Scaffold Modifications in Erythromycin Macrolide Antibiotics. A Chemical Minireview
Abstract
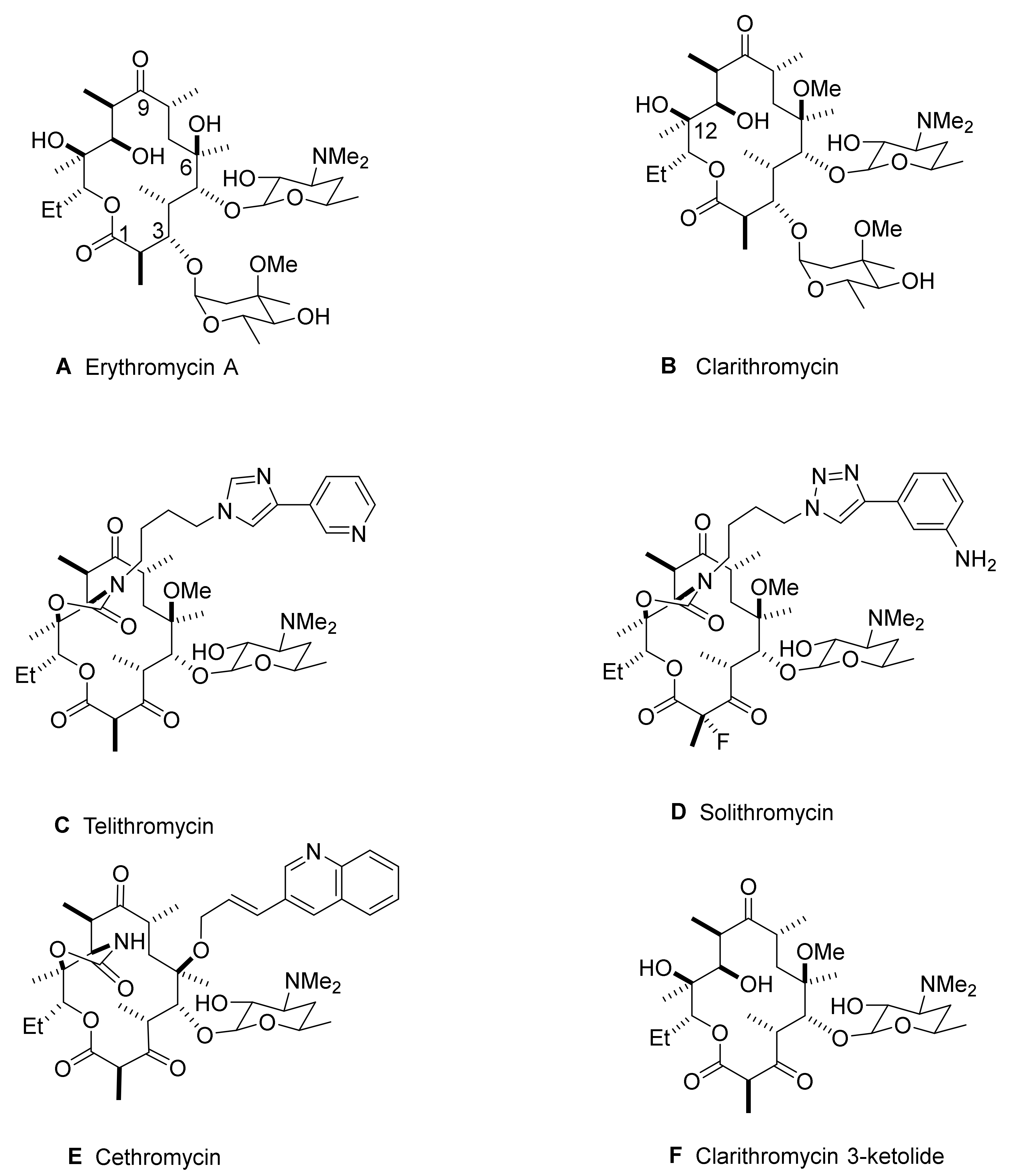
:1. Introduction
2. Synthesis
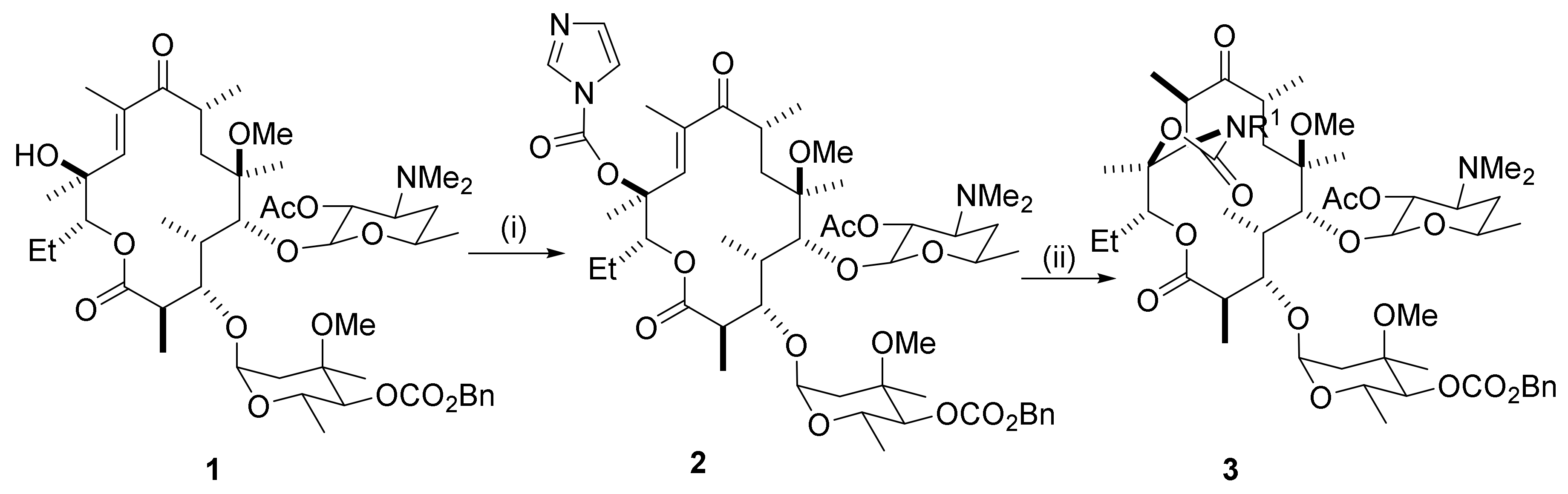
2.1. C2-Derivatives
2.2. Reactivity of the C2-Methyl Group
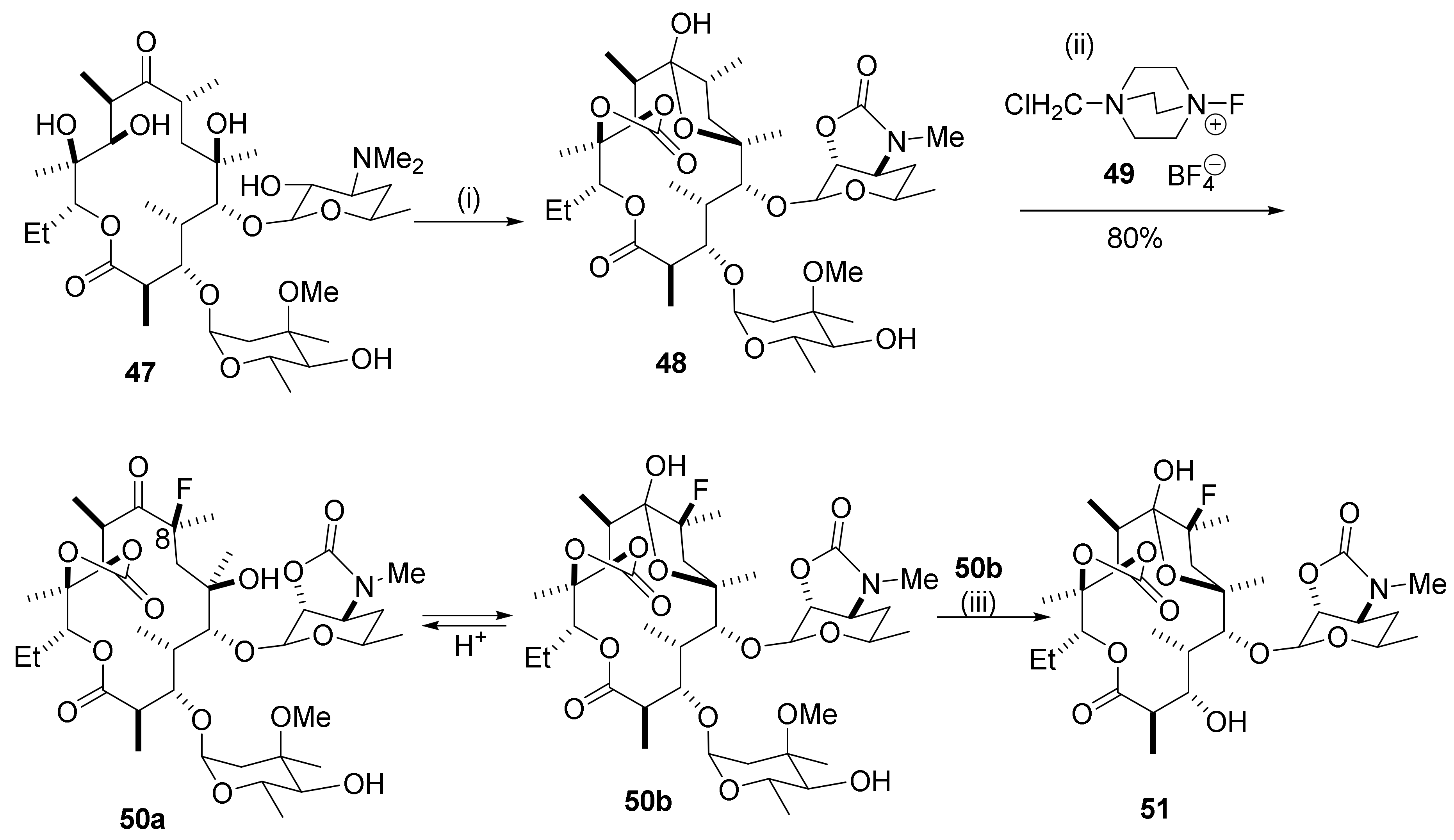
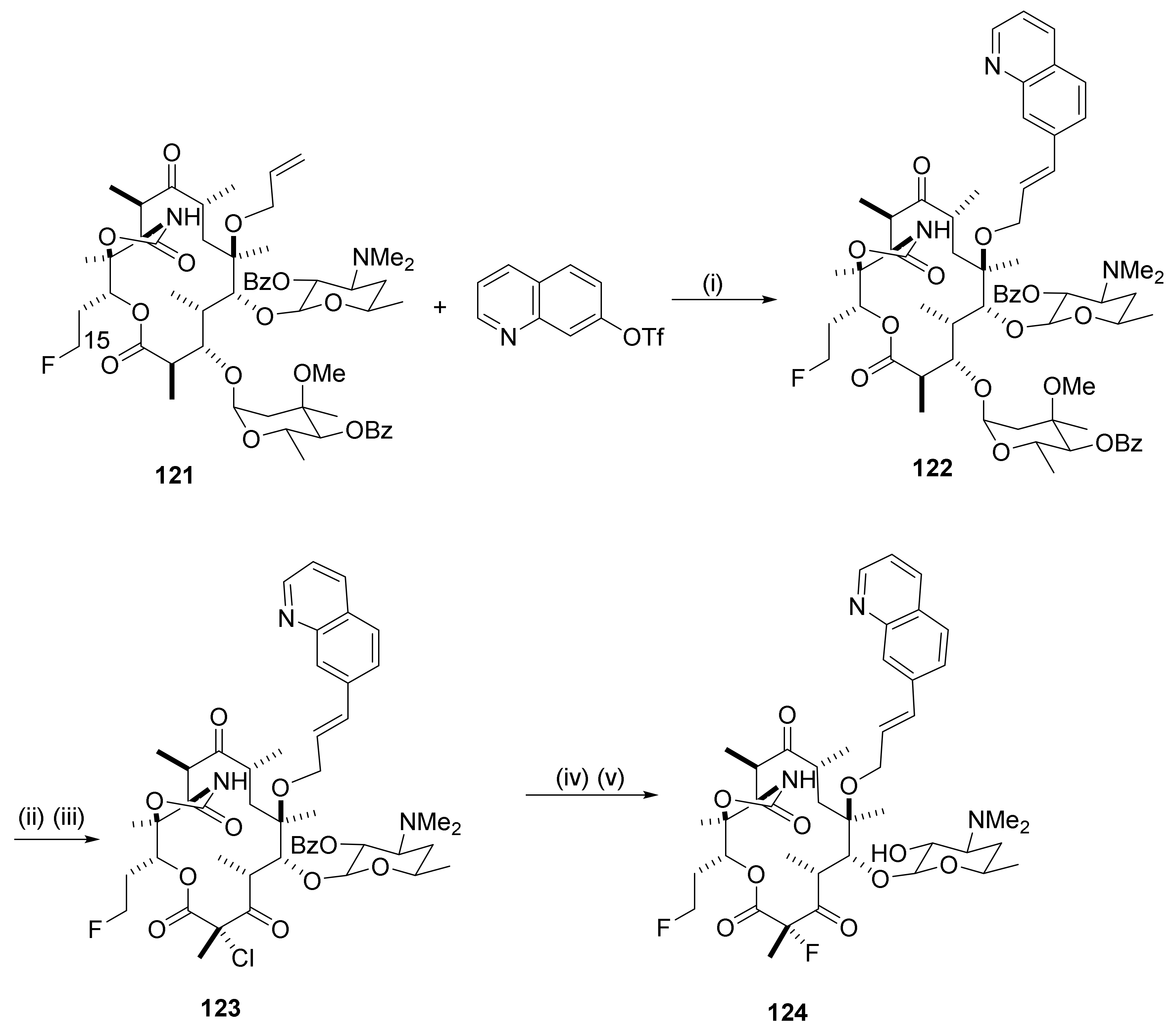
2.3. Fluoro Derivatives
2.4. 9-Aza Derivatives
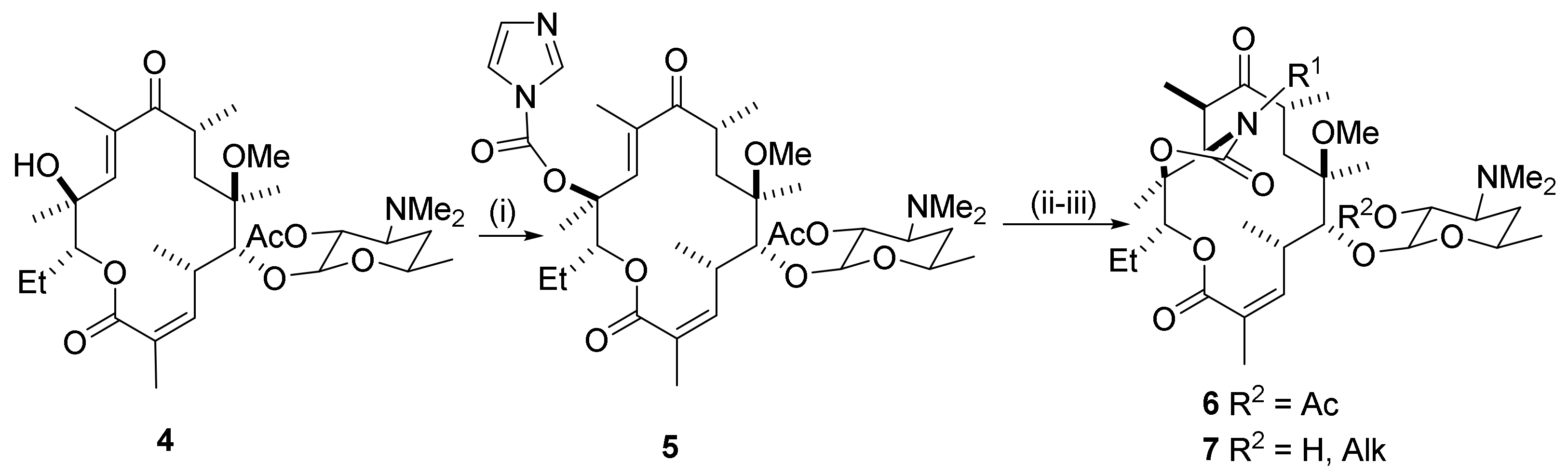
2.5. Synthesis of 4-Desmethyl
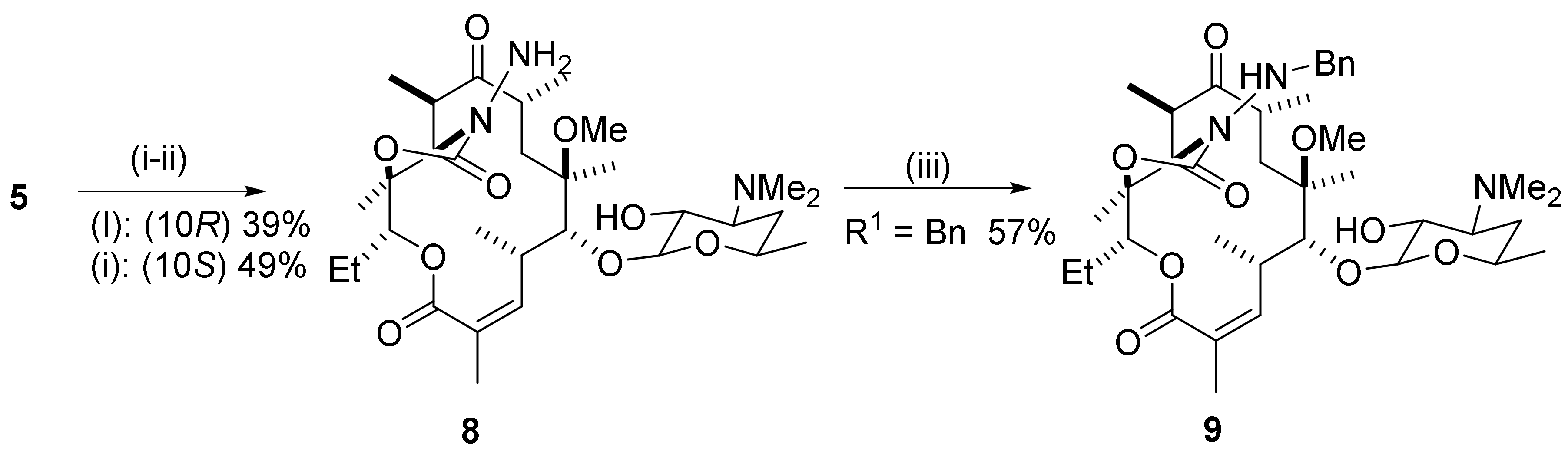
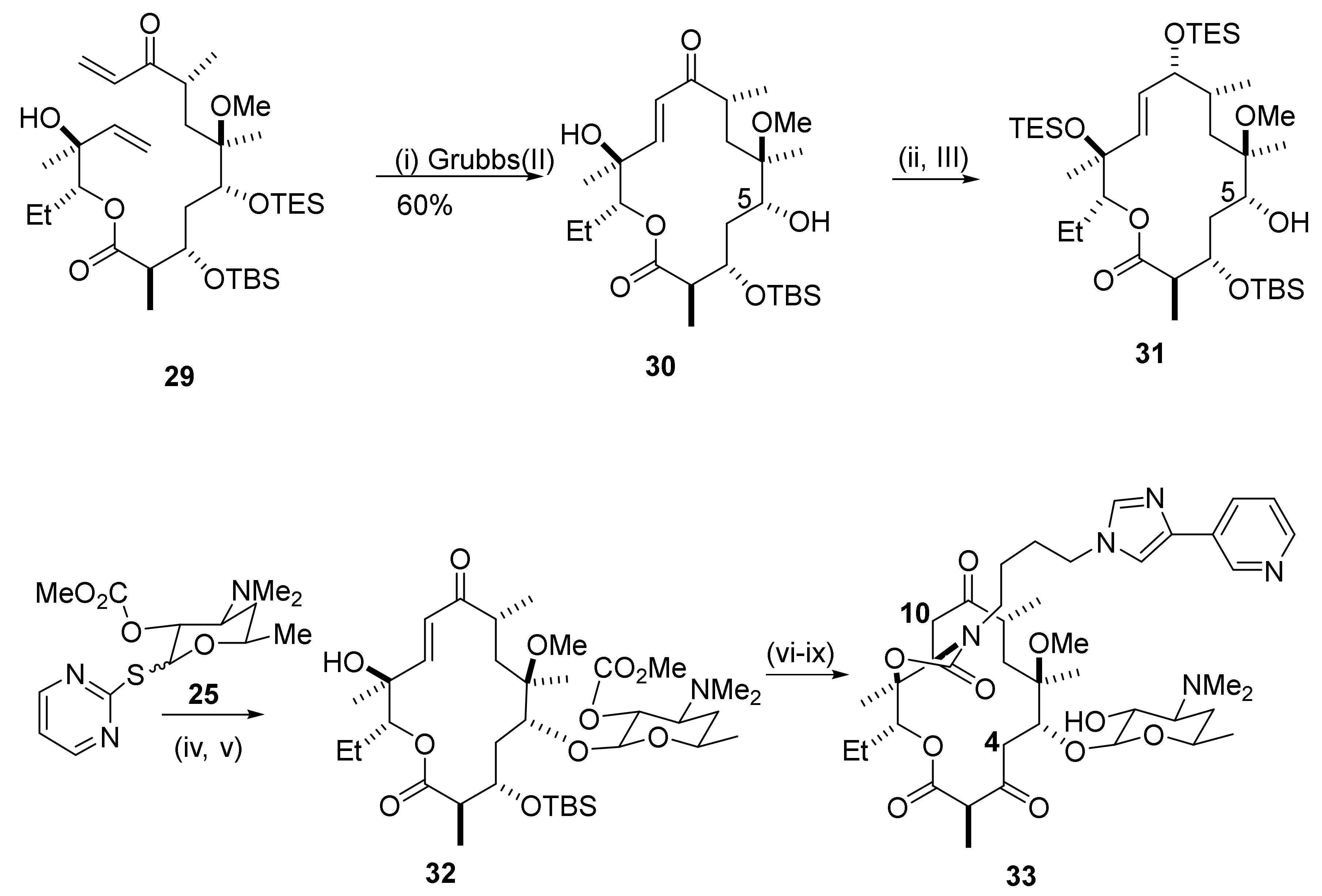
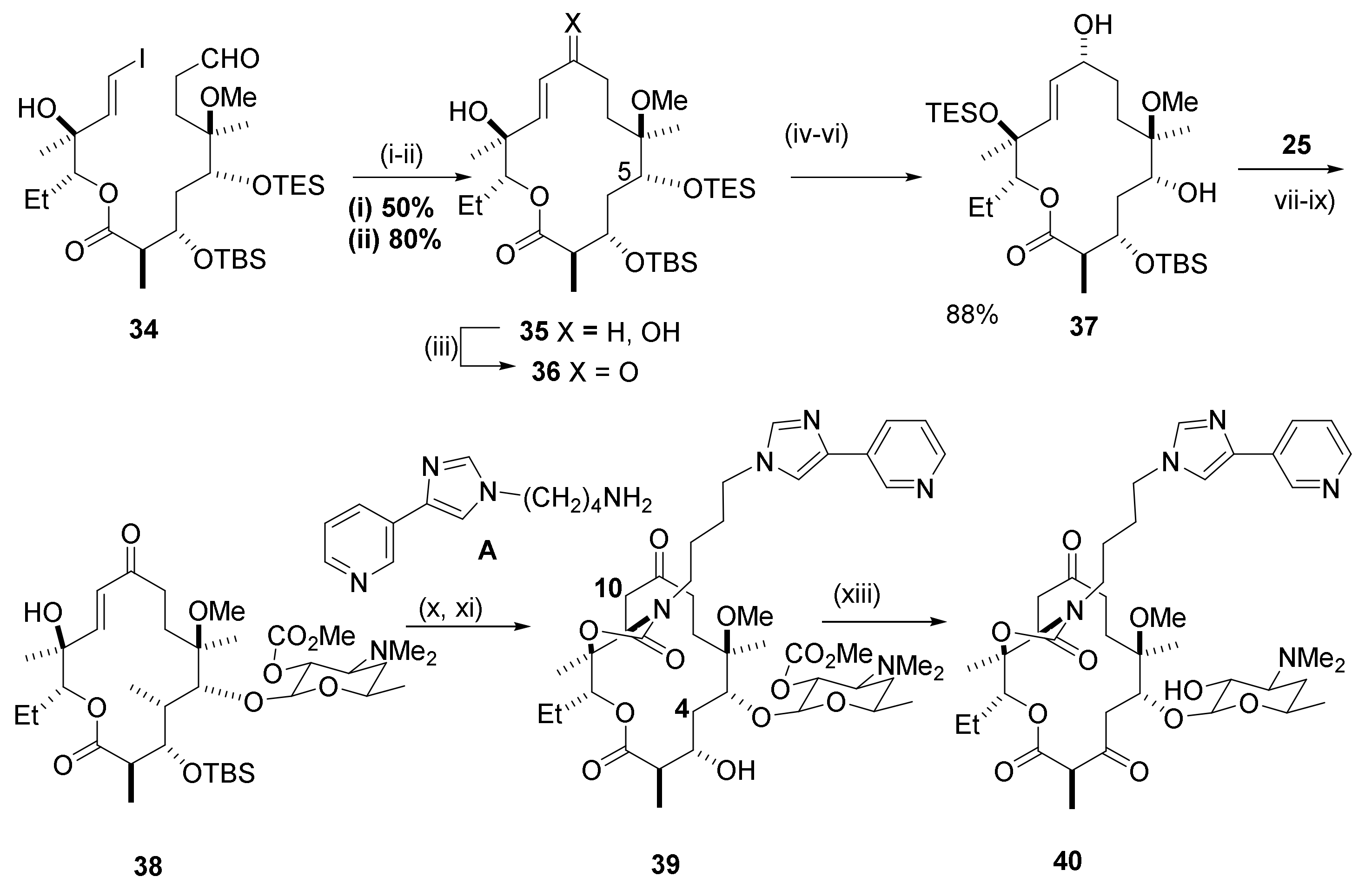
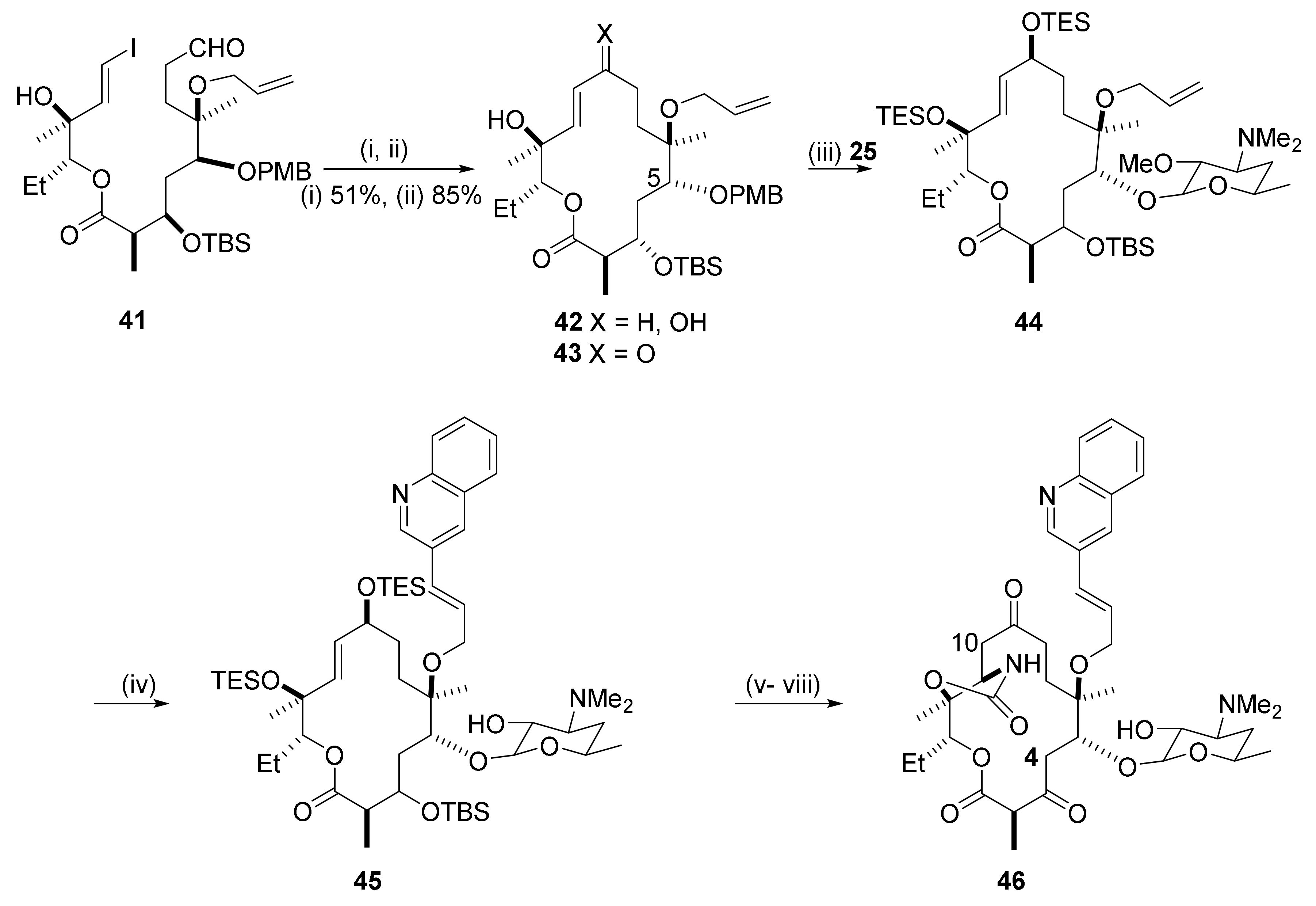
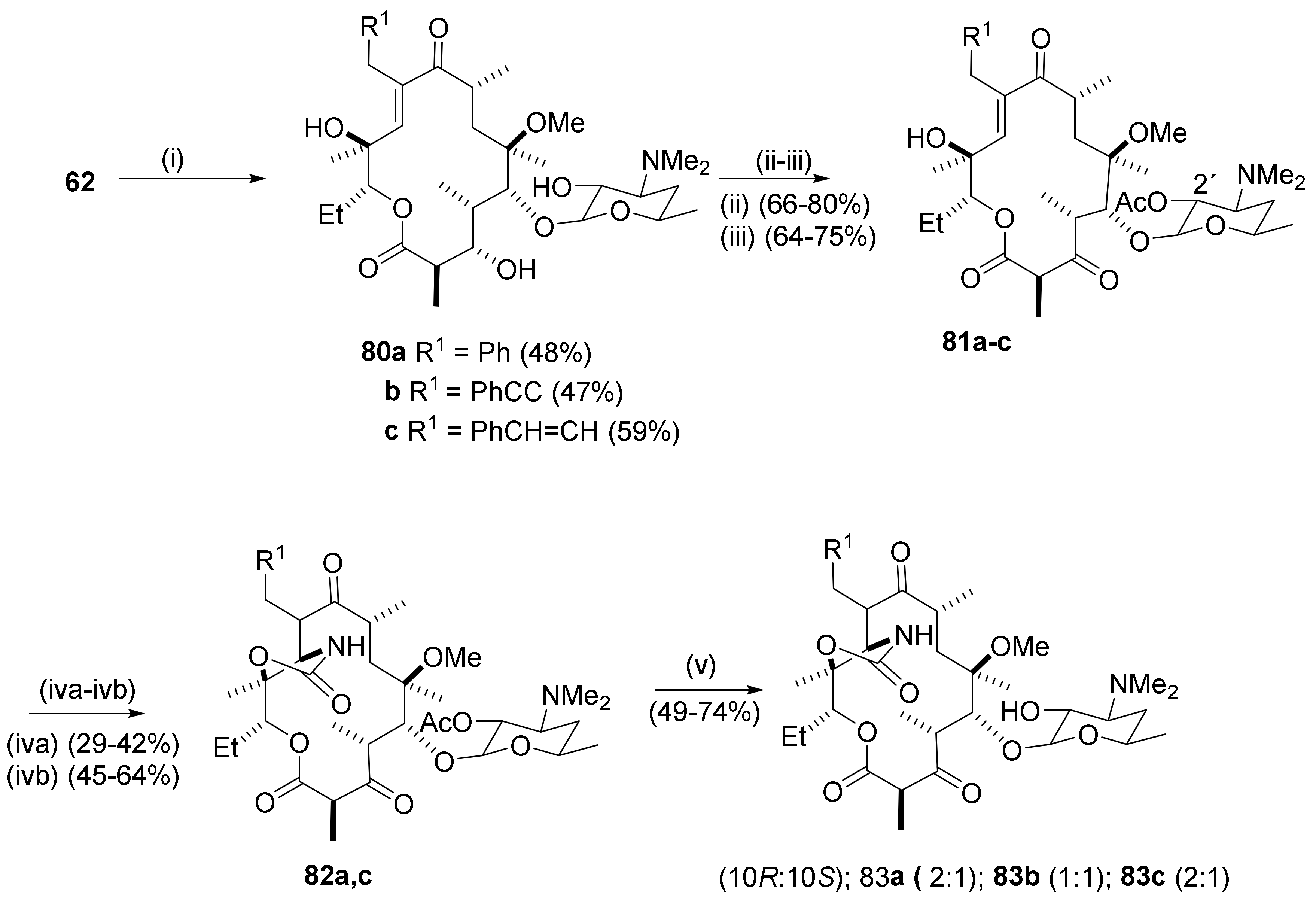
2.6. 4,10-Didesmethyl-Telithromycin Derivatives
2.7. 4,8,10-Tridesmethyl-Telithromycin Derivatives
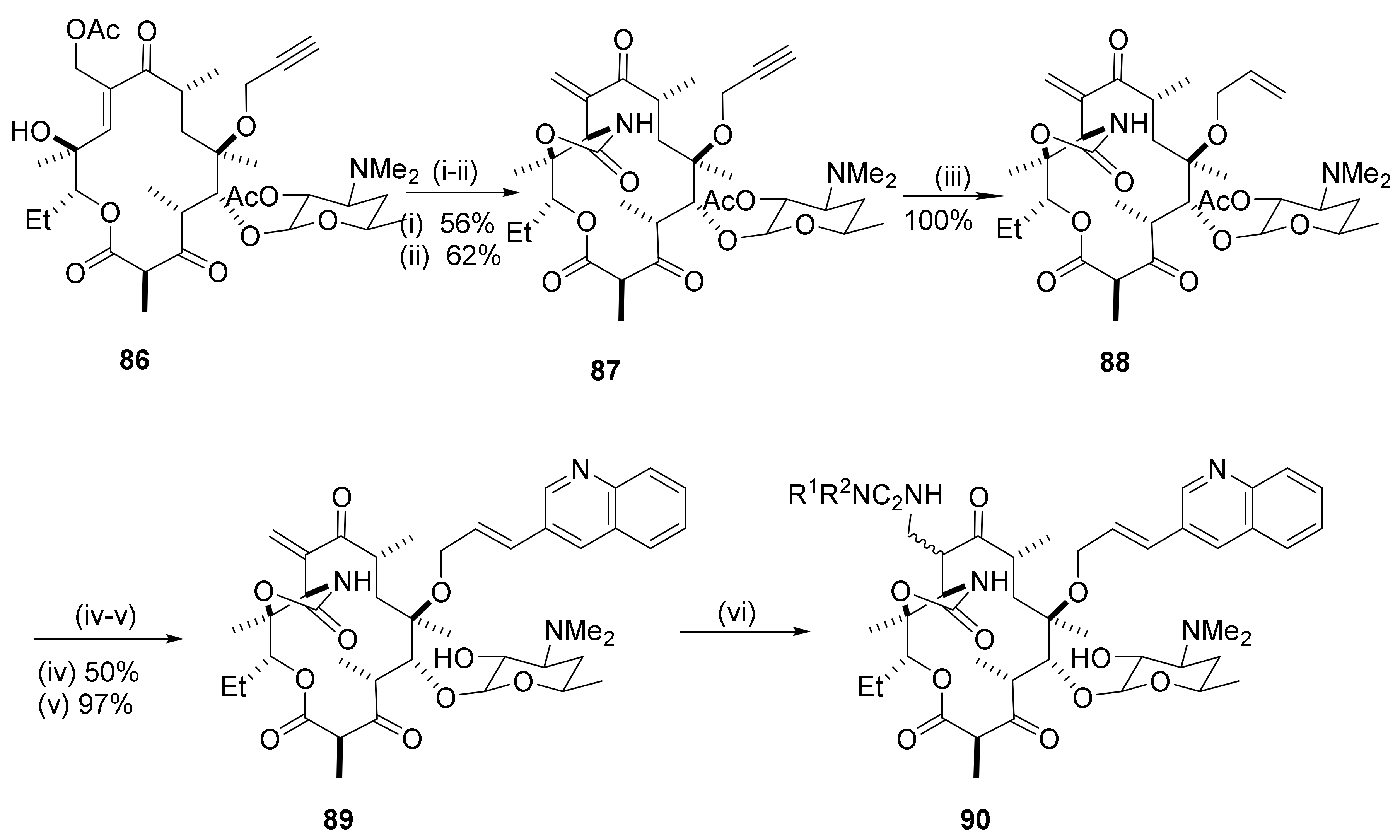
2.8. Cethromycin. Ketolides
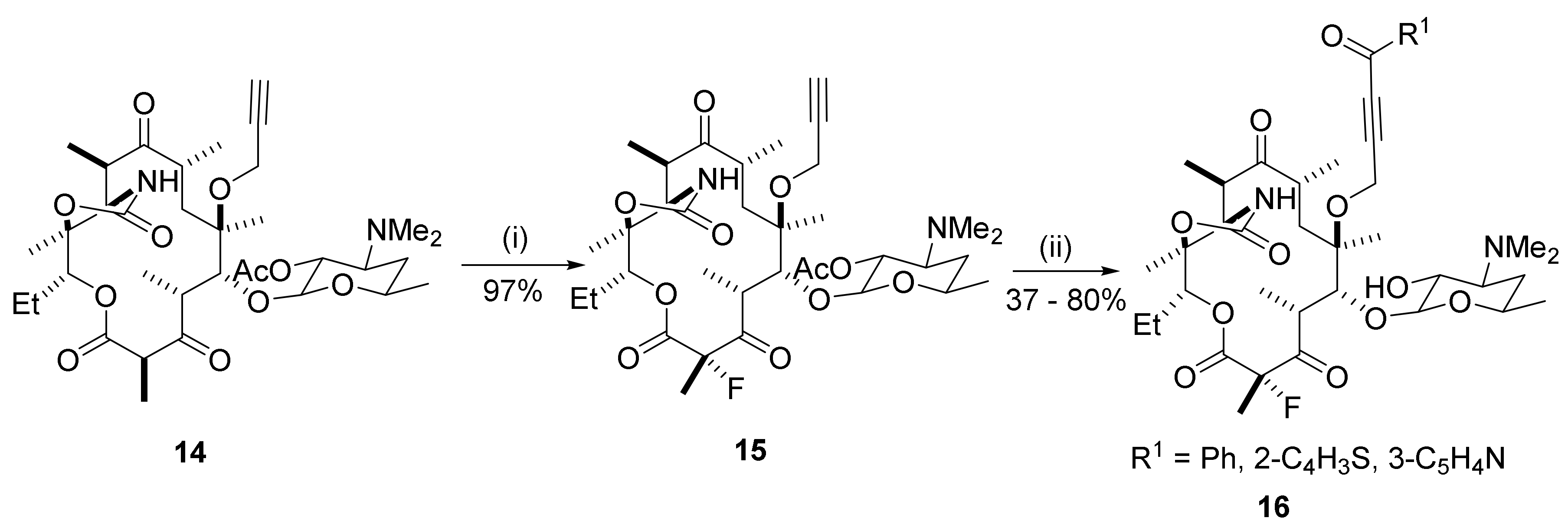
2.9. Synthesis of C8-Fluoro Derivatives
2.10. C9–C10 Unsaturation
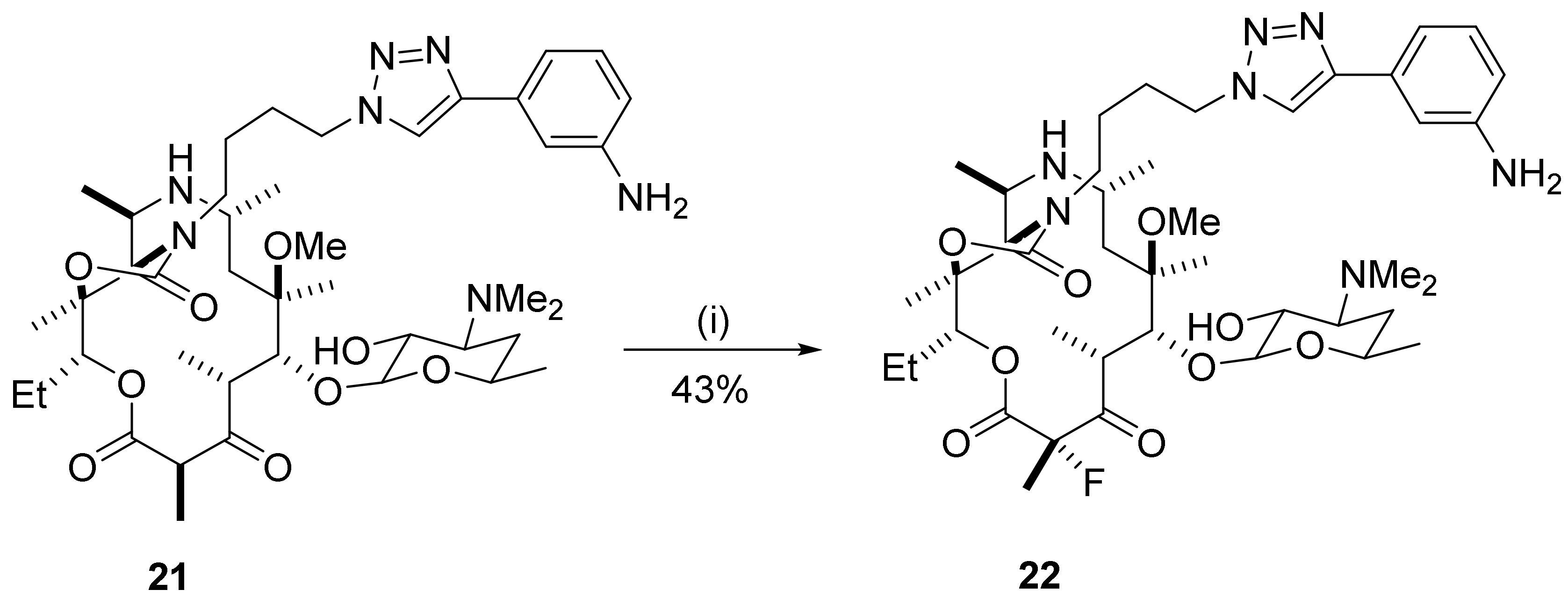
2.11. 9-Azamacrolides
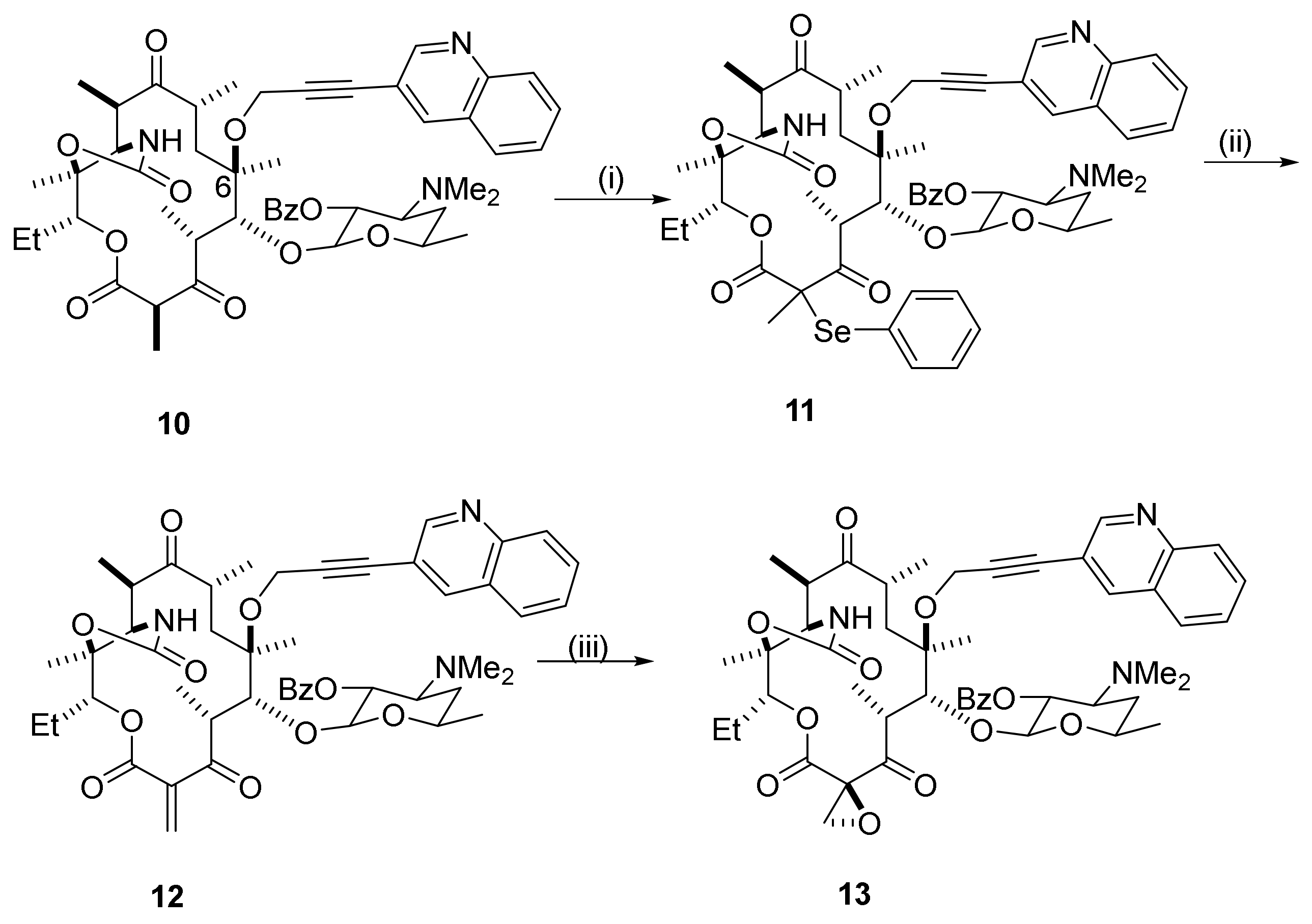
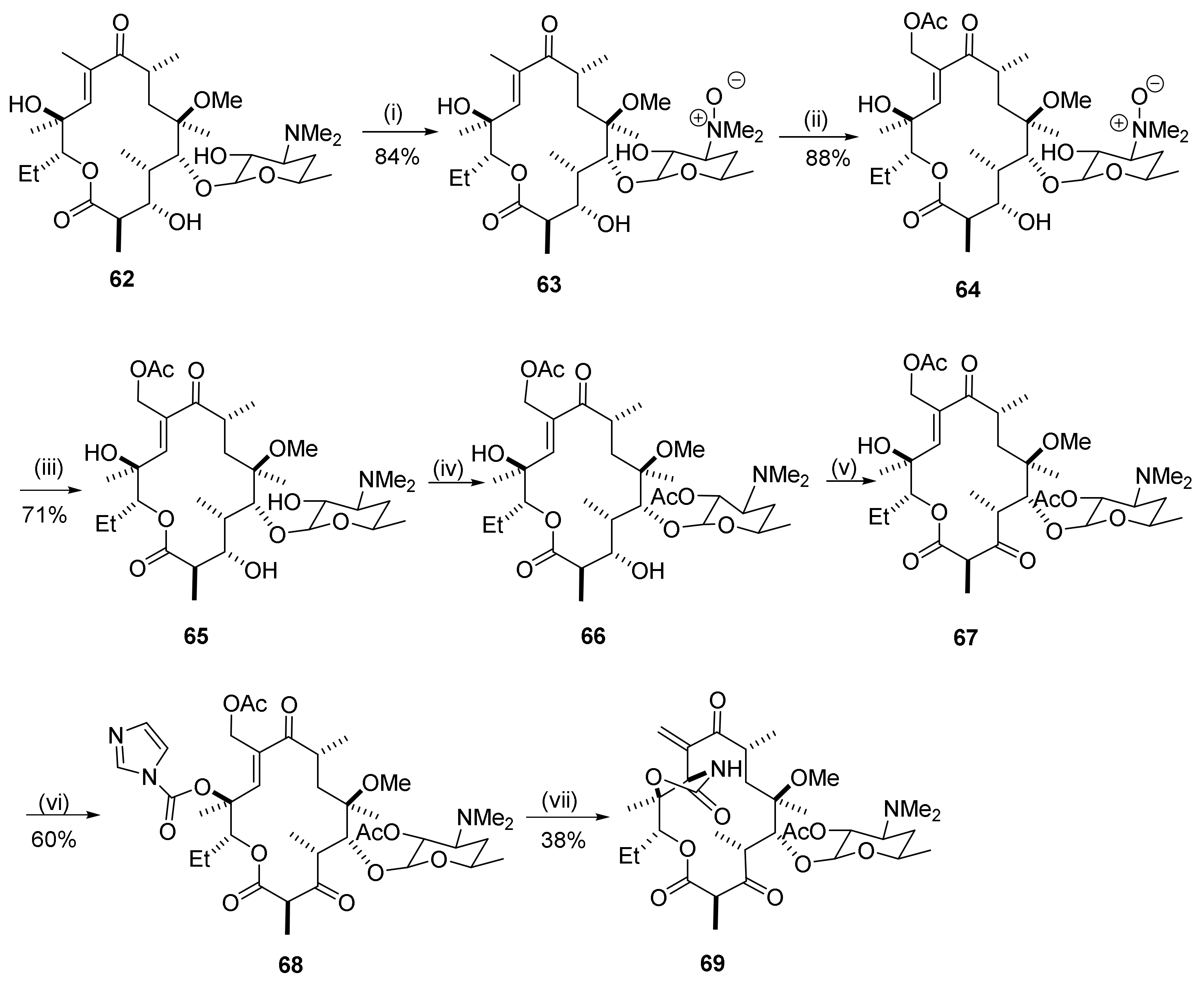
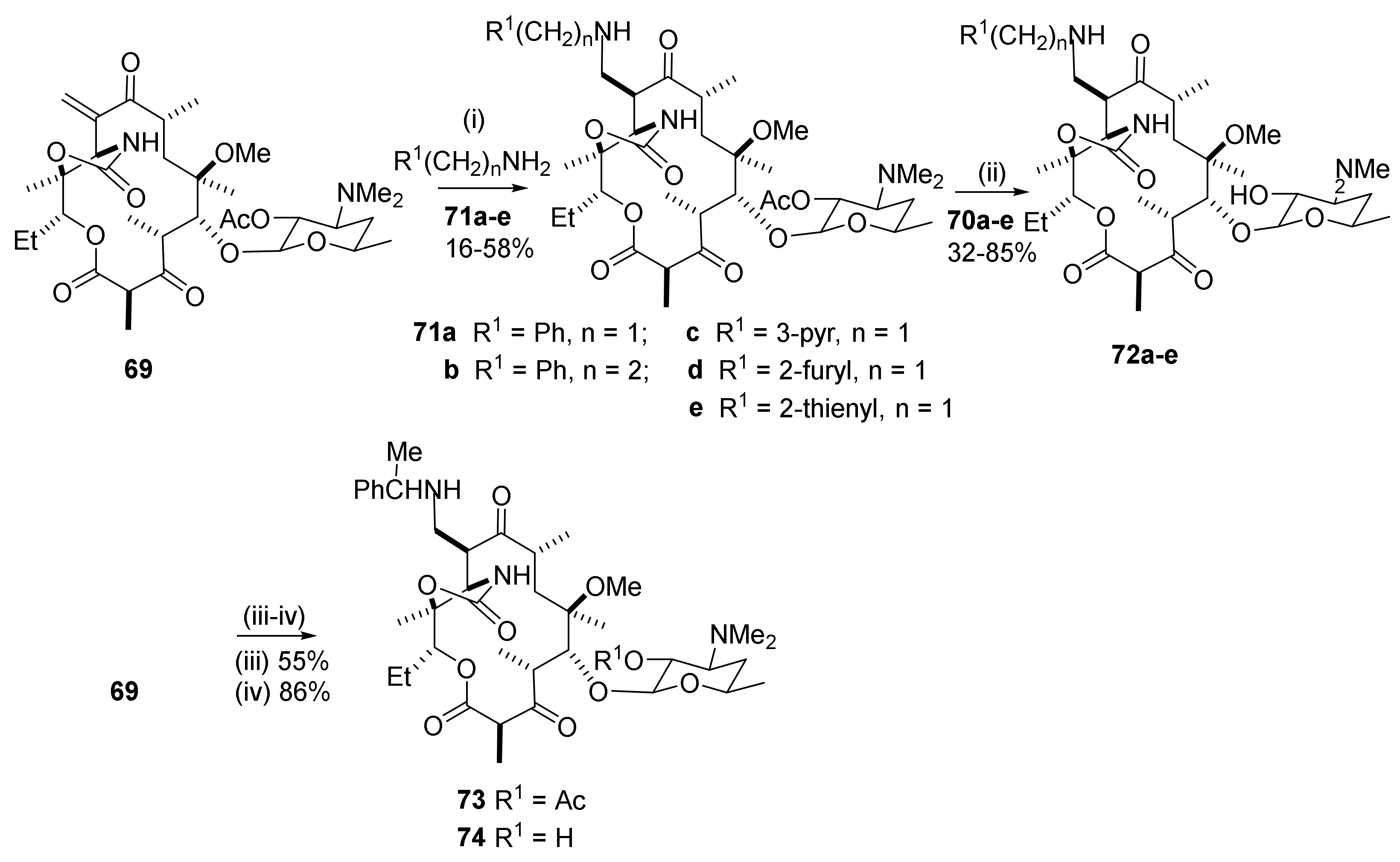
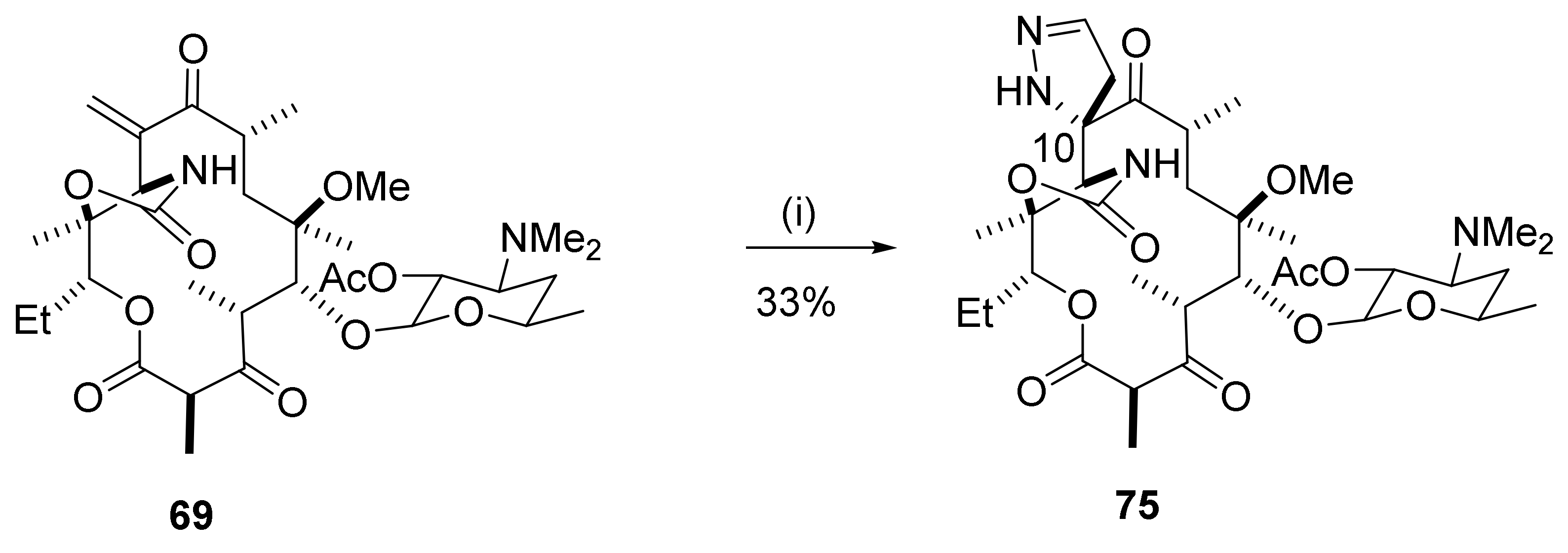
