Flavonoids and Related Members of the Aromatic Polyketide Group in Human Health and Disease: Do They Really Work?
Abstract
:1. Introduction
2. Flavonoids and Stilbenes
2.1. Role in Plants
2.2. Roles in Humans
2.2.1. Antioxidant Activity of Flavonoids and Stilbenes
2.2.2. Health Benefits of Common Dietary Flavonoids and Stilbenes via Non-Antioxidant Mechanisms
3. Aromatic Polyketides with More Restricted Distribution
3.1. Synthetic Flavonoids
3.2. Stilbenes
3.3. Styrylpyrones and Diarylheptanoids
3.4. Flavonolignans
3.5. Isoflavonoids
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Verpoorte, R. Exploration of nature’s chemodiversity: The role of secondary metabolites as leads in drug development. Drug Discov. Today 1998, 3, 232–238. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirumurugan, D.; Cholarajan, A.; Raja, S.S.S.; Vijayakumar, R. An Introductory Chapter: Secondary Metabolites. In Secondary Metabolites. Sources and Applications; Vijayakumar, R., Raja, S.S.S., Eds.; IntechOpen: London, UK, 2018; pp. 3–21. ISBN 978-1-78923-643-9. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.-T.; Kuhnle, G.G.C. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 1995, 12, 579–607. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Ayabe, S.-I.; Akashi, T. Cytochrome P450s in flavonoid metabolism. Phytochem. Rev. 2006, 5, 271–282. [Google Scholar] [CrossRef]
- Springob, K.; Nakajima, J.-I.; Yamazaki, M.; Saito, K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat. Prod. Rep. 2003, 20, 288–303. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Dixon, R.A. Proanthocyanidin biosynthesis - Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [Green Version]
- Harborne, J.B. The Flavonoids: Advances in Research since 1986, 1st ed.; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Boumendjel, A.; Di Pietro, A.; Dumontet, C.; Barron, D. Recent advances in the discovery of flavonoids and analogs with high-affinity binding to P-glycoprotein responsible for cancer cell multidrug resistance. Med. Res. Rev. 2002, 22, 512–529. [Google Scholar] [CrossRef]
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: Chemistry and biological activities. Nat. Prod. Res. 2019, 33, 3260–3272. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Introduction to Ecological Biochemistry, 4th ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S.J. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [Green Version]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Jaklová Dytrtová, J.; Straka, M.; Bělonožníková, K.; Jakl, M.; Ryšlavá, H. Does resveratrol retain its antioxidative properties in wine? Redox behaviour of resveratrol in the presence of Cu(II) and tebuconazole. Food Chem. 2018, 262, 221–225. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine–a diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.L.; Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Iannitti, R.; Palumbo, R. Quercetin: A pleiotropic kinase inhibitor against cancer. Cancer Treat. Res. 2014, 159. [Google Scholar] [CrossRef]
- Van Der Woude, H.; Ter Veld, M.G.R.; Jacobs, N.; Van Der Saag, P.T.; Murk, A.J.; Rietjens, I.M.C.M. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Mol. Nutr. Food Res. 2005, 49, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.L.; Mcfarland, M.; Niles, R.M. Molecular and physiological actions of quercetin: Need for clinical trials to assess its benefits in human disease. Nutr. Rev. 2014, 72, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, I.A. Warning Letter to Cape Fear Naturals. In Food and Drug Administration. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/cape-fear-naturals-512768-03022017 (accessed on 20 September 2019).
- Kim, S.-H.; Choi, K.-C. Anti-cancer effect and underlying mechanism(s) of Kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol. Res. 2013, 29, 229–234. [Google Scholar] [CrossRef]
- Thors, L.; Belghiti, M.; Fowler, C.J. Inhibition of fatty acid amide hydrolase by kaempferol and related naturally occurring flavonoids. Br. J. Pharmacol. 2008, 155, 244–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeresham, C.; Rama Rao, A.; Asres, K. Aldose reductase inhibitors of plant origin. Phytother. Res. 2014, 28, 317–333. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.-H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.A.; Tarahovsky, Y.S.; Gaidin, S.G.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoids determine the rate of fibrillogenesis and structure of collagen type I fibrils in vitro. Int. J. Biol. Macromol. 2017, 104, 631–637. [Google Scholar] [CrossRef]
