Synthesis and Antiplasmodial Evaluation of 4-Carboxamido- and 4-Alkoxy-2-Trichloromethyl Quinazolines
Abstract
:1. Introduction
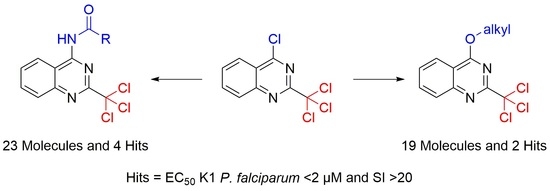
2. Results
2.1. Synthesis
2.1.1. Synthesis of 4-Carboxamido-2-Trichloromethylquinazoline Series
2.1.2. Synthesis of 4-Alkoxy-2-Trichloromethylquinazoline Series
2.2. Biological Evaluations
2.3. Structure-Activity Relationships (SAR)
2.3.1. SAR of 4-Carboxamido-2-Trichloromethylquinazoline Series
2.3.2. SAR of 4-Alkoxy-2-Trichloromethylquinazoline Series
3. Material and Methods
3.1. General
3.2. 4-Amino-2-Trichloromethylquinazoline (1)
3.3. 4-Chloro-N-(4-Chlorobenzoyl)-N-(2-Trichloromethylquinazolin-4-yl)benzamide (3)
3.4. 4-Chloro-2-Trichloromethylquinazoline (4)
3.5. General Procedure for the Preparation of Compounds (2), (5–25)
3.5.1. 4-Chloro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (2)
3.5.2. N-(2-Trichloromethylquinazolin-4-yl)benzamide (5)
3.5.3. 4-Nitro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (6)
3.5.4. 3-Nitro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (7)
3.5.5. 2-Nitro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (8)
3.5.6. 4-Fluoro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (9)
3.5.7. 3-Fluoro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (10)
3.5.8. 2-Fluoro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (11)
3.5.9. 3-Chloro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (12)
3.5.10. 2-Chloro-N-(2-Trichloromethylquinazolin-4-yl)benzamide (13)
3.5.11. 4-Bromo-N-(2-Trichloromethylquinazolin-4-yl)benzamide (14)
3.5.12. 4-Cyano-N-(2-Trichloromethylquinazolin-4-yl)benzamide (15)
3.5.13. 4-Methoxy-N-(2-Trichloromethylquinazolin-4-yl)benzamide (16)
3.5.14. N-(2-Trichloromethylquinazolin-4-yl)picolinamide (17)
3.5.15. N-(2-Trichloromethylquinazolin-4-yl)nicotinamide (18)
3.5.16. N-(2-Trichloromethylquinazolin-4-yl)isonicotinamide (19)
3.5.17. N-(2-Trichloromethylquinazolin-4-yl)acetamide (20)
3.5.18. N-(2-Trichloromethylquinazolin-4-yl)propionamide (21)
3.5.19. N-(2-Trichloromethylquinazolin-4-yl)isobutyramide (22)
3.5.20. N-(2-Trichloromethylquinazolin-4-yl)pivalamide (23)
3.5.21. N-(2-Trichloromethylquinazolin-4-yl)pentanamide (24)
3.5.22. N-(2-Trichloromethylquinazolin-4-yl)cyclohexanecarboxamide (25)
3.6. General Procedure for the Preparation of Compounds (26–44)
3.6.1. 4-Methoxy-2-Trichloromethylquinazoline (26)
3.6.2. 4-Ethoxy-2-Trichloromethylquinazoline (27)
3.6.3. 4-Propoxy-2-Trichloromethylquinazoline (28)
3.6.4. 4-Butoxy-2-Trichloromethylquinazoline (29)
3.6.5. 4-Isopropoxy-2-Trichloromethylquinazoline (30)
3.6.6. 4-(Prop-2-ynyloxy)-2-Trichloromethylquinazoline (31)
3.6.7. 4-(But-3-yn-2-yloxy)-2-Trichloromethylquinazoline (32)
3.6.8. 4-(4-Methylpent-1-yn-3-yloxy)-2-Trichloromethylquinazoline (33)
