Radioanalytical Techniques to Quantitatively Assess the Biological Uptake and In Vivo Behavior of Hazardous Substances
Abstract
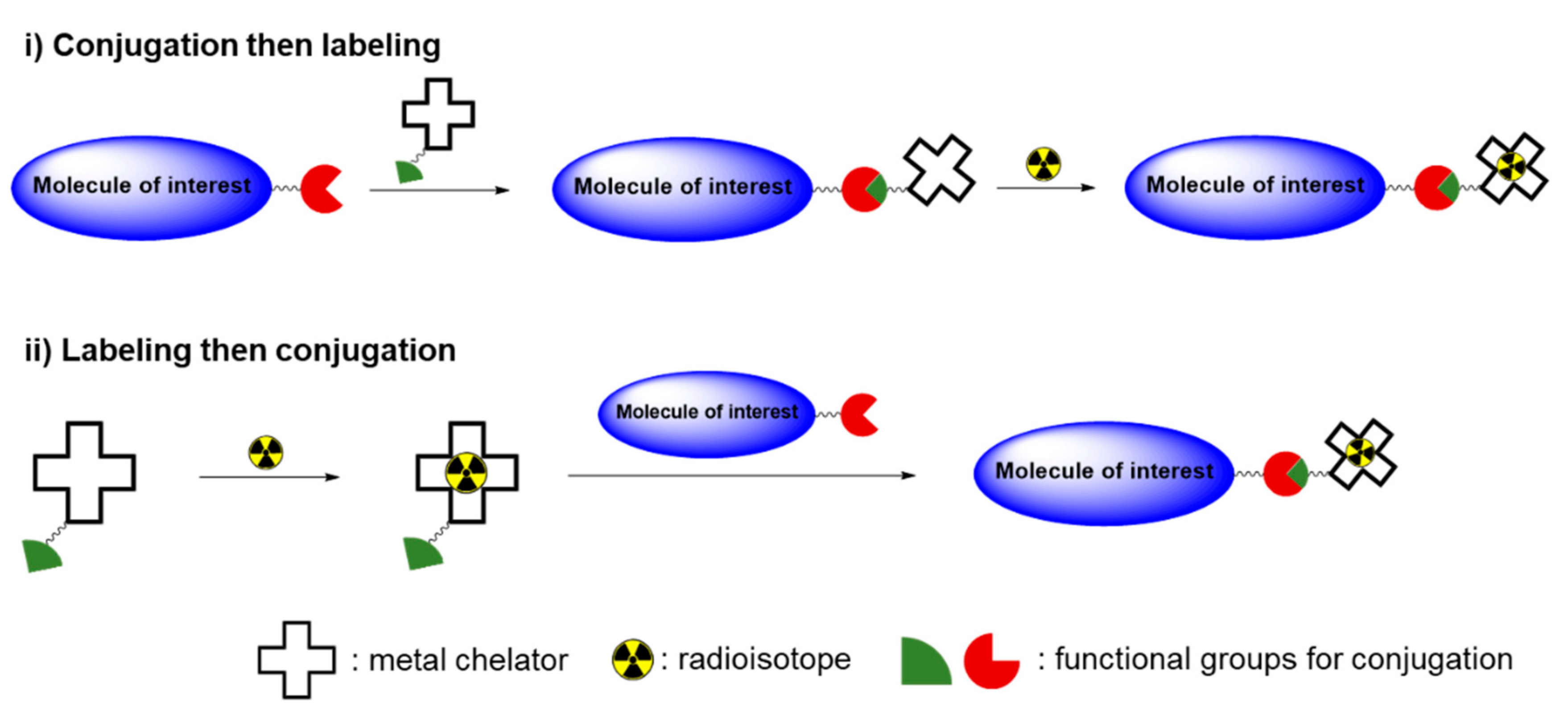
1. Introduction
2. Radioisotopes Used to Determine the Biodistributions of Toxic Materials
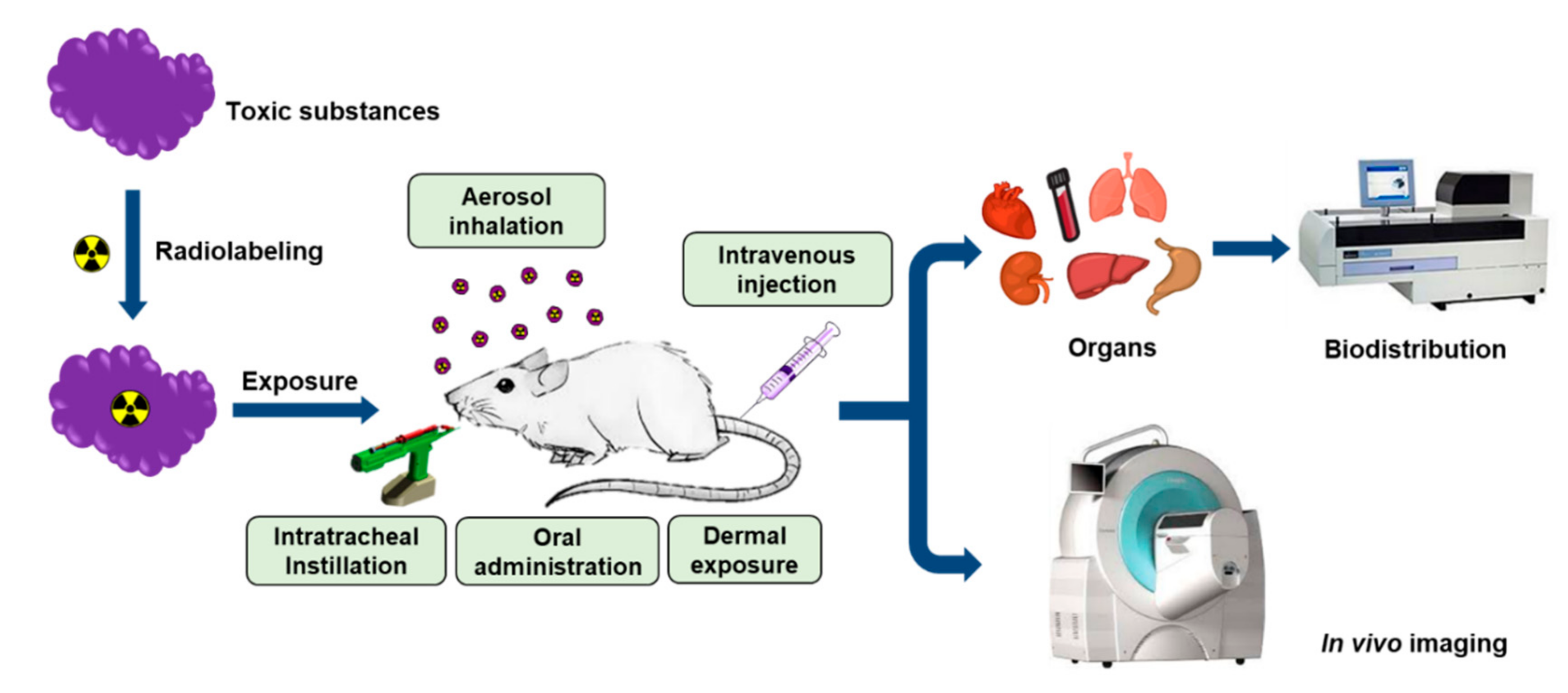
3. In Vivo Assessment of Hazardous Substances Using Radioanalytical Methods
3.1. Small Molecules
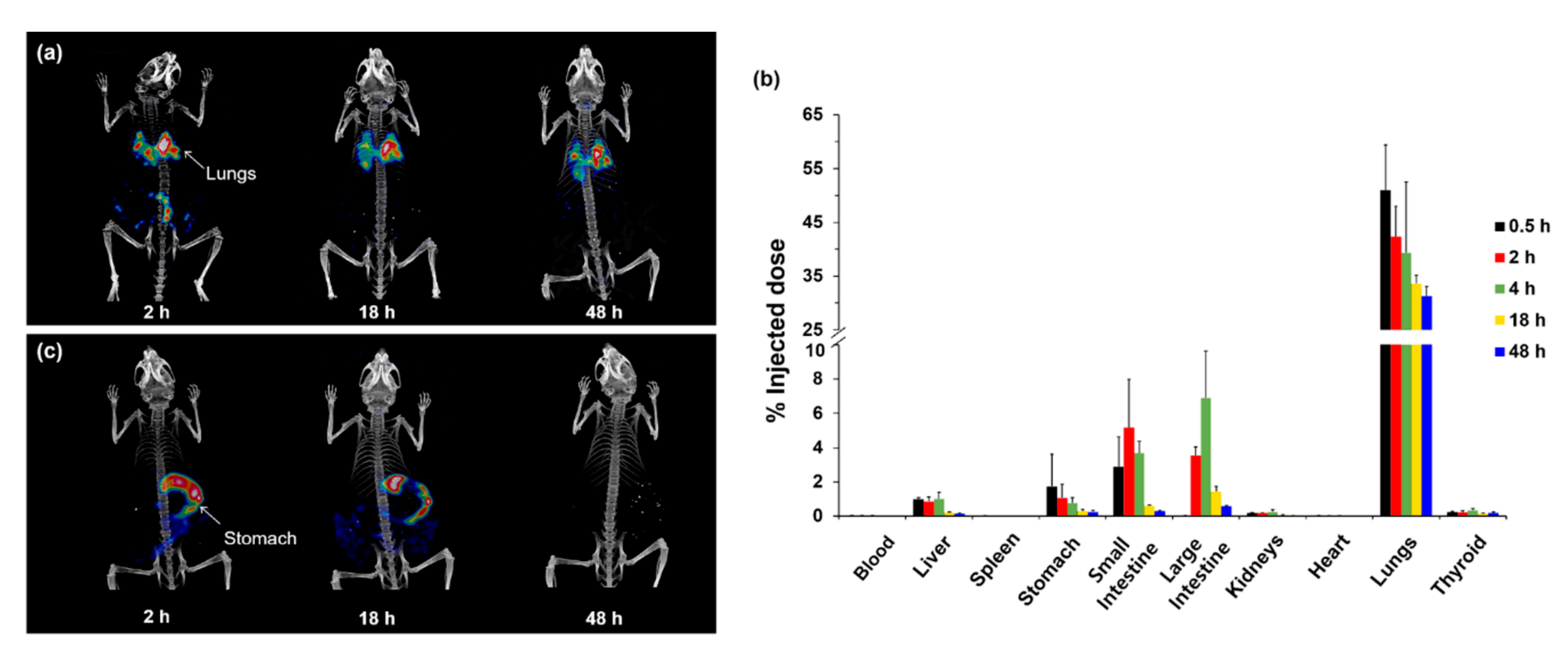
3.2. Nanomaterial
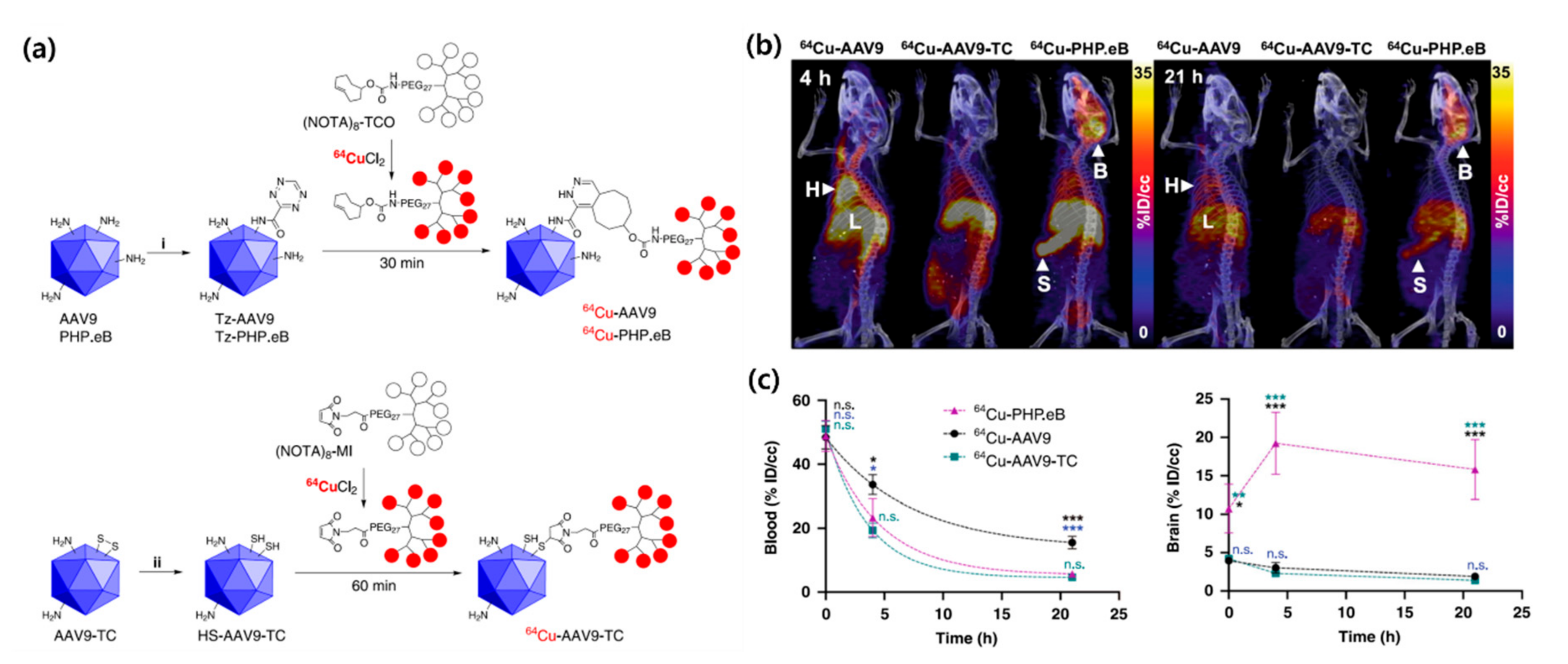
3.3. Macromolecules
3.4. Microorganisms
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PET | Positron emission tomography |
| SPECT | Single-photon emission computed tomography |
| CT | Computed tomography |
| LSC | Liquid scintillation counter |
| ID | Injected dose |
| PFASs | Perfluoroalkyl substances |
| PFOA | Perfluorooctanoic acid |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| PFOS | Perfluorooctane sulfonate |
| PFBS | Perfluorobutane sulfonate |
| BPA | Bisphenol A |
| NGS | Nanographene sheet |
| NGO | Nanoscale graphene oxide |
| FLG | Few-layer graphene |
| PHMG | Polyhexamethylene guanidine phosphate |
| LPS | Lipopolysaccharide |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| BODIPY | 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene |
| AAVs | Adeno-associated viruses |
References
- Chartres, N.; Bero, L.A.; Norris, S.L. A review of methods used for hazard identification and risk assessment of environmental hazards. Environ. Int. 2019, 123, 231–239. [Google Scholar] [CrossRef]
- Judson, R.; Richard, A.; Dix, D.J.; Houck, K.; Martin, M.; Kavlock, R.; Dellarco, V.; Henry, T.; Holderman, T.; Sayre, P.; et al. The toxicity data landscape for environmental chemicals. Environ. Health Persp. 2009, 117, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Folletti, I.; Zock, J.-P.; Moscato, G.; Siracusa, A. Asthma and rhinitis in cleaning workers: A systematic review of epidemiological studies. J. Asthma 2014, 51, 18–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelson, W.; Wang, Y.-X.; Sakwari, G.; Ding, Y.-B. Review of the effects of perinatal exposure to endocrine-disrupting chemicals in animals and humans. Rev. Environ. Contam. Toxicol. 2020, 251, 131–184. [Google Scholar] [PubMed]
- Witorsch, R.J.; Thomas, J.A. Personal care products and endocrine disruption: A critical review of the literature. Crit. Rev. Toxicol. 2010, 40, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Needham, L.L.; Sexton, K. Assessing children’s exposure to hazardous environmental chemicals: An overview of selected research challenges and complexities. J. Expo. Sci. Environ. Epidemiol. 2000, 10, 611–629. [Google Scholar] [CrossRef]
