 36, 270–286. [Google Scholar] [CrossRef] [Green Version]
- Mokri, N.A.; Oh, P.C.; Chew, T.L.; Mukhtar, H. Synthesis of Siliceous Mesoporous Mcm-48 Via Single and Binary Surfactant System. In Process Engineering and Advanced Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Guan, L.C.; Nur, H.; Endud, S. Bimodal Pore Size Mesoporous Mcm-48 Materials Prepared by Post-Synthesis Alumination. J. Phys. Sci. 2006, 17, 65–75. [Google Scholar]
- Qian, W.; Wang, H.; Chen, J.; Kong, Y. Spherical V-Fe-Mcm-48: The Synthesis, Characterization and Hydrothermal Stability. Materials 2015, 8, 1752–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlig, H.; Muenster, T.; Kloess, G.; Ebbinghaus, S.G.; Einicke, W.-D.; Gläser, R.; Enke, D. Synthesis of Mcm-48 Granules with Bimodal Pore Systems Via Pseudomorphic Transformation of Porous Glass. Microporous Mesoporous Mater. 2018, 257, 185–192. [Google Scholar] [CrossRef]
- Saputra, H.; Othman, R.; Sutjipto, A.G.E.; Muhida, R.; Ani, M.H. Gel-Like Properties of Mcm-41 Material and Its Transformation to Mcm-50 in a Caustic Alkaline Surround. Mater. Res. Bull. 2012, 47, 732–736. [Google Scholar] [CrossRef]
- Lin, C.; Zhu, W.; Li, J.; Liu, X.; Li, W.; Jiang, Y.; Yang, H.; Li, G. An Efficient Route to Rapidly Access Silica Materials with Differently Ordered Mesostructures through Counteranion Exchange. Chemistry (Weinheim an der Bergstrasse, Germany) 2013, 19, 10146–10149. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.-V.N. Advanced Synthesis Strategies of Mesoporous Sba-15 Supported Catalysts for Catalytic Reforming Applications: A State-of-the-Art Review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Jaramillo, L.Y.; Henao, W.A.; Pabón-Gelves, E. Synthesis of Sba-15/Mcm-41 Bimodal Mesoporous Silica. MRS Proc. 2016, 1817. [Google Scholar] [CrossRef]
- Pirez, C.; Morin, J.-C.; Manayil, J.C.; Lee, A.F.; Wilson, K. Sol-Gel Synthesis of Sba-15: Impact of Hcl on Surface Chemistry. Microporous Mesoporous Mater. 2018, 271, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Rahman, N.B.A.; Rasid, H.M.; Hassan, H.M.M.; Jalil, M.N. Synthesis and Characterization of Mesoporous Silica Mcm-41 and Sba-15 from Power Plant Bottom Ash. Malays. J. Anal. Sci. 2016, 20, 539–545. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A.; Jadach, B.; Piotrowska, H.; Murias, M.; Lulek, J.; Nowak, I. Synthesis and Characterization of Sba-16 Type Mesoporous Materials Containing Amine Groups. Microporous Mesoporous Mater. 2016, 220, 231–238. [Google Scholar] [CrossRef]
- Song, S.; Zhou, X.; Duan, A.; Zhao, Z.; Chi, K.; Zhang, M.; Jiang, G.; Liu, J.; Wang, X. Synthesis of Mesoporous Silica Material with Ultra-Large Pore Sizes and the Hds Performance of Dibenzothiophene. Microporous Mesoporous Mater. 2016, 226, 510–521. [Google Scholar] [CrossRef]
- Cao, Z.; Du, P.; Duan, A.; Guo, R.; Zhao, Z.; Zhang, H.L.; Zheng, P.; Xu, C.; Chen, Z. Synthesis of Mesoporous Materials Sba-16 with Different Morphologies and Their Application in Dibenzothiophene Hydrodesulfurization. Chem. Eng. Sci. 2016, 155, 141–152. [Google Scholar] [CrossRef]
- Renuka, N.K.; Anas, K.; Aniz, C.U. Synthesis, Characterisation and Activity of Sba-16 Supported Oxidation Catalysts for Co Conversion. Chin. J. Catal. 2015, 36, 1237–1241. [Google Scholar] [CrossRef]
- Meoto, S.; Kent, N.; Nigra, M.M.; Coppens, M.-O. Effect of Stirring Rate on the Morphology of Fdu-12 Mesoporous Silica Particles. Microporous Mesoporous Mater. 2017, 249, 61–66. [Google Scholar] [CrossRef]
- Carmona, D.; Balas, F.; Santamaría, J. Pore Ordering and Surface Properties of Fdu-12 and Sba-15 Mesoporous Materials and Their Relation to Drug Loading and Release in Aqueous Environments. Mater. Res. Bull. 2014, 59, 311–322. [Google Scholar] [CrossRef]
- Fan, J.; Du, P.; Wang, X.; Zheng, P.; Zhao, Z.; Duan, A.; Xu, C.; Li, J. Ultrasound-Assisted Synthesis of Ordered Mesoporous Silica Fdu-12 with a Hollow Structure. New J. Chem. 2018, 42, 2381–2384. [Google Scholar] [CrossRef]
- Li, W.K.; Xie, D.H.; Song, B.L.; Feng, L.; Pei, X.M.; Cui, Z.G. Synthesis and Characterization of Ordered Mesoporous Silica Using Rosin-Based Gemini Surfactants. J. Mater. Sci. 2018, 53, 2434–2442. [Google Scholar] [CrossRef]
- Travaglini, L.; De Cola, L. Morphology Control of Mesoporous Silica Particles Using Bile Acids as Cosurfactants. Chem. Mater. 2018, 30, 4168–4175. [Google Scholar] [CrossRef]
- Han, P.; Liu, T.C.; Ji, X.W.; Tang, S.K. Morphology-Controlled Synthesis of Mesoporous Silica with Co-Template of Surfactant P123 and Ionic Liquid [Dmim] Cl. Chin. Chem. Lett. 2018, 29, 1305–1309. [Google Scholar] [CrossRef]