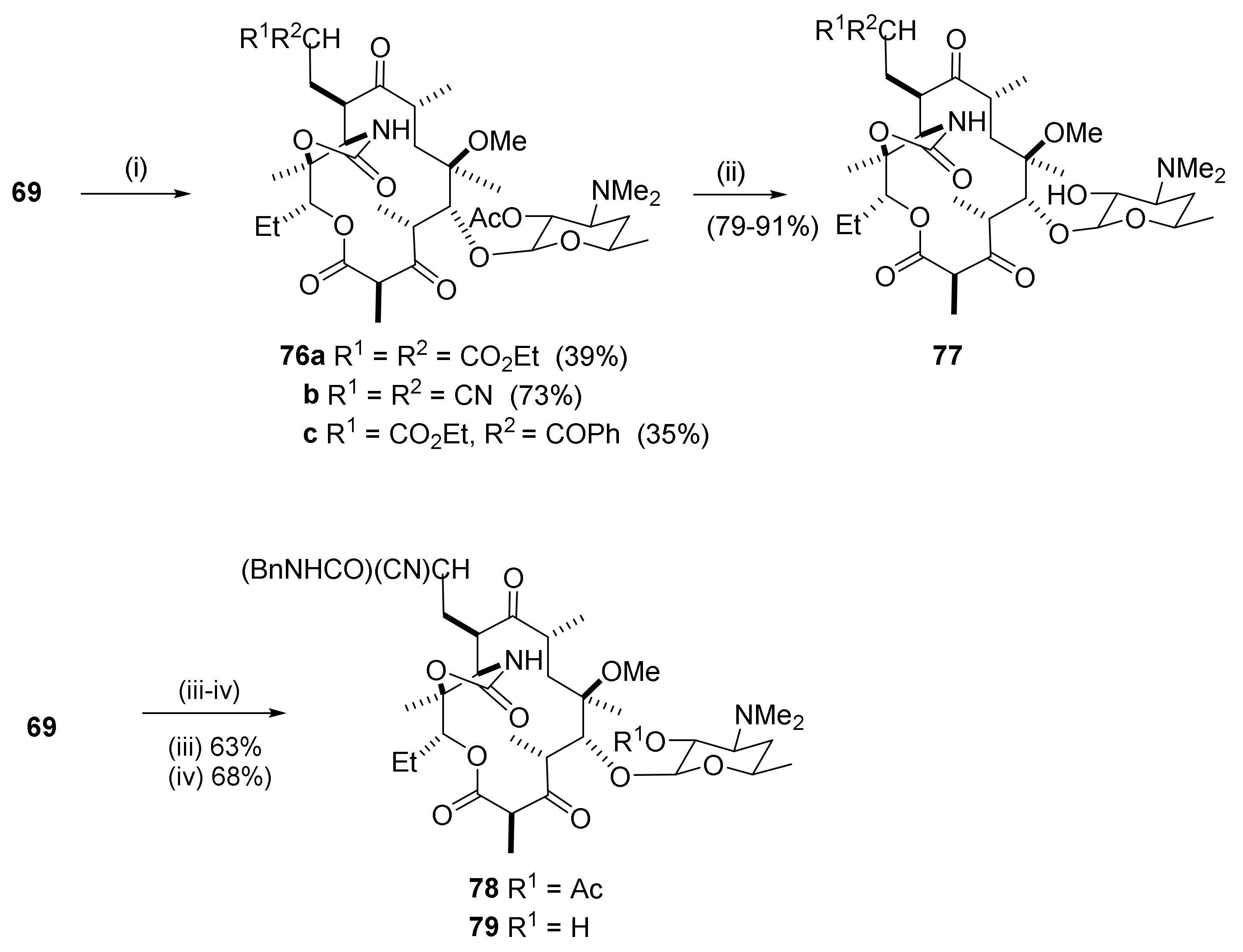
2.12. Transformations in the C10-Position
2.12.1. Activation of the C10-Methyl Group
2.12.2. C10-Methyl Aminations
2.12.3. C10-α-Heteraspirane Formation
2.12.4. C10-Carbylation Reactions
2.12.5. C10-Trans-Coupling by Transition Metal Catalysis
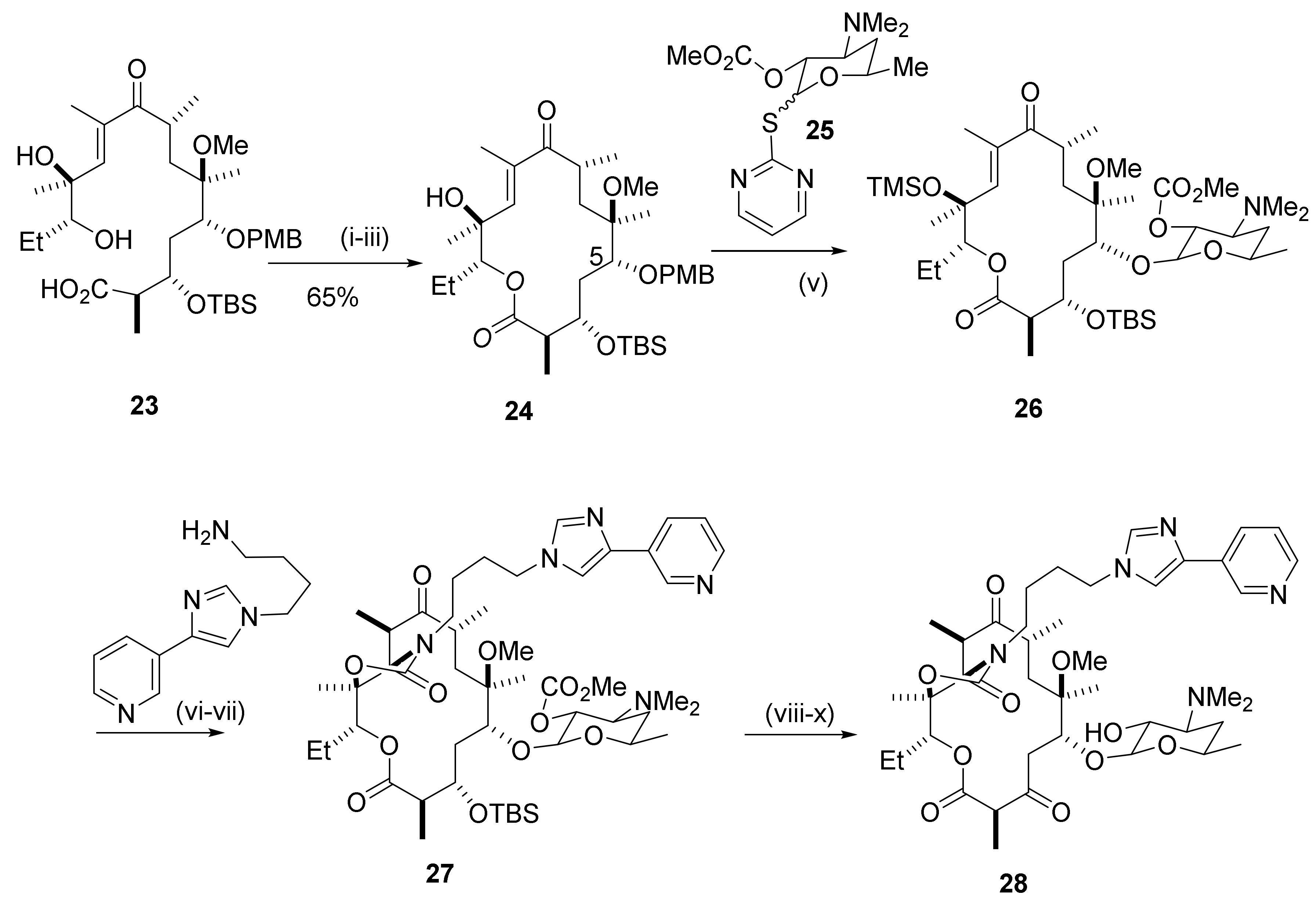
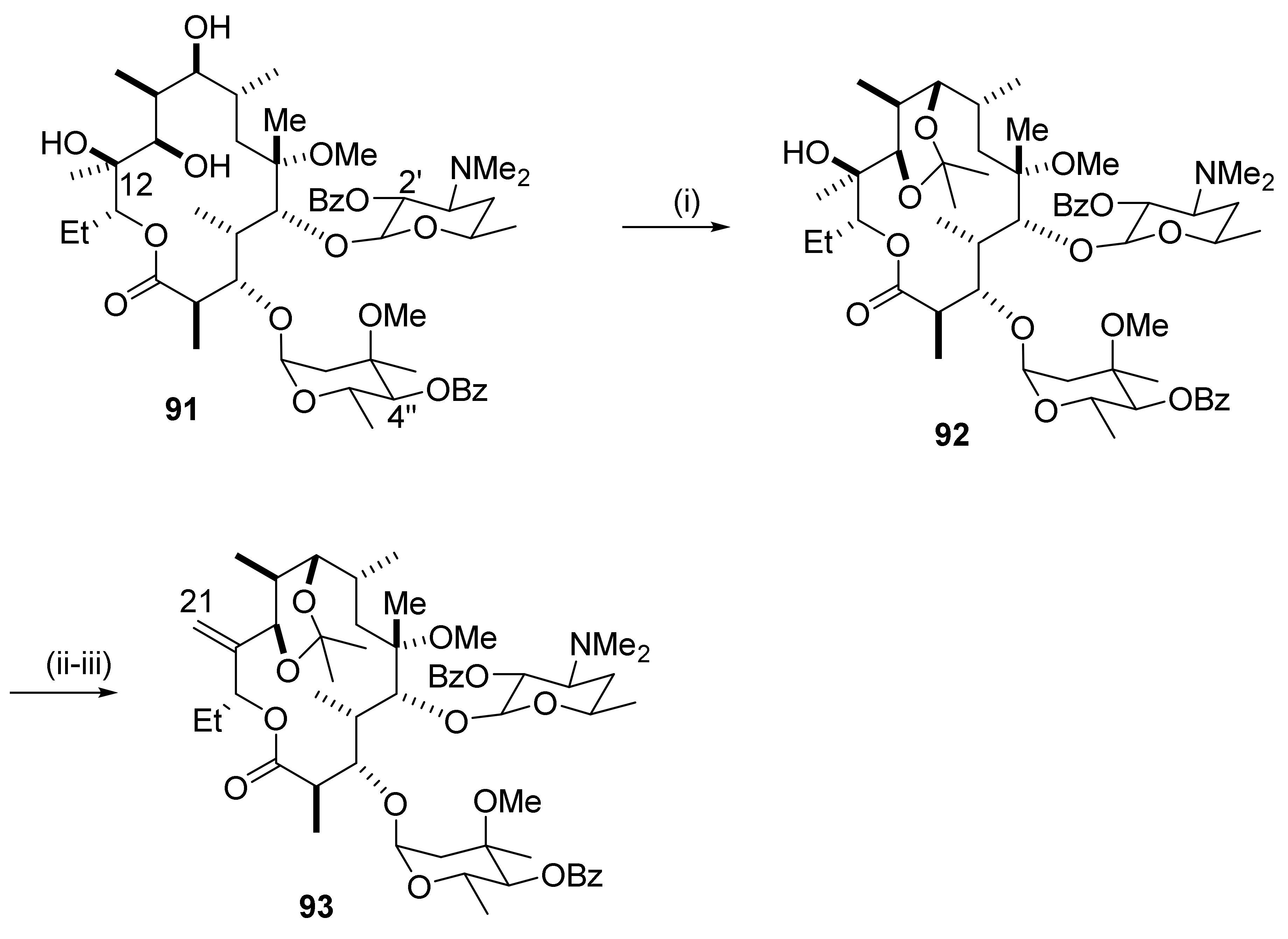
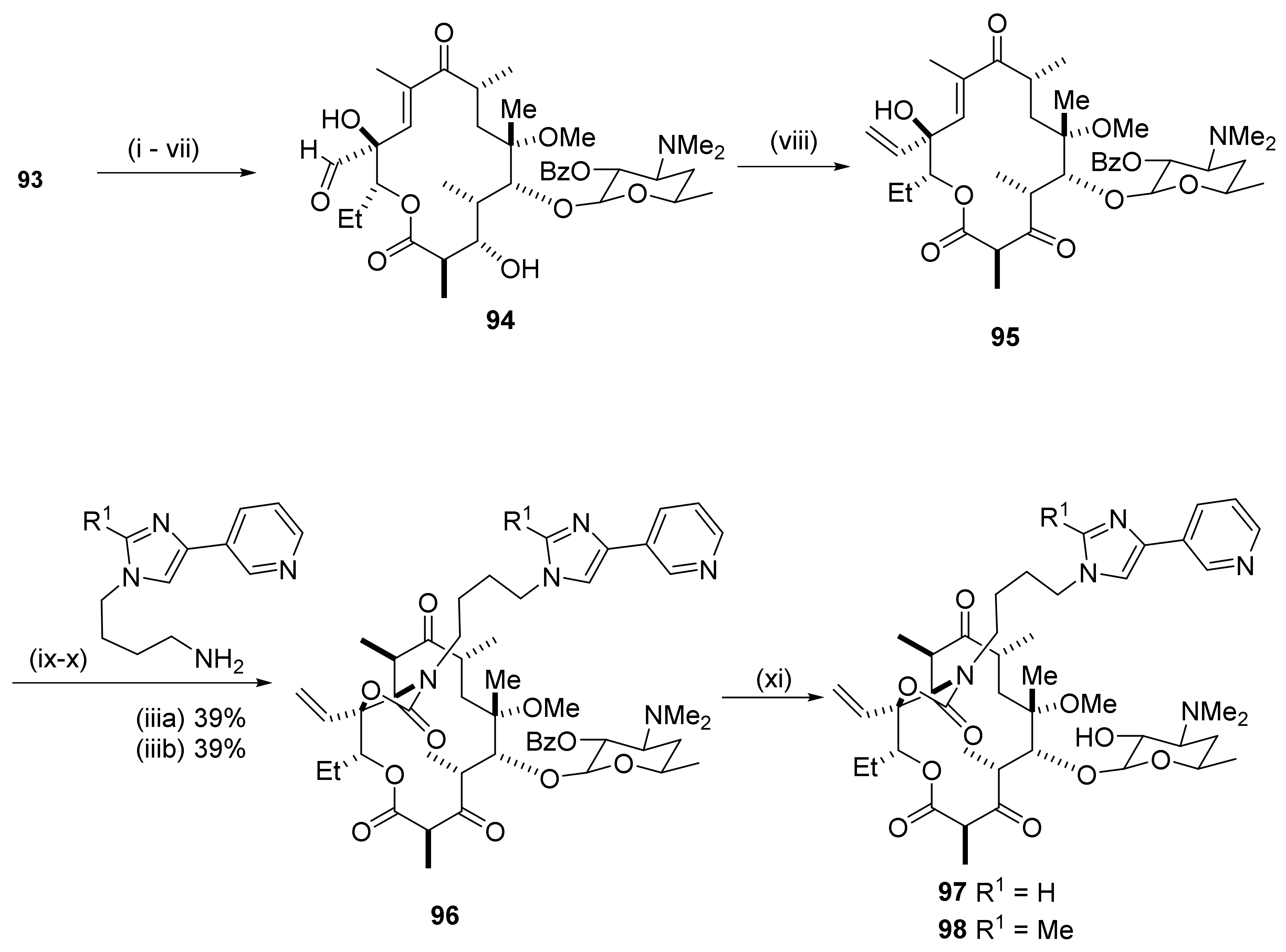
2.12.6. Reactions in the C12-Position
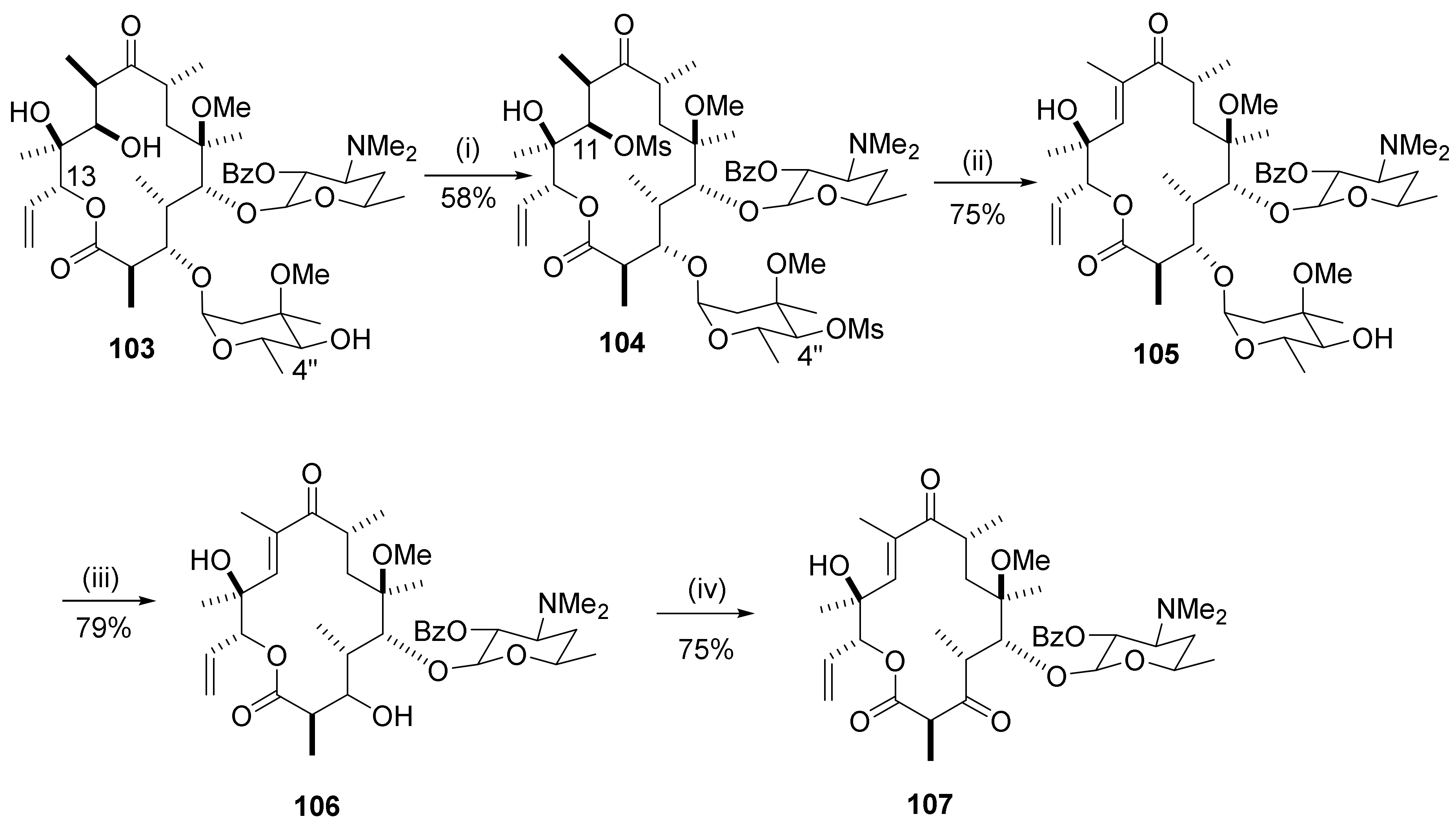
2.12.7. Reactions in the C13-Position
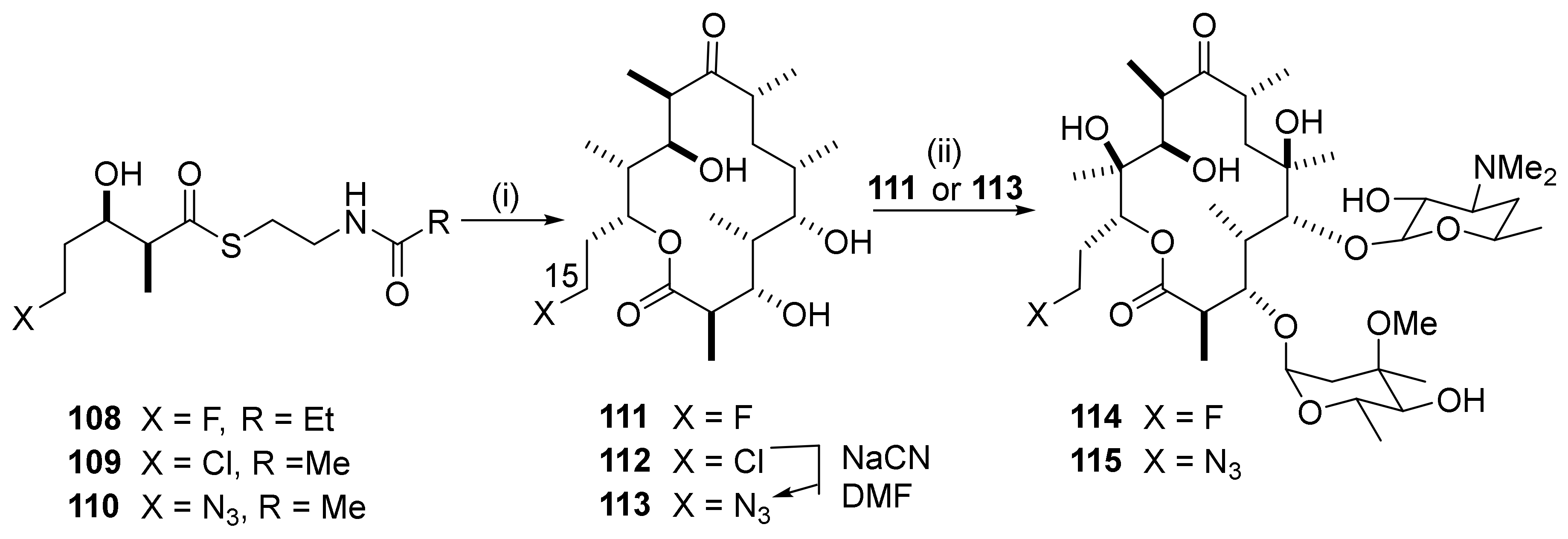
2.12.8. Reactions in the C15-Position
2.12.9. 15-Amino Macrolides
Funding
Conflicts of Interest
References
- Katz, L.; Ashley, G.W. Translation and protein synthesis: Macrolides. Chem. Rev. 2005, 105, 499–527. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Fernandes, P.; Martens, E.; Bertrand, D.; Pereira, D. The solithromycin journey: Its all in the chemistry. Bioorg. Med. Chem. 2016, 24, 6420–6428. [Google Scholar] [CrossRef] [Green Version]
- Wright, P.M.; Seiple, I.B.; Myers, A.G. The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chem. Int. Ed. 2014, 53, 8840–8869. [Google Scholar] [CrossRef] [Green Version]