- Meng, F.; Wang, J.; Ping, Q.; Yeo, Y. Quantitative Assessment of Nanoparticle Biodistribution by Fluorescence Imaging, Revisited. ACS Nano 2018, 12, 6458–6468. [Google Scholar] [CrossRef]
- Cilliers, C.; Nessler, I.; Christodolu, N.; Thurber, G.M. Tracking antibody distribution with near-infrared fluorescent dyes: Impact of dye structure and degree of labeling on plasma clearance. Pharmaceutics 2017, 14, 1623–1633. [Google Scholar] [CrossRef]
- Allen, K.J.H.; Jiao, R.; Malo, M.E.; Frank, C.; Dadachova, E. Biodistribution of a radiolabeled antibody in mice as an approach to evaluating antibody pharmacokinetics. Pharmaceutics 2018, 10, 262. [Google Scholar] [CrossRef]
- Khan, N.T. Radioisotopes and their biomedical applications. J. Biomol. Res. Ther. 2017, 6, 156. [Google Scholar] [CrossRef]
- Vaquero, J.J.; Kinahan, P. Positron emission tomography: Current challenges and opportunities for technological advances in clinical and preclinical imaging systems. Annu. Rev. Biomed. Eng. 2015, 17, 385–414. [Google Scholar] [CrossRef] [PubMed]
- Israel, O.; Pellet, O.; Biassoni, L.; De Palma, D.; Estrada-Lobato, E.; Gnanasegaran, G.; Kuwert, T.; la Fougère, C.; Mariani, G.; Massalha, S.; et al. Two decades of SPECT/CT—The coming of age of a technology: An updated review of literature evidence. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1990–2012. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Yun, S.J.; Jeon, J. Recent advances in bioorthogonal click chemistry for efficient synthesis of radiotracers and radiopharmaceuticals. Molecules 2019, 24, 3567. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J. Review of therapeutic applications of radiolabeled functional nanomaterials. Int. J. Mol. Sci. 2019, 20, 2323. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.R.; Sgouros, G.; Sofou, S. Targeted and nontargeted α-particle therapies. Annu. Rev. Biomed. Eng. 2018, 20, 73–93. [Google Scholar] [CrossRef]
- Lu, W.; Hong, H.; Cai, W. Radio-Nanomaterials for biomedical applications: State of the art. Eur. J. Nanomed. 2016, 8, 151–170. [Google Scholar] [CrossRef]
- Farzin, L.; Sheibani, S.; Moassesi, M.E.; Shamsipur, M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. A 2019, 107, 251–285. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjugate Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Boros, E.; Packard, A.B. Radioactive transition metals for imaging and therapy. Chem. Rev. 2019, 119, 870–901. [Google Scholar] [CrossRef]
