Biological Activity and Antibiofilm Molecular Profile of Citrus aurantium Essential Oil and Its Application in a Food Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Bitter Orange Essential Oil (Citrus aurantium L., CAEO)
2.2. Antioxidant Activity of CAEO
2.3. Antimicrobial Assay
2.4. Antibiofilm Activity of CAEO
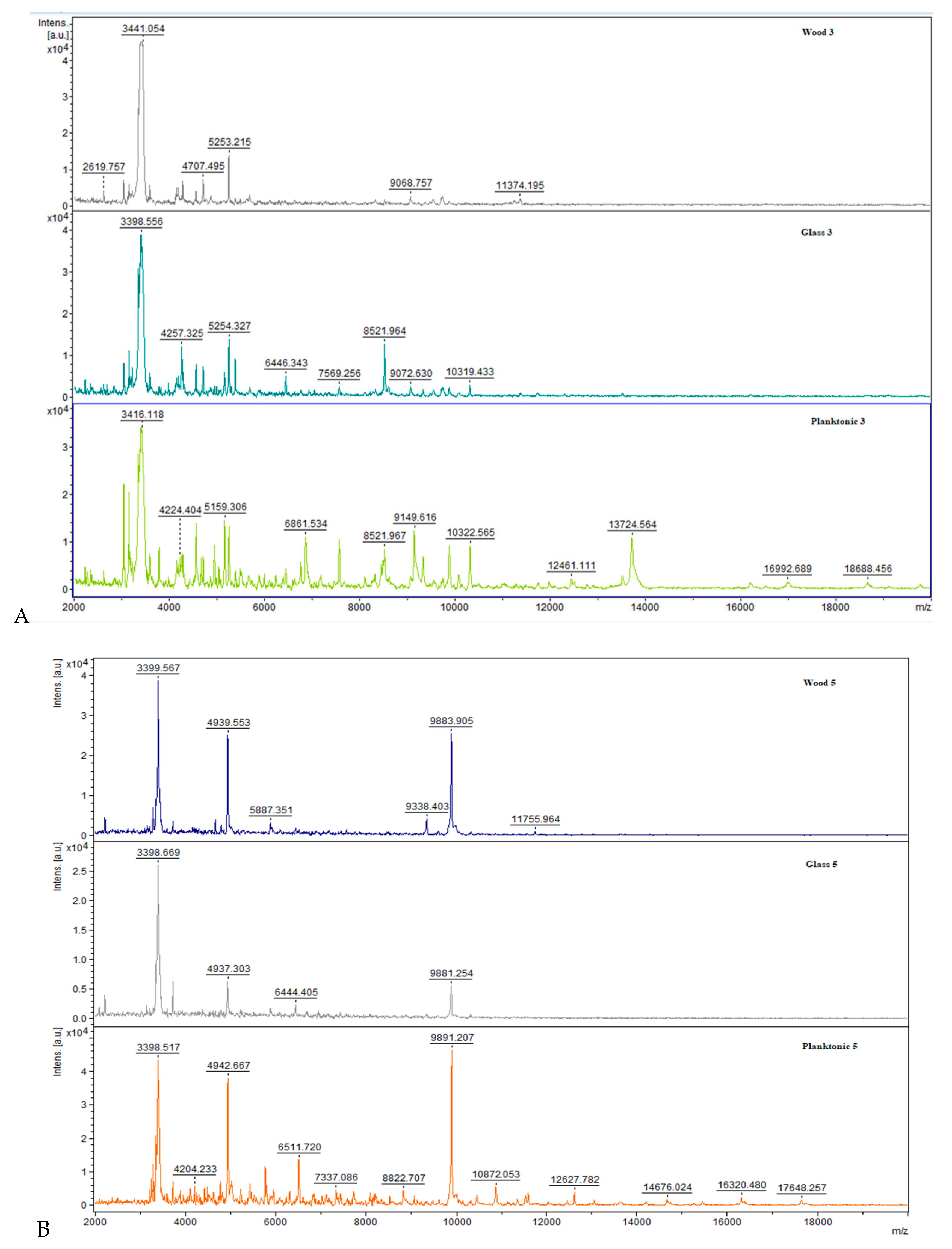
2.5. Studies on Biofilm Development and Molecular Differences on Surfaces after Treatment with C. aurantium EOs
2.6. Water Activity and Moisture Content
2.7. In Situ Antifungal Analysis on Bread
2.8. In Situ Antimicrobial Effect on Carrot
3. Materials and Methods
3.1. Essential Oil
3.2. Chemical Composition of Essential Oil
3.3. Radical Scavenging Activity-DPPH Method
3.4. Microorganisms
3.5. Antimicrobial Activity
3.6. Minimum Biofilm Inhibitory Concentration (MBIC)
3.7. Biofilm Development and Molecular Differences on Different Surfaces with MALDI-TOF MS Biotyper
3.8. Bread Making Process
3.9. Water Activity and Moisture Content
3.10. In Situ Antifungal Analysis on Bread
3.11. Vapor Phase of Antimicrobial Assay with Carrot
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Moraes Pultrini, A.; Almeida Galindo, L.; Costa, M. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. Life Sci. 2006, 78, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-I.; Park, H.-S.; Kim, M.-K.; Hong, G.-E.; Nagappan, A.; Lee, H.-J.; Yumnam, S.; Lee, W.-S.; Won, C.-K.; Shin, S.-C.; et al. Flavonoids identified from Korean Citrus aurantium L. inhibit Non-Small Cell Lung Cancer growth in vivo and in vitro. J. Funct. Foods 2014, 7, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Machado Rocha, L.R.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Fugh-Berman, A.; Myers, A. Citrus aurantium, an Ingredient of Dietary Supplements Marketed for Weight Loss: Current Status of Clinical and Basic Research. Exp. Biol. Med. 2004, 229, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.K.; George, N.I.; White, G.E.; Pellicore, L.S.; Abdel-Rahman, A.; Fabricant, D. Physiological effects following administration of Citrus aurantium for 28days in rats. Toxicol. Appl. Pharmacol. 2012, 261, 236–247. [Google Scholar] [CrossRef]
- Peixoto, J.S.; Comar, J.F.; Moreira, C.T.; Soares, A.A.; de Oliveira, A.L.; Bracht, A.; Peralta, R.M. Effects of Citrus aurantium (Bitter Orange) Fruit Extracts and p-Synephrine on Metabolic Fluxes in the Rat Liver. Molecules 2012, 17, 5854–5869. [Google Scholar] [CrossRef]
- Kang, S.R.; Park, K.I.; Park, H.S.; Lee, D.H.; Kim, J.A.; Nagappan, A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Park, M.K.; et al. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 2011, 129, 1721–1728. [Google Scholar] [CrossRef]
- Hamada, Y.; Nakajima, M.; Tsuzuki, K.; Amakura, Y.; Yoshimura, M.; Okuyama, S.; Furukawa, Y. Heptamethoxyflavone Reduces Phosphodiesterase Activity and T-Cell Growth in vitro. Int. Arch. Allergy Immunol. 2017, 174, 113–120. [Google Scholar] [CrossRef]
