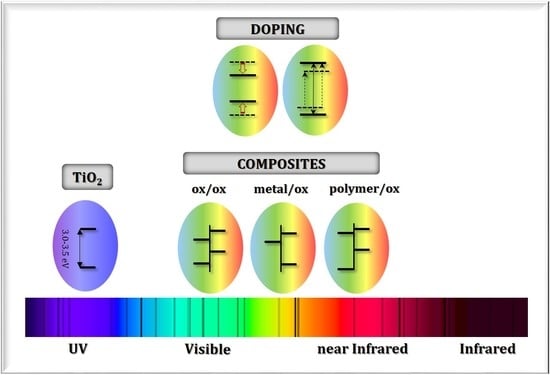
Sunlight-Operated TiO2-Based Photocatalysts
Abstract
:1. Introduction
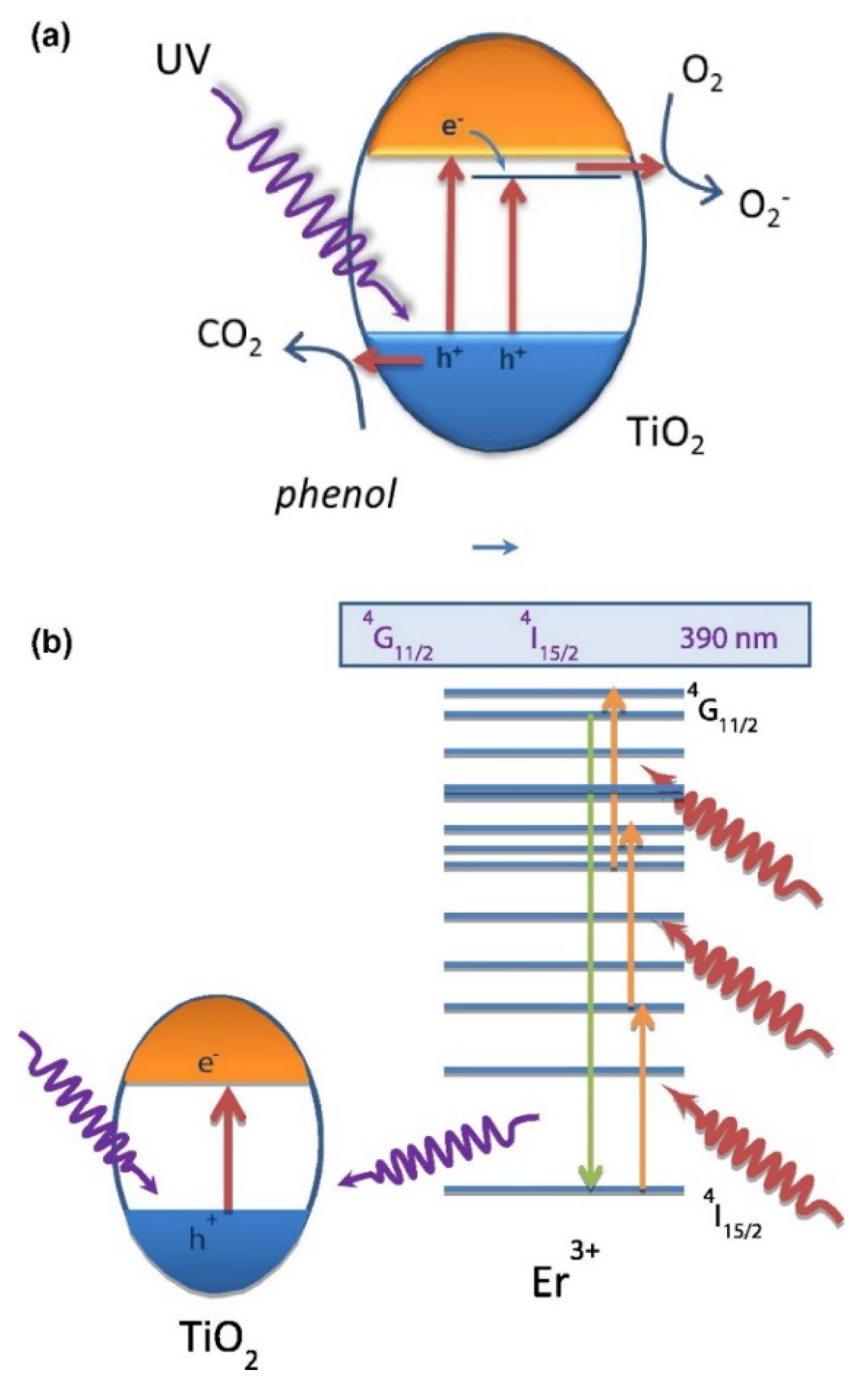
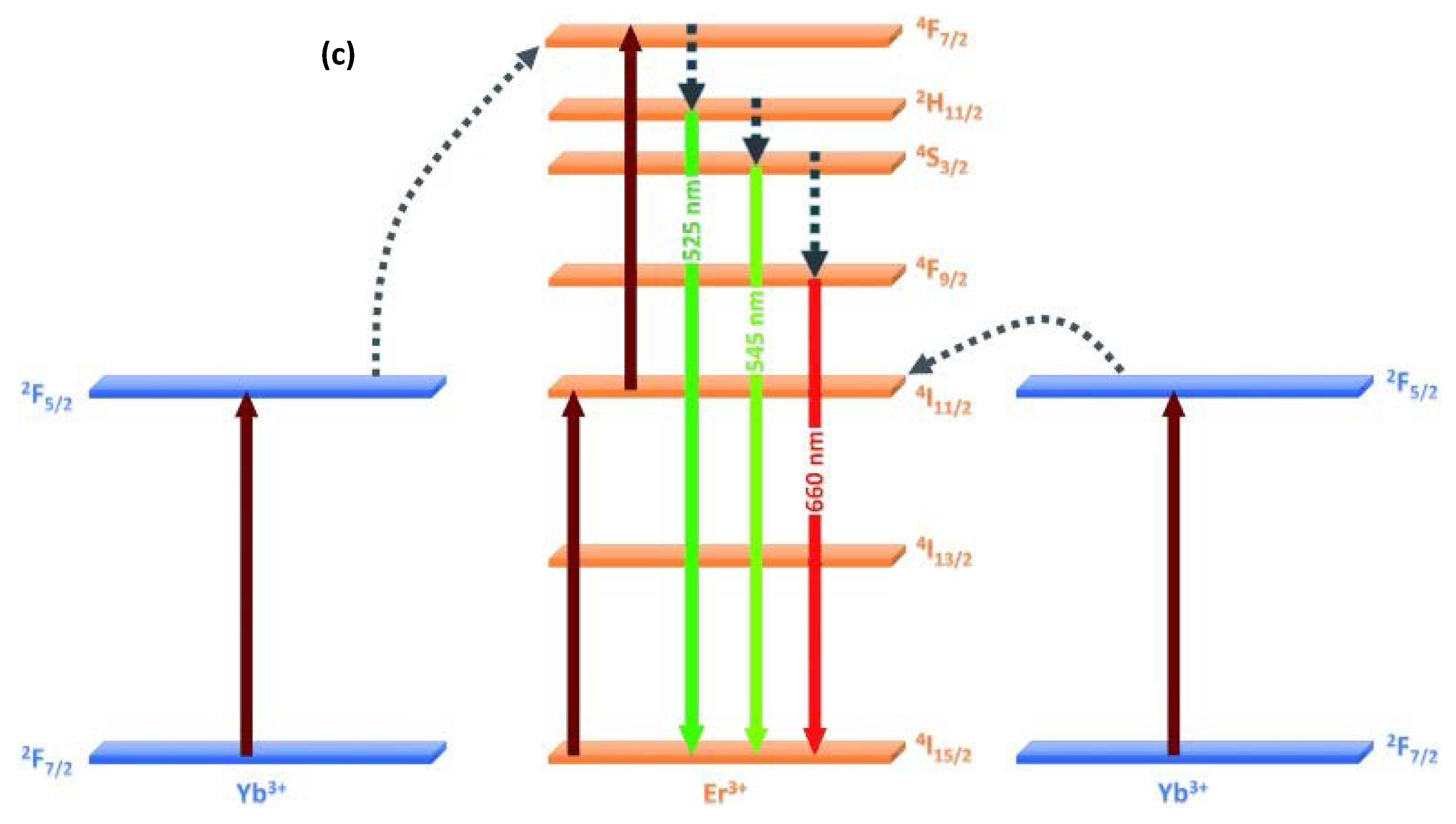
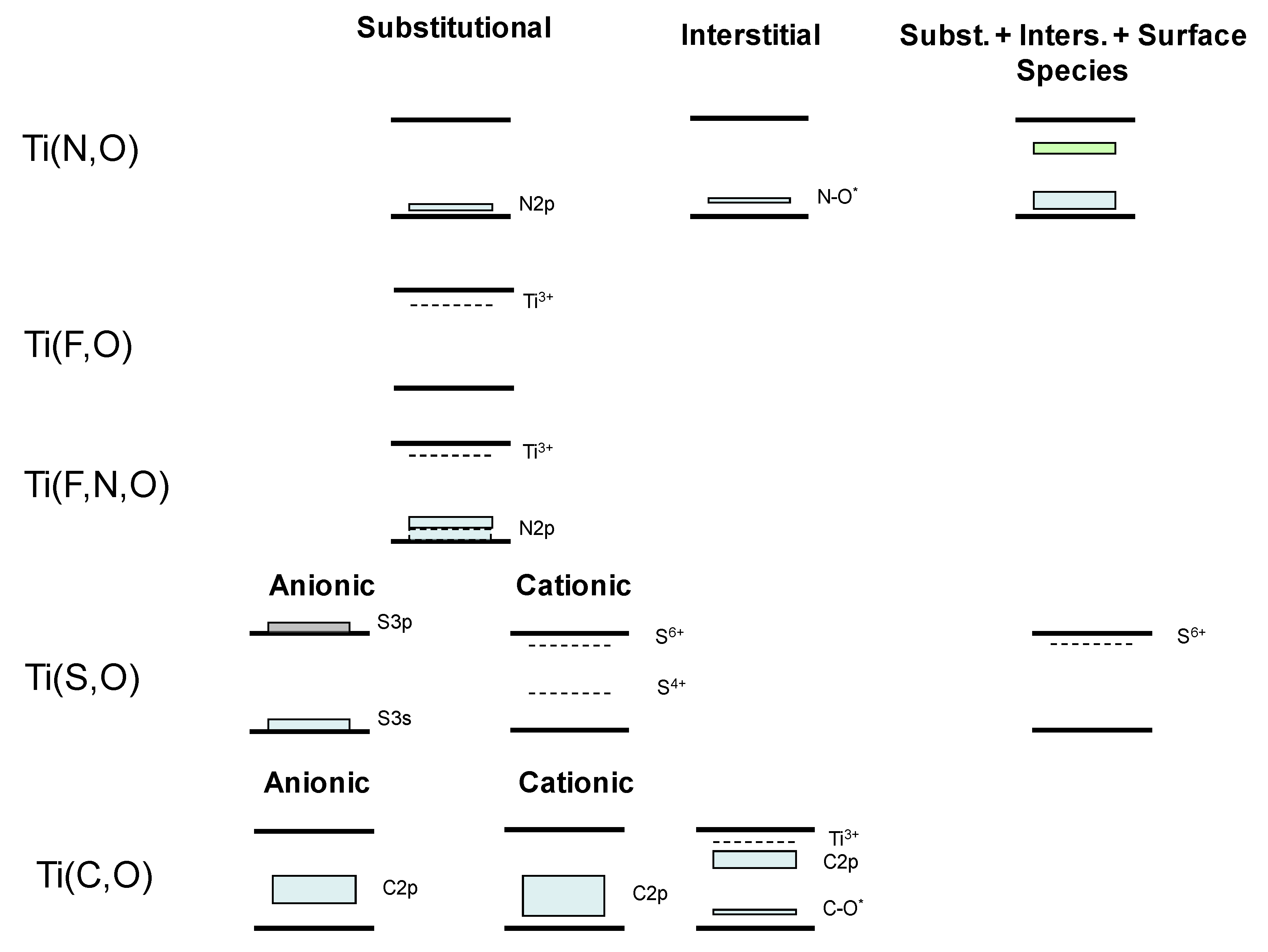
2. Doped Photocatalysts
3. Composite Photocatalysts
4. Conclusions and Outlook into the Future
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.H.A.; Li, K.; Zheng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.C.; Huang, H. Titanium Oxide Based Photocatalytic Materials Development and Their Role of in the Air Pollutants Degradation: Overview and Forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Yanjuan, C.; Yuxiong, W.; Hao, W.; Fangyan, C. Graphitic Carbon Nitrides: Modifications and Applications in Environmental Purification. Prog. Chem. 2016, 28, 428–437. [Google Scholar] [CrossRef]
- Leong, K.H.; Lim, P.F.; Sim, L.C.; Punia, V.; Pichiah, S. Improved solar light stimulated charge separation of g-C3N4 through self-altering acidic treatment. Appl. Surf. Sci. 2018, 430, 355–361. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Davey, K.; Qiao, S.-Z. Carbon, nitrogen and phosphorus containing metal-free photocatalysts for hydrogen production: Progress and challenges. J. Mater. Chem. A 2018, 6, 1305–1324. [Google Scholar] [CrossRef]
- Teixeira, I.F.; Barbosa, E.C.M.; Tang, S.C.E.; Camargo, P.H.C. Carbon nitrides and metal nanoparticles: From controlled synthesis to design principles for improved photocatalysis. Chem. Soc. Rev. 2018, 47, 7783–7817. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, C.; He, L. Recent advances of polyoxometalate-catalyzed selective oxidation based on structural classification. Acta Crys. 2018, C74, 1182–1201. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng, G. Modulation of Bi2MoO6-Based Materials for Photocatalytic Water Splitting and Environmental Application: A Critical Review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef] [PubMed]
- Caudillo-Flores, U.; Kubacka, A.; Berestok, T.; Zhang, T.; Llorca, J.; Arbiol, J.; Cabot, A.; Fernández-García, M. Hydrogen photogeneration using ternary CuGaS-TiO2-Pt composites. Int. J. Hydrogen Energy 2020, 45, 1510–1520. [Google Scholar] [CrossRef]
- Luca, V. Comparison of Size-Dependent Structural and Electronic Properties of Anatase and Rutile Nanoparticles. J. Phys. Chem. C 2009, 113, 6367–6380. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, S.; Zboril, R.; Schmuki, P. Photocatalysis with Reduced TiO2: From Black TiO2 to cocatalyst-free hydrogen photoproduction. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Wallenmeyer, P.; Anderson, A.; Murowchick, J.; Liu, L.; Chen, X. Hydrogenated black ZnO nanoparticles with enhanced photocatalytic performance. RSC Adv. 2014, 4, 41654–41658. [Google Scholar] [CrossRef]
- Wang, Q.; Edalati, K.; Koganemaru, Y.; Nakamura, S.; Watanabe, M.; Ishihara, T.; Horita, Z. Photocatalytic hydrogen generation in low-bandgap black zirconia produced by high pressure torsion. J. Mater. Chem. 2020, 8, 3643–3650. [Google Scholar] [CrossRef]
- Rajamaran, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Choi, W.Y.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Fuerte, A.; Hernández-Alonso, M.D.; Maira, A.J.; Martínez-Arias, A.; Fernández-García, M.; Conesa, J.C.; Soria, J. Visible light-activated nanosized doped-TiO2 photocatalysts. Chem. Commun. 2001, 2178–2179. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Nanostructured Ti–M mixed-metal oxides: Toward a visible light-driven photocatalyst. J. Catal. 2008, 254, 272–284. [Google Scholar] [CrossRef]
- Kubacka, A.; Colón, G.; Fernández-García, M. Cationic (V, Mo, Nb, W) doping of TiO2–anatase: A real alternative for visible light-driven photocatalysts. Catal. Today 2009, 143, 286–292. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Conesa, J.C.; Kubacka, A.; Fernández-García, M. UV and visible hydrogen photo-production using Pt promoted Nb-doped TiO2 photo-catalytsts: Interpreting quantum efficiency. Appl. Catal. B 2017, 216, 133–145. [Google Scholar] [CrossRef]