- Zhao, Y.; Detering, L.; Sultan, D.; Cooper, M.L.; You, M.; Cho, S.; Meier, S.L.; Leuhmann, H.; Sun, G.; Retting, M.; et al. Gold Nanoclusters Doped with 64Cu for CXCR4 Positron Emission Tomography Imaging of Breast Cancer and Metastasis. ACS Nano 2016, 10, 5959–5970. [Google Scholar] [CrossRef]
- Gómez-Vallejo, V.; Puigivila, M.; Plaza-García, S.; Szczupak, B.; Piñol, R.; Murillo, J.L.; Sorribas, V.; Lou, G.; Veintemillas, S.; Ramos-Cabrer, P.; et al. PEG-copolymer-coated iron oxide nanoparticles that avoid the reticuloendothelial system and act as kidney MRI contrast agents. Nanoscale 2018, 10, 14153–14164. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Jeon, J.; Shaheen, A.; Jang, S.J.; Park, S.H. Critical analysis of radioiodination techniques for micro and macro organic molecules. J. Radioanal. Nucl. Chem. 2016, 309, 859–889. [Google Scholar] [CrossRef]
- Nakayama, S.F.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. Trends Anal. Chem. 2019, 121, 115410. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Hoover, G.; Kar, S.; Guffey, S.; Leszczynski, J.; Sepúlveda, M.S. In vitro and in silico modeling of perfluoroalkyl substances mixture toxicity in an amphibian fibroblast cell line. Chemosphere 2019, 233, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lupton, S.J.; Huwe, J.K.; Smith, D.J.; Dearfield, K.L.; Johnston, J.J. Absorption and excretion of 14C-perfluorooctanoic acid (PFOA) in angus cattle (Bos taurus). J. Agric. Food Chem. 2012, 60, 1128–1134. [Google Scholar] [CrossRef]
- Sundström, M.; Bogdanska, J.; Pham, H.V.; Athanasios, V.; Nobel, S.; McAlees, A.; Eriksson, J.; DePierre, J.W.; Bergman, Å. Radiosynthesis of perfluorooctanesulfonate (PFOS) and perfluorobutanesulfonate(PFBS), including solubility, partition and adhesion studies. Chemosphere 2012, 87, 865–871. [Google Scholar] [CrossRef]
- Bogdanska, J.; Borg, D.; Sundström, M.; Bergström, U.; Halldin, K.; Abedi-Valugerdi, M.; Bergman, A.; Nelson, B.; Depierre, J.; Nobel, S. Tissue distribution of 3⁵S-labelled perfluorooctane sulfonate in adult mice after oral exposure to a low environmentally relevant dose or a high experimental dose. Toxicology 2011, 284, 54–62. [Google Scholar] [CrossRef]
- Borg, D.; Bogdanska, J.; Sundström, M.; Nobel, S.; Håkansson, H.; Bergman, Å.; DePierre, J.W.; Halldin, K.; Bergström, U. Tissue distribution of 3⁵S-labelled perfluorooctane sulfonate (PFOS) in C57Bl/6 mice following late gestational exposure. Reprod. Toxicol. 2010, 30, 558–565. [Google Scholar] [CrossRef]
