High-Performance Core/Shell of ZnO/TiO2 Nanowire with AgCl-Doped CdSe Quantum Dots Arrays as Electron Transport Layer for Perovskite Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM and HRTEM Studies
2.2. Photocurrent Density–Voltage (J–V) Characteristics
2.3. IPCE Spectra and Nyquist Plots Studies
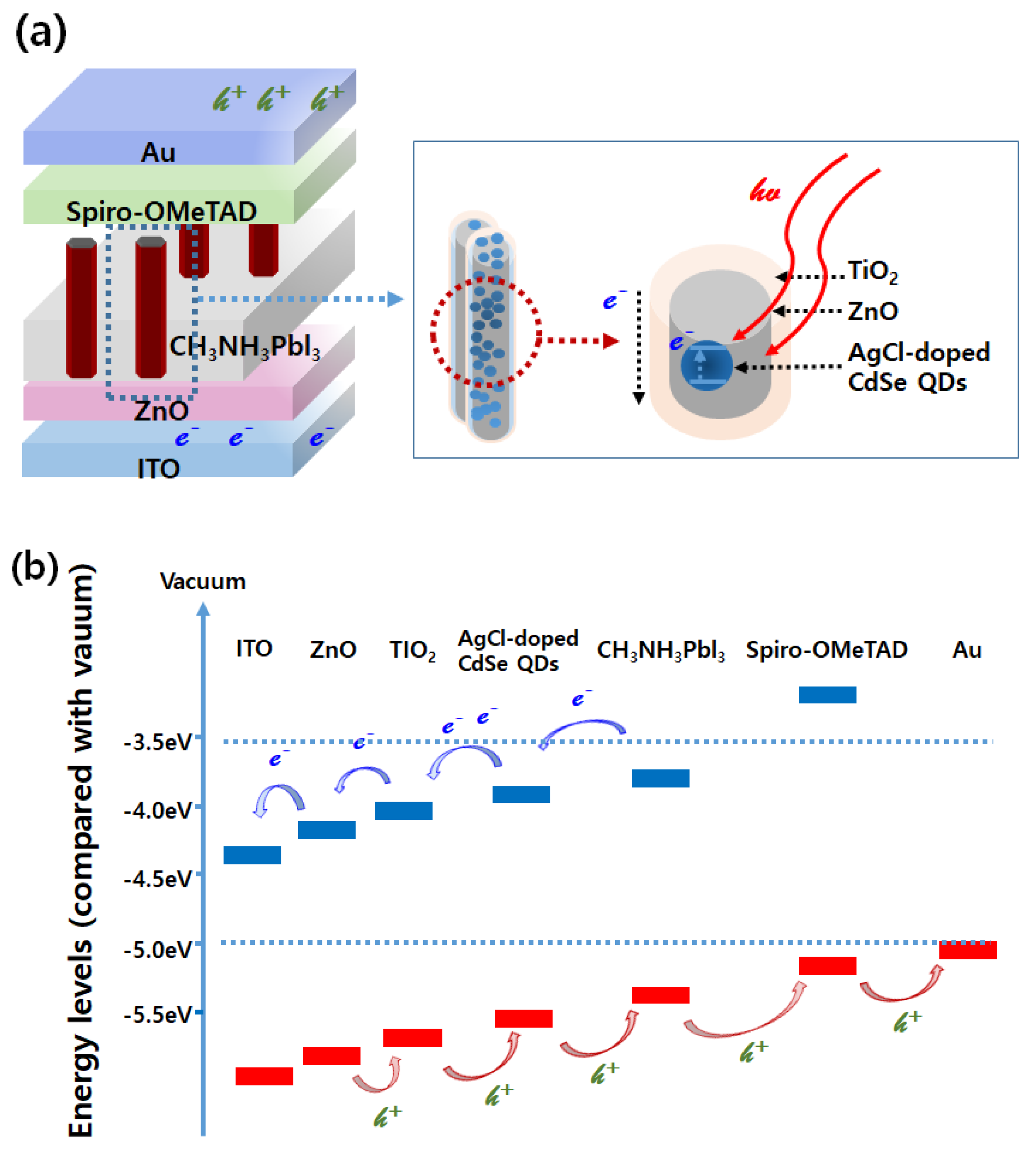
2.4. Efficient Photovoltaics Mechanism of Energy Band Diagram
3. Materials and Methods
3.1. Synthesis of NWs and Fabrication of Device
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oku, T.; Matsumoto, T.; Suzuki, A.; Suzuki, K. Fabrication and Characterization of a Perovskite-Type Solar Cell with a Substrate Size of 70 mm. Coatings 2015, 5, 646–655. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Buonassisi, T.; Egger, D.A.; Hodes, G.; Kronik, L.; Loo, Y.-L.; Lubomirsky, I.; Marder, S.R.; Mastai, Y.; Miller, J.S.; et al. Hybrid Organic-Inorganic Perovskites (HOIPs): Opportunities and Challenges. Adv. Mater. 2015, 27, 5102–5112. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Grätzel, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Colella, S.; Mosconi, E.; Fedeli, P.; Listorti, A.; Gazza, F.; Orlandi, F.; Ferro, P.; Besagni, T.; Rizzo, A.; Calestani, G.; et al. MAPbI3-xClxMixed Halide Perovskite for Hybrid Solar Cells: The Role of Chloride as Dopant on the Transport and Structural Properties. Chem. Mater. 2013, 25, 4613–4618. [Google Scholar] [CrossRef]
- Mosconi, E.; Ronca, E.; De, A.F. First principles investigation of the TiO2/Organohalide perovskites interface: The role of interfacial chlorine. J. Phys. Chem. Lett. 2014, 5, 2619–2625. [Google Scholar] [CrossRef]
- Ge, Y.; Nan, Y.; Chen, Y. Maximizing Information Transmission for Energy Harvesting Sensor Networks by an Uneven Clustering Protocol and Energy Management. KSII Trans. Internet Inf. Syst. 2020, 14, 1419–1436. [Google Scholar] [CrossRef]
- Strelcov, E.; Dong, Q.; Li, T.; Chae, J.; Shao, Y.; Deng, Y.; Gruverman, A.; Huang, J.; Centrone, A. CH3NH3PbI3 perovskites: Ferroelasticity revealed. Sci. Adv. 2017, 3, e1602165. [Google Scholar] [CrossRef] [Green Version]
- Hermes, I.M.; Bretschneider, S.A.; Bergmann, V.W.; Li, D.; Klasen, A.; Mars, J.; Tremel, W.; Laquai, F.; Butt, H.-J.; Mezger, M.; et al. Ferroelastic Fingerprints in Methylammonium Lead Iodide Perovskite. J. Phys. Chem. C 2016, 120, 5724–5731. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Xiao, J.; Sun, K.; Chen, L.; Hu, Y.; Ouyang, J.; Ong, K.P.; Zeng, K.; Wang, J. Ferroelectricity of CH3NH3PbI3 Perovskite. J. Phys. Chem. Lett. 2015, 6, 1155–1161. [Google Scholar] [CrossRef]