- Christoforidis, K.; Fernández-García, M. Photoactivity and charge trapping sites in copper and vanadium doped anatase TiO2 nano-materials. Catal. Sci. Technol. 2016, 6, 1094–1105. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, M.; Morales-Mendoza, G.; Rodriguez-Vargas, M.J.; Ibarra-Malo, C.C.; Rodriguez-Rodriguez, A.A.; Vela-Gonzalez, A.V.; Perez-García, S.A.; Gomez, R. Synthesis of Zn-doped TiO2 nanoparticles by the novel oil-in-water (O/W) microemulsion method and their use for the photocatalytic degradation of phenol. J. Environ. Chem. Eng. 2015, 3, 3037–3047. [Google Scholar] [CrossRef]
- Barba-Nieto, I.; Caudillo-Flores, U.; Gómez-Cerezo, M.N.; Kubacka, A.; Fernández-García, M. Boosting Pt/TiO2 hydrogen photoproduction through Zr doping of the anatase structure: A spectroscopic and mechanistic study. Chem. Eng. J. 2020, 398, 125665. [Google Scholar] [CrossRef]
- Yayapao, O.; Thongtem, S.; Phuruangrat, A.; Thongtem, T. Sonochemical synthesis, photocatalysis and photonic properties of 3% Ce-doped ZnO nanoneedles. Ceram. Int. 2013, 39, S563–S568. [Google Scholar] [CrossRef]
- Song, D.M.; Li, J.C. First principles study of band gap of Cu doped ZnO single-wall nanotube modulated by impurity concentration and concentration gradient. Comput. Mater. Sci. 2012, 65, 175–181. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Zhang, Y.; Khalid, N.R.; Xu, J.; Ullah, M.; Hong, Z. Preparation of highly efficient Al-doped ZnO photocatalyst by combustion synthesis. Curr. Appl. Phys. 2013, 13, 697–704. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, T.; Zhao, Y.; Zang, W.; Xing, L.; Xue, X. High-performance self-powered/ active humidity sensing of Fe-doped ZnO nanoarray nanogenerator. Sens. Actuators B Chem. 2015, 213, 382–389. [Google Scholar] [CrossRef]
- Wu, S.; Fang, J.; Xu, X.; Liu, Z.; Zhu, X.; Xu, W. Microemulsion synthesis, characterization of highly visible light responsive rare earth-doped Bi2O3. Photochem. Photobiol. 2012, 88, 1205–1210. [Google Scholar] [CrossRef]
- Xi, L.; Qian, D.; Huang, X.; Wang, H.-E. Fine nanoparticles of Al-SnO2 prepared by co-precipitation route in water/oil microemulsion. J. Alloys Compd. 2008, 462, 42–46. [Google Scholar] [CrossRef]
- Murtaza, G.; Osama, S.M.A.; Saleen, M.; Hassan, M.; Watoo, N.R.K. Structural, optical, and photocatalytic properties of Cd1−xS:Lax nanoparticles for optoelectronic applications. Appl. Phys. A 2018, 124, 778–787. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Liu, R.; Sun, Z.; Zhao, D.; Kou, C. Preparation of Ca-doped LaFeO3 nanopowders in a reverse microemulsion and their visible light photocatalytic activity. Mater. Lett. 2010, 64, 223–225. [Google Scholar] [CrossRef]
- Naccache, R.; Vetrone, F.; Capobianco, J.A. Lanthanide-Dope Upconverting Nanoparticles: Harvesting Light for Solar Cells. ChemSusChem 2013, 6, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, S.; Li, J.; Gao, W.; Deng, Y.; Dong, W.; Zhao, C.; Lu, G. Visible-to-ultraviolet upconverstion: Energy transfer, materials matrix, and synthesis strategies. Appl. Catal. B 2017, 206, 89–103. [Google Scholar] [CrossRef]
- Obregon, S.; Lee, S.W.; Colón, G. Exalted photocatalytic activity of tetragonal BiVO4by Er3+ doping though a luminescence cooperative mechanism. Dalton Trans. 2014, 43, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, W.; Yin, W.; Shang, M.; Wang, L.; Sun, S. Inducing photocatalysis by visible light beyong the absorption edge: Effect of upconversion agent on the photoactivity of Bi2WO6. Appl. Catal. B 2010, 101, 68–73. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, N.; Lou, Z.; Gu, L.; Miao, C.; Yuan, H.; Shan, A. Near-infrared photocatalysts of BiVO4/CaF:Er3+, Tm3+, Yb3+ with enhanced upconversion properties. Nanoscale 2014, 6, 1362–1368. [Google Scholar] [CrossRef]
- Obregon, S.; Kubacka, A.; Fernández-García, M.; Colón, G. High-performance Er3+-TiO2 system: Dual up-conversion and electronic role of the lanthanide. J. Catal. 2013, 299, 298–306. [Google Scholar] [CrossRef]
- Bhethanabotla, V.C.; Russel, D.R.; Kuhn, F.N. Assessment of mechanisms for enhanced performance of Yb/Er/titania photocatalysts for organic degradation: Role of rare earth elements in the titania phase. Appl. Catal. B 2017, 202, 156–164. [Google Scholar] [CrossRef]