- Burkemper, J.L.; Aweda, T.A.; Rosenberg, A.J.; Lunderberg, D.M.; Peaslee, G.F.; Lapi, S.E. Radiosynthesis and biological distribution of 18F-labeled perfluorinated alkyl substances. Environ. Sci. Technol. Lett. 2017, 4, 211–215. [Google Scholar] [CrossRef]
- Matuszczak, E.; Komarowska, M.D.; Debek, W.; Hermanowicz, A. The Impact of Bisphenol A on Fertility, Reproductive System, and Development: A Review of the Literature. Int. J. Endocrinol. 2019, 2019, 4068717. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, J.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.L.; Couto, R.; Oliveira, P.J. Bisphenol A as epigenetic modulator: Setting the stage for carcinogenesis? Eur. J. Clin. Investig. 2015, 45, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Liu, S. Low-Dose Bisphenol a Exposure: A Seemingly Instigating Carcinogenic Effect on Breast Cancer. Adv. Sci. 2016, 4, 1600248. [Google Scholar] [CrossRef] [PubMed]
- Marquet, F.; Payan, J.-P.; Beydon, D.; Wathier, L.; Grandclaude, M.-C.; Ferrari, E. In vivo and ex vivo percutaneous absorption of [14C]-bisphenol A in rats: A possible extrapolation to human absorption? Arch. Toxicol. 2011, 85, 1035–1043. [Google Scholar] [CrossRef]
- Tanaka, M.; Kawamoto, T.; Matsumoto, H. Distribution of 14C-bisphenol A in pregnant and newborn mice. Dent. Mater. 2010, 26, e181–e187. [Google Scholar] [CrossRef]
- Demierre, A.L.; Peter, R.; Oberli, A.; Bourqui-Pittet, M. Dermal penetration of bisphenol A in human skin contributes marginally to total exposure. Toxicol. Lett. 2012, 213, 305–308. [Google Scholar] [CrossRef]
- Wooten, A.L.; Aweda, T.A.; Lewis, B.C.; Gross, R.B.; Lapi, S.E. Biodistribution and PET Imaging of pharmacokinetics of manganese in mice using Manganese-52. PLoS ONE 2017, 12, e0174351. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, A.; Zhang, Y.; Lee, S.-T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Li, B.; Yang, J.; Huang, Q.; Zhang, Y.; Peng, C.; Zhang, Y.; He, Y.; Shi, J.; Li., W.; Hu., J.; et al. Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice. NPG Asia Mater. 2013, 5, e44. [Google Scholar] [CrossRef]
- Guo, X.; Dong, S.; Petersen, E.J.; Gao, S.; Huang, Q.; Mao, L. Biological uptake and depuration of radio-labeled graphene by daphnia magna. Environ. Sci. Technol. 2013, 46, 12524–12531. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Hu, M.; Pan, B.; Xie, Y.; Petersen, E.J. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part. Fibre Toxicol. 2016, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Warren, J.; Hodgson, A.; Marczylo, T.; Ignatyev, K.; Guo, C.; Smith, R. Slow lung clearance and limited translocation of four sizes of inhaled iridium nanoparticles. Part. Fibre Toxicol. 2017, 14, 5. [Google Scholar] [CrossRef]