- Yan, S.S.; Gao, Z.N.; Xia, Y.; Liao, X.M.; Chen, Y.F.; Han, J.; Pan, C.; Zhang, Y. A Tetraphenylethene Luminogen-Functionalized Gemini Surfactant for Simple and Controllable Fabrication of Hollow Mesoporous Silica Nanorods with Enhanced Fluorescence. Inorg. Chem. 2018, 57, 13653–13666. [Google Scholar] [CrossRef]
- Morsi, R.E.; Mohamed, R.S. Nanostructured Mesoporous Silica: Influence of the Preparation Conditions on the Physical-Surface Properties for Efficient Organic Dye Uptake. R. Soc. Open Sci. 2018, 5, 172021. [Google Scholar] [CrossRef] [Green Version]
- Zapelini, I.W.; Silva, L.L.; Cardoso, D. Effect of Hydrothermal Treatment on Structural and Catalytic Properties of [Cta]-Mcm-41 Silica. Materials 2018, 11, 860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naoko Igarashia, K.A.K.; Tanakac, Y.; Nakatac, S.; Hashimotoa, K.; Tatsumid, T. Investigation of the Factors Influencing the Structural Stability of Mesoporous Silica Molecular Sieves. Microporous Mesoporous Mater. 2003, 59, 43–52. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Yoon, S.B.; Park, Y.-J.; Lee, M.H.; Kwang-Yong, J. Mesoporous Silica Particles and Preparation Method Thereof. Google Patents US8057772B2, 15 December 2011. [Google Scholar]
- Satou, S.; Shimizu, T. Mesoporous Silica, Process for the Preparation of the Same, and Use Thereof. Google Patents EP1035073A1, 13 September 2000. [Google Scholar]
- Liong, M.; Lu, J.; Tamanoi, F.; Zink, J.I.; Nel, A. Mesoporous Silica Nanoparticles for Biomedical Applications. Google Patents US20100255103A1, 12 June 2018. [Google Scholar]
- DeShong, P.R.; Zachariah, M.R.; DeMuth, P.; Prakash, A.; Luckett, C.; English, D.S. Method for Forming Mesoporous Silica Nanoparticles, Mesoporous Silica Nanoparticles, and Applications Thereof. Google Patents US9271936B2, 1 March 2016. [Google Scholar]
- Chang, J.H.; Lee, H.S. Apparatus of Manufacturing Mesoporous Silica and Method of Manufacturing Mesoporous Silica Using the Same. Google Patents 2020. [Google Scholar]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous Silica Nanoparticles for Therapeutic/Diagnostic Applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Capeletti, L.B.; Loiola, L.M.D.; Picco, A.S.; da Silva Liberato, M.; Cardoso, M.B. 8—Silica Nanoparticle Applications in the Biomedical Field. In Smart Nanoparticles for Biomedicine; Ciofani, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–129. [Google Scholar]
- Du, X.; Li, X.; Xiong, L.; Zhang, X.; Kleitz, F.; Qiao, S.Z. Mesoporous Silica Nanoparticles with Organo-Bridged Silsesquioxane Framework as Innovative Platforms for Bioimaging and Therapeutic Agent Delivery. Biomaterials 2016, 91, 90–127. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.; Row, K.H.; Ahn, W.-S. Amine–Silica Composites for Co2 Capture: A Short Review. J. Energy Chem. 2017, 26, 868–880. [Google Scholar] [CrossRef] [Green Version]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous Silica Materials: From Physico-Chemical Properties to Enhanced Dissolution of Poorly Water-Soluble Drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.C.; Almeida, P.P.; Bermudez, V.Z. Ordered Mesoporous Sol—Gel Materials: From Molecular Sieves to Crystal-Like Periodic Mesoporous Organosilicas. In The Sol-Gel Handbook; Levy, D., Zayat, M., Eds.; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Diagboya, P.N.E.; Dikio, E.D. Silica-Based Mesoporous Materials; Emerging Designer Adsorbents for Aqueous Pollutants Removal and Water Treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, G.; Zhang, H. Synthesis, Functionalization, and Applications of Morphology-Controllable Silica-Based Nanostructures: A Review. Prog. Solid State Chem. 2016, 44, 1–19. [Google Scholar] [CrossRef]
- van Miltenburg, A.; Pawlesa, J.; Bouzga, A.M.; Žilková, N.; Čejka, J.; Stöcker, M. Alkaline Modification of Mcm-22 to a 3d Interconnected Pore System and Its Application in Toluene Disproportionation and Alkylation. Top. Catal. 2009, 52, 1190–1202. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot Economy and One-Pot Synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [Green Version]
- Da’na, E. Adsorption of Heavy Metals on Functionalized-Mesoporous Silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Darvishi, B.; Farahmand, L.; Majidzadeh-A, K. Stimuli-Responsive Mesoporous Silica Nps as Non-Viral Dual Sirna/Chemotherapy Carriers for Triple Negative Breast Cancer. Mol. Ther. Nucleic Acids 2017, 7, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Liu, D.; Guo, Z. Endogenous Stimuli-Responsive Nanocarriers for Drug Delivery. Chem. Lett. 2016, 45. [Google Scholar] [CrossRef] [Green Version]