- Rakita, Y.; Bar-Elli, O.; Meirzadeh, E.; Kaslasi, H.; Peleg, Y.; Hodes, G.; Lubomirsky, I.; Oron, D.; Ehre, D.; Cahen, D. Tetragonal CH3NH3PbI3is ferroelectric. Proc. Natl. Acad. Sci. USA 2017, 114, e5504–e5512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Poglitsch, A.; Weber, D. Dynamic disorder in methylammoniumtri- halogenoplumbates (II) observed by millimeter-wave spectroscopy. J. Chem. Phys. 1987, 87, 6373–6378. [Google Scholar] [CrossRef]
- Moehl, T.; Im, J.H.; Lee, Y.H.; Domanski, K.; Giordano, F.; Zakeeruddin, S.M.; Dar, M.I.; Heiniger, L.-P.; Nazeeruddin, M.K.; Park, N.-G.; et al. Strong Photocurrent Amplification in Perovskite Solar Cells with a Porous TiO2 Blocking Layer under Reverse Bias. J. Phys. Chem. Lett. 2014, 5, 3931–3936. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Chen, W.; Yue, Y.; Cai, M.; Xie, F.; Bi, E.; Islam, A.; Han, L. Perovskite solar cells with 18.21% efficiency and area over 1 cm2 fabricated by heterojunction engineering. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H. Highly reproducible, efficient hysteresis-less CH3NH3PbI3−xClxplanar hybrid solar cells without requiring heat-treatment. Nanoscale 2016, 8, 2554–2560. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, H.; Wang, D.; Li, Y.; He, X.; Zhang, H.; Shen, J. ZnO@TiO2 Core/Shell Nanowire Arrays with Different Thickness of TiO2 Shell for Dye-Sensitized Solar Cells. Crystals 2020, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Ou, H.; Hong, K.; Wang, L. Evidence of a strong electron-hole separation effect in ZnO@TiO2 core/shell nanowires. J. Alloy. Compd. 2018, 749, 217–220. [Google Scholar] [CrossRef]
- Zhao, R.; Zhu, L.; Cai, F.; Yang, Z.; Gu, X.; Huang, J.; Cao, L. ZnO/TiO2 core-shell nanowire arrays for enhanced dye-sensitized solar cell efficiency. Appl. Phys. A Mater. Sci. Process. 2013, 113, 67–73. [Google Scholar] [CrossRef]
- Demchenko, D.O.; Izyumskaya, N.; Feneberg, M.; Avrutin, V.; Özgür, Ü.; Goldhahn, R.; Morkoç, H. Optical properties of the organic-inorganic hybrid perovskite CH3NH3PbI3: Theory and experiment. Phys. Rev. B 2016, 94, 075206. [Google Scholar] [CrossRef]
- Berdiyorov, G.R.; El-Mellouhi, F.; Madjet, M.E.; Alharbi, F.H.; Peeters, F.M.; Kais, S. Effect of halide-mixing on the electronic transport properties of organometallic perovskites. Sol. Energy Mater. Sol. Cells 2016, 148, 2–10. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Lu, Z.; Zhou, Y.; Zhu, H. No-Reference Sharpness Index for Scanning Electron Microscopy Images Based on Dark Channel Prior. KSII Trans. Internet Inf. Syst. 2019, 13, 2529–2543. [Google Scholar] [CrossRef]
- Berdiyorov, G.R.; El-Mellouhi, F.; Madjet, M.E.; Alharbi, F.H.; Rashkeev, S.N. Electronic transport in organometallic perovskite CH3NH3PbI3: The role of organic cation orientations. Appl. Phys. Lett. 2016, 108, 053901. [Google Scholar] [CrossRef]