- Shim, H.E.; Lee, J.Y.; Lee, C.H.; Mushtaq, S.; Song, H.Y.; Song, L.; Choi, S.-J.; Lee, K.; Jeon, J. Quantification of inhaled aerosol particles composed of toxic household disinfectant using radioanalytical method. Chemosphere 2018, 207, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shim, H.E.; Song, L.; Moon, H.G.; Lee, K.; Yang, J.E.; Song, H.Y.; Choi, Y.J.; Choi, D.S.; Jeon, J. Efficient and stable radiolabeling of polycyclic aromatic hydrocarbon assemblies: In vivo imaging of diesel exhaust particulates in mice. Chem. Commun. 2019, 55, 447–450. [Google Scholar] [CrossRef]
- Duheron, V.; Moreau, M.; Collin, B.; Sali, W.; Bernhard, C.; Goze, C.; Gautier, T.; Pais de Barros, J.P.; Deckert, V.; Brunotte, F.; et al. Dual labeling of lipopolysaccharides for SPECT-CT imaging and fluorescence microscopy. ACS Chem. Biol. 2014, 9, 656–662. [Google Scholar] [CrossRef]
- Mushtaq, S.; Choi, M.H.; Yang, J.E.; Shim, H.E.; Song, L.; Song, H.Y.; Choi, Y.J.; Jeon, J. Technetium-99m-based simple and convenient radiolabeling of Escherichia coli for in vivo tracking of microorganisms. J. Radioanal. Nucl. Chem. 2018, 317, 997–1003. [Google Scholar] [CrossRef]
- Welling, M.M.; de Kome, C.M.; Spa, S.J.; van Willigen, D.M.; Hensbergen, A.W.; Bunschoten, A.; Duszenko, N.; Smits, W.K.; Roestenberg, M.; van Leeuwen, F.W.B. Multimodal Tracking of Controlled Staphylococcus aureus Infections in Mice. ACS Infect. Dis. 2019, 5, 1160–1168. [Google Scholar] [CrossRef]
- Kothari, P.; De, B.P.; He, B.; Chen, A.; Chiuchiolo, M.J.; Kim, D.; Nikolopoulou, A.; Amor-Coarasa, A.; Dyke, J.P.; Voss, H.U.; et al. Radioiodinated capsids facilitate in vivo non-invasive tracking of adeno-associated gene transfer vectors. Sci. Rep. 2017, 7, 39594. [Google Scholar] [CrossRef]
- Seo, J.W.; Ingham, E.S.; Mahakian, L.; Tumbale, S.; Wu, B.; Aghevlian, S.; Shams, S.; Baikoghli, M.; Jain, P.; Ding, X.; et al. Positron emission tomography imaging of novel AAV capsids maps rapid brain accumulation. Nat. Commun. 2020, 11, 2102. [Google Scholar] [CrossRef] [PubMed]
- Pietroiusti, A.; Stockmann-Juvala, H.; Lucaroni, F.; Savolainen, K. Nanomaterial exposure, toxicity, and impact on human health. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1513. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.M.; Liu, C.H.; Grodzinski, P. Nanomaterials innovation as an enabler for effective cancer interventions. Biomaterials 2020, 242, 119926. [Google Scholar] [CrossRef] [PubMed]