- Baker, W.R.; Clark, J.D.; Stephens, R.L.; Kim, K.H. Modification of macrolide antibiotics: Synthesis of 11-dioxy-11-(carboxyamino)-6-O-methylerythromycin A 11,12-(cyclic esters) via an Intramolecular Michael reaction of O-carbamates with an α,β-unsaturated ketone. J. Org. Chem. 1988, 53, 2340–2345. [Google Scholar] [CrossRef]
- Elliott, R.L.; Pireh, D.; Griesgraber, G.; Nilius, A.M.; Ewing, P.J.; Bui, M.H.; Raney, P.M.; Flamm, R.K.; Kim, K.; Henry, R.F.; et al. Anhydrolide macrolides: 1. Synthesis and antibacterial activity of 2,3-anhydro-6-O-methyl 11,12-carbamate erythromycin A analogues. J. Med. Chem. 1998, 41, 1651–1659. [Google Scholar] [CrossRef]
- Griesgraber, G.; Kramer, M.J.; Elliott, R.L.; Nilius, A.M.; Ewing, P.J.; Raney, P.M.; Bui, M.-H.; Flamm, R.K.; Chu, D.T.W.; Plattner, J.J.; et al. Anhydrolide macrolides. 2. Synthesis and antibacterial actity of 2,3-anhydro-6-O-methyl 11,12-carbazate erythromycin A analogues. J. Med. Chem. 1998, 41, 1660–1670. [Google Scholar] [CrossRef]