- Liu, L.; Shan, S.; Zhang, K.; Ning, Z.-Q.; Lu, X.-P.; Cheng, Y.-Y. Naringenin and hesperetin, two flavonoids derived from Citrus aurantium up-regulate transcription of adiponectin. Phyther. Res. 2008, 22, 1400–1403. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Xu, W.-F.; Chen, G.; Wang, H.-F.; Pei, Y.-H. Two new phenolic glycosides isolated from the fruits of Citrus aurantium. Chin. J. Nat. Med. 2017, 15, 41–44. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M. High-performance liquid chromatography methods for the analysis of adrenergic amines and flavanones in Citrus aurantium L. var.amara. Phytochem. Anal. 2004, 15, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Bagatela, B.S.; Lopes, A.P.; Cabral, E.C.; Perazzo, F.F.; Ifa, D.R. High-performance thin-layer chromatography/desorption electrospray ionization mass spectrometry imaging of the crude extract from the peels of Citrus aurantium L. (Rutaceae). Rapid Commun. Mass Spectrom. 2015, 29, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Ryu, K.-H.; Lee, J.-M.; Kim, H.-K.; Seol, G.H. Endothelium- and smooth muscle-dependent vasodilator effects of Citrus aurantium L. var. amara: Focus on Ca2+ modulation. Biomed. Pharmacother. 2016, 82, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, Z.; Therios, I.; Roumeliotis, E.; Kanellis, A.K.; Molassiotis, A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant. Physiol. Biochem. 2015, 86, 155–165. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S. Erratum to “Chromatographic and electrophoretic methods for the analysis of phenethylamine alkaloids in Citrus aurantium” [J. Chromatogr. A 1161 (2007) 71–88]. J. Chromatogr. A 2007, 1164, 334. [Google Scholar] [CrossRef]
- Kamal, G.M.; Anwar, F.; Hussain, A.I.; Sarri, N.; Ashraf, M.Y. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Int. Food Res. J. 2011, 18, 1275. [Google Scholar]
- Nunes Wolffenbuttel, A.; Zamboni, A.; Kerpel dos Santos, M.; Tassi Borille, B.; Americo Augustin, O.; de Cassia Mariotti, K.; Bainy Leal, M.; Pereira Limberger, R. Chemical Components of Citrus Essential Oils from Brazil. Nat. Prod. J. 2015, 5, 14–27. [Google Scholar] [CrossRef]
- Amorim, J.L.; Simas, D.L.R.; Pinheiro, M.M.G.; Moreno, D.S.A.; Alviano, C.S.; da Silva, A.J.R.; Dias Fernandes, P. Anti-Inflammatory Properties and Chemical Characterization of the Essential Oils of Four Citrus Species. PLoS ONE 2016, 11, e0153643. [Google Scholar] [CrossRef] [Green Version]
- Ouedrhiri, W.; Bouhdid, S.; Balouiri, M.; Lalami, A.E.O.; Moja, S.; Chahdi, F.O.; Greche, H. Chemical composition of Citrus aurantium L. leaves and zest essential oils, their antioxidant, antibacterial single and combined effects. J. Chem. Pharm. Res. 2015, 7, 78–84. [Google Scholar]
- Khakpour, S.; Khosravi, M.; Mashayekhipour, Z.; Jahromy, M.H. Effect of Citrus aurantium L. Essential Oil and Haloperidol on Anxiety in Male Mice. World J. Neurosci. 2014, 04, 427–433. [Google Scholar] [CrossRef]
- Azhdarzadeh, F.; Hojjati, M. Chemical composition and antimicrobial activity of leaf, ripe and unripe peel of bitter orange (Citrus aurantium) essential oils. Nutr. Food Sci. Res. 2016, 3, 43–50. [Google Scholar] [CrossRef]
- Radan, M.; Parčina, A.; Burčul, F. Chemical Composition and Antioxidant Activity of Essential Oil Obtained from Bitter Orange Peel (Citrus aurantium L.) Using Two Methods. Croat. Chem. Acta 2018, 91. [Google Scholar] [CrossRef]