- Ray, S. Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew: Norwich, NY, USA, 2015; Volume 2, pp. 39–55. ISBN 978-0-323-37521-4. [Google Scholar]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Hong, H.; Cai, W.; Liu, Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 2013, 8, 2392–2403. [Google Scholar] [CrossRef]
- Choi, M.H.; Shim, H.E.; Yun, S.J.; Kim, H.R.; Mushtaq, S.; Lee, C.H.; Park, S.H.; Choi, D.S.; Lee, D.E.; Byun, E.B.; et al. Highly efficient method for 125I-radiolabeling of biomolecules using inverse-electron-demand Diels-Alder reaction. Bioorg. Med. Chem. 2016, 16, 30274–30277. [Google Scholar] [CrossRef]
- Choi, M.H.; Shim, H.E.; Nam, Y.R.; Kim, H.R.; Kang, J.A.; Lee, D.E.; Park, S.H.; Choi, D.S.; Jang, B.S.; Jeon, J. Synthesis and evaluation of an (125)I-labeled azide prosthetic group for efficient and bioorthogonal radiolabeling of cyclooctyne-group containing molecules using copper-free click reaction. Bioorg. Med. Chem. Lett. 2016, 26, 875–878. [Google Scholar] [CrossRef]
- Jeon, J.; Kang, J.A.; Shim, H.E.; Nam, Y.R.; Yoon, S.; Kim, H.R.; Lee, D.E.; Park, S.H. Efficient method for iodine radioisotope labeling of cyclooctyne-containing molecules using strain-promoted copper-free click reaction. Bioorg. Med. Chem. 2015, 23, 3303–3308. [Google Scholar] [CrossRef]
- Choi, M.H.; Rho, J.K.; Kang, J.A.; Shim, H.E.; Nam, Y.R.; Yoon, S.; Kim, H.R.; Lee, D.E.; Park, S.H.; Jang, B.S.; et al. Efficient radiolabeling of rutin with 125I and biodistribution study of radiolabeled rutin. J. Radioanal. Nucl. Chem. 2016, 308, 477–483. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeon, J.; Hong, S.H.; Rhim, W.K.; Lee, Y.S.; Youn, H.; Chung, J.K.; Lee, M.C.; Lee, D.S.; Kang, K.W.; et al. Tumor targeting and imaging using cyclic RGD-PEGylated gold nanoparticle probes with directly conjugated iodine-125. Small 2011, 7, 2052–2060. [Google Scholar] [CrossRef]
- Steiner, S.; Bisig, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Diesel exhaust: Current knowledge of adverse effects and underlying cellular mechanisms. Arch. Toxicol. 2016, 90, 1541–1553. [Google Scholar] [CrossRef]
- McClellan, R.O.; Hesterberg, T.W.; Wall, J.C. Evaluation of carcinogenic hazard of diesel engine exhaust needs to consider revolutionary changes in diesel technology. Regul. Toxicol. Pharmacol. 2012, 63, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [PubMed]