- Anu, S.M.; Kim, H.J.; Kim, J.-E.; Boo, Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother. Res. 2008, 22, 1200–1207. [Google Scholar] [CrossRef]
- Katavic, P.L.; Lamb, K.; Navarro, H.; Prisinzano, T.E. Flavonoids as opioid receptor ligands: Identification and preliminary structure-activity relationships. J. Nat. Prod. 2007, 70, 1278–1282. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Kuboyama, T.; Tohda, C. A systematic strategy for discovering a therapeutic drug for Alzheimer’s disease and its target molecule. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.E.; Vauzour, D.; Rendeiro, C. Flavonoids and cognition: The molecular mechanisms underlying their behavioural effects. Arch. Biochem. Biophys. 2009, 492, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Sekar, S.; Barathidasan, R.; Manivasagam, T.; Thenmozhi, A.J.; Sevanan, M.; Chidambaram, S.B.; Essa, M.M.; Guillemin, G.J.; Sakharkar, M.K. Naringenin Decreases α-Synuclein Expression and Neuroinflammation in MPTP-Induced Parkinson’s Disease Model in Mice. Neurotox. Res. 2018, 33, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Venigalla, M.; Gyengesi, E.; Münch, G. Curcumin and apigenin–Novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural Regen. Res. 2015, 10, 1181–1185. [Google Scholar] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The therapeutic potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Si, D.; Wang, Y.; Zhou, Y.-H.; Guo, Y.; Wang, J.; Zhou, H.; Li, Z.-S.; Fawcett, J.P. Mechanism of CYP2C9 inhibition by flavones and flavonols. Drug Metab. Dispos. 2009, 37, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.C.; Khoo, H.-E. Biological effects of myricetin. Gen. Pharmacol. 1997, 29, 121–126. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y. Minireview: Therapeutic potential of myricetin in diabetes mellitus. Food Sci. Hum. Wellness 2012, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Strobel, P.; Allard, C.; Perez-Acle, T.; Calderon, R.; Aldunate, R.; Leighton, F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem. J. 2005, 386, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zheng, X.; Yi, X.; Liu, C.; Kong, D.; Zhang, J.; Gong, M. Myricetin: A potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 2603–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Lindemeyer, K.; Gonzalez, C.; Shao, X.M.; Spigelman, I.; Olsen, R.W.; Liang, J. Dihydromyricetin as a novel anti-alcohol intoxication medication. J. Neurosci. 2012, 32, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Ellinger, S.; Reusch, A.; Stehle, P.; Helfrich, H.-P. Epicatechin ingested via cocoa products reduces blood pressure in humans: A nonlinear regression model with a Bayesian approach. Am. J. Clin. Nutr. 2012, 95, 1365–1377. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809–2830. [CrossRef] [Green Version]
- Vogiatzoglou, A.; Mulligan, A.A.; Bhaniani, A.; Lentjes, M.A.H.; McTaggart, A.; Luben, R.N.; Heiss, C.; Kelm, M.; Merx, M.W.; Spencer, J.P.E.; et al. Associations between flavan-3-ol intake and CVD risk in the Norfolk cohort of the European Prospective Investigation into Cancer (EPIC-Norfolk). Free Radic. Biol. Med. 2015, 84, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Zięba, K.; Makarewicz-Wujec, M.; Kozłowska-Wojciechowska, M. Cardioprotective Mechanisms of Cocoa. J. Am. Coll. Nutr. 2019, 38, 564–575. [Google Scholar] [CrossRef]
- Martinez, S.E.; Davies, N.M.; Reynolds, J.K. Toxicology and Safety of Flavonoids. In Flavonoid Pharmacokinetics: Methods of Analysis, Preclinical and Clinical Pharmacokinetics, Safety, and Toxicology; Davies, N.M., Yáñez, J.A., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 249–280. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1489–1551. [CrossRef] [Green Version]
- Schoonees, A.; Visser, J.; Musekiwa, A.; Volmink, J. Pycnogenol(®) for the treatment of chronic disorders. Cochrane Database Syst. Rev. Online 2012, 2, CD008294. [Google Scholar]
- Riegsecker, S.; Wiczynski, D.; Kaplan, M.J.; Ahmed, S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013, 93, 307–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignes, M. Anxiolytic Properties of the Green Tea Polyphenol (-)-Epigallocatechin Gallate. In Tea in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 1399–1409. [Google Scholar]