- Trabelsi, D.; Ammar, A.H.; Bouabdallah, F.; Zagrouba, F. Antioxidant and Antimicrobial Activities of Essential Oils and Methanolic Extracts of Tunisian Citrus aurantium L. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 18–27. [Google Scholar] [CrossRef]
- Periyanayagam, K.; Dhanalakshmi, S.; Karthikeyan, V.; Magesh, M. Antibacterial activity of Citrus aurantium leaf essential oil against S. aureus and MRSA. J. Drug Discov. Ther. 2014, 2, 54–60. [Google Scholar]
- Abderrezak, M.K.; Abaza, I.; Aburjai, T.; Kabouche, A.; Kabouche, Z. Comparative compositions of essential oils of Citrus aurantium growing in different soils. J. Mater. Environ. Sci. 2014, 5, 1913–1918. [Google Scholar]
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef] [Green Version]
- Leja, K.; Szudera-Kończal, K.; Świtała, E.; Juzwa, W.; Kowalczewski, P.Ł.; Czaczyk, K. The Influence of Selected Plant Essential Oils on Morphological and Physiological Characteristics in Pseudomonas Orientalis. Foods 2019, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Jaglic, Z.; Desvaux, M.; Weiss, A.; Nesse, L.L.; Meyer, R.L.; Demnerova, K.; Schmidt, H.; Giaouris, E.; Sipailiene, A.; Teixeira, P.; et al. Surface adhesins and exopolymers of selected foodborne pathogens. Microbiology 2014, 160, 2561–2582. [Google Scholar] [CrossRef] [Green Version]
- Crossman, L.C.; Gould, V.C.; Dow, J.M.; Vernikos, G.S.; Okazaki, A.; Sebaihia, M.; Saunders, D.; Arrowsmith, C.; Carver, T.; Peters, N.; et al. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 2008, 9, R74. [Google Scholar] [CrossRef] [Green Version]
- Brooke, J.S. Stenotrophomonas maltophilia: An. Emerging Global Opportunistic Pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [Green Version]
- Goss, C.H.; Otto, K.; Aitken, M.L.; Rubenfeld, G.D. Detecting Stenotrophomonas maltophilia Does Not Reduce Survival of Patients with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2002, 166, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Karpati, F.; Malmborg, A.-S.; Alfredsson, H.; Hjelte, L.; Strandvik, B. Bacterial colonisation with Xanthomonas maltophilia-a retrospective study in a cystic fibrosis patient population. Infection 1994, 22, 258–263. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira-Garcia, D.; Dall’Agnol, M.; Rosales, M.; Azzuz, A.C.G.S.; Martinez, M.B.; Girón, J.A. Characterization of Flagella Produced by Clinical Strains of Stenotrophomonas maltophilia. Emerg. Infect. Dis. 2002, 8, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Figueirêdo, P.M.S.; Furumura, M.T.; Santos, A.M.; Sousa, A.C.T.; Kota, D.J.; Levy, C.E.; Yano, T. Cytotoxic activity of clinical Stenotrophomonas maltophilia. Lett. Appl. Microbiol. 2006, 43, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, G.; Spedicato, I.; D’Antonio, D.; Robuffo, I.; Piccolomini, R. Biofilm Formation by Stenotrophomonas maltophilia: Modulation by Quinolones, Trimethoprim-Sulfamethoxazole, and Ceftazidime. Antimicrob. Agents Chemother. 2004, 48, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bonaventura, G.; Pompilio, A.; Zappacosta, R.; Petrucci, F.; Fiscarelli, E.; Rossi, C.; Piccolomini, R. Role of Excessive Inflammatory Response to Stenotrophomonas maltophilia Lung Infection in DBA/2 Mice and Implications for Cystic Fibrosis. Infect. Immun. 2010, 78, 2466–2476. [Google Scholar] [CrossRef] [Green Version]