- Geva-Zatorsky, N.; Alvarez, D.; Hudak, J.E.; Reading, N.C.; Erturk-Hasdemir, D.; Dasgupta, S.; von Andrian, U.H.; Kasper, D.L. In vivo imaging and tracking of host-microbiota interactions via metabolic labeling of gut anaerobic bacteria. Nat. Med. 2015, 21, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Faintuch, J.; Faintuch, S. Microbiome and Metabolome in Diagnosis, Therapy, and other Strategic Applications; Academic Press: Cambridge, MA, USA, 2019; Volume 46, pp. 435–449. ISBN 978-0-12-815249-2. [Google Scholar]
- Jürgens, S.; Wolfgang, A.; Küh, F.E. Rhenium and technetium based radiopharmaceuticals: Development and recent advances. J. Org. Chem. 2014, 751, 83–89. [Google Scholar] [CrossRef]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]


| Radioisotope | Decay Half-Life | Decay Mode | Detection Instrument(s) | Radiolabeling Method |
|---|---|---|---|---|
| 14C | 5730 y | β− | LSC 1 | Incorporation into an organic molecule |
| 18F | 109.8 min | β+ | γ counter, PET 2 (imaging) | Nucleophilic or electrophilic substitution |
| 35S | 87.3 d | β− | LSC | Addition reaction, metabolic labeling (for amino acids) |
| 52Mn | 5.6 d | β+ | γ counter, PET (imaging) | Chelation |
| 64Cu | 12.7 h | β+, β−, EC 3 | γ counter, PET (imaging) | Chelation |
| 99mTc | 6.0 h | IT 4 | γ counter, SPECT 5 (imaging) | Chelation |
| 111In | 2.80 d | EC | γ counter, SPECT (imaging) | Chelation |
| 123I | 13.2 d | EC | γ counter, SPECT (imaging) | Electrophilic substitution |
| 124I | 4.18 d | β+, EC | γ counter, PET (imaging) | Electrophilic substitution |
| 125I | 59.4 d | EC | γ counter, SPECT (imaging) | Electrophilic substitution |
| 192Ir | 73.8 d | β−, EC | γ radiography | - |
| Substance | Radioisotope and Labeling Method | Exposure Route | Animal Model | Results | Ref. |
|---|---|---|---|---|---|
| Perfluorooctanoic acid | 14C, incorporation | Oral exposure | Angus cows |
| [26] |
| Perfluorooctane sulfonate | 35S, nucleophilic addition to [35S]SO2 | Oral exposure | C57BL/6 mice |
| [28] |
| 35S, nucleophilic addition to [35S]SO2 | Gestational exposure | Pregnant C57BL/6 mice |
| [29] | |
| Perfluorinated alkyl compounds | 18F, isotopic exchange (19F → 18F) | Intravenous injection | CD1 mice |
| [30] |
| Bisphenol A | 14C, incorporation | Intravenous and topical injection, percutaneous absorption | SD rats (in vivo), human skin (in vitro) |
| [35] |
| 14C, incorporation | Intraperitoneal injection | Pregnant mice |
| [36] | |
| 14C, incorporation | Percutaneous absorption | Human skin (in vitro) |