3.6.9. 2-(2-Trichloromethylquinazolin-4-yloxy)ethanol (34)
3.6.10. 4-(2-Methoxyethoxy)-2-Trichloromethylquinazoline (35)
3.6.11. 4-(2-Chloroethoxy)-2-Trichloromethylquinazoline (36)
3.6.12. 4-(3-Chloropropoxy)-2-Trichloromethylquinazoline (37)
3.6.13. 4-(4-Chlorobutoxy)-2-Trichloromethylquinazoline (38)
3.6.14. 4-(2-Fluoroethoxy)-2-Trichloromethylquinazoline (39)
3.6.15. 4-(2-Bromoethoxy)-2-Trichloromethylquinazoline (40)
3.6.16. N,N-Diethyl-2-[(2-Trichloromethylquinazolin-4-yl)oxy]ethanamine (41)
3.6.17. N-{2-[(2-Trichloromethylquinazolin-4-yl)oxy]ethyl}acetamide (42)
3.6.18. 4-[2-(Piperidin-1-yl)ethoxy]-2-Trichloromethylquinazoline (43)
3.6.19. 4-[2-(Pyrrolidin-1-yl)ethoxy]-2-Trichloromethylquinazoline (44)
3.7. General Procedure for the Preparation of Compounds (45–46)
3.7.1. 4-methoxy-N-(2-Methylquinazolin-4-yl)benzamide (45)
3.7.2. 4-methoxy-N-(2-Trifluoromethylquinazolin-4-yl)benzamide (46)
3.8. General Procedure for the Preparation of Compounds (47–49)
3.8.1. N,N-Diethyl-2-[(2-Methylquinazolin-4-yl)oxy]ethanamine (47)
3.8.2. N,N-Diethyl-2-[(2-Trifluoromethylquinazolin-4-yl)oxy]ethan-1-Amine (48)
3.8.3. N,N-Diethyl-2-(Quinazolin-4-yloxy)ethanamine (49)
3.9. Biology
3.9.1. In Vitro Cytotoxicity Evaluation
3.9.2. In Vitro Antiplasmodial Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). World Malaria Report 2019. Available online: https://www.who.int/publications-detail-redirect/world-malaria-report-2019 (accessed on 30 June 2020).
- Mbengue, A.; Bhattacharjee, S.; Pandharkar, T.; Liu, H.; Estiu, G.; Stahelin, R.V.; Rizk, S.S.; Njimoh, D.L.; Ryan, Y.; Chotivanich, K.; et al. Molecular Mechanism of Artemisinin Resistance in Plasmodium falciparum Malaria. Nature 2015, 520, 683–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, O.; Lu, G.Y.; von Seidlein, L. Geographic Expansion of Artemisinin Resistance. J. Travel Med. 2019, 26, taz030. [Google Scholar] [CrossRef] [PubMed]
- Ocan, M.; Akena, D.; Nsobya, S.; Kamya, M.R.; Senono, R.; Kinengyere, A.A.; Obuku, E. K13-Propeller Gene Polymorphisms in Plasmodium falciparum Parasite Population in Malaria Affected Countries: A Systematic Review of Prevalence and Risk Factors. Malar. J. 2019, 18, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Ueno, M.; Suzuki, R.; Ishitani, H.; Kim, H.-S.; Wataya, Y. Catalytic Asymmetric Synthesis of Antimalarial Alkaloids Febrifugine and Isofebrifugine and Their Biological Activity. J. Org. Chem. 1999, 64, 6833–6841. [Google Scholar] [CrossRef] [PubMed]
