Hydrothermal Conversion of Spent Sugar Beets into High-Value Platform Molecules
Abstract
:1. Introduction
2. Results and Discussion
2.1. Feedstock Characterization
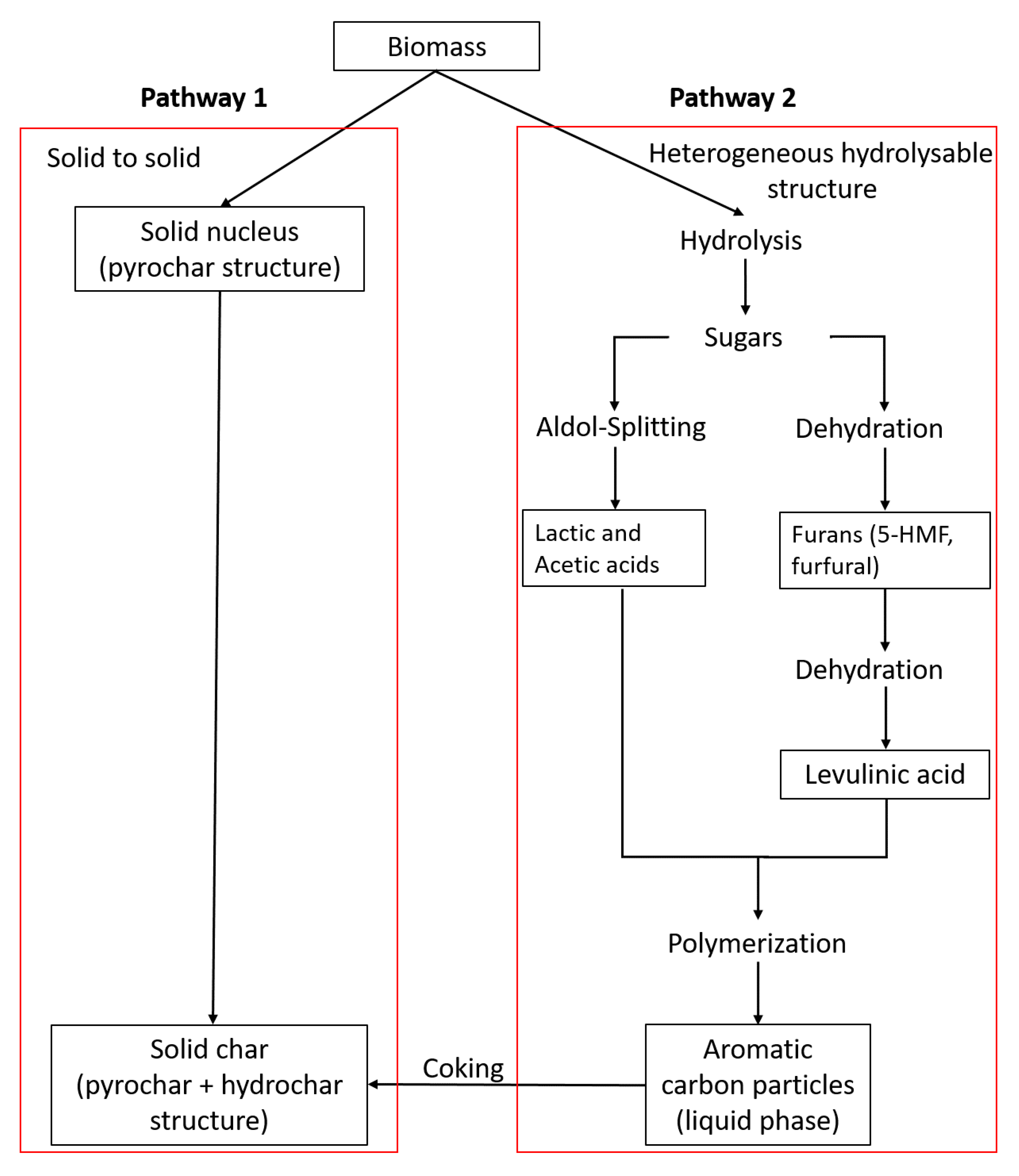
2.2. Solid Characterization
2.3. Liquid Characterization
3. Materials and Methods
3.1. Material
3.2. Hydrothermal Treatment
3.3. Characterization of Biomass and Products
3.3.1. Solid Fraction
3.3.2. Liquid Fraction
3.4. Error Estimation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryan, L.; Convery, F.; Ferreira, S. Stimulating the use of biofuels in the European Union: Implications for climate change policy. Energy Policy 2006, 34, 3184–3194. [Google Scholar] [CrossRef] [Green Version]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwald, W.; Bobleter, O. Hydrothermolysis of cellulose under static and dynamic conditions at high temperatures. J. Carbohydr. Chem. 1989, 8, 565–578. [Google Scholar] [CrossRef]
- Titirici, M.-M. Sustainable Caron Materials from Hydrothermal Processes; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Paksung, N.; Pfersich, J.; Arauzo, P.J.; Jung, D.; Kruse, A. Structural effects of cellulose on hydrolysis and carbonization behavior during hydrothermal treatment. ACS Omega 2020, 5, 12210–12223. [Google Scholar] [CrossRef]
- Körner, P.; Jung, D.; Kruse, A. Influence of the pH value on the hydrothermal degradation of fructose. ChemistryOpen 2019, 8, 1121–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körner, P.; Jung, D.; Kruse, A. The effect of different Brønsted acids on the hydrothermal conversion of fructose to HMF. Green Chem. 2018, 20, 2231–2241. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Ulbrich, M.; Preßl, D.; Fendt, S.; Gaderer, M.; Spliethoff, H. Impact of HTC reaction conditions on the hydrochar properties and CO2 gasification properties of spent grains. Fuel Process. Technol. 2017, 167, 663–669. [Google Scholar] [CrossRef]
- Xia, H.; Xu, S.; Hu, H.; An, J.; Li, C. Efficient conversion of 5-hydroxymethylfurfural to high-value chemicals by chemo- and bio-catalysis. RSC Adv. 2018, 8, 30875–30886. [Google Scholar] [CrossRef] [Green Version]