- Griesgraber, G.; Or, Y.-S.; Chu, D.T.W.; Nilius, A.M.; Johnson, R.K.; Flamm, R.K.; Henry, R.F.; Plattner, J.J. 3-Keto-11,12-carbazate derivatives of 6-O-methylerythromycin A: Synthesis and in vitro activity. J. Antibiot. 1996, 49, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Phan, L.T.; Or, Y.S.; Ma, Z. Preparation of macrolide erythromycin 6-O-alkyl-2-nor-2-substituted agents. Patent WO2001040241, 7 June 2001. [Google Scholar]
- Phan, L.T.; Clark, R.F.; Rupp, M.; Or, Y.S.; Chu, D.T.W.; Ma, Z. Synthesis of 2-fluoro-6-O-propargyl-11,12-carbamate ketolides: A novel class of antibiotics. Org. Lett. 2000, 2, 2951–2954. [Google Scholar] [CrossRef] [PubMed]
- Daher, S.; Jin, X.; Patel, J.; Freundlich, J.S.; Buttaro, B.; Andrade, R.B. Synthesis and biological evaluation of solithromycin analogues against multidrug resistant pathogens. Bioorg. Med. Chem. Lett. 2019, 29, 1386–1389. [Google Scholar] [CrossRef] [PubMed]
- Glassford, I.; Teijaro, C.N.; Daher, S.S.; Weil, A.Q.; Small, M.C.; Redhu, S.K.; Colussi, D.J.; Jacobson, M.A.; Childers, W.E.; Buttaro, B.; et al. Ribozome-templated azide alkyne cycloadditions: Synthesis of potent macrolide antibiotics by in situ click chemistry. J. Am. Chem. Soc. 2016, 138, 