- Momose, Y.; Maeda-Yamamoto, M.; Nabetani, H. Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int. J. Food Sci. Nutr. 2016, 67, 606–613. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults–Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Dou, J.; Temple, R.; Agarwal, R.; Wu, K.-M.; Walker, S. New therapies from old medicines. Nat. Biotechnol. 2008, 26, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Maeda-Yamamoto, M.; Usui, S.; Fujisawa, T. “Benifuuki” green tea containing O-methylated catechin reduces symptoms of Japanese cedar pollinosis: A randomized, double- blind, placebo-controlled trial. Allergol. Int. 2014, 63, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, D.J.; Lu, G.P.; Cai, N.S.; Wu, Z.G.; Li, Y.H.; Chen, H.; Zhu, J.Q.; Jin, X.J.; Wouters, B.C.; Zhao, J. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: A randomized controlled trial. Arch. Intern. Med. 2003, 163, 1448–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trautwein, E.A.; Du, Y.; Meynen, E.; Yan, X.; Wen, Y.; Wang, H.; Molhuizen, H.O.F. Purified black tea theaflavins and theaflavins/catechin supplements did not affect serum lipids in healthy individuals with mildly to moderately elevated cholesterol concentrations. Eur. J. Nutr. 2010, 49, 27–35. [Google Scholar] [CrossRef]
- Steptoe, A.; Gibson, E.L.; Vounonvirta, R.; Williams, E.D.; Hamer, M.; Rycroft, J.A.; Erusalimsky, J.D.; Wardle, J. The effects of tea on psychophysiological stress responsivity and post-stress recovery: A randomised double-blind trial. Psychopharmacology 2007, 190, 81–89. [Google Scholar] [CrossRef]
- Saito, A.; Nakazato, R.; Suhara, Y.; Shibata, M.; Fukui, T.; Ishii, T.; Asanuma, T.; Mochizuki, K.; Nakayama, T.; Osakabe, N. The impact of theaflavins on systemic-and microcirculation alterations: The murine and randomized feasibility trials. J. Nutr. Biochem. 2016, 32, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, H.; Zhao, Q.; He, Y.; Niu, J.; Debnath, A.K.; Wu, S.; Jiang, S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim. Biophys. Acta Gen. Subj. 2005, 1723, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zapata, M.J.; Vernooij, R.W.; Uriona Tuma, S.M.; Stein, A.T.; Moreno, R.M.; Vargas, E.; Capellà, D.; Bonfill Cosp, X. Phlebotonics for venous insufficiency. Cochrane Database Syst. Rev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Morling, J.R.; Broderick, C.; Yeoh, S.E.; Kolbach, D.N. Rutosides for treatment of post-thrombotic syndrome. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Ramezani-Jolfaie, N.; Lorzadeh, E.; Khoshbakht, Y.; Salehi-Abargouei, A. Hesperidin, a major flavonoid in orange juice, might not affect lipid profile and blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. Phytother. Res. 2019, 33, 534–545. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A.; Perry, C.M. Micronised purified flavonoid fraction: A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs 2003, 63, 71–100. [Google Scholar] [CrossRef]
- Astashov, V.; Timchenko, D. Benefits of micronized purified flavonoid fraction in the reduction of symptoms after operation for hemorrhoidal disease. Phlebolymphology 2014, 21, 95–99. [Google Scholar]
- Edwards, D.J.; Bernier, S.M. Naringin and naringenin are not the primary CYP3A inhibitors in grapefruit juice. Life Sci. 1996, 59, 1025–1030. [Google Scholar] [CrossRef]
- Pirmohamed, M. Drug-grapefruit juice interactions. BMJ Online 2013, 346. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, K.; Zhou, Y.; An, Y.; Hu, T.; Lu, J.; Huang, S.; Pei, G. Naringin dihydrochalcone ameliorates cognitive deficits and neuropathology in APP/PS1 transgenic mice. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Kashani-Amin, E.; Larijani, B.; Ebrahim-Habibi, A. Neohesperidin dihydrochalcone: Presentation of a small molecule activator of mammalian alpha-amylase as an allosteric effector. FEBS Lett. 2013, 587, 652–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waalkens-Berendsen, D.H.; Kuilman-Wahls, M.E.M.; Bär, A. Embryotoxicity and teratogenicity study with neohesperidin dihydrochalcone in rats. Regul. Toxicol. Pharmacol. 2004, 40, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jamshidi, A.; Nikbakht-Nasrabadi, E.; Khosravi-Boroujeni, H. Green tea catechins and blood pressure: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Nutr. 2014, 53, 1299–1311. [Google Scholar] [CrossRef]