- Gellis, A.; Kieffer, C.; Primas, N.; Lanzada, G.; Giorgi, M.; Verhaeghe, P.; Vanelle, P. A New DMAP-Catalyzed and Microwave-Assisted Approach for Introducing Heteroarylamino Substituents at Position-4 of the Quinazoline Ring. Tetrahedron 2014, 70, 8257–8266. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.; Suzanne, P.; Lancelot, J.-C.; Verhaeghe, P.; Lesnard, A.; Basmaciyan, L.; Hutter, S.; Laget, M.; Dumètre, A.; Paloque, L.; et al. Discovery of New Thienopyrimidinone Derivatives Displaying Antimalarial Properties toward Both Erythrocytic and Hepatic Stages of Plasmodium. Eur. J. Med. Chem. 2015, 95, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Desroches, J.; Kieffer, C.; Primas, N.; Hutter, S.; Gellis, A.; El-Kashef, H.; Rathelot, P.; Verhaeghe, P.; Azas, N.; Vanelle, P. Discovery of New Hit-Molecules Targeting Plasmodium falciparum through a Global SAR Study of the 4-Substituted-2-Trichloromethylquinazoline Antiplasmodial Scaffold. Eur. J. Med. Chem. 2017, 125, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, P.; Azas, N.; Gasquet, M.; Hutter, S.; Ducros, C.; Laget, M.; Rault, S.; Rathelot, P.; Vanelle, P. Synthesis and Antiplasmodial Activity of New 4-Aryl-2-Trichloromethylquinazolines. Bioorg. Med. Chem. Lett. 2008, 18, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Castera-Ducros, C.; Azas, N.; Verhaeghe, P.; Hutter, S.; Garrigue, P.; Dumètre, A.; Mbatchi, L.; Laget, M.; Remusat, V.; Sifredi, F.; et al. Targeting the Human Malaria Parasite Plasmodium falciparum: In Vitro Identification of a New Antiplasmodial Hit in 4-Phenoxy-2-Trichloromethylquinazoline Series. Eur. J. Med. Chem. 2011, 46, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Gellis, A.; Primas, N.; Hutter, S.; Lanzada, G.; Remusat, V.; Verhaeghe, P.; Vanelle, P.; Azas, N. Looking for New Antiplasmodial Quinazolines: DMAP-Catalyzed Synthesis of 4-Benzyloxy- and 4-Aryloxy-2-Trichloromethylquinazolines and Their in Vitro Evaluation toward Plasmodium falciparum. Eur. J. Med. Chem. 2016, 119, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Primas, N.; Verhaeghe, P.; Cohen, A.; Kieffer, C.; Dumètre, A.; Hutter, S.; Rault, S.; Rathelot, P.; Azas, N.; Vanelle, P. A New Synthetic Route to Original Sulfonamide Derivatives in 2-Trichloromethylquinazoline Series: A Structure-Activity Relationship Study of Antiplasmodial Activity. Molecules 2012, 17, 8105–8117. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, P.; Rathelot, P.; Gellis, A.; Rault, S.; Vanelle, P. Highly Efficient Microwave Assisted α-Trichlorination Reaction of α-Methylated Nitrogen Containing Heterocycles. Tetrahedron 2006, 62, 8173–8176. [Google Scholar] [CrossRef]
- Verhaeghe, P.; Azas, N.; Hutter, S.; Castera-Ducros, C.; Laget, M.; Dumètre, A.; Gasquet, M.; Reboul, J.-P.; Rault, S.; Rathelot, P.; et al. Synthesis and in Vitro Antiplasmodial Evaluation of 4-Anilino-2-Trichloromethylquinazolines. Bioorg. Med. Chem. 2009, 17, 4313–4322. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, P.; Dumètre, A.; Castera-Ducros, C.; Hutter, S.; Laget, M.; Fersing, C.; Prieri, M.; Yzombard, J.; Sifredi, F.; Rault, S.; et al. 4-Thiophenoxy-2-Trichloromethyquinazolines Display in Vitro Selective Antiplasmodial Activity against the Human Malaria Parasite Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2011, 21, 6003–6006. [Google Scholar] [CrossRef] [PubMed]