- Saravanamurugan, S.; Pandey, A.; Hu, L.; Riisager, A. Biomass, Biofuels, Biochemicals: Recent Advances in Devvelopment of Platform Chemicals; Elsevier B.V.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Li, Y.M.; Zhang, X.Y.; Li, N.; Xu, P.; Luo, W.-Y.; Zong, M.-H. Biocatalytic Reduction of HMF to 2,5-Bis(hydroxymethyl)furan by HMF-Tolerant Whole Cells. ChemSusChem 2017, 10, 372–378. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Li, F.; Li, X.L.; Li, C.; Shi, J.; Fu, Y. Aerobic oxidative esterification of 5-hydroxymethylfurfural to dimethyl furan-2,5-dicarboxylate by using homogeneous and heterogeneous PdCoBi/C catalysts under atmospheric oxygen. Green Chem. 2018, 20, 3050–3058. [Google Scholar] [CrossRef]
- Nguyen, H.; Nikolakis, V.; Vlachos, D.G. Mechanistic insights into lewis acid metal salt-catalyzed glucose chemistry in aqueous solution. ACS Catal. 2016, 6, 1497–1504. [Google Scholar] [CrossRef]
- Tong, Z.; Pullammanappallil, P.; Teixeira, A.A. How Ethanol Is Made from Cellulosic Biomass. Available online: https://edis.ifas.ufl.edu (accessed on 3 April 2020).
- Kessler, M.T.; Scholten, J.D.; Prechtl, M.H.G. Metal Catalysts immobilised in Ionic Liquids: A Couple with Opportunities for Fine Chemicals Derived from Biomass. In New and Future Development in Catalysis: Hybrid Materials, Composites, and Organocatalysts; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, M.G.; Li, Z.; Mu, T. Advances in the conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts. RSC Adv. 2016, 6, 98874–98892. [Google Scholar] [CrossRef]
- Kruse, A.; Zevaco, T.A. Properties of hydrochar as function of feedstock, reaction conditions and post-treatment. Energies 2018, 11, 674. [Google Scholar] [CrossRef] [Green Version]
- Karayildirim, T.; Sinaǧ, A.; Kruse, A. Char and coke formation as unwanted side reaction of the hydrothermal biomass gasification. Chem. Eng. Technol. 2008, 31, 1561–1568. [Google Scholar] [CrossRef]
- Fasahat, P.; Aghaeezadeh, M.; Jabbari, L.; Sadeghzadeh Hemayati, S.; Townson, P. Sucrose accumulation in sugar beet: From fodder beet selection to genomic selection. Sugar Tech. 2018, 20, 635–644. [Google Scholar] [CrossRef]
- Risser, D.; Baltruschat, N. Suedzucker Reports Strong 3rd Quarter in Fiscal 2019/2020. Available online: https://www.suedzucker.de/en/press/sudzucker-reports-strong-3rd-quarter-fiscal-201920 (accessed on 27 April 2020).