- Azzini, E.; Giacometti, J.; Russo, G.L. Antiobesity Effects of Anthocyanins in Preclinical and Clinical Studies. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Blachly, J.S.; Byrd, J.C. Emerging drug profile: Cyclin-dependent kinase inhibitors. Leuk. Lymphoma 2013, 54, 2133–2143. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Resveratrol chemosensitizes TNF-β-induced survival of 5-FU-treated colorectal cancer cells. Nutrients 2018, 10, 888. [Google Scholar] [CrossRef] [Green Version]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Induction of the epithelial-to-mesenchymal transition of human colorectal cancer by human TNF-β (Lymphotoxin) and its reversal by resveratrol. Nutrients 2019, 11, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, M.M.; Jørgensen, J.O.L.; Jessen, N.; Richelsen, B.; Pedersen, S.B. Resveratrol in metabolic health: An overview of the current evidence and perspectives. Ann. N. Y. Acad. Sci. 2013, 1290, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol in primary and secondary prevention of cardiovascular disease: A dietary and clinical perspective. Ann. N. Y. Acad. Sci. 2013, 1290, 37–51. [Google Scholar] [CrossRef]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [Green Version]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus-systematic review and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef]
- Alarcón De La Lastra, C.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.C.; Veiga, F.; Ribeiro, A.J. New delivery systems to improve the bioavailability of resveratrol. Expert Opin. Drug Deliv. 2011, 8, 973–990. [Google Scholar] [CrossRef]
- Ahmed, T.; Javed, S.; Javed, S.; Tariq, A.; Šamec, D.; Tejada, S.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Resveratrol and Alzheimer’s Disease: Mechanistic Insights. Mol. Neurobiol. 2017, 54, 2622–2635. [Google Scholar] [CrossRef]
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F.G. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1605–1618. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.; Dempsey, R.J.; Vemuganti, R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem. Int. 2015, 89, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, H.C.; Hunt, K.M.; Diamond, A.; Elmets, C.A.; Afaq, F. Phytochemicals for the management of melanoma. Mini-Rev. Med. Chem. 2016, 16, 953–979. [Google Scholar]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Pettit, G.R.; Singh, S.B.; Niven, M.L.; Hamei, E.; Schmidt, J.M. Isolation, structure, and synthesis of combretastatins A-l and B-l, potent new inhibitors of microtubule assembly, derived from combretum caffrum. J. Nat. Prod. 1987, 50, 119–131. [Google Scholar] [CrossRef]
- Cirla, A.; Mann, J. Combretastatins: From natural products to drug discovery. Nat. Prod. Rep. 2003, 20, 558–564. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Sorba, G.; Pagliai, F.; Busacca, S.; Genazzani, A.A. Medicinal chemistry of combretastatin A4: Present and future directions. J. Med. Chem. 2006, 49, 3033–3044. [Google Scholar] [CrossRef]
- Nam, N.-H. Combretastatin A-4 analogues as antimitotic antitumor agents. Curr. Med. Chem. 2003, 10, 1697–1722. [Google Scholar] [CrossRef]
- Nagaiah, G.; Remick, S.C. Combretastatin A4 phosphate: A novel vascular disrupting agent. Future Oncol. 2010, 6, 1219–1228. [Google Scholar] [CrossRef]
- Young, S.L.; Chaplin, D.J. Combretastatin A4 phosphate: Background and current clinical status. Expert Opin. Investig. Drugs 2004, 13, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Grisham, R.; Ky, B.; Tewari, K.S.; Chaplin, D.J.; Walker, J. Clinical trial experience with CA4P anticancer therapy: Focus on efficacy, cardiovascular adverse events, and hypertension management. Gynecol. Oncol. Res. Pract. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Hori, K.; Saito, S.; Kubota, K. A novel combretastatin A-4 derivative, AC7700, strongly stanches tumour blood flow and inhibits growth of tumours developing in various tissues and organs. Br. J. Cancer 2002, 86, 1604–1614. [Google Scholar] [CrossRef] [Green Version]