- Pompilio, A.; Piccolomini, R.; Picciani, C.; D’Antonio, D.; Savini, V.; Di Bonaventura, G. Factors associated with adherence to and biofilm formation on polystyrene by Stenotrophomonas maltophilia: The role of cell surface hydrophobicity and motility. FEMS Microbiol. Lett. 2008, 287, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against Infection of Arabidopsis Roots by Pseudomonas syringae Is Facilitated by Biofilm Formation and Surfactin Production. Plant. Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef] [Green Version]
- Demain, A.L.; Elander, R.P. The beta-lactam antibiotics: Past, present, and future. Antonie Van Leeuwenhoek 1999, 75, 5–19. [Google Scholar] [CrossRef]
- Chakravarti, R.; Sahai, V. Compactin-A Review. Appl. Microbiol. Biotechnol. 2004, 64, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Ciavatta, M.L.; Buommino, E.; Tufano, M.A. Antitumor extrolites produced by Penicillium species. Int. J. Biomed. Pharm. Sci. 2008, 2, 1–23. [Google Scholar]
- Frisvad, J.C.; Smedsgaard, J.; Larsen, T.O.; Samson, R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004, 49, e41. [Google Scholar]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices? A mini review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 353–368. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and Antifungal Activities of Spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felšöciová, S.; Vukovic, N.; Jeżowski, P.; Kačániová, M. Antifungal activity of selected volatile essential oils against Penicillium sp. Open Life Sci. 2020, 15, 511–521. [Google Scholar] [CrossRef]
- Sessou, P.; Farougou, S.; Ahounou, S.; Hounnankpo, Y.; Azokpota, P.; Youssao, I.; Sohounhlou, D. Comparative Study of Antifungal Activities of Six Selected Essential Oils against Fungal Isolates from Cheese Wagashi in Benin. Pakistan J. Biol. Sci. 2013, 16, 1751–1757. [Google Scholar] [CrossRef] [Green Version]
- Gandomi, H.; Misaghi, A.; Basti, A.A.; Bokaei, S.; Khosravi, A.; Abbasifar, A.; Javan, A.J. Effect of Zataria multiflora Boiss. essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food Chem. Toxicol. 2009, 47, 2397–2400. [Google Scholar] [CrossRef]
- Bourgou, S.; Rahali, F.Z.; Ourghemmi, I.; Saïdani Tounsi, M. Changes of Peel Essential Oil Composition of Four Tunisian Citrus during Fruit Maturation. Sci. World J. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, A.; Hashemi, P.; Talei, G.R.; Borzuei, M.; Ghiasvand, A.R. Comparative analyses of the volatile components of Citrus aurantium L. flowers using ultrasonic-assisted headspace SPME and hydrodistillation combined with GC-MS and evaluation of their antimicrobial activities. Anal. Bioanal. Chem. Res. 2014, 1, 83–91. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile Constituents and Antioxidant Activity of Peel, Flowers and Leaf Oils of Citrus aurantium L. Growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrad, K.; Hamouda, A.B.; Chaieb, I.; Laarif, A.; Jemâa, J.M.-B. Chemical composition, fumigant and anti-acetylcholinesterase activity of the Tunisian Citrus aurantium L. essential oils. Ind. Crops Prod. 2015, 76, 121–127. [Google Scholar] [CrossRef]