- Rahikkala, A.; Pereira, S.A.P.; Figueiredo, P.; Passos, M.L.C.; Araújo, A.R.T.S.; Saraiva, M.L.M.F.S.; Santos, H.A. Mesoporous Silica Nanoparticles for Targeted and Stimuli-Responsive Delivery of Chemotherapeutics: A Review. Adv. Biosyst. 2018, 2, 1800020. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous Silica Nanoparticles for Stimuli-Responsive Controlled Drug Delivery: Advances, Challenges, and Outlook. Int. J. Nanomed. 2016, 12, 87–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugan, B.; Krishnan, U.M. Chemoresponsive Smart Mesoporous Silica Systems—An Emerging Paradigm for Cancer Therapy. Int. J. Pharm. 2018, 553, 310–326. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, M.-C.; Lin, H.-C.; Sun, X.-Y.; Li, Y.-Y.; Yan, S.-Q. Synthesis and Drug Delivery Applications for Mesoporous Silica Nanoparticles. J. Med. Biotechnol. 2017, 1, 1–8. [Google Scholar]
- Moreira, A.F.; Dias, D.R.; Correia, I.J. Stimuli-Responsive Mesoporous Silica Nanoparticles for Cancer Therapy: A Review. Microporous Mesoporous Mater. 2016, 236, 141–157. [Google Scholar] [CrossRef]
- López-Saucedo, F.; Flores-Rojas, G.G.; Meléndez-Ortiz, H.I.; Morfín-Gutierrez, A.; Luna-Straffon, M.A.; Bucio, E. Chapter 14—Stimuli-Responsive Nanomaterials for Drug Delivery. In Characterization and Biology of Nanomaterials for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–424. [Google Scholar]
- Zhu, J.; Niu, Y.; Li, Y.; Gong, Y.; Shi, H.; Huo, Q.; Liu, Y.; Xu, Q. Stimuli-Responsive Delivery Vehicles Based on Mesoporous Silica Nanoparticles: Recent Advances and Challenges. J. Mater. Chem. B 2017, 5, 1339–1352. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, C. Advances in Silica Based Nanoparticles for Targeted Cancer Therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 317–332. [Google Scholar] [CrossRef]
- Feng, Y.; Panwar, N.; Tng, D.J.H.; Tjin, S.C.; Wang, K.; Yong, K.-T. The Application of Mesoporous Silica Nanoparticle Family in Cancer Theranostics. Coord. Chem. Rev. 2016, 319, 86–109. [Google Scholar] [CrossRef]
- Saroj, S.; Rajput, S.J. Composite Smart Mesoporous Silica Nanoparticles as Promising Therapeutic and Diagnostic Candidates: Recent Trends and Applications. J. Drug Deliv. Sci. Technol. 2018, 44, 349–365. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.X.; Meng, H. Mesoporous Silica Nanoparticles: A Multifunctional Nano Therapeutic System. Integr. Biol. UK 2013, 5, 19–28. [Google Scholar] [CrossRef]
- Pan, Y.; Xue, P.; Liu, S.; Zhang, L.; Guan, Q.; Zhu, J.; Tian, X. Metal-Based Hybrid Nanoparticles as Radiosensitizers in Cancer Therapy. Colloid Interface Sci. Commun. 2018, 23, 45–51. [Google Scholar] [CrossRef]
- Freitas, L.B.O.; Corgosinho, L.M.; Faria, J.A.Q.A.; dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; De Souza, E.M.B. Multifunctional Mesoporous Silica Nanoparticles for Cancer-Targeted, Controlled Drug Delivery and Imaging. Microporous Mesoporous Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- Ambrogio, M.W.; Thomas, C.R.; Zhao, Y.L.; Zink, J.I.; Stoddartt, J.F. Mechanized Silica Nanoparticles: A New Frontier in Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 903–913. [Google Scholar] [CrossRef] [Green Version]
- Mamaeva, V.; Sahlgren, C.; Linden, M. Mesoporous Silica Nanoparticles in Medicine--Recent Advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Mamaeva, M.J.; Mamaeva, V.; Sahlgren, C.; Linden, M. Nanopartricles in Targeted Cancer Therapy—Mesoporous Silica Nanopartciles Entering Preclinical Development Stage. Nanomed. Nanotechnol. Biol. Med. 2012, 7, 111–120. [Google Scholar] [CrossRef]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguia, J.R.; Martinez-Manez, R.; Sancenon, F. Gated Materials for on-Command Release of Guest Molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef]
- Lei, C.; Chen, B.; Li, X.; Qi, W.; Liu, J. Non-Destructively Shattered Mesoporous Silica for Protein Drug Delivery. Microporous Mesoporous Mater. Off. J. Int. Zeolite Assoc. 2013, 175, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Cheng, J.J. Nonporous Silica Nanoparticles for Nanomedicine Application. Nano Today 2013, 8, 290–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Miller, M.L.; Di Pasqua, A.J. Biocompatibility of Mesoporous Silica Nanoparticles? Comments Inorg. Chem. 2015, 36, 61–80. [Google Scholar] [CrossRef]