- Reszczyńka, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible light activity of rare earth metal doped (Er3+, Yb3+, or Er3+/Yb3+) titania photocatalysts. Appl. Catal. B 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Sompalli, N.K.; Das, A.; De Syamal, S.; Deivasijamani, P. Mesoporous monolith design of mixed phased titania doped Sm3+/Er3+ composites: A super responsive visible light photocatalyst for organic pollutant clean-up. Appl. Surf. Sci. 2020, 504, 144350. [Google Scholar] [CrossRef]
- Kubacka, A.; Muñoz-Batista, M.; Ferrer, M.; Fernández-García, M. Er-W codoping of TiO2-anatase: Structural and electronic characterization and disinfection capability under UV-vis, and near-IR excitation. Appl. Catal. B Environ. 2018, 228, 113–129. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Li, Z.; Liu, X.; Zhu, S.; Liang, Y.; Yeung, K.W.K.; Wu, S. Ce and Er co-doped TiO2 for rapid bacteria killing using visible light. Bioactive Mater. 2020, 5, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Belver, C.; Bellod, R.; Fuerte, A.; Fernández-García, M. Nitrogen-containing TiO2 photocatalysts. Part 1. Synthesis and solid characterization. Appl. Catal. B Environ. 2006, 65, 301–308. [Google Scholar] [CrossRef]
- Belver, C.; Bellod, R.; Stewart, S.J.; Requejo, F.G.; Fernández-García, M. Nitrogen-containing TiO2 photocatalysts. Part 2. Photocatalytic behavior under sunlight excitation. Appl. Catal. B Environ. 2006, 65, 309–314. [Google Scholar] [CrossRef]
- Stewart, S.J.; Fernández-García, M.; Belver, C.; Simon Mun, B.; Requejo, F.G. Influence of N-Doping on the Structure and Electronic Properties of Titania Nanoparticle Photocatalysts. J. Phys. Chem. B 2006, 110, 16482–16486. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and Characterization of Nitrogen-Doped TiO2 Nanophotocatalyst with High Visible Light Activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-doped titanium oxide: An overview of material design dimensionality effect over moder applications. J. Photochem. Photobiol. C 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Wang, W.; Tadé, M.O.; Shao, Z. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018, 92, 33–63. [Google Scholar] [CrossRef]
- Kubacka, A.; Bachiller-Baeza, B.; Colón, G.; Fernández-García, M. Doping level effect on sunlight-driven W, N-co-doped TiO2-anatase photo-catalysts for aromatic hydrocarbon partial oxidation. Appl. Catal. B Environ. 2010, 93, 274–281. [Google Scholar] [CrossRef]
- Kubacka, A.; Colón, G.; Fernández-García, M. N- and/or W-(co)doped TiO2-anatase catalysts: Effect of the calcination treatment on photoactivity. Appl. Catal. B Environ. 2010, 95, 238–244. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Labhsetwar, N.K.; Hishita, S.; Ohashi, N. Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies. Chem. Phys. Lett. 2005, 401, 579–584. [Google Scholar] [CrossRef]
- Czoska, A.M.; Livraghi, S.; Chiesa, M.; Giamello, E.; Agnoli, S.; Granozzi, G.; Finazzi, E.; Di Valentin, C.; Pacchioni, G. The Nature of Defects in Fluorine-Doped TiO2. J. Phys. Chem. C 2008, 112, 8951–8956. [Google Scholar] [CrossRef]
- Chen, C.; Rong, H.; Kaiguang, M.; Zhimin, R.; Wang, H.; Qian, G.; Wang, Z. Shape evolution of highly crystalline anatase TiO2 nanobipyramids. Crys. Growth Design 2011, 11, 5221–5226. [Google Scholar] [CrossRef]
- Xu, L.; Ming, L.; Chen, F. TiO2 with “fluorine-occupied” surface oxygen vacancies and its stability enhanced photoactivity performance. ChemCatChem 2015, 7, 1797–1800. [Google Scholar] [CrossRef]
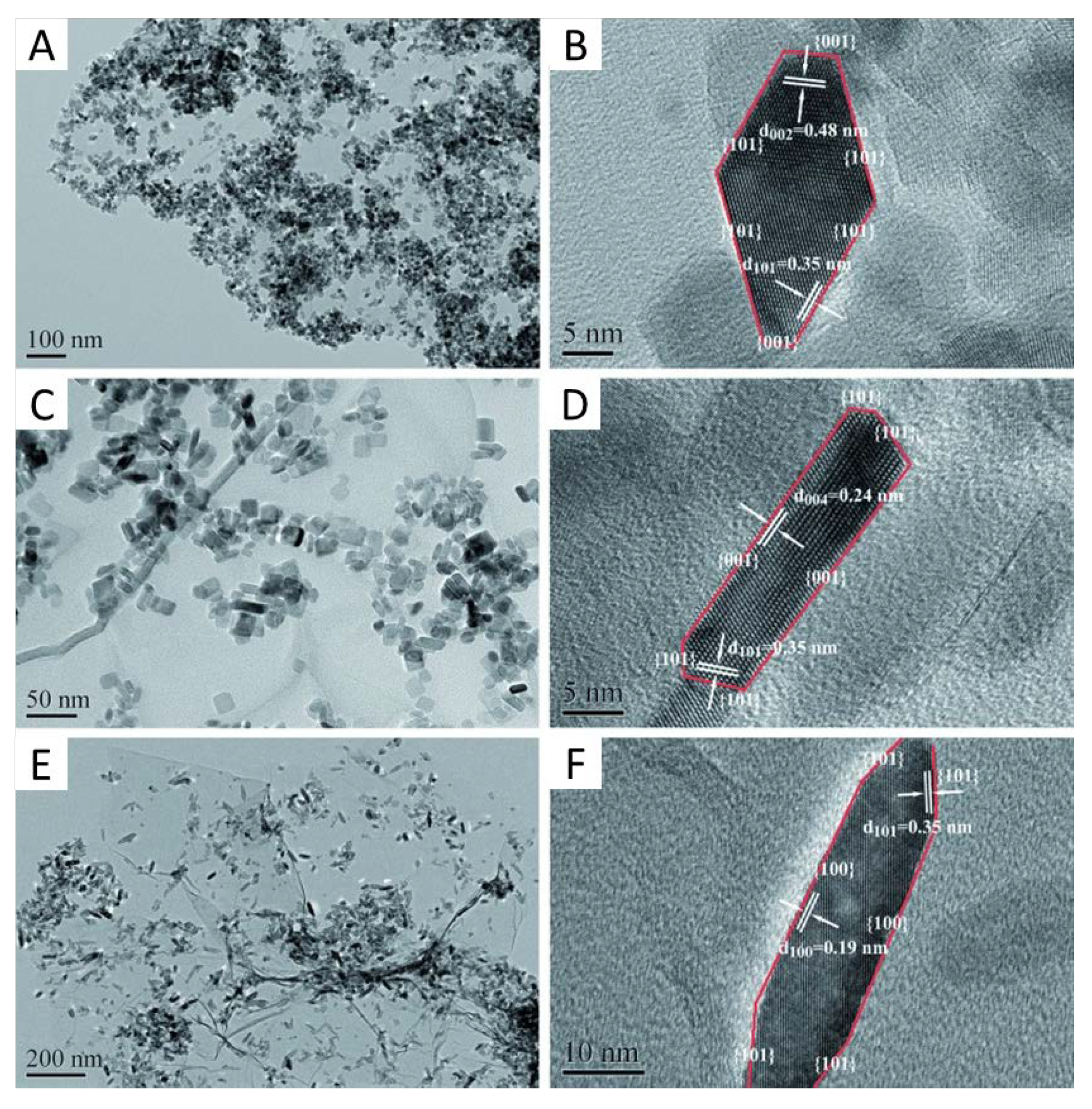
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cagnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous Synthesis of TiO2 Nanocrystals Using TiF4 to Engineer Morphology, Oxygen Vacancy Concentration, and Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef]