- Zheng, F.; Takenaka, H.; Wang, F.; Koocher, N.Z.; Rappe, A.M. First-Principles Calculation of the Bulk Photovoltaic Effect in CH3NH3PbI3 and CH3NH3PbI3–xClx. J. Phys. Chem. Lett. 2014, 6, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soler, J.M.; Artacho, E.; Gale, J.D.; Garcıa, A.; Junquera, J.; Ordej, P. The Siesta method for ab initio order-N materials simulation. J. Phys. Condens. Matter. 2002, 14, 2745–2779. [Google Scholar] [CrossRef] [Green Version]
- Ordejón, P.; Artacho, E.; Soler, J.M. Self-Consistent order-Ndensity-functional calculations for very large systems. Phys. Rev. B 1996, 53, R10441–R10444. [Google Scholar] [CrossRef] [Green Version]
- Brandbyge, M.; Mozos, J.-L.; Ordejón, P.; Taylor, J.; Stokbro, K. Density-Functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 16540. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Gao, X.; Abtew, T.A.; Sun, Y.-Y.; Zhang, S.; Zhang, P. Quasiparticle band gap of organic-inorganic hybrid perovskites: Crystal structure, spin-orbit coupling, and self-energy effects. Phys. Rev. B 2016, 93, 085202. [Google Scholar] [CrossRef] [Green Version]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.K.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A 2013, 1, 5628. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (ZnO/TiO2 core-shell hybrid arrays with AgCl-doped CdSe quantum dots) are available from the authors. |





| Samples | Voc (V) | Jsc (mA cm−2) | FF (%) | H (%) |
|---|---|---|---|---|
| ZnO NWs arrays | 1.024 | 21.32 | 0.631 | 12.04 |
| ZnO/TiO2 core/shell arrays | 1.057 | 22.01 | 0.674 | 13.25 |
| ZnO/TiO2 core/shell arrays with QDs | 1.138 | 22.18 | 0.697 | 14.34 |
| ZnO/TiO2 core/shell arrays with AgCl doped QDs | 1.179 | 22.71 | 0.712 | 15.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Lee, B.S.; Hwang, S.W. High-Performance Core/Shell of ZnO/TiO2 Nanowire with AgCl-Doped CdSe Quantum Dots Arrays as Electron Transport Layer for Perovskite Solar Cells. Molecules 2020, 25, 3969. https://doi.org/10.3390/molecules25173969
Kim JM, Lee BS, Hwang SW. High-Performance Core/Shell of ZnO/TiO2 Nanowire with AgCl-Doped CdSe Quantum Dots Arrays as Electron Transport Layer for Perovskite Solar Cells. Molecules. 2020; 25(17):3969. https://doi.org/10.3390/molecules25173969
Chicago/Turabian StyleKim, Jin Mo, Bong Soo Lee, and Sung Won Hwang. 2020. "High-Performance Core/Shell of ZnO/TiO2 Nanowire with AgCl-Doped CdSe Quantum Dots Arrays as Electron Transport Layer for Perovskite Solar Cells" Molecules 25, no. 17: 3969. https://doi.org/10.3390/molecules25173969