- Shao, D.; Lu, M.M.; Zhao, Y.W.; Zhang, F.; Tan, Y.F.; Zheng, X.; Pan, Y.; Xiao, X.-A.; Wang, Z.; Dong, W.-F.; et al. The Shape Effect of Magnetic Mesoporous Silica Nanoparticles on Endocytosis, Biocompatibility and Biodistribution. Acta Biomater. 2017, 49, 531–540. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, N.; Chen, L.; Xie, L.; Cui, M.; Li, S.; Xu, L. Effect of Shape on Mesoporous Silica Nanoparticles for Oral Delivery of Indomethacin. Pharmaceutics 2018, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Hernandez Montoto, A.; Montes, R.; Samadi, A.; Gorbe, M.; Terres, J.M.; Cao-Milan, R.; Aznar, E.; Ibanez, J.; Masot, R.; Marcos, M.D.; et al. Gold Nanostars Coated with Mesoporous Silica Are Effective and Nontoxic Photothermal Agents Capable of Gate Keeping and Laser-Induced Drug Release. ACS Appl. Mater. Interfaces 2018, 10, 27644–27656. [Google Scholar] [CrossRef]
- Adhikari, C.; Mishra, A.; Nayak, D.; Chakraborty, A. Metal Organic Frameworks Modified Mesoporous Silica Nanoparticles (Msn): A Nano-Composite System to Inhibit Uncontrolled Chemotherapeutic Drug Delivery from Bare-Msn. J. Drug Deliv. Sci. Technol. 2018, 47, 1–11. [Google Scholar] [CrossRef]
- Li, E.; Yang, Y.; Hao, G.; Yi, X.; Zhang, S.; Pan, Y.; Xing, B.; Gao, M. Multifunctional Magnetic Mesoporous Silica Nanoagents for in Vivo Enzyme-Responsive Drug Delivery and Mr Imaging. Nanotheranostics 2018, 2, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.H.; Liu, Z.N.; Bai, Y.K.; Zhou, Y.S.; Chen, X. Multiple-Responsive Mesoporous Silica Nanoparticles for Highly Accurate Drugs Delivery to Tumor Cells. Acs Omega 2018, 3, 4306–4315. [Google Scholar] [CrossRef] [Green Version]
- Angiolini, L.; Valetti, S.; Cohen, B.; Feiler, A.; Douhal, A. Fluorescence Imaging of Antibiotic Clofazimine Encapsulated within Mesoporous Silica Particle Carriers: Relevance to Drug Delivery and the Effect on Its Release Kinetics. Phys. Chem. Chem. Phys. 2018, 20, 11899–11911. [Google Scholar] [CrossRef]
- Sonmez, M.; Ficai, D.; Ficai, A.; Alexandrescu, L.; Georgescu, M.; Trusca, R.; Gurau, D.; Titu, M.A.; Andronescu, E. Applications of Mesoporous Silica in Biosensing and Controlled Release of Insulin. Int. J. Pharm. 2018, 549, 179–200. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M. Bone Tissue Engineering Using Silica-Based Mesoporous Nanobiomaterials:Recent Progress. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, Y.; Ren, L.; Zhao, N.; Gong, Y.; Wang, D.A. Novel Mesoporous Silica-Based Antibiotic Releasing Scaffold for Bone Repair. Acta Biomater. 2009, 5, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Chiari-Andréo, B.G.; Almeida-Cincotto, M.G.J.; Oshiro, J.A.; Taniguchi, C.Y.Y.; Chiavacci, L.A.; Isaac, V.L.B. Chapter 5—Nanoparticles for Cosmetic Use and Its Application. In Nanoparticles in Pharmacotherapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Cambridge, UK, 2019; pp. 113–146. [Google Scholar]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Schäfer-Korting, M.; Maibach, H.I. Measuring Silica Nanoparticles in the Skin. In Agache’s Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants; Humbert, P., Fanian, F., Maibach, H.I., Agache, P.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1141–1164. [Google Scholar]
- Poland, C.; Larsen, P.; Read, S.; Varet, J.; Hankin, S.; Lam, H.J. Assessment If Nano-Enabled Technologies in Cosmetics; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2006. [Google Scholar]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-Based Cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef]
- Petit, J.L.V.; Gonzalez, R.D.; Botello, A.F. Lipid Nanoparticle Capsules. Google Patents US20130017239A1, 17 January 2013. [Google Scholar]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered Silica: Properties, Applications and Toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef]
- Ambrogi, V.; Latterini, L.; Marmottini, F.; Pagano, C.; Ricci, M. Mesoporous Silicate Mcm-41 as a Particulate Carrier for Octyl Methoxycinnamate: Sunscreen Release and Photostability. J. Pharm. Sci. 2013, 102, 1468–1475. [Google Scholar] [CrossRef]
- Nafisi, S. Silica Nanoparticles for Increased Cosmetic Ingredient Efficacy. Cosmet. Toilet. 2015. Available online: https://www.cosmeticsandtoiletries.com/research/chemistry/Silica-Nanoparticles-for-Increased-Cosmetic-Ingredient-Efficacy--300987651.html (accessed on 19 August 2020).