- Stumpfe, D.; Bajorath, J. Exploring Activity Cliffs in Medicinal Chemistry. J. Med. Chem. 2012, 55, 2932–2942. [Google Scholar] [CrossRef] [PubMed]
- Caballero-García, G.; Romero-Ortega, M.; Barroso-Flores, J. Reactivity of Electrophilic Chlorine Atoms Due to σ-Holes: A Mechanistic Assessment of the Chemical Reduction of a Trichloromethyl Group by Sulfur Nucleophiles. Phys. Chem. Chem. Phys. 2016, 18, 27300–27307. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.J. Falcipain Cysteine Proteases of Malaria Parasites: An Update. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140362. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Guiguemde, W.A.; Shelat, A.A.; Bouck, D.; Duffy, S.; Crowther, G.J.; Davis, P.H.; Smithson, D.C.; Connelly, M.; Clark, J.; Zhu, F.; et al. Chemical Genetics of Plasmodium falciparum. Nature 2010, 465, 311–315. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2, 9, 16, 24, 41 and 44 are available from the authors. |





 |  |  |  | |
|---|---|---|---|---|
| A | B | C | Series D | |
| Antiplasmodial activity EC50 P. falciparum K1 (µM) | 2.5 | 1.1 | 1.8 | >10 |
| Cytotoxicity CC50 HepG2 (µM) | >125 | 50 | 19.4 | 38–136 |
| Selectivity Index (SI) | >50 | 45 | 11 | - |
 | ||||||
|---|---|---|---|---|---|---|
| N° | Base (equiv.) | Acyl Chloride (equiv.) | Solvent | Temp. | Time | LC-MS Estimated Conversion |
| 1 | NaH (1.0 equiv) | 1.0 equiv | DMF | 0 °C → R. T | 12 h | (1) 80%, (3) 20% |
| 2 | NaH (1.0 equiv) | 1.0 equiv | DMF | 0 °C | 24 h | (1) 95%, (3) 5% |
| 3 | NaH (1.5 equiv) | 1.5 equiv | DMF | 0 °C → R. T | 24 h | (1) 75%, (3) 25% |
| 4 | NaH (2.0 Equiv) | 2.0 equiv | DMF | 0 °C → R. T | 12 h | (1) 60%, (3) 40% |
| 5 | NaH (1.0 Equiv) | 1.0 equiv | THF | 0 °C → R. T | 12 h | (1) 90%, (3) 10% |
| 6 | tBuOK (1.1 equiv) | 1.5 equiv | DMF | 0 °C → R. T | 16 h | (1) 85%, (3) 10% |
| 7 | NaHMDS (1.1 equiv) | 1.0 equiv | THF | 0 °C → R. T | 16 h | (1) 50%, (2) <5%, (3) 45% |
| 8 | Et3N (2.0 equiv) | 3.0 equiv | Dioxane | 105 °C | 30 min | (1) >95% |
| 9 | Et3N (5.0 equiv) | 3.0 equiv | Dioxane | 105 °C | 24 h | (1) >90% |
| 10 | NaH (5.0 Equiv) | 2.0 equiv | DMF | 0 °C → R. T | 24 h | (3) 90% * |
 | |||||
|---|---|---|---|---|---|
| Molecule | R- | Yield (%) | HepG2 CC50 (µM) | PfK1 EC50 (µM) | SI d |
| 3 |  | 90 | 20.0 | 1.46 | 13.7 |
| 5 |  | 60 | 24.2 | 1.76 | 13.8 |
| 6 |  | 67 | 22.1 | 4.18 | 5.3 |
| 7 |  | 60 | 24.8 | 3.37 | 7.4 |
| 8 |  | 54 | >15.6 c | 3.0 | >5.2 |
| 9 |  | 88 | 29.4 | 1.34 | 21.9 |