- Donovan, M. Refining of Sugarbeet and Sugarcane; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Ziemiński, K.; Romanowska, I.; Kowalska-Wentel, M.; Cyran, M. Effects of hydrothermal pretreatment of sugar beet pulp for methane production. Bioresour. Technol. 2014, 166, 187–193. [Google Scholar] [CrossRef]
- Rempe, C.; Maschkowski, G.; Lobitz, R. Zucker. 2020. Available online: https://www.bzfe.de/inhalt/zucker-31842.html (accessed on 2 April 2020).
- Negahdar, L.; Delidovich, I.; Palkovits, R. Aqueous-phase hydrolysis of cellulose and hemicelluloses over molecular acidic catalysts: Insights into the kinetics and reaction mechanism. Appl. Catal. B Environ. 2016, 184, 285–298. [Google Scholar] [CrossRef]
- Schnepf, E. Structure of cell walls and cellulose fibrils in glaucocystis. Planta 1965, 67, 213–224. [Google Scholar] [CrossRef]
- Tieke, B.; Lechner, M.D.; Gehrke, K.; Nordmeier, E.H. Makromolekulare Chemie. Chemistry and Physics; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Sjöström, E. Wood Chemistry: Fundamentals and Applications; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Deguchi, S.; Tsujii, K.; Horikoshi, K. Crystalline-to-amorphous transformation of cellulose in hot and compressed water and its implications for hydrothermal conversion. Green Chem. 2008, 10, 191–196. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, H. Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water. Ind. Eng. Chem. Res. 2010, 49, 3902–3909. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef] [Green Version]
- Dinjus, E.; Kruse, A.; Tröger, N. Hydrothermale karbonisierung: 1. einfluss des lignins in lignocellulosen. Chem. Ing. Tech. 2011, 83, 1734–1741. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B. Quantification in X-ray Fluorescence Spectrometry. In X-ray Spectroscopy; Sharma, S.K., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Ok, Y.S.; Tsang, D.C.W.; Bolan, N.; Novak, J.M. Biochar from Biomass and Waste: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef] [Green Version]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal degradation of cellulose at temperature from 200 to 300 °C. Ind. Eng. Chem. Res. 2018, 57, 6576–6584. [Google Scholar] [CrossRef]
- Reza, M.T.; Becker, W.; Sachsenheimer, K.; Mumme, J. Hydrothermal carbonization (HTC): Near infrared spectroscopy and partial least-squares regression for determination of selective components in HTC solid and liquid products derived from maize silage. Bioresour. Technol. 2014, 161, 91–101. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldforb, G.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Fiori, L. Hydrothermal carbonization kinetics of lignocellulosic agro-wastes: Experimental data and modeling. Energies 2019, 12, 516. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Jatzwauck, M.; Schumpe, A. Kinetics of hydrothermal carbonization (HTC) of soft rush. Biomass Bioenergy 2015, 75, 94–100. [Google Scholar] [CrossRef]
- Baratieri, M.; Basso, D.; Patuzzi, F.; Castello, D.; Fiori, L. Kinetic and thermal modeling of hydrothermal carbonization applied to grape marc. Chem. Eng. Trans. 2015, 43, 505–510. [Google Scholar]
- Kannan, S.; Gariepy, Y.; Raghavan, G.S.V. Optimization and characterization of hydrochar produced from microwave hydrothermal carbonization of fish waste. Waste Manag. 2017, 65, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Zimmermann, M.; Kruse, A. Hydrothermal carbonization of fructose: Growth mechanism and kinetic model. ACS Sustain. Chem. Eng. 2018, 6, 13877–13887. [Google Scholar] [CrossRef]
- Fan, F.; Yang, Z.; Li, H.; Shi, Z.; Kan, H. Preparation and properties of hydrochars from macadamia nut shell via hydrothermal carbonization. R. Soc. Open Sci. 2018, 5, 181126. [Google Scholar] [CrossRef] [Green Version]
- Orem, W.H.; Finkelman, R.B. Coal Formation and Geochemistry. In Treatise on Geochemistry; Elsevier–Pergamon: Oxford, UK, 2003; pp. 191–222. [Google Scholar]
- Carrasco, S.; Silva, J.; Pino-Cortés, E.; Gómez, J.; Vallejo, F.; Díaz-Robles, L.; Campos, V.; Cubillos, F.; Pelz, S.; Paczkowski, S.; et al. Experimental study on hydrothermal carbonization of lignocellulosic biomass with magnesium chloride for solid fuel production. Processes 2020, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Arauzo, P.J.; Olszewski, M.P.; Wang, X.; Pfersich, J.; Sebastian, V.; Manyà, J.; Hedin, N.; Kruse, A. Assessment of the effects of process water recirculation on the surface chemistry and morphology of hydrochar. Renew. Energy 2020, 155, 1173–1180. [Google Scholar] [CrossRef]
- Brebbia, C.A.; Sendra, J.J. Energy and Sustainability VII; WIT Press: Southampton, UK, 2017. [Google Scholar]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J. Hydrothermal carbonization of loblolly pine: Reaction chemistry and water balance. Biomass Convers. Biorefinery 2014, 4, 311–321. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified Correlation for Estimating HHV of Solid, Liquid and Gaseous Fuels. Available online: http://www.fuel (accessed on 21 May 2020).