- Khettal, B.; Kadri, N.; Tighilet, K.; Adjebli, A.; Dahmoune, F.; Maiza-Benabdeslam, F. Phenolic compounds from Citrus leaves: Antioxidant activity and enzymatic browning inhibition. J. Complement. Integr. Med. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Bendaha, H.; Bouchal, B.; El Mounsi, I.; Salhi, A.; Berrabeh, M.; El Bellaoui, M.; Mimouni, M. Chemical composition, antioxidant, antibacterial and antifungal activities of peel essential oils of Citrus aurantium grown in Eastern Morocco. Der Pharm. Lett. 2016, 8, 239–245. [Google Scholar]
- Choi, H.-S.; Song, H.S.; Ukeda, H.; Sawamura, M. Radical-Scavenging Activities of Citrus Essential Oils and Their Components: Detection Using 1,1-Diphenyl-2-picrylhydrazyl. J. Agric. Food Chem. 2000, 48, 4156–4161. [Google Scholar] [CrossRef]
- Madhuri, S.; Hegde, A.U.; Srilakshmi, N.S.; Prashith Kekuda, T.R. Antimicrobial activity of citrus sinensis and Citrus aurantium peel extracts. J. Pharm. Sci. Innov. 2014, 3, 366–368. [Google Scholar] [CrossRef]
- Teneva, D.; Denkova-Kostova, R.; Goranov, B.; Hristova-Ivanova, Y.; Slavchev, A.; Denkova, Z.; Kostov, G. Chemical composition, antioxidant activity and antimicrobial activity of essential oil from Citrus aurantium L. zest against some pathogenic microorganisms. Z. Naturforsch. C 2019, 74, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Guarrasi, V.; Vargas, M.; Germanà, M.A.; Moschetti, G. Antilisterial effect of citrus essential oils and their performance in edible film formulations. Food Control. 2016, 59, 750–758. [Google Scholar] [CrossRef] [Green Version]
- Settanni, L.; Palazzolo, E.; Guarrasi, V.; Aleo, A.; Mammina, C.; Moschetti, G.; Germanà, M.A. Inhibition of foodborne pathogen bacteria by essential oils extracted from citrus fruits cultivated in Sicily. Food Control. 2012, 26, 326–330. [Google Scholar] [CrossRef]
- Fathi, H.; Paknejad, S.; Ahanjan, M. Evaluating antimicrobial effects of different orange blossom extract (Citrus aurantium L.) on microbial species in vitro. Health Biotechnol. Biopharma 2017, 1, 25–36. [Google Scholar]
- Gniewosz, M.; Kraśniewska, K.; Kosakowska, O.; Pobiega, K.; Wolska, I. Chemical compounds and antimicrobial activity of petitgrain (Citrus aurantium L. var. amara) essential oil. Herba Pol. 2017, 63, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.D.E.S.; Bonatto, C.C.; Lopes, C.A.P.; Pereira, A.L.; Silva, L.P. Use of MALDI-TOF mass spectrometry to analyze the molecular profile of Pseudomonas aeruginosa biofilms grown on glass and plastic surfaces. Microb. Pathog. 2015, 86, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, C.; Zhao, Q.; Xiao, S. The Impact of spgM, rpfF, rmlA Gene Distribution on Biofilm Formation in Stenotrophomonas maltophilia. PLoS ONE 2014, 9, e108409. [Google Scholar] [CrossRef] [PubMed]