- Mino, L.; Pellegrino, F.; Rades, S.; Radnik, J.; Hodoroaba, V.-D.; Spoto, G.; Maurino, V.; Martra, G. Beyond Shape Engineering of TiO2 Nanoparticles: Post-Synthesis Treatment Dependence of Surface Hydration, Hydroxylation, Lewis Acidity and Photocatalytic Activity of TiO2 Anatase Nanoparticles with Dominant {001} or {101} Facets. ACS Appl. Nano Mater. 2018, 1, 5355–5365. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhang, J.; Gao, P.; Wang, Y.; Li, X.; Wu, T.; Wang, Y.; Chen, Y.; Yang, P. Full visible light absorption of TiO2 nanotubes indiced by anionic S22− doping and their greatly enhanced photocatalytic H2 production abilities. Appl. Catal. B 2017, 206, 168–174. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, Z.; Li, Y.; Wang, W.; Li, J.; Li, J.; Ao, Y.; He, J.; Sharma, V.K.; Wang, J. Morphology- and Phase- Controlled Synthesis of Visible-Light-Activated Sdoped TiO2 with Tunable S4+/S6+ Ratio. Chem. Eng. J. 2020, 125549. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Figueroa, J.S.A.; Fernández-García, M. Iron-Sulfur codoped TiO2 anatase nano-materials: UV and sunlight activity for toluene degradation. Appl. Catal. B Environ. 2012, 117–118, 310–316. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Carbon-doped Anatase TiO2 Powders as a Visible-light Sensitive Photocatalyst. Chem. Lett. 2003, 32, 772–773. [Google Scholar] [CrossRef]
- Treschev, S.Y.; Chou, P.-W.; Tseng, Y.-H.; Wang, J.-B.; Perevedentseva, E.V.; Cheng, C.-L. Photoactivities of the visible-light-activated mixed-phase carbon-containing titanium dioxide: The effect of carbon incorporation. Appl. Catal. B 2008, 79, 8–16. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, J.; Peng, T. New insights into the enhanced photocatalytic activity of N, C, and S-doped ZnO photocatalysts. Appl. Catal. B 2016, 181, 220–227. [Google Scholar] [CrossRef]
- Leviand, A.B.; Bhalu, M.N.; Malghe, Y.S. Visible light photocatalytic degradation of malachite green using modified titania. J. Mater. Res. Technol. 2019, 8, 299–358. [Google Scholar] [CrossRef]
- Lavand, A.B.; Malghe, Y.S. Synthesis, characterization and visible light photocatalytic activity of carbon and iron modified ZnO. J. King Saud Univ. 2018, 30, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Pei, C.; Gong, J. Insights into interface engineering in steam reforming reactions for hydrogen production. Energ. Environ. Sci. 2019, 12, 3473–3495. [Google Scholar] [CrossRef]
- Hong, S.S.; Lee, M.S.; Park, S.S.; Lee, G.D. Synthesis of nanosized TiO2/SiO2 particles in the microemulsion and their photocatalytic activity on the decomposition of p-nitrophenol. Catal. Today 2003, 87, 99–105. [Google Scholar] [CrossRef]
- Manurung, P.; Situmeang, R.; Ginting, E.; Pardede, I. Synthesis and Characterization of Titania-Rice Husk Silica Composites as photocatalyst. Indones. J. Chem. 2015, 15, 36–42. [Google Scholar] [CrossRef]
- Kubacka, A.; Muñoz-Batista, M.J.; Ferrer, M.; Fernández-García, M. UV and visible light optimization of anatase TiO2 antimicrobial properties: Surface deposition of metal and oxide /(Cu, Zn, Ag) species. Appl. Catal. B Environ. 2013, 140–141, 680–690. [Google Scholar] [CrossRef]
- Kubacka, A.; Muñoz-Batista, M.J.; Fernández-García, M.; Obregón, S.; Colón, G. Evolution of H2 photoproduction with Cu content on CuOx-TiO2 composite catalysts prepared by a microemulsion method. Appl. Catal. B Environ. 2015, 163, 214–222. [Google Scholar] [CrossRef]
- Ali, M.E.M.; Alanezi, A.A.; Azeez, F.A.; Ghaly, M.Y. Photoassisted mineralization of remazole red F3B over NiO/TiO2 and CdO/TiO2 nanoparticles under simulated sunlight. Sep. Sci. Technol. 2018, 53, 170–180. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Dimitropoulos, M.; Govatsi, A.; Sygellou, L.; Tsakiroglou, C.D.; Yannopoulos, S.N. Influence of thesurface-to-bulk defects ratio of ZnO and TiO2 on their UV-mediated photocatalytic activity. Appl. Catal. B 2017, 205, 292–301. [Google Scholar] [CrossRef]
- Xu, J.-J.; Chen, M.-D.; Fu, D. Preparation of bismuth oxide/titania composite particles and their photocatalytic activity to degradation of 4-chlorophenol. Trans. Nonferr. Met. Soc. China 2011, 21, 340–345. [Google Scholar] [CrossRef]
- Gómez-Cerezo, M.N.; Muñoz-Batista, M.J.; Tudela, D.; Fernández-García, M.; Kubacka, A. Composite Bi2O3–TiO2 catalysts for toluene photo-degradation: Ultraviolet and visible light performances. Appl. Catal. B Environ. 2014, 156–157, 307–313. [Google Scholar] [CrossRef]
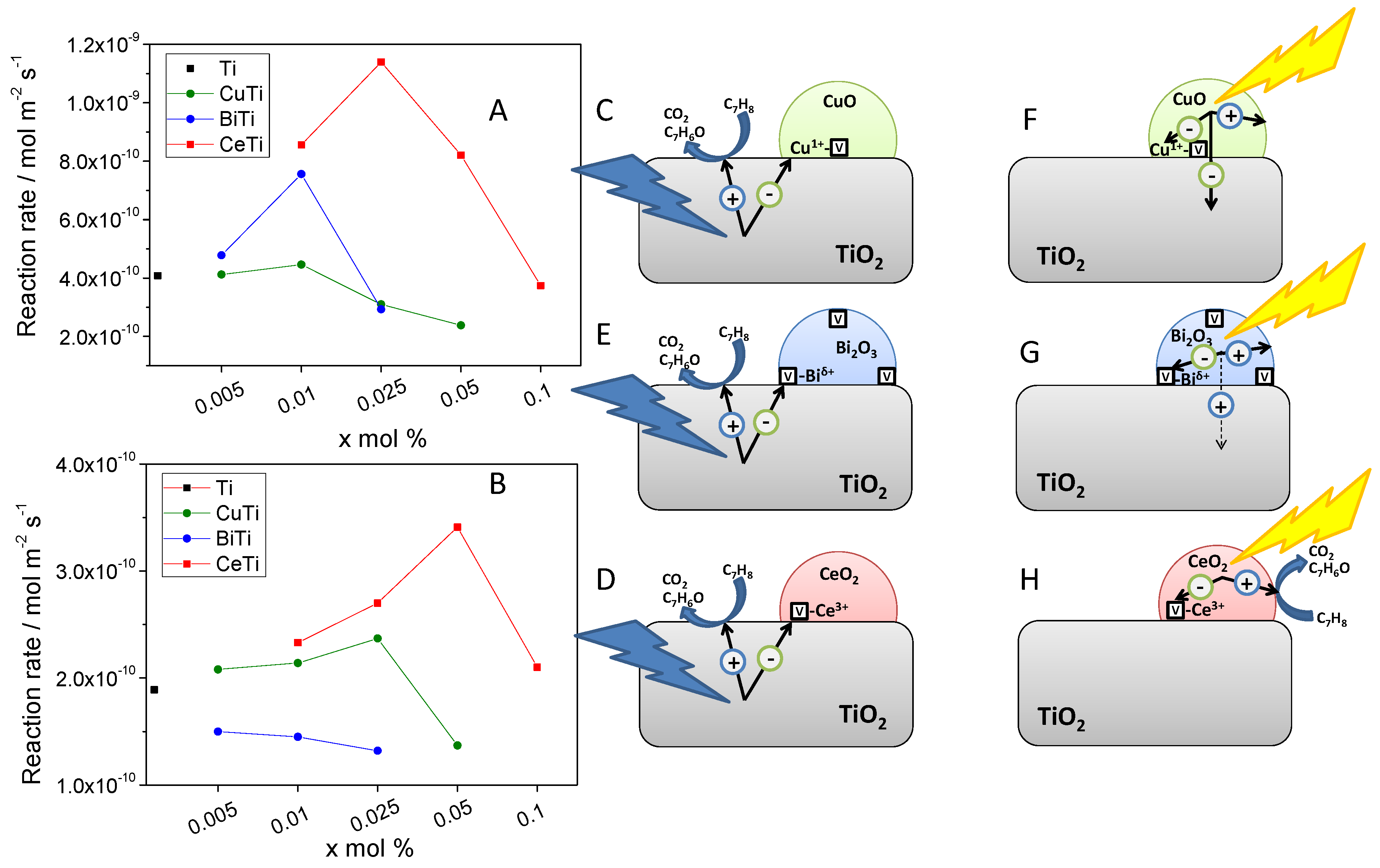