- O’Boyle, N.M.; Carr, M.; Greene, L.M.; Bergin, O.; Nathwani, S.M.; McCabe, T.; Lloyd, D.G.; Zisterer, D.M.; Meegan, M.J. Synthesis and evaluation of azetidinone analogues of combretastatin A-4 as tubulin targeting agents. J. Med. Chem. 2010, 53, 8569–8584. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Sun, L.; Lou, H.; Ji, M. Synthesis and biological evaluation of Combretastatin A-4 derivatives containing a 3′-O-substituted carbonic ether moiety as potential antitumor agents. Chem. Cent. J. 2013, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauchen, J. Natural products and their (semi-)synthetic forms in treatment of migraine: History and current status. Curr. Med. Chem. 2019, 27, 3784–3808. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Stough, C.; Bousman, C.A.; Wahid, Z.T.; Murray, G.; Teschke, R.; Savage, K.M.; Dowell, A.; Ng, C.; Schweitzer, I. Kava in the treatment of generalized anxiety disorder: A double-blind, randomized, placebo-controlled study. J. Clin. Psychopharmacol. 2013, 33, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Ooi, S.L.; Henderson, P.; Pak, S.C. Kava for generalized anxiety disorder: A review of current evidence. J. Altern. Complement. Med. 2018, 24, 770–780. [Google Scholar] [CrossRef]
- Ligresti, A.; Villano, R.; Allarà, M.; Ujváry, I.; Di Marzo, V. Kavalactones and the endocannabinoid system: The plant-derived yangonin is a novel CB 1 receptor ligand. Pharmacol. Res. 2012, 66, 163–169. [Google Scholar] [CrossRef]
- Whitton, P.A.; Lau, A.; Salisbury, A.; Whitehouse, J.; Evans, C.S. Kava lactones and the kava-kava controversy. Phytochemistry 2003, 64, 673–679. [Google Scholar] [CrossRef]
- Dasgupta, A. Effect of Herbal Remedies on Clinical Laboratory Tests. In Accurate Results in the Clinical Laboratory: A Guide to Error Detection and Correction; Dasgupta, A., Sepulveda, J.L., Eds.; Elsevier: London, UK, 2013; pp. 75–92. [Google Scholar]
- Teschke, R.; Sarris, J.; Schweitzer, I. Kava hepatotoxicity in traditional and modern use: The presumed Pacific kava paradox hypothesis revisited. Br. J. Clin. Pharmacol. 2012, 73, 170–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teschke, R. Kava hepatotoxicity: Pathogenetic aspects and prospective considerations. Liver Int. 2010, 30, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.N. Potential for interaction of kava and St. John’s wort with drugs. J. Ethnopharmacol. 2005, 100, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of EMT. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [Green Version]
- Toden, S.; Okugawa, Y.; Buhrmann, C.; Nattamai, D.; Anguiano, E.; Baldwin, N.; Shakibaei, M.; Boland, C.R.; Goel, A. Novel evidence for curcumin and boswellic acid-induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev. Res. 2015, 8, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. Curcumin May (Not) Defy Science. ACS Med. Chem. Lett. 2017, 8, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Bisson, J.; McAlpine, J.B.; Friesen, J.B.; Chen, S.-N.; Graham, J.; Pauli, G.F. Can Invalid Bioactives Undermine Natural Product-Based Drug Discovery? J. Med. Chem. 2016, 59, 1671–1690. [Google Scholar] [CrossRef]
- Baker, M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017, 541, 144–145. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Cheng, A.-L. Clinical studies with curcumin. Adv. Exp. Med. Biol. 2007, 595, 471–480. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef]
- Lasoff, D.R.; Cantrell, F.L.; Ly, B.T. Death associated with intravenous turmeric (Curcumin) preparation. Clin. Toxicol. 2018, 56, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Timmermann, B.N. Comparative effects of two gingerol-containing zingiber officinale extracts on experimental Rheumatoid arthritis. J. Nat. Prod. 2009, 72, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarnell, E. Herbal medicine and migraine. Altern. Complement. Ther. 2017, 23, 1–10. [Google Scholar] [CrossRef]
- Hitomi, S.; Ono, K.; Terawaki, K.; Matsumoto, C.; Mizuno, K.; Yamaguchi, K.; Imai, R.; Omiya, Y.; Hattori, T.; Kase, Y.; et al. [6]-gingerol and [6]-shogaol, active ingredients of the traditional Japanese medicine hangeshashinto, relief oral ulcerative mucositis-induced pain via action on Na+ channels. Pharmacol. Res. 2017, 117, 288–302. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Gažák, R.; Walterová, D.; Křen, V. Silybin and silymarin - New and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef]