- Gingichashvili, S.; Duanis-Assaf, D.; Shemesh, M.; Featherstone, J.D.B.; Feuerstein, O.; Steinberg, D. Bacillus subtilis Biofilm Development–A Computerized Study of Morphology and Kinetics. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, S.A.C.; Zambrana, J.R.M.; Di Iorio, F.B.R.; Pereira, C.A.; Jorge, A.O.C. The antimicrobial effects of Citrus limonum and Citrus aurantium essential oils on multi-species biofilms. Braz. Oral Res. 2014, 28, 22–27. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, Antimicrobial and Antibiofilm Activity of Coriander (Coriandrum sativum L.) Essential Oil for Its Application in Foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Lombard, G.E.; Weinert, I.A.G.; Minnaar, A.; Taylor, J.R.N. Preservation of South African Steamed Bread Using Hurdle Technology. LWT-Food Sci. Technol. 2000, 33, 138–143. [Google Scholar] [CrossRef]
- Barbosa-Cnovas, G.V.; Fontana, A.J.; Schmidt, S.J.; Labuza, T.P. Water Activity in Foods: Fundamentals and Applications; Blackwell Publishing Ltd.: Oxford, UK, 2007; ISBN 9780470376454. [Google Scholar]
- Cazier, J.-B.; Gekas, V. Water activity and its prediction: A review. Int. J. Food Prop. 2001, 4, 35–43. [Google Scholar] [CrossRef]
- Labuzo, T.P.; Mcnally, L.; Gallagher, D.; Hawkes, J.; Hurtado, F. Stability of intermediate moisture foods. 1. Lipid oxidation. J. Food Sci. 1972, 37, 154–159. [Google Scholar] [CrossRef]
- Roos, Y.H.; Finley, J.W.; DeMan, J.M. Water. In Principles of Food Chemistry; DeMan, J.M., Finley, J., Hurst, W.J., Lee, C., Eds.; Springer International Publishing: Gaithersburg, MD, USA, 1999; p. 606. [Google Scholar]
- Lahlali, R.; Serrhini, M.N.; Jijakli, M.H. Studying and modelling the combined effect of temperature and water activity on the growth rate of P. expansum. Int. J. Food Microbiol. 2005, 103, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Day, L. Cereal Food Production with Low Salt. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Nederlands, 2016; pp. 396–402. [Google Scholar]
- Lee, M.-R.; Swanson, B.G.; Baik, B.-K. Influence of Amylose Content on Properties of Wheat Starch and Breadmaking Quality of Starch and Gluten Blends. Cereal Chem. J. 2001, 78, 701–706. [Google Scholar] [CrossRef]
- Jaekel, L.Z.; daSilva, C.B.; Steel, C.J.; Chang, Y.K. Influence of xylanase addition on the characteristics of loaf bread prepared with white flour or whole grain wheat flour. Food Sci. Technol. 2012, 32, 844–849. [Google Scholar] [CrossRef] [Green Version]
- Saranraj, P.; Geetha, M. Microbial spoilage of bakery products and its control by preservatives. Int. J. Pharm. Biol. Arch. 2012, 3, 38–48. [Google Scholar]
- Quaglia, M.; Ederli, L.; Pasqualini, S.; Zazzerini, A. Biological control agents and chemical inducers of resistance for postharvest control of Penicillium expansum Link. on apple fruit. Postharvest Biol. Technol. 2011, 59, 307–315. [Google Scholar] [CrossRef]
- Metoui, N.; Gargouri, S.; Amri, I.; Fezzani, T.; Jamoussi, B.; Hamrouni, L. Activity antifungal of the essential oils; aqueous and ethanol extracts from Citrus aurantium L. Nat. Prod. Res. 2015, 29, 2238–2241. [Google Scholar] [CrossRef]
- Ellouze, I.; Abderrabba, M.; Sabaou, N.; Mathieu, F.; Lebrihi, A.; Bouajila, J. Season’s Variation Impact on Citrus aurantium Leaves Essential Oil: Chemical Composition and Biological Activities. J. Food Sci. 2012, 77, T173–T180. [Google Scholar] [CrossRef]
- Fabio, A.; Cermelli, C.; Fabio, G.; Nicoletti, P.; Quaglio, P. Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. Phyther. Res. 2007, 21, 374–377. [Google Scholar] [CrossRef]