- Muñoz-Batista, M.J.; Kubacka, A.; Fontelles-Carceller, O.; Tudela, D.; Fernández-García, M. Surface CuO, Bi2O3, and CeO2 Species Supported in TiO2-Anatase: Study of Interface Effects in Toluene Photodegradation Quantum Efficiency. ACS Appl. Mater. Interfaces 2016, 8, 13934–13945. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Batista, M.J.; Ballari, M.M.; Kubacka, A.; Cassano, A.E.; Alfano, O.M.; Fernández-García, M. Acetaldehyde degradation under UV and visible irradiation using CeO2–TiO2 composite systems: Evaluation of the photocatalytic efficiencies. Chem. Eng. J. 2014, 255, 297–306. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Li, Y.; Yue, X.; Wang, C. Phase-dependent enhancement for CO2 photocatalytic reduction over CeO2/TiO2 catalysts. Catal. Sci. Technol. 2016, 6, 7967–7975. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Kubacka, A.; Rachwalik, R.; Bachiller-Baeza, B.; Fernández-García, M. Green photo-oxidation of styrene over W–Ti composite catalysts. J. Catal. 2014, 309, 428–438. [Google Scholar] [CrossRef]
- Wodka, D.; Socha, R.P.; Bielańska, E.; Elżbieciak-Wodka, M.; Nowak, P.; Warszyński, P. Photocatalytic activity of titanium dioxide modified by Fe2O3. Appl. Surf. Sci. 2014, 319, 173–180. [Google Scholar] [CrossRef]
- Lu, N.; Zhao, Y.; Liu, H.; Guo, Y.; Yuan, X.; Xu, H.; Peng, H.; Qin, H. Design of polyoxometallate–titania composite film (H3PW12O40/TiO2) for the degradation of an aqueous dye Rhodamine B under the simulated sunlight irradiation. J. Hazard Mater. 2012, 199–200, 1–8. [Google Scholar] [CrossRef]
- Bertolini, G.R.; Pizzio, L.R.; Kubacka, A.; Muñoz-Batista, M.J.; Fernández-García, M. Composite H3PW12O40–TiO2 catalysts for toluene selective photo-oxidation. Appl. Catal. B Environ. 2018, 225, 100–109. [Google Scholar] [CrossRef]
- Obregón, S.; Colón, G. Er3+-BiVO4/TiO2 complex heterostructure with excellent photocatalytic performance. RSC Adv. 2014, 4, 6920–6926. [Google Scholar] [CrossRef] [Green Version]
- Jian, Z.; Huang, S.; Cao, Y.; Zhang, Y. Hydrothermal Preparation and Characterization of TiO2/BiVO4 Composite Catalyst and its Photolysis of Water to Produce Hydrogen. Photochem. Photobiol. 2016, 92, 363–370. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Meira, D.; Colón, G.; Kubacka, A.; Fernández-García, M. Phase-Contact Engineering in Mono- and Bimetallic Cu-Ni co-catalysts for Hydrogen Photocatalytic Materials. Angew. Chem. Int. Ed. 2018, 57, 1199–1203. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, Y.; Sun, Y.; Wang, J.; Bie, L.; Duan, Y. Sunlight-activated AlFeO3/TiO2 photocatalyst. Sci. China Ser. B 2006, 49, 67–74. [Google Scholar] [CrossRef]
- Emsaki, M.; Hassanzadeh-Tabrizi, S.A.; Saffar-Teluri, A. Microemulsion synthesis of ZnO–ZnWO4 nanoparticles for superior photodegradation of organic dyes in water. J. Mater. Sci. Mater Electron. 2018, 29, 2384–2391. [Google Scholar] [CrossRef]
- Falak, P.; Hassanzadeh-Tabrizi, S.A.; Saffar-Teluri, A. Synthesis, characterization, and magnetic properties of ZnO-ZnFe2O4 nanoparticles with high photocatalytic activity. J. Magn. Magn. Mater. 2017, 441, 98–104. [Google Scholar] [CrossRef]
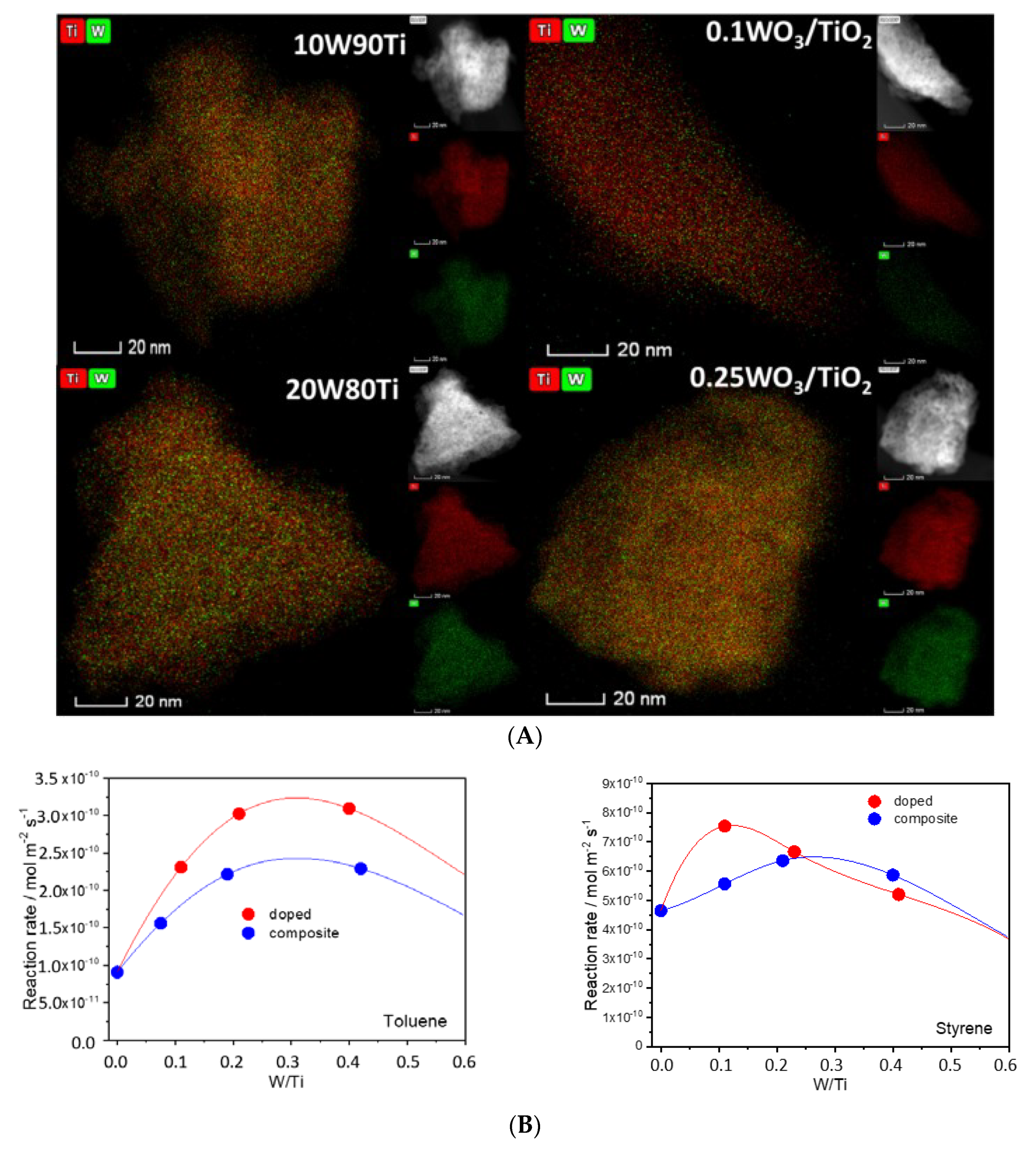
- Caudillo-Flores, U.; Muñoz-Batista, M.J.; Hungría, A.B.; Haro, M.L.; Fernández-García, M.; Kubacka, A. Toluene and styrene photo-oxidation quantum efficiency: Comparison between doped and composite tungsten-containing anatase-based catalysts. Appl. Catal. B Environ. 2019, 245, 49–61. [Google Scholar] [CrossRef]
- Kondo, K.; Murakami, N.; Ye, C.; Tsubota, T.; Ohno, T. Development of highly efficient sulfur-doped TiO2 photocatalysts hybridized with graphitic carbon nitride. Appl. Catal. B 2013, 142–143, 362–367. [Google Scholar] [CrossRef]
- Miranda, C.; Mansilla, H.; Yáñez, J.; Obregón, S.; Colón, G. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. J. Photochem. Photobiol. A 2013, 253, 16–21. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Kubacka, A.; Fernández-García, M. Effect of g-C3N4 loading on TiO2-based photocatalysts: UV and visible degradation of toluene. Catal. Sci. Technol. 2014, 4, 2006–2015. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Kubacka, A.; Fernández-García, M. Effective Enhancement of TiO2 Photocatalysis by Synergistic Interaction of Surface Species: From Promoters to Co-catalysts. ACS Catal. 2014, 4, 4277–4288. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Wu, X.; Lee, P.; Shih, K. Fabrication of heterostructured g-C3N4/Ag-TiO2 hybrid photocatalyst with enhanced performance in photocatalytic conversion of CO2 under simulated sunlight irradiation. Appl. Surf. Sci. 2017, 402, 198–207. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Li, K.; Xiong, J.; Chen, T.; Yan, L.; Dai, Y.; Song, D.; Lv, Y.; Zeng, Z. Preparation of graphene/TiO2 composites by nonionic surfactant strategy and their simulated sunlight and visible light photocatalytic activity towards representative aqueous POPs degradation. J. Hazard. Mater. 2013, 250–251, 19–28. [Google Scholar] [CrossRef]