| [37] | |
| Manganese | 52Mn | Nasal inhalation, intravenous injection | CD1 mice |
| [38] |
| Graphene | 125I, electrophilic substitution | Intravenous injection | BALB/c mice |
| [39] |
| Graphene oxide | 125I, electrophilic substitution | Intratracheal instillation | Kunming mice |
| [40] |
| Graphene | 14C, graphitization with 14C-labeled phenol | Intake | Daphnia magna |
| [41] |
| Graphene | 14C, graphitization with 14C-labeled phenol | Intratracheal instillation, oral exposure | ICR mice |
| [42] |
| Iridium NPs | 192Ir, incorporation | Nasal inhalation | SD rats |
| [43] |
| PHMG | 111In, DOTA chelation | Whole-body inhalation, intratracheal instillation | SD rats |
| [44] |
| DEP | 125I, self-assembly with 125I-labeled pyrene | Intratracheal instillation, oral exposure | ICR mice |
| [45] |
| LPS | 111In, DOTA-BODIPY conjugate chelation | Intravenous injection | C57BL/6 mice |
| [46] |
| E. Coli | 99mTc, [99mTc(CO)3] chelation with intracellular proteins | Intravenous injection, oral exposure | ICR mice |
| [47] |
| S. Aureus | 99mTc, radiolabeled peptide attached to the cell membrane | Intramuscular injection | Swiss mice |
| [48] |
| AAV | 124I, electrophilic substitution/modified Bolton–Hunter reagent | Intraparenchymal injection | CD-1 mice |
| [49] |
| AAV | 64Cu, NOTA chelation followed by conjugation on the AAV surface | Intravenous injection | C57BL/6 mice |
| [50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Mushtaq, S.; Park, J.E.; Shin, H.S.; Lee, S.-Y.; Jeon, J. Radioanalytical Techniques to Quantitatively Assess the Biological Uptake and In Vivo Behavior of Hazardous Substances. Molecules 2020, 25, 3985. https://doi.org/10.3390/molecules25173985
Lee JY, Mushtaq S, Park JE, Shin HS, Lee S-Y, Jeon J. Radioanalytical Techniques to Quantitatively Assess the Biological Uptake and In Vivo Behavior of Hazardous Substances. Molecules. 2020; 25(17):3985. https://doi.org/10.3390/molecules25173985
Chicago/Turabian StyleLee, Jae Young, Sajid Mushtaq, Jung Eun Park, Hee Soon Shin, So-Young Lee, and Jongho Jeon. 2020. "Radioanalytical Techniques to Quantitatively Assess the Biological Uptake and In Vivo Behavior of Hazardous Substances" Molecules 25, no. 17: 3985. https://doi.org/10.3390/molecules25173985
APA StyleLee, J. Y., Mushtaq, S., Park, J. E., Shin, H. S., Lee, S.-Y., & Jeon, J. (2020). Radioanalytical Techniques to Quantitatively Assess the Biological Uptake and In Vivo Behavior of Hazardous Substances. Molecules, 25(17), 3985. https://doi.org/10.3390/molecules25173985