3136–3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiple, I.B.; Zhang, Z.; Jakubec, P.; Langlois-Mercier, A.; Wright, P.M.; Hog, D.T.; Yabu, K.; Allu, S.R.; Fukuzaki, T.; Carlsen, P.N.; et al. A platform for the discovery of new macrolide antibiotics. Nature 2016, 533, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Glassford, I.; Lee, M.; Wagh, B.; Velvadupu, V.; Paul, T.; Sandelin, G.; Debrosse, C.; Klepacki, D.; Small, M.C.; Nackerell, A.D.; et al. Desmethyl macrolides: Synthesis and evaluation of 4-desmethyl telithromycin. Med. Chem. Lett. 2014, 5, 1021–1026. [Google Scholar] [CrossRef]
- Velvadapu, V.; Glassford, I.; Lee, M.; Paul, T.; Debrosse, C.; Klepacki, D.; Small, M.C.; MacKerell, A.D.; Andrade, R.B. Desmethyl macrolides: Synthesis and evaluation of 4,10-didesmethyl telithromycin. Med. Chem. Lett. 2012, 3, 211–215. [Google Scholar] [CrossRef]
- Velvadapu, V.; Paul, T.; Wagh, B.; Glassford, I.; DeBrosse, C.; Andrade, R.B. Total Synthesis of (−)−2,8,10-tridesmethyl telithromycin. J. Org. Chem. 2011, 76, 7516–7527. [Google Scholar] [CrossRef] [Green Version]
- Wagh, B.; Paul, T.; DeBrosse, C.; Klepacki, D.; Small, M.C.; MacKerell, A.D.; Andrade, R.B. Desmethyl macrolides: Synthesis and evaluation of 4,8,10-tridesmetyl cetromycin. Med. Chem. Lett. 2013, 4, 1114–1118. [Google Scholar] [CrossRef]