- Laranjeira, M.; Shirosaki, Y.; Yasutomi, S.Y.; Miyazaki, T.; Monteiro, F.J. Enhanced Biosafety of Silica Coated Gadolinium Based Nanoparticles. J. Mater. Sci. Mater. Med. 2017, 28, 46. [Google Scholar] [CrossRef]
- Park, Y.-H.; Kim, J.N.; Jeong, S.H.; Choi, J.E.; Lee, S.-H.; Choi, B.H.; Lee, J.P.; Sohn, K.H.; Kim, M.-K.; Son, S.W. Assessment of Dermal Toxicity of Nanosilica Using Cultured Keratinocytes, a Human Skin Equivalent Model and an in Vivo Model. Toxicology 2010, 267, 178–181. [Google Scholar] [CrossRef]
- Liu, L.; Fu, X.; Zhang, H.; Ma, W.; Zhang, L.; Zhang, Y.; Liu, M.; Liang, K.; Hou, S.; Chen, A. Luminogen-Functionalized Mesoporous Sba-15 for Fluorescent Detection of Antibiotic Cefalexin. J. Mater. Res. 2018, 33, 1442–1448. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.L.; Wang, B.L.; Li, D.D.; Yu, J.H. Fluorescent Sensors Based on Aiegen-Functionalised Mesoporous Silica Nanoparticles for the Detection of Explosives and Antibiotics. Inorg. Chem. Front. 2018, 5, 2183–2188. [Google Scholar] [CrossRef]
- Erami, R.S.; Ovejero, K.; Meghdadi, S.; Filice, M.; Amirnasr, M.; Rodriguez-Dieguez, A.; de La Orden, M.U.; Gomez-Ruiz, S. Applications of Nanomaterials Based on Magnetite and Mesoporous Silica on the Selective Detection of Zinc Ion in Live Cell Imaging. Nanomaterials 2018, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, S.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Lavaee, P.P.; Nejabat, M.; Abnous, K.; Taghdisi, S.M. A Label-Free Fluorescent Aptasensor for Detection of Kanamycin Based on Dsdna-Capped Mesoporous Silica Nanoparticles and Rhodamine, B. Anal. Chim. Acta 2018, 1030, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, M.; Wang, K.; Chen, Z. Stable Mesoporous Silica Nanoparticles Incorporated with Mos2 and Aie for Targeted Fluorescence Imaging and Photothermal Therapy of Cancer Cells. Colloids Surf. B Biointerfaces 2019, 174, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Nanoporous Silica-Dye Microspheres for Enhanced Colorimetric Detection of Cyclohexanone. Chemosensors 2018, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Begum, G.; Oschatz, C.; Oschatz, M.; Kaskel, S.; Brunner, E.; Kröger, N. Influence of Silica Architecture on the Catalytic Activity of Immobilized Glucose Oxidase. Bioinspired Biomim. Nanobiomater. 2019, 8, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Aghayan, M.; Mahmoudi, A.; Sazegar, M.R.; Hajiagha, N.G.; Nazari, K. Enzymatic Activity of Fe-Grafted Mesoporous Silica Nanoparticles: An Insight into H2O2 and Glucose Detection. New J. Chem. 2018, 42, 16060–16068. [Google Scholar] [CrossRef]
- Gunduz, O.; Yetmez, M.; Sonmez, M.; Georgescu, M.; Alexandrescu, L.; Ficai, A.; Ficai, D.; Andronescu, E. Mesoporous Materials Used in Medicine and Environmental Applications. Curr. Top. Med. Chem. 2015, 15, 1501–1515. [Google Scholar] [CrossRef]
- Lehman, S.; Larsen, S. Zeolite and Mesoporous Silica Nanomaterials: Greener Syntheses, Environmental Applications and Biological Toxicity. Environ. Sci. Nano 2014, 1, 200. [Google Scholar] [CrossRef]
- Chen, H.; Huang, G.; Zhou, H.; Zhou, X.; Xu, H. Highly Efficient Triazolone/Metal Ion/Polydopamine/Mcm-41 Sustained Release System with Ph Sensitivity for Pesticide Delivery. R. Soc. Open Sci. 2018, 5, 180658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Chen, H.; Shi, J. In Vivo Bio-Safety Evaluations and Diagnostic/Therapeutic Applications of Chemically Designed Mesoporous Silica Nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Echiburu-Chau, C.; Roy, D. Organophosphorous Pesticides and Estrogen Induce Transformation of Breast Cells Affecting P53 and C-Ha-Ras Genes. Int. J. Oncol. 2009, 35, 1061–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrani, Z.; Ebrahimzadeh, H.; Asgharinezhad, A.A. Synthesis and Characterization of a Poly (P-Phenylenediamine)-Based Electrospun Nanofiber for the Micro-Solid-Phase Extraction of Organophosphorus Pesticides from Drinking Water and Lemon and Orange Juice Samples. J. Sep. Sci. 2018, 41, 3477–3485. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Gao, L.; Jiang, Q.; Hou, Q.; Hong, Y.; Shen, W.; Wang, Y.; Zhu, J.H. Fabricating Efficient Porous Sorbents to Capture Organophosphorus Pesticide in Solution. Microporous Mesoporous Mater. 2020, 294, 109911. [Google Scholar] [CrossRef]