- Poppe, L.; Petersen, M. Variation in the flavonolignan composition of fruits from different Silybum marianum chemotypes and suspension cultures derived therefrom. Phytochemistry 2016, 131, 68–75. [Google Scholar] [CrossRef]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition… and why does it matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Ferenci, P. Silymarin in the treatment of liver diseases: What is the clinical evidence? Clin. Liver Dis. 2016, 7, 8–10. [Google Scholar] [CrossRef]
- Mengs, U.; Pohl, R.-T.; Mitchell, T. Legalon® SIL: The antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr. Pharm. Biotechnol. 2012, 13, 1964–1970. [Google Scholar] [CrossRef]
- Loguercio, C.; Andreone, P.; Brisc, C.; Brisc, M.C.; Bugianesi, E.; Chiaramonte, M.; Cursaro, C.; Danila, M.; De Sio, I.; Floreani, A.; et al. Silybin combined with phosphatidylcholine and vitamin e in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 1658–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapčík, O. Isoflavonoids in non-leguminous taxa: A rarity or a rule? Phytochemistry 2007, 68, 2909–2916. [Google Scholar] [CrossRef]
- Mikšátková, P.; Lanková, P.; Huml, L.; Lapčík, O. Isoflavonoids in the Amaryllidaceae family. Nat. Prod. Res. 2014, 28, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Botta, B.; Menendez, P.; Zappia, G.; De Lima, R.A.; Torge, R.; Delle Monache, G. Prenylated isoflavonoids: Botanical distribution, structures, biological activities and biotechnological studies. An update (1995–2006). Curr. Med. Chem. 2009, 16, 3414–3468. [Google Scholar] [CrossRef] [PubMed]
- Miadoková, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Tit, D.M.; Bungau, S.; Iovan, C.; Nistor Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef] [Green Version]
- Lethaby, A.; Marjoribanks, J.; Kronenberg, F.; Roberts, H.; Eden, J.; Brown, J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst. Rev. 2013, 2013. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Bentz, E.-K.; Leodolter, S.; Tscherne, G.; Reuss, F.; Cross, H.S.; Huber, J.C. Phytoestrogens in clinical practice: A review of the literature. Fertil. Steril. 2007, 87, 1243–1249. [Google Scholar] [CrossRef]
- Wuttke, W.; Jarry, H.; Seidlová-Wuttke, D. Isoflavones-Safe food additives or dangerous drugs? Ageing Res. Rev. 2007, 6, 150–188. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Peñalvo, J.L.; Gil, J.I.; Medina, S.; Horcajada, M.N.; Lafay, S.; Silberberg, M.; Llorach, R.; Zafrilla, P.; García-Mora, P.; et al. Soy isoflavones and cardiovascular disease epidemiological, clinical and-omics perspectives. Curr. Pharm. Biotechnol. 2012, 13, 624–631. [Google Scholar] [CrossRef]
- Bilal, I.; Chowdhury, A.; Davidson, J.; Whitehead, S. Phytoestrogens and prevention of breast cancer: The contentious debate. World J. Clin. Oncol. 2014, 5, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Cawood, E.; Kinniburgh, D.; Provan, A.; Collins, A.R.; Irvine, D.S. Effect of a phytoestrogen food supplement on reproductive health in normal males. Clin. Sci. 2001, 100, 613–618. [Google Scholar] [CrossRef] [Green Version]
- Hamilton-Reeves, J.M.; Vazquez, G.; Duval, S.J.; Phipps, W.R.; Kurzer, M.S.; Messina, M.J. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: Results of a meta-analysis. Fertil. Steril. 2010, 94, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strom, B.L.; Schinnar, R.; Ziegler, E.E.; Barnhart, K.T.; Sammel, M.D.; Macones, G.A.; Stallings, V.A.; Drulis, J.M.; Nelson, S.E.; Hanson, S.A.; et al. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. J. Am. Med. Assoc. 2001, 286, 807–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merritt, R.J.; Jenks, B.H. Safety of Soy-Based Infant Formulas Containing Isoflavones: The Clinical Evidence. J. Nutr. 2004, 134, 1220S–1224S. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Padhye, S. Lesson learned from nature for the development of novel anti-cancer agents: Implication of isoflavone, curcumin, and their synthetic analogs. Curr. Pharm. Des. 2010, 16, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Johnson-Ajinwo, O.R.; Uche, F.I. Advances of plant-derived natural products in ovarian cancer therapy. Int. J. Cancer Res. Prev. 2016, 9, 81–105. [Google Scholar]
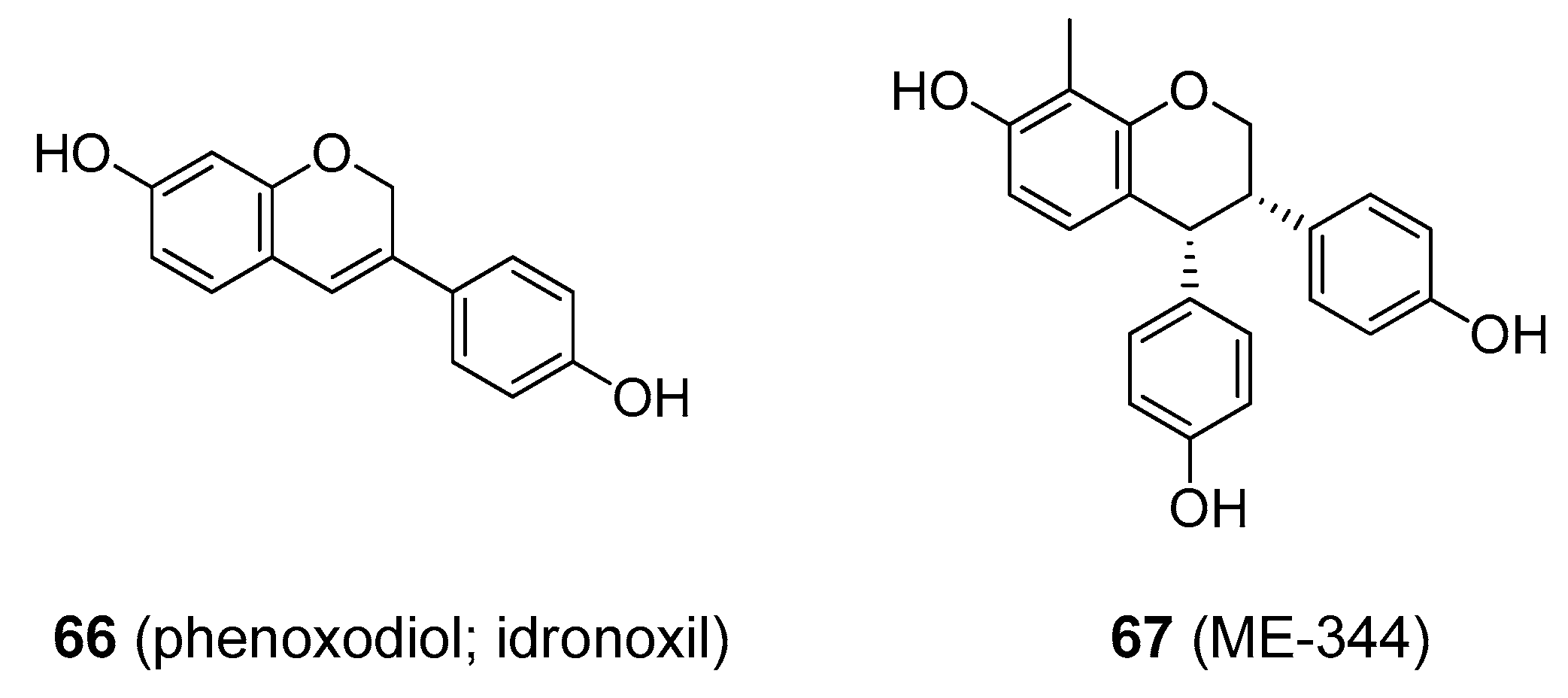