- Du, J.; Lai, X.; Yang, N.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically Ordered Macro−Mesoporous TiO2−Graphene Composite Films: Improved Mass Transfer, Reduced Charge Recombination, and Their Enhanced Photocatalytic Activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef]
- Jain, S.M.; Biedrzychi, J.J.; Maurino, V.; Zecchina, A.; Mino, L.; Spoto, G. Acetylene oligomerization on the surface of TiO2: A step forward in the in situ synthesis of nanostructured carbonaceous structures on the surface of photoactive oxides. J. Mater. Chem. A 2014, 2, 12247–12254. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y.; Tang, Z.; Zhao, H.; Li, Y.; Wang, D. Photocatalytic Properties of Graphdiyne and Graphene Modified TiO2: From Theory to Experiment. ACS Nano 2013, 7, 1504–1512. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Y.; Chen, Y.; Feng, Y.; Ju, C.; Zhang, B.; Liu, H.; Xu, J. Graphdiyne-hybridized N-doped TiO2 nanosheets for enhanced visible light photocatalytic activity. J. Mater. Sci. 2018, 53, 8921–8932. [Google Scholar] [CrossRef]
- Ren, Y.; Dong, Y.; Feng, Y.; Xu, J. Compositing Two-Dimensional Materials with TiO2 for Photocatalysis. Catalysts 2018, 8, 590. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Li, Y.; Yang, H.; Li, M.; Huang, Y.; Zhang, S.; Ji, H. An ultrathin carbon layer activated CeO2 heterojunction nanorods for photocatalytic degradation of organic pollutants. Appl. Catal. B 2019, 259, 118085. [Google Scholar] [CrossRef]
- Liu, C.; Mao, D.; Pan, J.; Qian, J.; Zhang, W.; Chen, F.; Chen, Z.; Song, Y. Fabrication of highly efficient heterostructured Ag-CeO2/g-C3N4 hybrid photocatalyst with enhanced visible-light photocatalytic activity. J. Rare Earths 2019, 37, 1269–1278. [Google Scholar] [CrossRef]
- Liu, L.; Lui, Z.; Liu, A.; Gu, X.; Ge, C.; Gao, F.; Dong, L. Engineering the TiO2-Graphene interface to enhance photocatalytic H2 production. ChemSusChem 2014, 7, 618–626. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.; Tian, J.; Wang, X.; Cui, H. TiO2 nanobelts decorated with In2S3 nanoparticles as photocatalysts with enhanced full-solar-spectrum photocatalytic activity toward degradation of tetracycline. Part. Part. Syst. Charact. 2017, 34, 1700127. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Sun, H.; Ng, Y.H.; Zhang, K.-Q.; Lai, Y. MoS2 quantum dots @TiO2 nanotube arrays: En extended spectrum-driven Photocatalyst for solar hydrogen evolution. ChemSusChem 2018, 11, 1708–1721. [Google Scholar] [CrossRef]
- Gannoruva, A.; Niroshan, K.; Ileperuma, O.A.; Bandara, J. Infrared radiation active, novel compoposte for water splitting. Int. J. Hydrogen Energy 2014, 39, 15411–15415. [Google Scholar] [CrossRef]
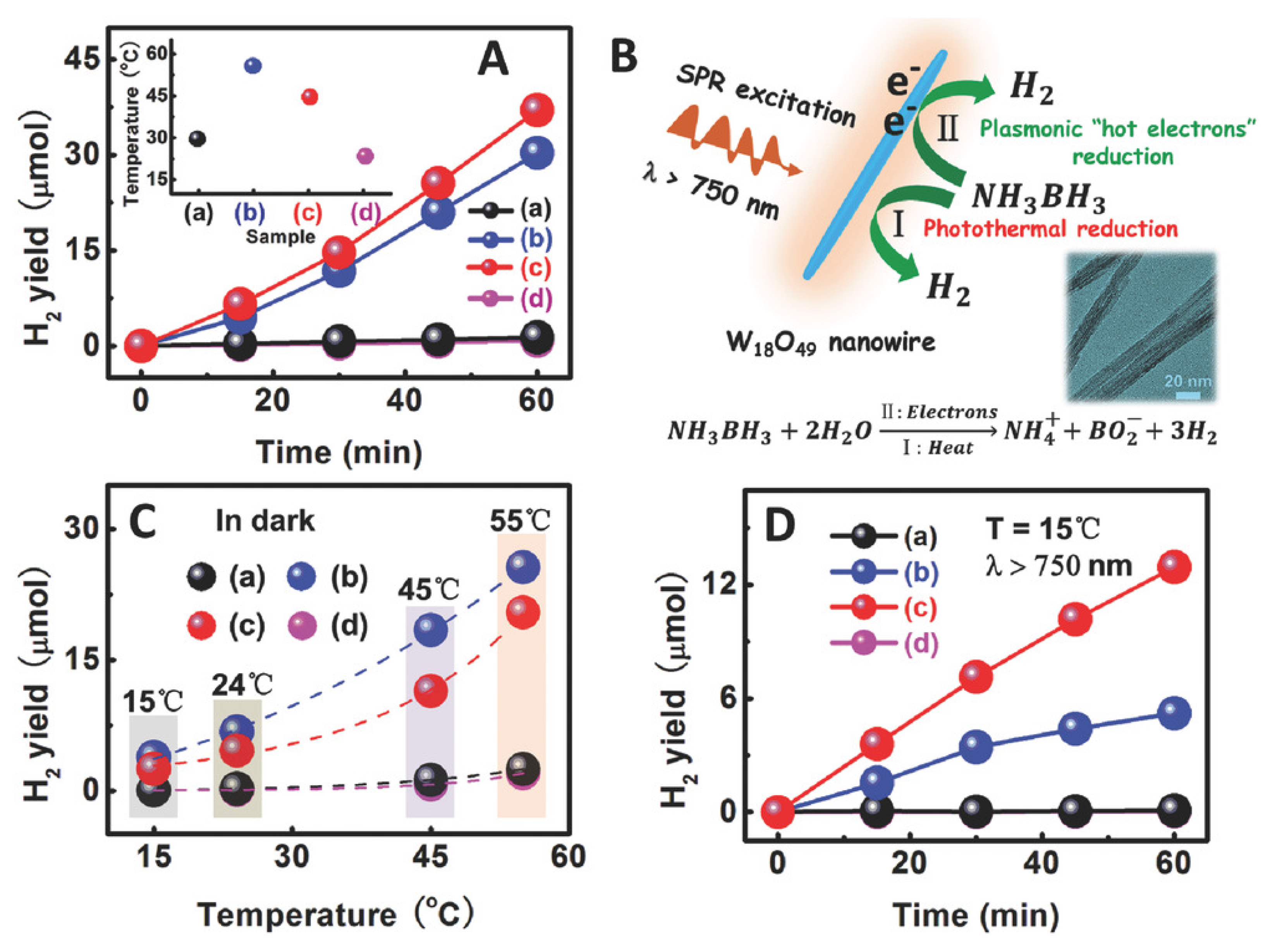
- Yan, M.; Li, G.; Guo, C.; Gou, W.; Ding, D.D.; Zhang, S.; Liu, S. WO3−x sensitized TiO2 sphere with full-spectrum photocatalytic activity from UV to near infrared. Nanoscale 2016, 8, 17828–17835. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, X.; Liu, B.; Guo, L.; Lu, N.; Wang, L.; Huang, J.; Liu, K.; Dong, B. IR-driven untrafast transfer of plasmonic hot electrons in nonmetallic branched heterostructures for enhanced H2 generation. Adv. Mater. 2018, 30, 1705221. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, X.; Matras-Postolek, K.; Huang, B.; Yang, P. Z-scheme reduced graphene oxide/TiO2-bronze/W18O49 ternary heterostructure towards efficient full solar-spectrum. Carbon 2018, 139, 415–426. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Agostini, G.; Marini, C.; Kubacka, A.; Fernández-García, M. Hydrogen thermos-photo production using Ru/TiO2: Heat and light synergistic effects. Appl. Catal. B Environ. 2019, 256, 117790. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, L.; Duan, Y.; Chen, Y. Synergetic effect of thermo-photocatalytic oxidation of benzene on Pt-TiO2/Ce-MnOx. J. Rare Earths 2012, 30, 1106–1111. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, W.; Zhang, L.; Qiu, R.; Sun, S.; Zheng, Y. A strategy for improving deactivation of catalytic combustion at low temperature via synergistic photocatalysis. Appl. Catal. B Environ. 2015, 165, 399–407. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Eslava-Castillo, A.M.; Kubacka, A.; Fernández-García, M. Thermo-photo degradation of 2-propanol using a composite ceria-titania catalyst: Physico-chemical interpretation from a kinetic model. Appl. Catal. B Environ. 2018, 225, 298–306. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Yang, Y.; Schi, Z.; Zhang, Q.; Wu, S.; Zhao, X. Formation of CeMnxOy/OMS-2 nanocomposite significantly enhances UV–vis-infrared light-driven catalytic activity. Catal. Today 2019, 326, 46–53. [Google Scholar] [CrossRef]
- Wang, W.; Ding, M.; Lu, C.; Ni, Y.; Zu, Z. A study on upconversion UV-visi-NIR responsible photocatalytic activity and mechanisms of hexagonal phase NaYF4:Yb3+,Tm3+@TiO2 core-shell structured catalyst. Appl. Catal. B 2014, 144, 379–385. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Mei, Y.; Wang, L.; Dua, G.; Xiong, Y. Synthesis of Rhombic Hierarchical YF3 Nanocrystals and Their Use as Upconversion Photocatalysts after TiO2 Coating. Nanoscale 2013, 5, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, W.; Ni, Y.; Lu, C.; Xu, Z. Different upconversion properties of β-NaYF4:Yb3+, Tm3+/Er3+-TiO2 in affecting the near-infrared-driven photocatalytic activity of high-reactive TiO2. ACS Appl. Mater. Interfaces 2014, 6, 340–348. [Google Scholar] [CrossRef]
- Huang, H.; Li, H.; Wang, Z.; Wang, P.; Zheng, Z.; Liu, Y.; Dai, Y.; Li, Y.; Huang, B. Efficient near-infrared photocatalysts based on NaYF4:Yb+3,Nd+3 @ TiO2 core-shell nanoparticles. Chem. Eng. J. 2019, 361, 1089–1097. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Dong, Q.; Liu, B.; Wang, Y.; Sekino, T.; Lee, S.W.; Sato, T. UV, visible and near-infrared lights induced NOx destruction of (Yb,Er)-NaYF4/C-TiO2 composite. Sci. Rep. 2013, 3, 2918–2925. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yin, S.; Dong, Q.; Sato, T. Blue/green/red colour emitting up-conversion phosphors coupled C-TiO2 with UV, visible and NIR responsive photocatalytic performance. Appl. Catal. B 2014, 156–157, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Tair, M.; Tasleem, S.; Tahir, B. Recent development in band engineering of binary semiconductor materials for solar driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Liu, L.; Duy Dao, T.; Kodiyath, R.; Kang, Q.; Abe, H.; Nagao, T.; Ye, J. Plasmonic Janus-Composite Photocatalyst Comprising Au and C–TiO2 for Enhanced Aerobic Oxidation over a Broad Visible-Light Range. Adv. Funct. Mater. 2014, 24, 7754–7762. [Google Scholar] [CrossRef]
- Puga, A.V.; Forneli, A.; García, H.; Corma, A. Production of H2 by Ethanol Photoreforming on Au/TiO2. Adv. Funct. Mater. 2014, 24, 241–248. [Google Scholar] [CrossRef]
- Xu, Z.; Quintanilla, M.; Vetrone, F.; Govorov, A.O.; Chaker, M.; Ma, D. Harvesting lost photons: Plasmon and upconversion enhanced broadband photocatalytic activity in core@shell microspheres based in Lanthanide-doped NaYF4, TiO2 and Au. Adv. Funct. Mater. 2015, 25, 2950–2960. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Jian, X.; Xu, J.; Gao, Z.; Song, Y.-Y. NIR Light-Driven Photocatalysis on Amphiphilic TiO2 Nanotubes for Controllable Drug Release. ACS Appl. Mater. Interfaces 2020, 12, 23606–23616. [Google Scholar] [CrossRef]
- Zielińska, A.; Kowalska, E.; Sobczak, J.W.; Łącka, I.; Gazda, M.; Ohtani, B.; Hupka, J.; Zaleska, A. Silver-doped TiO2 prepared by microemulsion method: Surface properties, bio- and photoactivity. Sep. Purif. Technol. 2013, 72, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Liang, W.; Guo, J.; Tang, H.; Liu, S. MoS2 quantum dots decorated g-C3N4/Ag heterostructures for enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2018, 430, 234–242. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Han, Z.; Yang, L.; Liu, J. Non-ultraviolet photocatalytic kinetics of NaYF4:Yb,Tm@TiO2/Ag core@comby shell nanostructures. J. Mater. Chem. A 2015, 3, 14642–14650. [Google Scholar] [CrossRef]