- Andrade, R.B. Total synthesis of desmethyl macrolide antibiotics. Synlett 2015, 26, 2199–2215. [Google Scholar] [CrossRef]
- Heggelund, A.; Undheim, K. Synthesis of 8-fluorinated erythromycin cyclic 2′,3′-carbamates. Synth. Commun. 2009, 39, 1903–1913. [Google Scholar] [CrossRef]
- Phan, L.T.; Farmer, J.J.; Or, Y.S. Preparation of antibiotic macrolide erythromycin 11-C-Substituted ketolides. Patent WO2004000864, 31 December 2004. [Google Scholar]
- Gunnes, S.; Undheim, K. Chemoselective synthesis of erythromycin A ketolides substituted in the C10-methyl group. Bioorg. Med. Chem. 2007, 15, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Gunnes, S.; Rømming, C.; Undheim, K. Selective substitutions in the C10-methyl group in erythromycin derivatives. Tetrahedron 2006, 62, 6090–6099. [Google Scholar] [CrossRef]
- Anwar, H.F.; Andrei, M.; Undheim, K. Synthesis of clarithromuycin ketolides chemically modified at the unreactive C10-methyl group. Bioorg. Med. Chem. 2017, 25, 2313–2326. [Google Scholar] [CrossRef] [PubMed]