- Aydin, S. Removal of Organophosphorus Pesticides from Aqueous Solution by Magnetic Fe3o4/Red Mud-Nanoparticles. Water Environ. Res. 2016, 88, 2275–2284. [Google Scholar] [CrossRef]
- Wei, M.; Yan, X.; Liu, S.; Liu, Y. Preparation and Evaluation of Superparamagnetic Core–Shell Dummy Molecularly Imprinted Polymer for Recognition and Extraction of Organophosphorus Pesticide. J. Mater. Sci. 2017, 53, 4897–4912. [Google Scholar] [CrossRef]
- Yaguchi, H.; Inoue, T.; Sasaki, K.; Maekawa, K. Dopamine Regulates Termite Soldier Differentiation through Trophallactic Behaviours. R. Soc. Open Sci. 2016, 3, 150574. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Lin, T.; Wu, H.; Guo, L.; Ye, P.; Hao, Y.; Guo, Q.; Jiang, J.; Fu, F.; Chen, G. Mussel-Inspired Polydopamine Coated Mesoporous Silica Nanoparticles as Ph-Sensitive Nanocarriers for Controlled Release. Int. J. Pharm. 2014, 463, 22–26. [Google Scholar] [CrossRef]
- Altenburger, R.; Backhaus, T.; Boedeker, W.; Faust, M.; Scholze, M. Simplifying Complexity: Mixture Toxicity Assessment in the Last 20 Years. Environ. Toxicol. Chem. 2013, 32, 1685–1687. [Google Scholar] [CrossRef]
- Nazir, A.; Cedergreen, N. Quantifying Synergy: A Systematic Review of Mixture Toxicity Studies within Environmental Toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef]
- Martin, T.M.; Young, D.M.; Lilavois, C.R.; Barron, M.G. Comparison of Global and Mode of Action-Based Models for Aquatic Toxicity. SAR QSAR Environ. Res. 2015, 26, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, S.-S.; Qu, R.; Liu, H.-L. Global Concentration Additivity and Prediction of Mixture Toxicities, Taking Nitrobenzene Derivatives as an Example. Ecotoxicol. Environ. Saf. 2017, 144, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Li, K.; Li, T.; Qu, R. Comments on “the Synergistic Toxicity of the Multi Chemical Mixtures: Implications for Risk Assessment in the Terrestrial Environment”. Environ. Int. 2016, 94, 396–398. [Google Scholar] [CrossRef]
- Stork, L.G.; Gennings, C.; Carter, W.H.; Johnson, R.E.; Mays, D.P.; Simmons, J.E.; Wagner, E.D.; Plewa, M.J. Testing for Additivity in Chemical Mixtures Using a Fixed-Ratio Ray Design and Statistical Equivalence Testing Methods. J. Agric. Biol. Environ. Stat. 2007, 12, 514–533. [Google Scholar] [CrossRef]
- Su, L.; Meng, Q.; Yuan, X. Joint Toxicity of Aniline and Nitroanilines to Daphnia Magna. Res. Environ. Sci. 2002, 15, 42–44. [Google Scholar]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals ((*) Oh). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Liu, T.; Sun, C.; Ta, N.; Hong, J.; Yang, S.; Chen, C. Effect of Copper on the Degradation of Pesticides Cypermethrin and Cyhalothrin. J. Environ. Sci. 2007, 19, 1235–1238. [Google Scholar] [CrossRef]
- Kim, E.Y.; Chae, H.J.; Chu, K.H. Enzymatic Oxidation of Aqueous Pentachlorophenol. J. Environ. Sci. 2007, 19, 1032–1036. [Google Scholar] [CrossRef]
- Diao, J.; Zhao, G.; Li, Y.; Huang, J.; Sun, Y. Carboxylesterase from Spodoptera Litura: Immobilization and Use for the Degradation of Pesticides. Procedia Environ. Sci. 2013, 18, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Glenn, H.; Bradley, F.; Chmelka, F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Am. Assoc. Adv. Sci. 2016, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbs, A.J.; Williams, N. Indoor Air Pollution from Pesticides Used in Wood Remedial Treatments. Environ. Pollut. 1983, 6, 271–296. [Google Scholar] [CrossRef]