- Tao, N.; Liu, Y.; Zhang, M. Chemical composition and antimicrobial activities of essential oil from the peel of bingtang sweet orange (Citrus sinensis Osbeck). Int. J. Food Sci. Technol. 2009, 44, 1281–1285. [Google Scholar] [CrossRef]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef]
- Van Hung, P.; Chi, P.T.L.; Phi, N.T.L. Comparison of antifungal activities of Vietnamese citrus essential oils. Nat. Prod. Res. 2013, 27, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Morck, D.W.; Storey, D.G. Minimal biofilm eradication concentration (MBEC) assay: Susceptibility testing for biofilms. Biofilms Infect. 2006, 257. [Google Scholar]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Brazilian J. Infect. Dis. 2011, 15, 305–311. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds of CAEO and microorganisms are available from the authors. |









| Name | Synonyms | TIC% Area a |
|---|---|---|
| sabinene | 4(10)-thujene | 0.32 ± 0.06 |
| 3-carene | 0.47 ± 0.09 | |
| β-myrcene | 2.32 ± 0.31 | |
| d-limonene | 1.57 ± 0.11 | |
| 1,8-cineol | eucalyptol | 2.70 ± 0.28 |
| cis-ocimene | 0.88 ± 0.07 | |
| β-cis-ocimene | ocimene-X | 2.39 ± 0.21 |
| α-terpinolene | 0.58 ± 0.09 | |
| linalyl acetate | 63.37 ± 2.78 | |
| caryophyllene | 1.34 ± 0.13 | |
| (−)-α-terpineol | p-menth-1-en-8-ol | 8.84 ± 0.67 |
| bicyclogermacrene | isobicyclogermacrene, lepidozene, isolepidozene | 0.33 ± 0.06 |
| neryl acetate | 3.77 ± 0.44 | |
| geranyl acetate | 6.02 ± 0.52 | |
| cis-geraniol | Nerol neryl alcohol | 1.63 ± 0.07 |
| geraniol | trans-geraniol lemonol geranyl alcohol | 3.68 ± 0.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kačániová, M.; Terentjeva, M.; Galovičová, L.; Ivanišová, E.; Štefániková, J.; Valková, V.; Borotová, P.; Kowalczewski, P.Ł.; Kunová, S.; Felšöciová, S.; et al. Biological Activity and Antibiofilm Molecular Profile of Citrus aurantium Essential Oil and Its Application in a Food Model. Molecules 2020, 25, 3956. https://doi.org/10.3390/molecules25173956
Kačániová M, Terentjeva M, Galovičová L, Ivanišová E, Štefániková J, Valková V, Borotová P, Kowalczewski PŁ, Kunová S, Felšöciová S, et al. Biological Activity and Antibiofilm Molecular Profile of Citrus aurantium Essential Oil and Its Application in a Food Model. Molecules. 2020; 25(17):3956. https://doi.org/10.3390/molecules25173956
Chicago/Turabian StyleKačániová, Miroslava, Margarita Terentjeva, Lucia Galovičová, Eva Ivanišová, Jana Štefániková, Veronika Valková, Petra Borotová, Przemysław Łukasz Kowalczewski, Simona Kunová, Soňa Felšöciová, and et al. 2020. "Biological Activity and Antibiofilm Molecular Profile of Citrus aurantium Essential Oil and Its Application in a Food Model" Molecules 25, no. 17: 3956. https://doi.org/10.3390/molecules25173956
APA StyleKačániová, M., Terentjeva, M., Galovičová, L., Ivanišová, E., Štefániková, J., Valková, V., Borotová, P., Kowalczewski, P. Ł., Kunová, S., Felšöciová, S., Tvrdá, E., Žiarovská, J., Benda Prokeinová, R., & Vukovic, N. (2020). Biological Activity and Antibiofilm Molecular Profile of Citrus aurantium Essential Oil and Its Application in a Food Model. Molecules, 25(17), 3956. https://doi.org/10.3390/molecules25173956