- Zhang, L.; Zhang, J.; Ye, Z.; Townsend, D.M.; Tew, K.D. Pharmacology of ME-344, a novel cytotoxic isoflavone. Adv. Cancer Res. 2019, 142, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Wrangel, C.V.; Schwabe, K.; John, N.; Krauss, J.K.; Alam, M. The rotenone-induced rat model of Parkinson’s disease: Behavioral and electrophysiological findings. Behav. Brain Res. 2015, 279, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Rupert, A.S.; Poi, M.; Phelps, M.A.; Andritsos, L.; Baiocchi, R.; Benson, D.M.; Blum, K.A.; Christian, B.; Flynn, J.; et al. Flavopiridol can be safely administered using a pharmacologically derived schedule and demonstrates activity in relapsed and refractory non-Hodgkin’s lymphoma. Am. J. Hematol. 2014, 89, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemann, D.W.; Chaplin, D.J.; Walicke, P.A. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P). Expert Opin. Investig. Drugs 2009, 18, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Mekhail, T.; Hutson, T.E.; Ganapathi, R.; Kelly, G.E.; Bukowski, R.M. Phase I trial of phenoxodiol delivered by continuous intravenous infusion in patients with solid cancer. Ann. Oncol. 2006, 17, 860–865. [Google Scholar] [CrossRef] [PubMed]

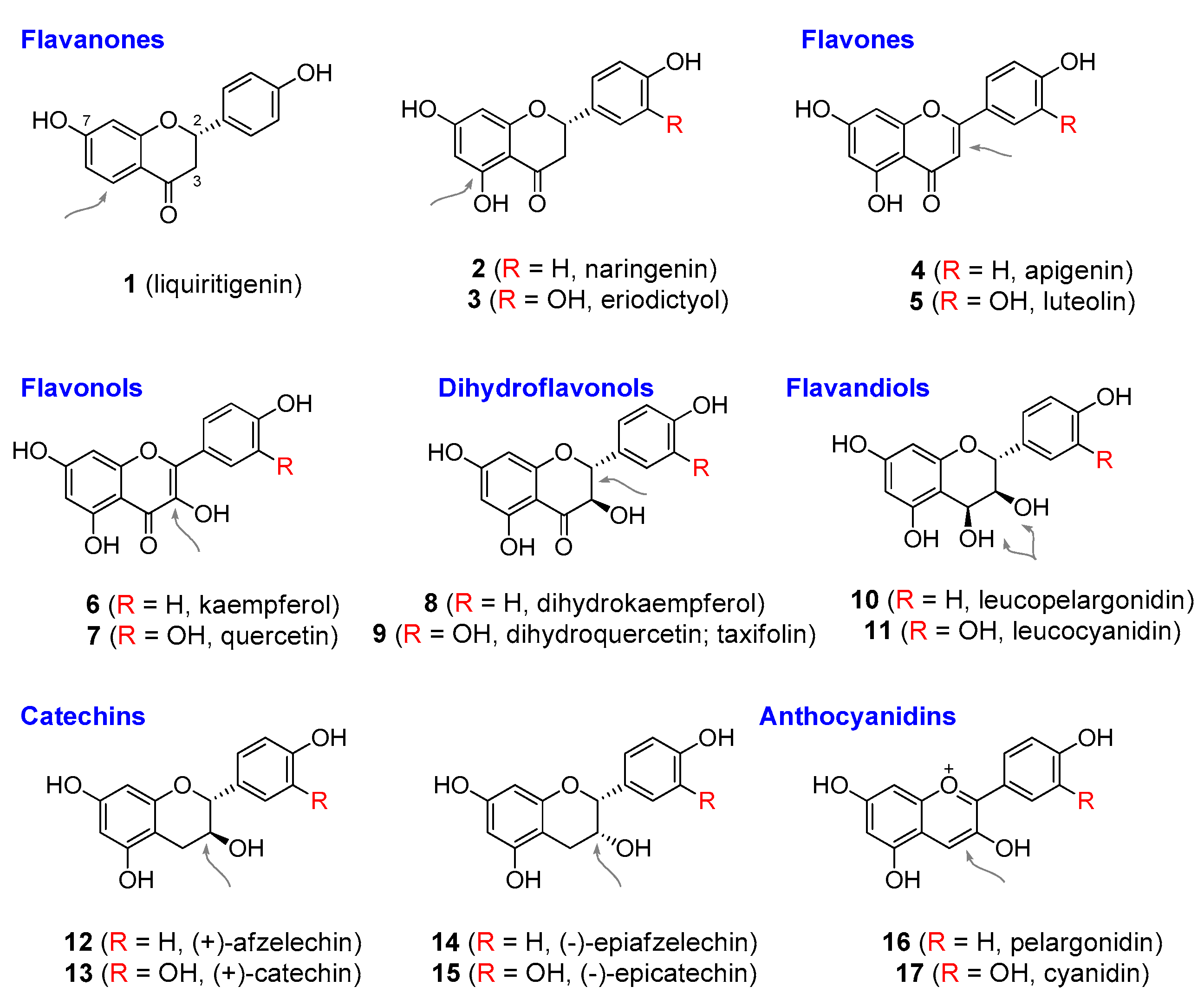
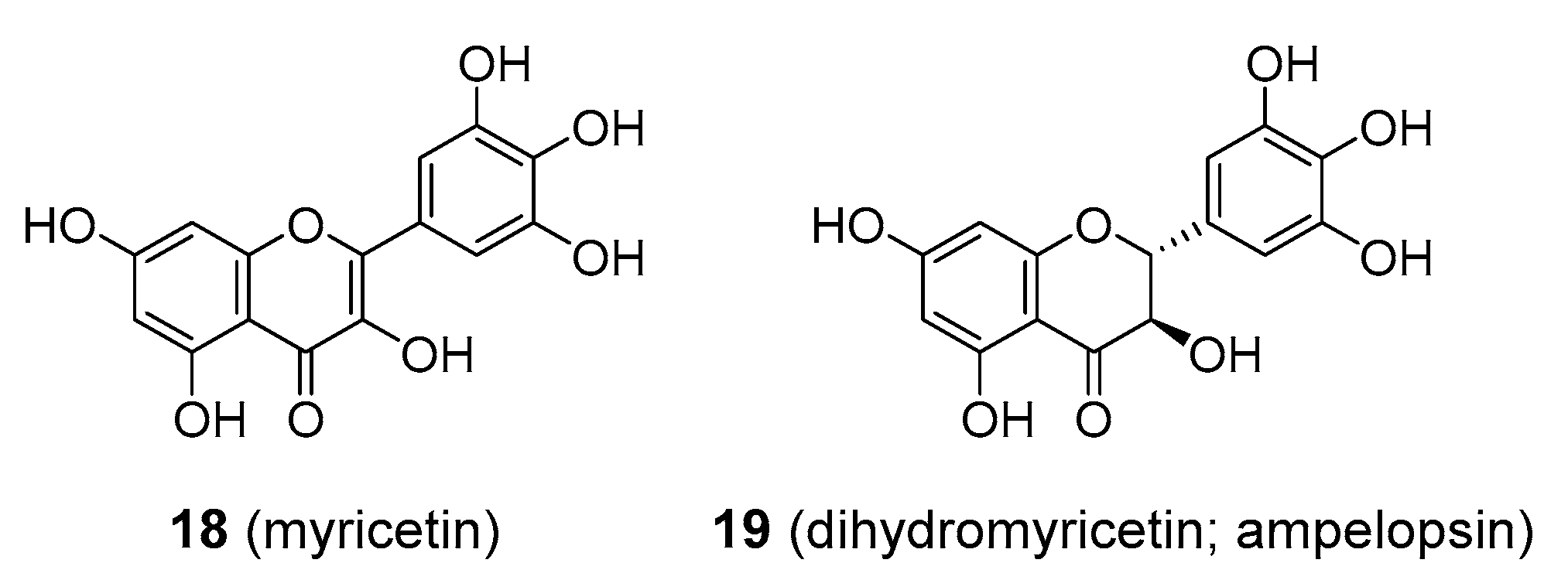

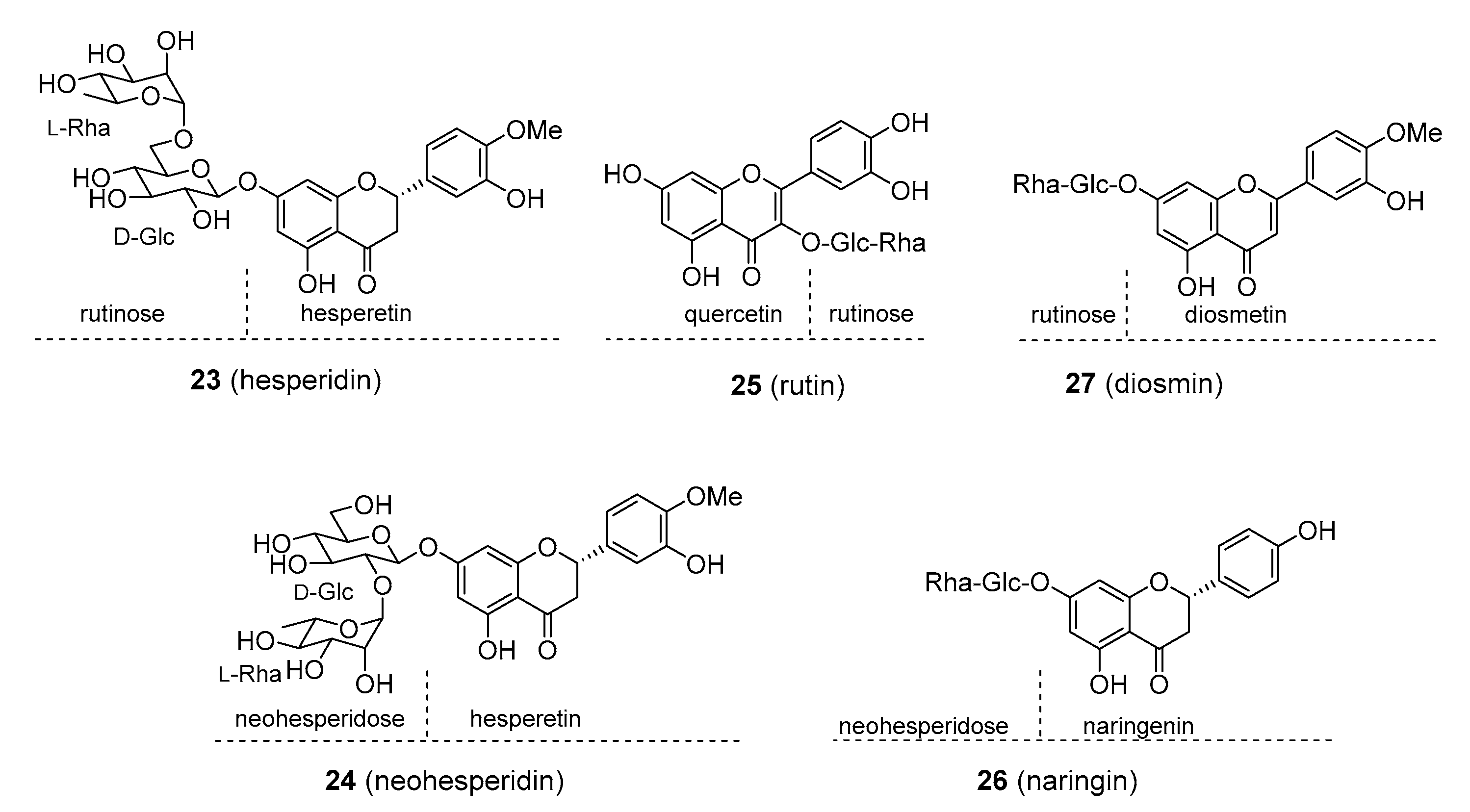
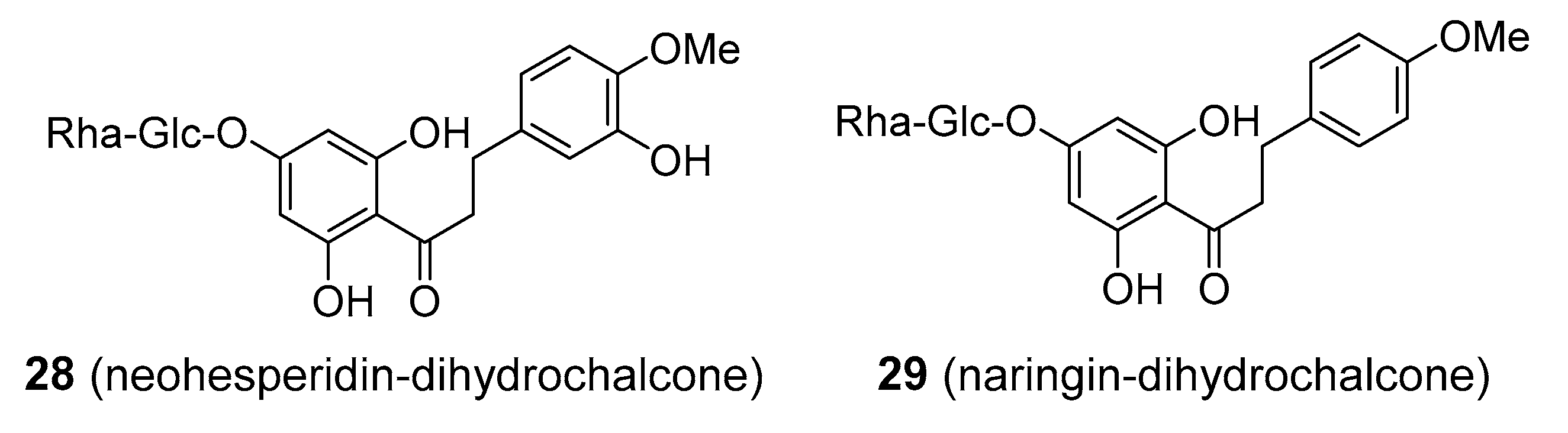
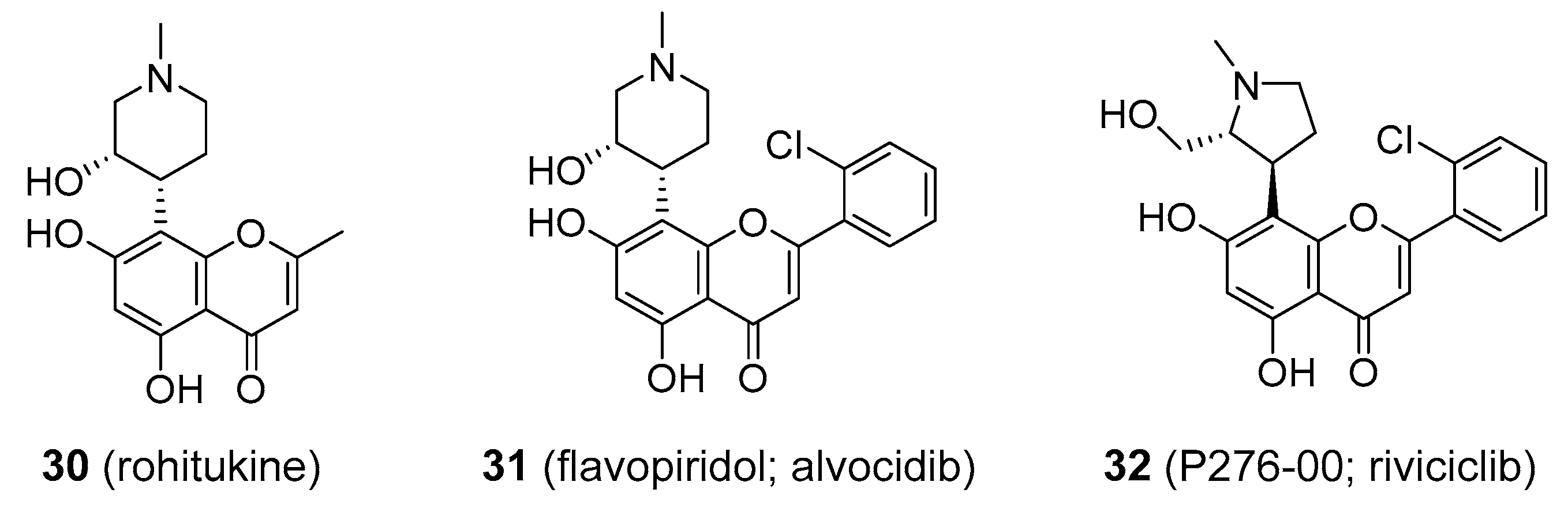
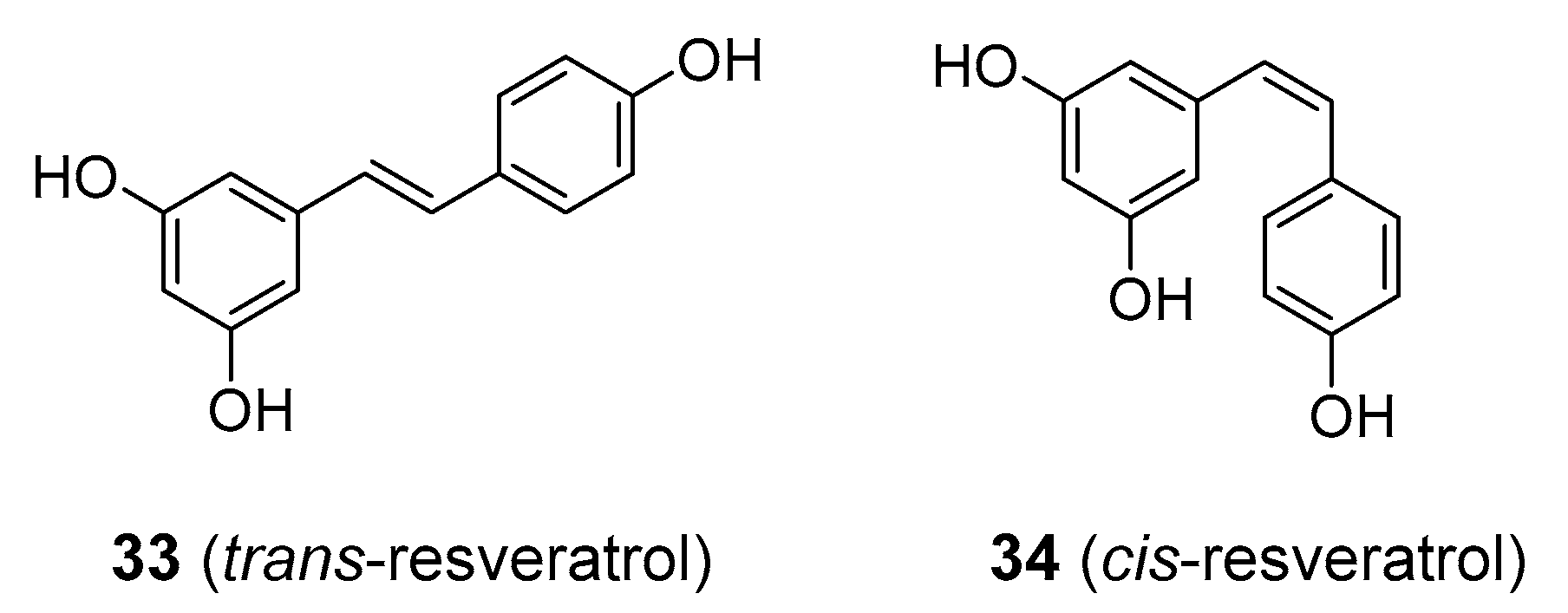
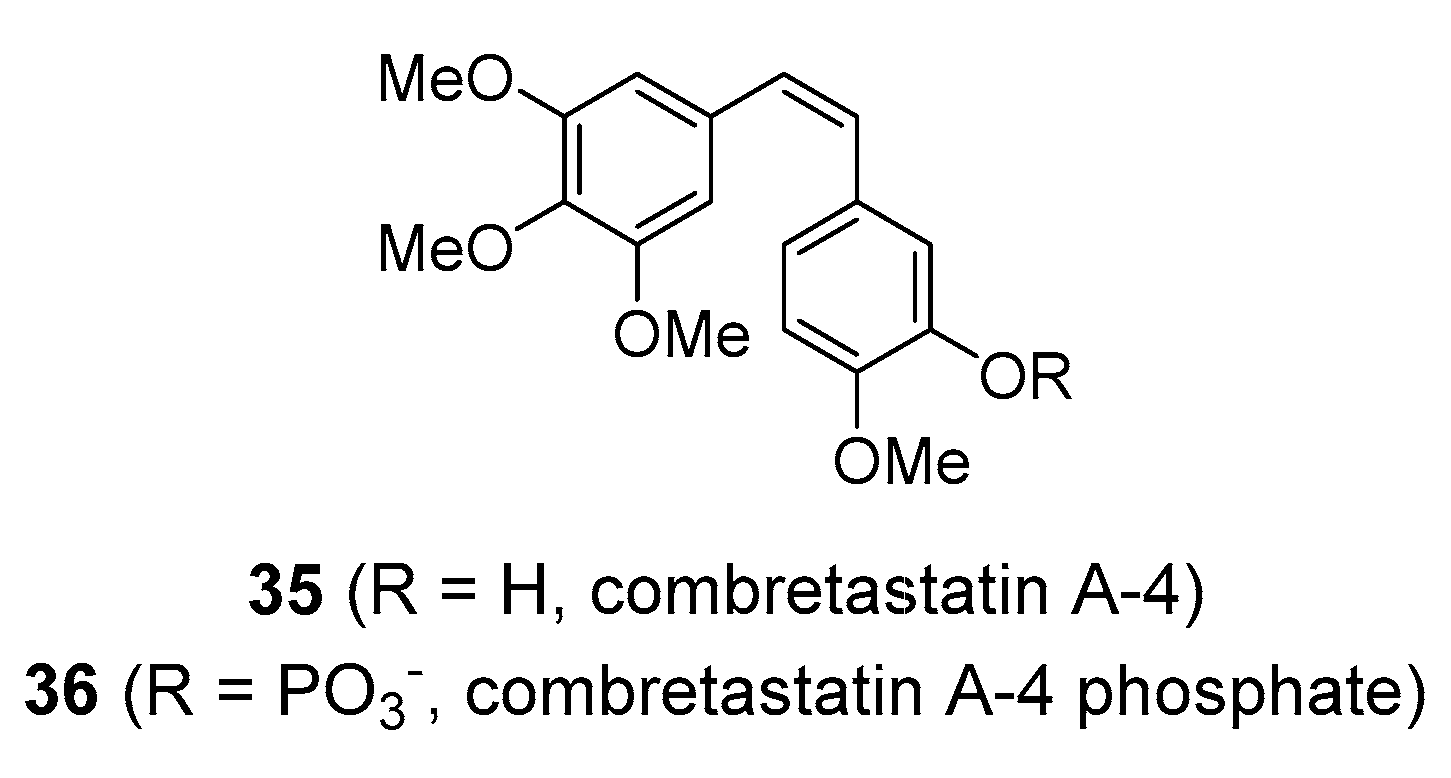
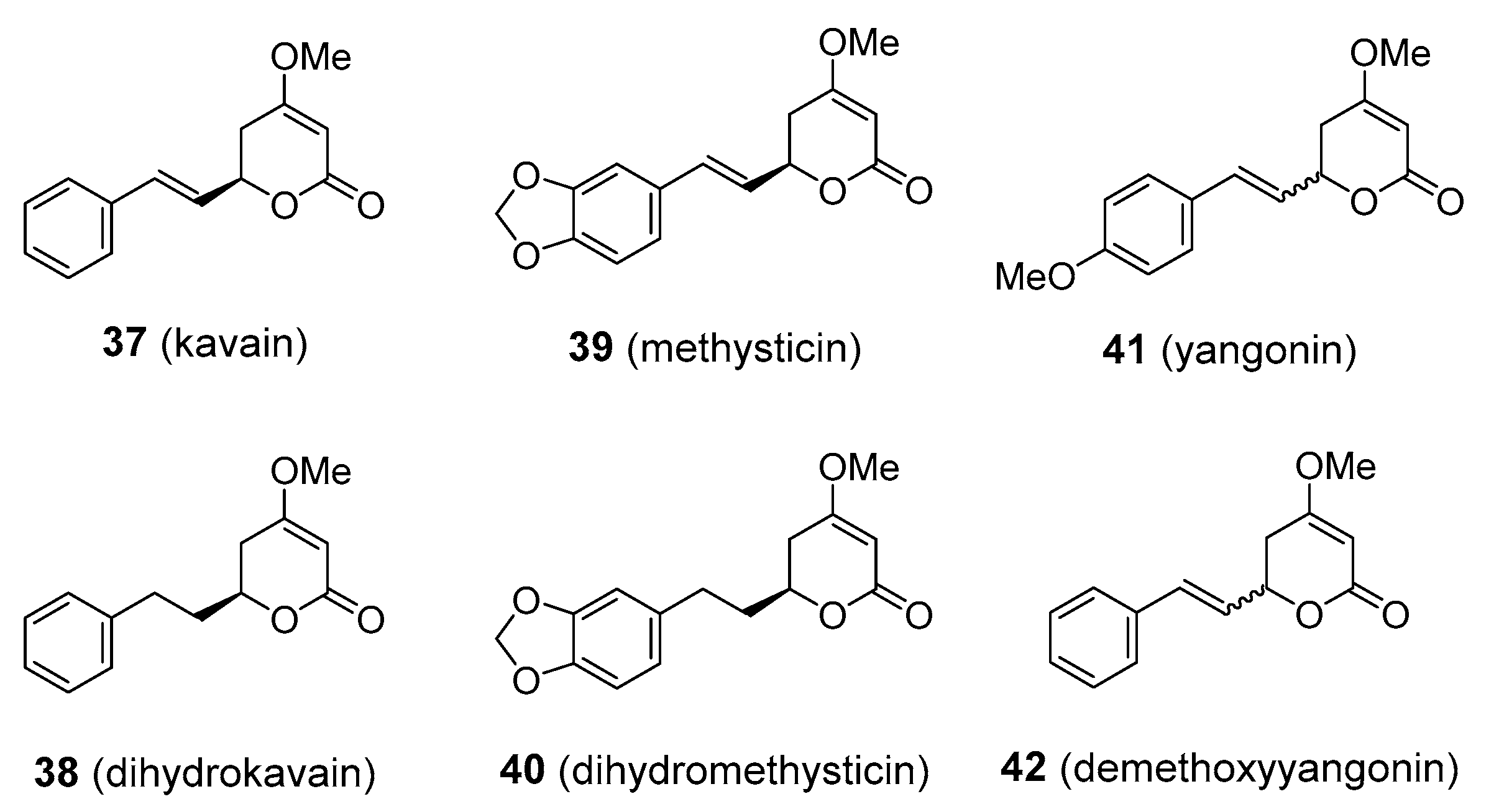
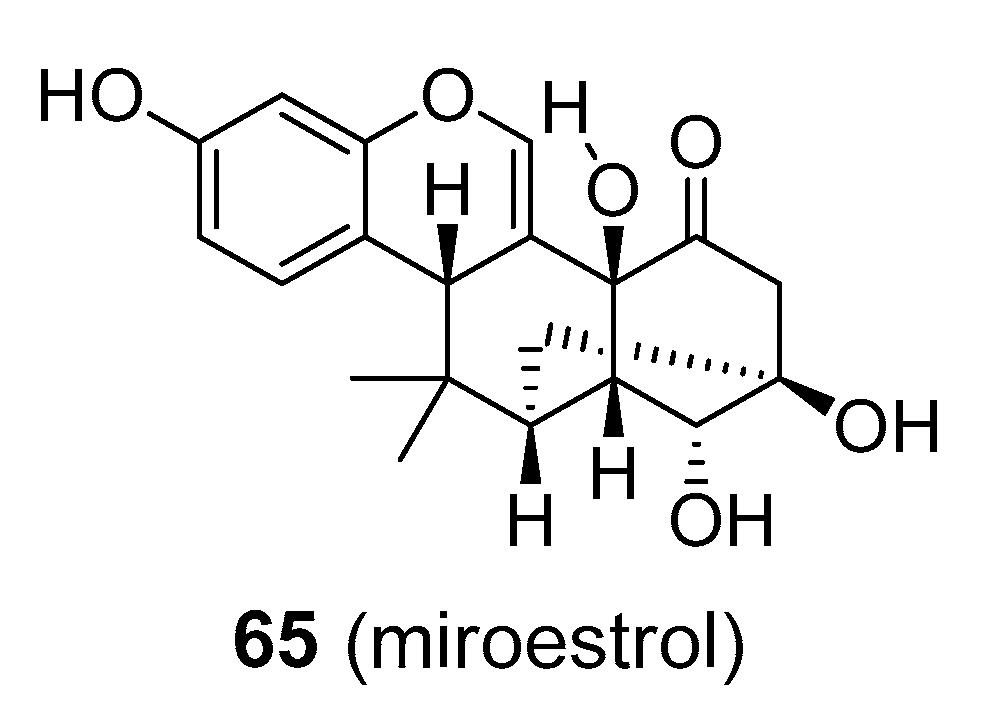
| Compound | Common Sources |
|---|---|
| apigenin | Vegetables of the Apiaceae family, such as parsley and celery |
| luteolin | |
| kaempferol | Fruits (apples, cherries, berries), brassicaceous vegetables (broccoli, Brussels sprouts, cabbage), amaryllidaceous plants (onions, leeks), beverages (tea, red wine) |
| quercetin | |
| myricetin | |
| rutin | |
| catechin | Green tea, cocoa, chocolate, alcoholic beverages (red wine), some fruits (apples) |
| epicatechin | |
| epigallocatechin gallate | |
| theaflavin | Black tea |
| cyanidin | Fruits and beverages (berries, cherries, grapes, red wine) |
| pelargonidin | |
| hesperidin | Citrus fruits (lemons, oranges, grapefruits), grapes and some vegetables (e.g., tomatoes) |
| neohesperidin | |
| naringenin | |
| naringin | |
| taxifolin |
| Group/Compound | Source |
|---|---|
| Stilbenes | |
| resveratrol | Grapes (Vitis vinifera; Vitaceae), cherries (various Prunus species; Rosaceae), groundnuts (Arachis hypogaea; Fabaceae), Japanese knotweed (Reynoutria japonica; Polygonaceae) |
| combretastatin A-4 | Eastern Cape South African bushwillow tree (Combretum caffrum; Combretaceae) |
| Styrylpyrones | |
| kavalactones | Kava kava (Piper methysticum; Piperaceae) |
| Diarylheptanoids | |
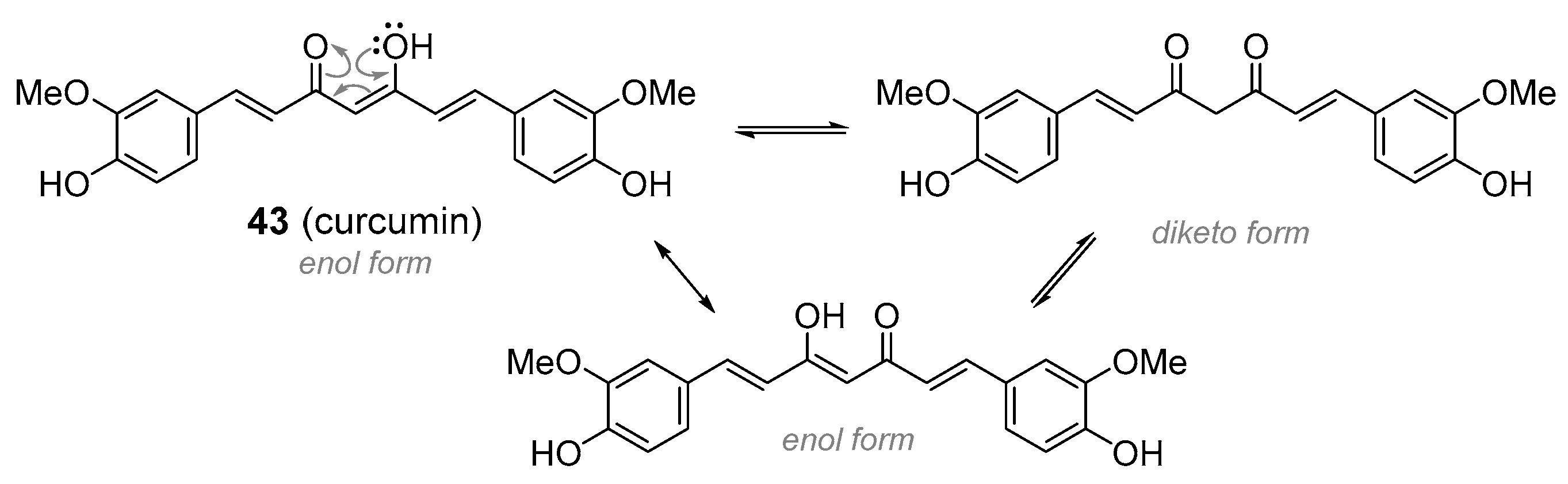
| curcumin | Turmeric (Curcuma longa; Zingiberaceae) |
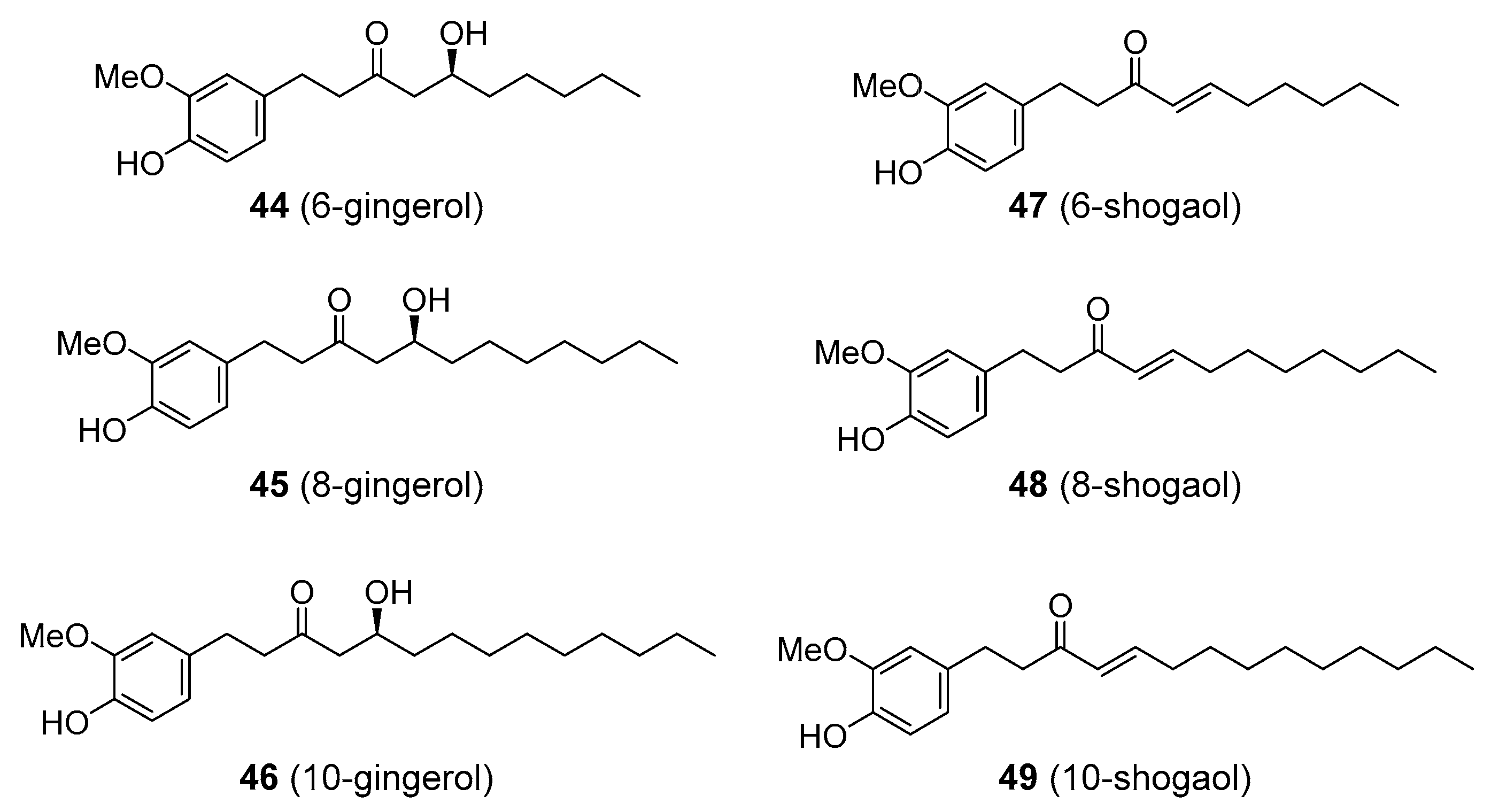
| gingerols | Ginger (Zingiber officinale; Zingiberaceae) |
| shogaols | |
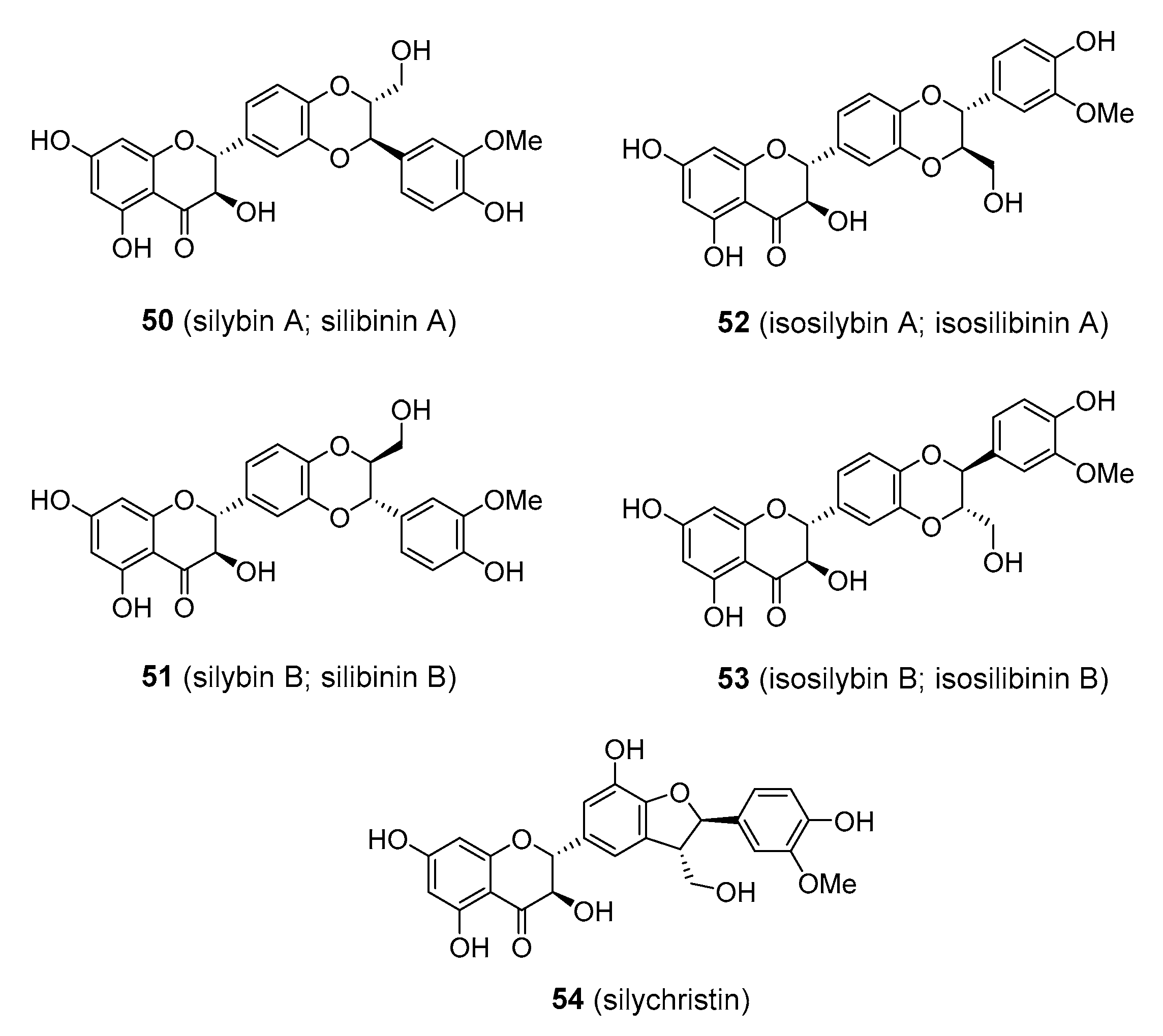
| Flavonolignans | |
| Silymarin | Milk thistle (Silybum marianum; Asteraceae) |
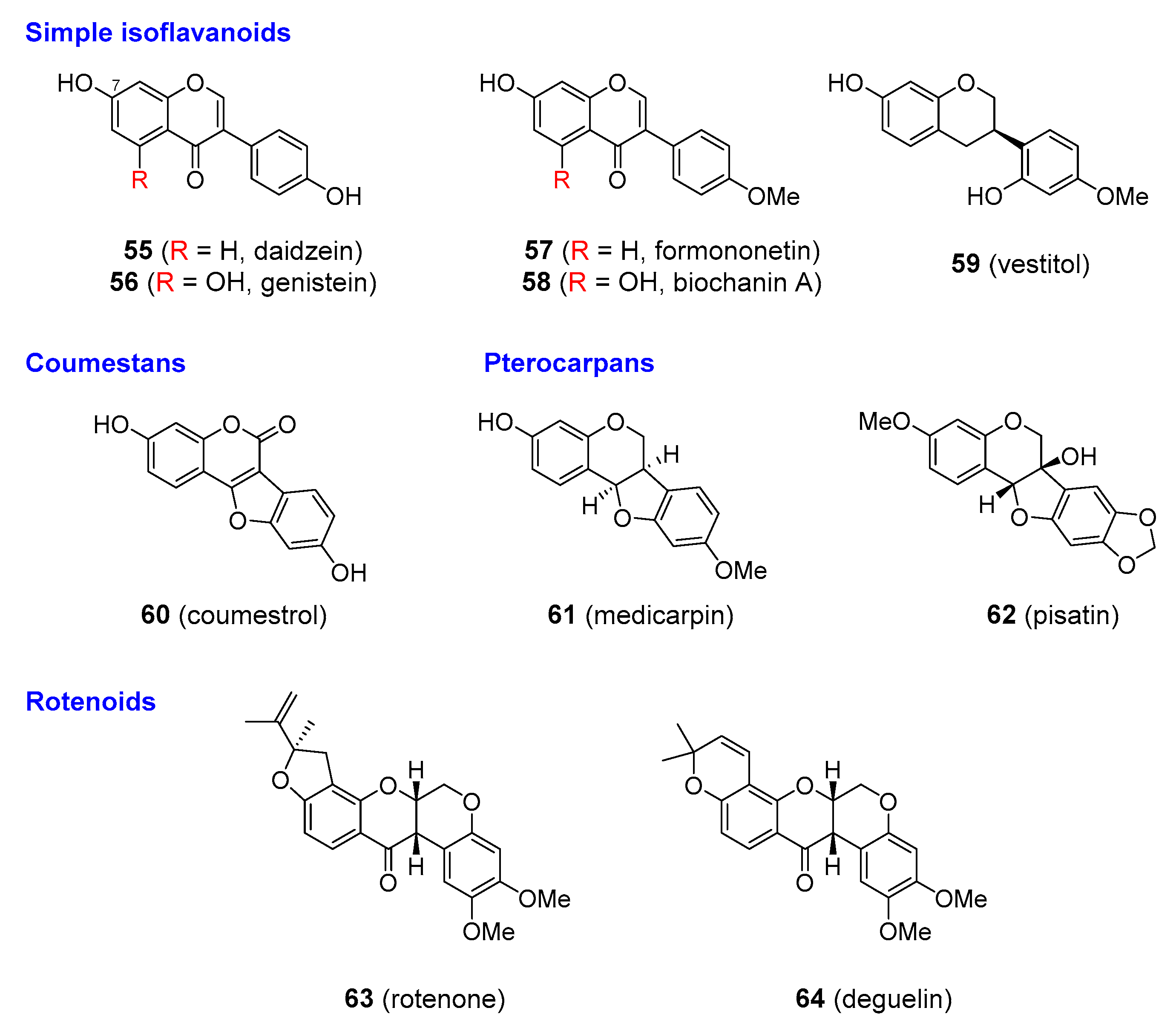
| Isoflavonoids | |
| daidzein | Leguminous plants (such as soybean, Glycine max; Fabaceae) |
| genistein | |
| coumestrol | Lucerne and clovers (Medicago sativa and Trifolium spp; Fabaceae) |
| medicarpin | Lucerne |
| vestitol | |
| pisatin | Pea (Pisum sativum; Fabaceae) |
| rotenoids (e.g., rotenone, degueline) | Various Derris and Deguelia species (Fabaceae) |
| Compound | Condition at Which It Might Be Particularly Helpful a | Doses at Which It Was Tested (mg/kg Body Weight)/Type of Study | Doses at Which It Showed Toxic Effects (mg/kg Body Weight; Oral Doses in Animals) b | Daily Doses Recommended by the Dietary Supplement Retailer (mg/kg Body Weight) c | Is There Clinical Evidence That It Has, or Will Have, Therapeutic Benefit in Humans? |
|---|---|---|---|---|---|
| quercetin | cancer | 1.6–4000/animal studies up to 14.3 c/human studies | 159 | 0.3–7.1 | Some i [27] |
| kaempferol | cancer | 1–200/animal studies | 1000 | 1.4–5.7 | Very limited [81] |
| taxifolin | cancer | ~ 50 mg/animal studies | 985–1200 IP d | 0.14–0.2 | No |
| naringenin/ naringin | CVD | 5–200 mg/animal studies 2.9 c/human studies | 0.2 | Very limited [82] | |
| apigenin | AD, cancer | 7.5–50/animal studies | Data not available | 0.7 | No good data [43] |
| luteolin | cancer, inflammatory conditions | 10–100/animal studies | >2500–5000 | 1.4–4.3 | No [83] |
| myricetin | inflammatory conditions, diabetes | 50–500/animal studies | 1000 IP d | 1.4 | Limited for humans [46] |