| 10 |  | 80 | 19.9 | 2.86 | 6.9 |
| 11 |  | 98 | >15.6 c | 5.23 | >3.0 |
| 2 |  | 74 | 21.0 | 0.99 | 21.2 |
| 12 |  | 82 | 16.4 | 1.54 | 10.6 |
| 13 |  | 83 | 27.3 | 9.04 | 4.1 |
| 14 |  | 62 | 19.5 | 1.30 | 15.0 |
| 15 |  | 95 | 31.4 | 3.50 | 9.0 |
| 16 |  | 75 | 27.7 | 0.94 | 29.5 |
| 17 |  | 81 | >7.8 c | 14.5 | >0.5 |
| 18 |  | 31 | 72.9 | 3.9 | 18.7 |
| 19 |  | 22 | 22.8 | 1.8 | 12.7 |
| 20 |  | 31 | 31.2 | >10 | >3.1 |
| 21 |  | 32 | >62.5 c | 4.9 | >12.8 |
| 22 |  | 52 | >31.2 c | 3.5 | >9.0 |
| 23 |  | 55 | 34.9 | 1.8 | 19.4 |
| 24 |  | 43 | 42.3 | 1.6 | 26.4 |
| 25 |  | 51 | >15.6 c | 2.4 | >6.5 |
| Doxorubicin a | 0.2 | - | - | ||
| Chloroquine b | 30 | 0.8 | 37.5 | ||
| Doxycycline b | 20 | 6.0 | 3.3 | ||
 | |||||
|---|---|---|---|---|---|
| Molecule | R- | Yield (%) | HepG2 CC50 (µM) | PfK1 EC50 (µM) | SI e |
| 26 |  | 81 | 39.3 | 7.1 | 5.5 |
| 27 |  | 79 | 47.1 | 4.7 | 10 |
| 28 |  | 75 | 54.8 | 3.5 | 15 |
| 29 |  | 79 | 82.4 | 5.8 | 14.2 |
| 30 |  | 53 | 101.5 | >10 d | <10 |
| 31 |  | 87 | 49.6 | 3.4 | 14.6 |
| 32 |  | 89 | 45.0 | >10 d | <4.5 |
| 33 |  | 90 | 30.6 | >10 d | <3 |
| 34 |  | 42 | 7.9 | 10.0 | 0.8 |
| 35 |  | 97 | 53.2 | 2.3 | 23.1 |
| 36 |  | 79 | >62.5 | 2.2 | >28 |
| 37 |  | 70 | 60.4 | 4.2 | 14.4 |
| 38 |  | 28 | 50.5 | 4.3 | 11.7 |
| 39 |  | 71 | 29.5 | 8.0 | 3.7 |
| 40 |  | 71 | >62.5 c | 2.2 | >28 |
| 41 |  | 67 | 32.3 | 0.9 | 35.9 |
| 42 |  | 79 | >99.7 c | 3.3 | >30 |
| 43 |  | 20 | 23.4 | 1.4 | 16.5 |
| 44 |  | 47 | 43.7 | 1.3 | 33.6 |
| Doxorubicin a | 0.2 | - | - | ||
| Chloroquine b | 30 | 0.8 | 37.5 | ||
| Doxycycline b | 20 | 6 | 3.3 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrane, D.; Gellis, A.; Hutter, S.; Prieri, M.; Verhaeghe, P.; Azas, N.; Vanelle, P.; Primas, N. Synthesis and Antiplasmodial Evaluation of 4-Carboxamido- and 4-Alkoxy-2-Trichloromethyl Quinazolines. Molecules 2020, 25, 3929. https://doi.org/10.3390/molecules25173929
Amrane D, Gellis A, Hutter S, Prieri M, Verhaeghe P, Azas N, Vanelle P, Primas N. Synthesis and Antiplasmodial Evaluation of 4-Carboxamido- and 4-Alkoxy-2-Trichloromethyl Quinazolines. Molecules. 2020; 25(17):3929. https://doi.org/10.3390/molecules25173929
Chicago/Turabian StyleAmrane, Dyhia, Armand Gellis, Sébastien Hutter, Marion Prieri, Pierre Verhaeghe, Nadine Azas, Patrice Vanelle, and Nicolas Primas. 2020. "Synthesis and Antiplasmodial Evaluation of 4-Carboxamido- and 4-Alkoxy-2-Trichloromethyl Quinazolines" Molecules 25, no. 17: 3929. https://doi.org/10.3390/molecules25173929
APA StyleAmrane, D., Gellis, A., Hutter, S., Prieri, M., Verhaeghe, P., Azas, N., Vanelle, P., & Primas, N. (2020). Synthesis and Antiplasmodial Evaluation of 4-Carboxamido- and 4-Alkoxy-2-Trichloromethyl Quinazolines. Molecules, 25(17), 3929. https://doi.org/10.3390/molecules25173929