- Morris, T.-A.; Huddersman, K. Gas and Liquid Phase Sorption Studies of Lindane on Nay and Mcm-41 Molecular Sieves. Phys. Chem. 1999, 1, 4673E80. [Google Scholar] [CrossRef]
- Chanda, W.; Manyepa, M.; Chikwanda, E.; Daka, V.; Chileshe, J.; Tembo, M.; Kasongo, J.; Chipipa, A.; Handema, R.; Mulemena, J.A. Evaluation of Antibiotic Susceptibility Patterns of Pathogens Isolated from Routine Laboratory Specimens at Ndola Teaching Hospital: A Retrospective Study. PLoS ONE 2019, 14, e0226676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, P.V. Chapter 19—Medical Biotechnology: Techniques and Applications. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 449–469. [Google Scholar]
- Ouwehand, A.C.; Tennilä, J. Chapter 18—Probiotics and Antibiotic Use. In Probiotics, Prebiotics, and Synbiotics; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 271–277. [Google Scholar]
- Vallero, D.A. Chapter 1—Environmental Biotechnology: An overview. In Environmental Biotechnology, 2nd ed.; Vallero, D.A., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 1–40. [Google Scholar]
- Stefanakis, D.; Ghanotakis, D. Synthesis and Characterization of Nanoparticles, Consisting of a Gadolinium Paramagnetic Core and a Mesoporous Silica Shell, for Controlled Delivery of Hydrophobic Drugs. J. Nanopart. Res. 2013, 16, 2211. [Google Scholar] [CrossRef]
- Namazi, H.; Rakhshaei, R.; Hamishehkar, H.; Kafil, H.S. Antibiotic Loaded Carboxymethylcellulose/Mcm-41 Nanocomposite Hydrogel Films as Potential Wound Dressing. Int. J. Biol. Macromol. 2016, 85, 327–334. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H. A Potential Bioactive Wound Dressing Based on Carboxymethyl Cellulose/Zno Impregnated Mcm-41 Nanocomposite Hydrogel. Mat. Sci. Eng. C Mater. 2017, 73, 456–464. [Google Scholar] [CrossRef]
- Zeng, Z.-W.; Tan, X.-F.; Liu, Y.-G.; Tian, S.-R.; Zeng, G.-M.; Jiang, L.-H.; Yin, Z.-H.; Liu, N.; Liu, S.-B.; Li, J. Comprehensive Adsorption Studies of Doxycycline and Ciprofloxacin Antibiotics by Biochars Prepared at Different Temperatures. Front. Chem. 2018, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.A.; Janjani, H. Antibiotics Adsorption from Aqueous Solutions Using Carbon Nanotubes: A Systematic Review. Toxin Rev. 2020, 39, 87–98. [Google Scholar] [CrossRef]
- Wikandari, R.; Sanjaya, A.P.; Millati, R.; Karimi, K.; Taherzadeh, M.J. Chapter 20—Fermentation Inhibitors in Ethanol and Biogas Processes and Strategies to Counteract Their Effects. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 461–499. [Google Scholar]
- Wang, S.; Wang, H. Adsorption Behavior of Antibiotic in Soil Environment: A Critical Review. Front. Environ. Sci. Eng. 2015, 9, 565–574. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Mokhtari, M.; Babaei, F.; Malek Ahmadi, R.; Ehrampoush, M.H.; Faramarzian, M. Removal Methods of Antibiotic Compounds from Aqueous Environments–a Review. J. Environ. Health Sustain. Dev. 2016, 1, 43–62. [Google Scholar]
- Li, D.; Liu, S. Chapter 13—Detection of Industrial Water Quality. In Water Quality Monitoring and Management; Li, D., Liu, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 329–349. [Google Scholar]
- García Lozano, M.; Peña García, Y.; Silva Gonzalez, J.A.; Ochoa Bañuelos, C.V.; Luevanos Escareño, M.P.; Balagurusamy, N. Chapter 40—Biosensors for Food Quality and Safety Monitoring: Fundamentals and Applications. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 691–709. [Google Scholar]