| catechin e | CVD | 50–2000/animal studies 1.4–7.1 c/human studies | >10,000 | 0.7–8.6 | Some i [84] |
| epicatechin e | 1000 | ||||
| epigallocatechin gallate e | 2170 | ||||
| theaflavin f | CVD and cancer | 250–3000/animal studies 1.4–7.1 c/human studies | 562 IP d | 0.7–1.4 | Limited [66] |
| anthocyanins | diabetes | 10–2000/animal studies 0.3–16.4 c/human studies | Data not available | 0.02–1.4 | Limited [85] |
| pycnogenol® g | venous insufficiency | 10–40/animal studies 2.1–5.1 c/human studies | 2000–4000 | 0.3–1.4 | Limited [59] |
| rutin | venous insufficiency | 10–150/animal studies 7.1 c/human studies | 2000 IPd | usually 7.1 | Some i [71] |
| daflon h | venous insufficiency | 7.1–14.2 c/human studies | >10,000 for diosmetin 1000 IP d for hesperidin | 7.1–14.2 | Some i [74] |
| Compound | Medicinal Application a | Mode of Action | Dose and Mode of Application a | Is There Clinical Evidence That It Has, or Will Have, Therapeutic Benefit in Humans? a | Comment a |
|---|---|---|---|---|---|
| flavopiridol | cancer | cyclin-dependent kinase inhibitor | 60–100 mg mg/m2 IV | Yes [160] | Replaced by more efficient agents |
| resveratrol | inflammatory conditions, CVD, cancer | remains to be established | 1.4–4.2 mg/kg bw b orally in the form of dietary supplements | Not enough data [92,100] | Still not accepted as a medicinal agent |
| combretastatin A-4 posphate | cancer | vascular disrupting agent; mitotic poison | 5–120 mg/m2 IV | Yes [161] | Still in clinical development |
| kava and kavalactones | anxiolytic | interaction with GABA, glutamate, dopamine, serotonin and cannabinoid systems | 1–3.5 mg/kg bw b of kavalactones orally in the form of standardized kava root extract | Yes [116] | Due to severe hepatotoxicity no longer marketed in many countries |
| curcumin | inflammatory conditions, cancer | remains to be established | 7.14–14.2 mg/kg bw b of turmeric extract orally in the form of dietary supplements | Despite large number of clinical trials, no evidence have been observed as of yet [127] | There are few reports of deaths after administration of IV curcumin |
| gingerols and shogaols | kinetosis, migraine, headache, rheumatism | presumably via interaction with serotonin receptors | 3.5–14.2 mg/kg bw b of ginger powder orally | Limited data in humans [132] | Ginger contains other compounds which may also contribute to observed effects |
| silymarin | liver damage and injury | inhibition of toxin absorption | 2–10.2 mg/kg bw b of silymarin orally in the form of standardized Silybum seed extract 20 mg/kg bw b IV c | Yes, e.g., IV form of silymarinc is used clinically in treatment of mushroom poisoning. Oral products (e.g Silybum infusions) seems to have low effectivity [138] | Orally active derivatives would perhaps expand the therapeutic applicability |
| daidzein/genistein | menopause symptoms | interaction with oestrogen receptors | 0.05 - 0.7 mg/kg bw b of isoflavonoids orally in the form of standardized soy extracts | Conflicting results are observed, more studies are required [144] | Oestrogens are planar molecules, whereas isoflavonoids not–this feature may impede their interaction with oestrogenic receptors |
| phenoxodiol | cancer | inhibition of NADH oxidase | up to 27 mg/kg bw b IV | Yes [162] | More efficient agents are in clinical development |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauchen, J.; Huml, L.; Rimpelova, S.; Jurášek, M. Flavonoids and Related Members of the Aromatic Polyketide Group in Human Health and Disease: Do They Really Work? Molecules 2020, 25, 3846. https://doi.org/10.3390/molecules25173846
Tauchen J, Huml L, Rimpelova S, Jurášek M. Flavonoids and Related Members of the Aromatic Polyketide Group in Human Health and Disease: Do They Really Work? Molecules. 2020; 25(17):3846. https://doi.org/10.3390/molecules25173846
Chicago/Turabian StyleTauchen, Jan, Lukáš Huml, Silvie Rimpelova, and Michal Jurášek. 2020. "Flavonoids and Related Members of the Aromatic Polyketide Group in Human Health and Disease: Do They Really Work?" Molecules 25, no. 17: 3846. https://doi.org/10.3390/molecules25173846
APA StyleTauchen, J., Huml, L., Rimpelova, S., & Jurášek, M. (2020). Flavonoids and Related Members of the Aromatic Polyketide Group in Human Health and Disease: Do They Really Work? Molecules, 25(17), 3846. https://doi.org/10.3390/molecules25173846