- Delbecq, F.; Wang, Y.; Muralidhara, A.; Ouardi, K.E.; Marlair, G.; Len, C. Hydrolysis of Hemicellulose and Derivatives—A Review of Recent Advances in the Production of Furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef]
- Pelley, J.W. Elsevier’s Integrated Review Biochemistry; Elsevier Inc.: Philadelphia, PA, USA, 2012. [Google Scholar]
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular self-assembled chaos: Polyphenolic lignin’s barrier to cost-effective lignocellulosic biofuels. Molecules 2010, 15, 8641–8688. [Google Scholar] [CrossRef] [Green Version]
- Möller, M.; Harnisch, F.; Schröder, U. Hydrothermal liquefaction of cellulose in subcritical water-the role of crystallinity on the cellulose reactivity. RSC Adv. 2013, 3, 11035–11044. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant. Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Tao, F.; Song, H.; Chou, L. Catalytic conversion of cellulose to chemicals in ionic liquid. Carbohydr. Res. 2011, 346, 58–63. [Google Scholar] [CrossRef]
- de Bruyn, C.A.L.; van Ekenstein, W.A. Action des alcalis sur les sucres, II. Transformation réciproque des uns dans les autres des sucres glucose, fructose et mannose. Recl. Trav. Chim. Pays-Bas 1895, 14, 203–216. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Zhang, D.; Liu, C. Theoretical Elucidation of Glucose Dehydration to 5-Hydroxymethylfurfural Catalyzed by a SO3H-Functionalized Ionic Liquid. J. Phys. Chem. B 2015, 119, 13398–13406. [Google Scholar] [CrossRef] [PubMed]
- Carraher, J.M.; Fleitman, C.N.; Tessonnier, J.P. Kinetic and mechanistic study of glucose isomerization using homogeneous organic brønsted base catalysts in water. ACS Catal. 2015, 5, 3162–3173. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, B.G.; Silva, M.A.P.; Moraes, C. Synthesis of hmf from glucose in aqueous medium using niobium and titanium oxides. Braz. J. Pet. Gas. 2013, 7, 71–82. [Google Scholar] [CrossRef]
- de Souza, R.L.; Yu, H.; Rataboul, F.; Essayem, N. 5-Hydroxymethylfurfural (5-HMF) production from hexoses: Limits of heterogeneous catalysis in hydrothermal conditions and potential of concentrated aqueous organic acids as reactive solvent system. Challenges 2012, 3, 212–232. [Google Scholar] [CrossRef]
- Yang, L.; Tsilomelekis, G.; Caratzoulas, S.; Vlachos, D.G. Mechanism of brønsted acid-catalyzed glucose dehydration. ChemSusChem 2015, 8, 1334–1341. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, G.; Tang, X.; Wu, Z.; Xu, J.; Lin, L.; Liu, S. Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural over cellulose-derived carbonaceous catalyst in ionic liquid. Bioresour. Technol. 2013, 148, 501–507. [Google Scholar] [CrossRef]
- Tschirner, S.; Weingart, E.; Teevs, L.; Prüße, U. Catalytic dehydration of fructose to 5-hydroxymethylfurfural (hmf) in low-boiling solvent hexafluoroisopropanol (HFIP). Molecules 2018, 23, 1866. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, D.; Kruse, A.; Sauer, J. Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production—A review. Biomass Convers. Biorefinery 2017, 7, 247–274. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Lund, C.R.F. Formation and growth of humins via aldol addition and condensation during acid-catalyzed conversion of 5-hydroxymethylfurfural. Energy Fuels 2011, 25, 4745–4755. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Xing, R.; Conner, W.C.; Huber, G.W. Kinetics and reaction engineering of levulinic acid production from aqueous glucose solutions. ChemSusChem 2012, 5, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Rodríguez Correa, C.; Stollovsky, M.; Hehr, T.; Rauscher, Y.; Rolli, B.; Kruse, A. Influence of the Carbonization Process on Activated Carbon Properties from Lignin and Lignin-Rich Biomasses. ACS Sustain. Chem. Eng. 2017, 5, 8222–8233. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses; USDA Agricultural Research Service: Washington, DC, USA, 1970; pp. 1–24. [Google Scholar]
- Jung, H.-J.G. Analysis of forage fiber and cell walls in ruminant nutrition. J. Nutr. 1997, 127, 810S–813S. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef]
- Wüst, D.; Correa, C.R.; Jung, D.; Zimmermann, M.; Kruse, A.; Fiori, L. Understanding the influence of biomass particle size and reaction medium on the formation pathways of hydrochar. Biomass Convers. Biorefinery 2019, 1–4. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Afzal, W.; Cheng, K.; Liu, N.; Pauly, M.; Bell, A.T.; Liu, Z.; Prausnitz, J.M. Nitric-acid hydrolysis of Miscanthus giganteus to sugars fermented to bioethanol. Biotechnol. Bioprocess. Eng. 2015, 20, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.; Körner, P.; Kruse, A. Kinetic study on the impact of acidity and acid concentration on the formation of 5-hydroxymethylfurfural (HMF), humins, and levulinic acid in the hydrothermal conversion of fructose. Biomass Convers. Biorefinery 2019, 1–16. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Compound | Content [wt.%] |
|---|---|
| cellulose | 17.3 |
| hemicellulose | 20.0 |
| lignin | 0.6 |
| others | 62.1 |
| Sample | Proximate Analysis [wt.% db] | Ultimate Analysis Dry [wt.% db] | ||||||
|---|---|---|---|---|---|---|---|---|
| Volatile Matter | Ash | Fixed Carbon | N | C | H | S | O | |
| raw | 77.6 | 5.7 | 16.6 | 1.7 | 42.4 | 6.2 | 0.2 | 43.7 |
| S_160 | 73.5 | 5.6 | 20.9 | 2.0 | 45.3 | 5.8 | 0.2 | 41.1 |
| S_160_0.5 | 72.1 | 5.8 | 22.1 | 2.2 | 47.8 | 6.1 | 0.2 | 37.9 |
| S_160_1 | 69.6 | 5.8 | 24.2 | 2.4 | 52.3 | 5.8 | 0.2 | 33.5 |
| S_200_0 | 65.8 | 6.1 | 28.1 | 2.3 | 54.2 | 5.5 | 0.2 | 31.7 |
| S_200_0.5 | 64.9 | 5.6 | 29.5 | 2.3 | 55.0 | 5.5 | 0.2 | 31.5 |
| S_200_1 | 64.5 | 5.6 | 30.0 | 2.1 | 55.8 | 5.4 | 0.2 | 30.8 |
| Sample | HHV [MJ/kg] 1 | Ed | Yhydrochar [wt.%] |
|---|---|---|---|
| raw | 17.6 | ||
| S_160_0 | 18.4 | 1.0 | 63.2 |
| S_160_0.5 | 19.9 | 1.1 | 55.7 |
| S_160_1 | 21.6 | 1.2 | 53.1 |
| S_200_0 | 22.1 | 1.3 | 50.3 |
| S_200_0.5 | 22.4 | 1.3 | 51.6 |
| S_200_1 | 22.7 | 1.3 | 52.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfersich, J.; Arauzo, P.J.; Lucian, M.; Modugno, P.; Titirici, M.-M.; Fiori, L.; Kruse, A. Hydrothermal Conversion of Spent Sugar Beets into High-Value Platform Molecules. Molecules 2020, 25, 3914. https://doi.org/10.3390/molecules25173914
Pfersich J, Arauzo PJ, Lucian M, Modugno P, Titirici M-M, Fiori L, Kruse A. Hydrothermal Conversion of Spent Sugar Beets into High-Value Platform Molecules. Molecules. 2020; 25(17):3914. https://doi.org/10.3390/molecules25173914
Chicago/Turabian StylePfersich, Jens, Pablo J. Arauzo, Michela Lucian, Pierpaolo Modugno, Maria-Magdalena Titirici, Luca Fiori, and Andrea Kruse. 2020. "Hydrothermal Conversion of Spent Sugar Beets into High-Value Platform Molecules" Molecules 25, no. 17: 3914. https://doi.org/10.3390/molecules25173914