- Pruden, A. Chapter 8—Antibiotics and Antibiotic Resistance: Closing the Loop between Hospitals and the Environment. In Health Care and Environmental Contamination; Boxall, A.B.A., Kookana, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 137–148. [Google Scholar]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive Removal of Antibiotics from Water and Wastewater: Progress and Challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.J.; Zhao, Z.W.; Sun, T.Y.; Shi, W.X.; Cui, F.Y. Adsorption of Quinolone Antibiotics in Spherical Mesoporous Silica: Effects of the Retained Template and Its Alkyl Chain Length. J. Hazard. Mater. 2016, 305, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Otalvaro, J.O.; Avena, M.; Brigante, M. Adsorption of Norfloxacin on a Hexagonal Mesoporous Silica: Isotherms, Kinetics and Adsorbent Reuse. Adsorption 2019, 25, 1375–1385. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, Y.M.; Li, L.S.; Liu, P.H.; Li, X.K.; Chen, W.R.; Xue, Y. The Correlation of Adsorption Behavior between Ciprofloxacin Hydrochloride and the Active Sites of Fe-Doped Mcm-41. Front. Chem. 2018, 6, 17. [Google Scholar] [CrossRef]
- Panahi, A.H.; Ashrafi, S.D.; Kamani, H.; Khodadadi, M.; Lima, E.C.; Mostafapour, F.K.; Mahvi, A.H. Removal of Cephalexin from Artificial Wastewater by Mesoporous Silica Materials Using Box-Behnken Response Surface Methodology. Desalin. Water Treat. 2019, 159, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Yadav, H.K.S.; Raizaday, A. Chapter 12—Inorganic Nanobiomaterials for Medical Imaging. In Nanobiomaterials in Medical Imaging; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 365–401. [Google Scholar]
- Mysen, B.; Richet, P. Chapter 5—Silica. In Silicate Glasses and Melts, 2nd ed.; Mysen, B., Richet, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–183. [Google Scholar]







| MSN Type | Dimensionality and Crystal System | Space Group | Pore Size [nm] | Surface Area [m2/g] | Pore Volume [cm3/g] | Reference |
|---|---|---|---|---|---|---|
| MCM-41 | 2D hexagonal | P6mm | 1.5–8 | 900–2100 | >1 | [15,23,24,25] |
| MCM-48 | cubic | Ia3d | 1.5–6.5 | 900–1500 | >1 | [15,23,26] |
| MCM-50 | lamellar | p2 | 2–5 | n.a. | >1 | [15,23,27] |
| SBA-11 | cubic | Pm3m | 2.1–3.6 | n.a. | 0.68 | [15,23,27] |
| SBA-12 | 3D hexagonal | P63/mmc | 3.1 | n.a. | 0.83 | [15,23,27] |
| SBA-15 | 2D hexagonal | p6mm | 6–10 | 662 | 1.17 | [15,23,28] |
| SBA-16 | cubic | Im3m | 5–15 | 1000 | 0.91 | [15,23,27] |
| FSM-16 | 2D hexagonal | p6mm | 3.2–3.9 | 500–900 | 0.96 | [29,30,31] |
| TUD-1 | disordered | - | 2.5–25 | 300–1000 | 0.5–1.7 | [32,33,34] |
| HMM-33 | disordered | - | 4–15 | - | - | [35] |
| COK-12 | hexagonal | P6m | 5.5–6 | 860 | 0.45–1.23 | [15,35,36] |
| FDU-2 | cubic | Fd3m | 2.3–3 | 960 | 0.98 | [37] |
| FDU-11 | tetragonal | P4/mmm | 2.7 | 1490 | 1.88 | [38] |
| FDU-12 | cubic | Fm3m | 36 | 250–450 | 0.27–0.48 | [39] |
| FDU-13 | orthorhombic | Pmmm | 1.7 | 1210 | 1.83 | [38] |
| MSN Type | pH | Silica Precursor | Surfactant | Additives |
|---|---|---|---|---|
| MCM-41 | basic | TEOS, TMOS | CTAB, cetyltrimethylammonium tosylate, cetyltrimethylammonium chloride, Pluronic F68 | - |
| MCM-48 | basic | TEOS | CTAB | - |
| MCM-50 | basic | TEOS, TMOS | Gemini surfactants | |
| SBA-15 | acid | TEOS, TMOS | Pluronic P123 | - |
| SBA-16 | acid | TEOS | Pluronic P123, Pluronic F127 | potassium chloride |
| FDU-12 | acid | TEOS | Pluronic F123 | 1,3,5-trimethylbenzene, xylene, toluene, potassium chloride |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chircov, C.; Spoială, A.; Păun, C.; Crăciun, L.; Ficai, D.; Ficai, A.; Andronescu, E.; Turculeƫ, Ș.C. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules 2020, 25, 3814. https://doi.org/10.3390/molecules25173814
Chircov C, Spoială A, Păun C, Crăciun L, Ficai D, Ficai A, Andronescu E, Turculeƫ ȘC. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules. 2020; 25(17):3814. https://doi.org/10.3390/molecules25173814
Chicago/Turabian StyleChircov, Cristina, Angela Spoială, Cătălin Păun, Luminița Crăciun, Denisa Ficai, Anton Ficai, Ecaterina Andronescu, and Ștefan Claudiu Turculeƫ. 2020. "Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents" Molecules 25, no. 17: 3814. https://doi.org/10.3390/molecules25173814