- Lociuro, S.; Ege, T.; Andreotti, D.; Arena, A.; Gagliardi, S.; Palombi, G.; Presenti, C. Erythromycin ketolide derivatives bearing C-10 modifications. Patent WO2014049356, 3 April 2014. [Google Scholar]
- Burger, M.T.; Lin, X.; Chu, D.T.; Hiebert, C.; Rico, A.C.; Seid, M.; Caroll, G.L.; Barker, L.; Huh, K.; Langhorne, M.; et al. Synthesis and antibacterial activity of novel C12 vinyl ketolides. J. Med. Chem. 2006, 49, 1730–1743. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.T.; Hiebert, C.; Seid, M.; Chu, D.T.; Barker, L.; Langhorne, M.; Shawar, R.; Kidney, J.; Desai, M.C.; Plattner, J.J. Synthesis and antibacterial activity of novel C12 ethyl ketolides. Bioorg. Med. Chem. 2006, 14, 5592–5604. [Google Scholar] [CrossRef]
- Fardis, M.; Ashley, G.W.; Carney, J.R.; Chu, D.T. Synthesis of 14,15-dehydroerythromycin A ketolides: Effect of the 13-substituent on erythromycin tautomerism. J. Antibiot. 2001, 54, 278–284. [Google Scholar] [CrossRef]
- Ashley, G.W.; Burlingame, M.; Desai, R.; Fu, H.; Leaf, T.; Licari, P.J.; Tran, C.; Abbanat, D.; Bush, K.; Macielag, M. Preparation of erythromycin analogs having functional groups at C-15. J. Antibiot. 2006, 59, 392–401. [Google Scholar] [CrossRef]
- Ashley, G.; Shaw, S.J.; Li, Y. Preparation of erythromycin A derived amido-macrolides for use in treatment of bacterial infections. Patent WO2003061671, 31 July 2003. [Google Scholar]
- Shaw, S.J.; Ashley, G.W.; Burlingame, M.A. Preparation of 7-quinolyl 15-fluoro-ketolide erythromycin derivatives as antibacterial agents. Patent WO2007070621, 21 June 2007. [Google Scholar]







© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Undheim, K. Scaffold Modifications in Erythromycin Macrolide Antibiotics. A Chemical Minireview. Molecules 2020, 25, 3941. https://doi.org/10.3390/molecules25173941
Undheim K. Scaffold Modifications in Erythromycin Macrolide Antibiotics. A Chemical Minireview. Molecules. 2020; 25(17):3941. https://doi.org/10.3390/molecules25173941
Chicago/Turabian StyleUndheim, Kjell. 2020. "Scaffold Modifications in Erythromycin Macrolide Antibiotics. A Chemical Minireview" Molecules 25, no. 17: 3941. https://doi.org/10.3390/molecules25173941
APA StyleUndheim, K. (2020). Scaffold Modifications in Erythromycin Macrolide Antibiotics. A Chemical Minireview. Molecules, 25(17), 3941. https://doi.org/10.3390/molecules25173941