- Prakash, M.; Thangaruju, D.; Karthikeyan, R.; Arivanandhan, M.; Shimura, Y.; Hayakawa, Y. UV-visible and near-infrared active NaGdF4:Yb:Er/Ag/TiO2 composite for enhanced photocatalytic applications. RSC Adv. 2016, 6, 80655–80665. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, P.; Liu, M. Graphene Oxide Enwrapped Ag/AgX (X = Br, Cl) Nanocomposite as a Highly Efficient Visible-Light Plasmonic Photocatalyst. ACS Nano 2011, 5, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Lin, S.; Liu, L.; Hu, J.; Cui, W. Oil-in-water self-assembled Ag@AgCl QDs sensitized Bi2WO6: Enhanced photocatalytic degradation under visible light irradiation. Appl. Catal. B Environ. 2015, 164, 192–203. [Google Scholar] [CrossRef]
- Wang, W.; Jing, L.; Qu, Y.; Luan, Y.; Fu, H.; Xiao, Y. Facile fabrication of efficient AgBr-TiO2 nanoheterostructured photocatalyst for degrading pollutants and its photogenerated charge transfer mechanism. J. Hazard. Mater. 2012, 243, 169–178. [Google Scholar] [CrossRef]
- Shah, Z.H.; Ge, Y.; Ye, W.; Lin, X.-J.; Zhang, S.; Lu, R. Visible light activation of SrTiO3 by loading Ag/AgX (X = Cl, Br) for highly efficient plasmon-enhanced photocatalysis. Mater. Chem. Phys. 2017, 198, 73–82. [Google Scholar] [CrossRef]
- Chavadej, S.; Phuaphromyod, P.; Gulari, E.; Rangsunvigit, P.; Sreethawong, T. Photocatalytic degradation of 2-propanol by using Pt/TiO2 prepared by microemulsion technique. Chem. Eng. J. 2008, 137, 489–495. [Google Scholar] [CrossRef]
- Lin, W.-C.; Yang, W.-D.; Huang, I.-L.; Wu, T.-S.; Chung, Z.-J. Hydrogen Production from Methanol/Water Photocatalytic Decomposition Using Pt/TiO2−xNx Catalyst. Energy Fuels 2009, 23, 2192–2196. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Wei, Z.; Janczarek, M.; Wysocka, I.; Kowalska, E. Size-Controlled Synthesis of Pt Particles on TiO2 Surface: Physicochemical Characteristic and Photocatalytic Activity. Catalysts 2019, 9, 940. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, F.; Sordello, F.; Mino, L.; Minero, C.; Hororoaba, V.-D.; Martra, G.; Maurino, V. Formic Acid Photoreforming for Hydrogen Production on Shape-Controlled Anatase TiO2 Nanoparticles: Assessment of the Role of Fluorides, {101}/{001} Surfaces Ratio, and Platinization. ACS Catal. 2019, 9, 6692–6697. [Google Scholar] [CrossRef]
- Długokęcka, M.; Łuczak, J.; Polkowska, Ż.; Zaleska-Medynska, A. The effect of micro-emulsion composition on the morphology of Pd nanoparticles deposited at the surface of TiO2 and photoactivity of Pd-TiO2. Appl. Surf. Sci. 2017, 405, 220–230. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and characterization of monometallic (Au) and bimetallic (Ag/Au) modified-titania photocatalysts activated by visible light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Gołąbiewska A, Lisowski W, Jarek M, Nowaczyk G, Zielińska-Jurek A, Zaleska A: Visible light photoactivity of TiO2 loaded with monometallic (Au or Pt) and bimetallic (Au/Pt) nanoparticles. Appl. Surf. Sci. 2014, 317, 1131–1142. [CrossRef]
- Cybula, A.; Priebe, J.B.; Pohl, M.-M.; Sobczak, J.W.; Schneider, M.; Zielińska-Jurek, A.; Brückner, A.; Zaleska, A. The effect of calcination temperature on structure and photocatalyticproperties of Au/Pd nanoparticles supported on TiO2. Appl. Catal. B Environ. 2014, 152–153, 202–211. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Kong, X.; Jiang, H.; Zhang, F.; Shi, J. Novel Ag-Au bimetallic alloy decorated near-infrared responsive three-dimensionalrod-like architectures for efficient photocatalytic water purification. J. Colloid Interface Sci. 2018, 522, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Caudillo-Flores, U.; Muñoz-Batista, M.J.; Fernández-García, M.; Kubacka, A. Bimetallic Pt-Pd co-catalyst Nb-doped TiO2 materials for H2 photo-production under UV and Visible light illumination. Appl. Catal. B Environ. 2018, 238, 533–545. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Barba-Nieto, I.; Gómez-Cerezo, M.N.; Martínez-Arias, A.; Fernández-García, M.; Kubacka, A. Toward the Green Production of H2: Binary Pt-Ru Promoted Nb-TiO2 Based Photocatalysts. ACS Sustain. Chem. Eng. 2019, 7, 15671–15683. [Google Scholar] [CrossRef]
- Sharma, G.; García-Peñas, A.; Kumar, A.; Naushad, M.; Tessema Mola, G.; Alshehri, S.M.; Ahmedd, J.; Alhokbany, N.; Stadler, F.J. Fe/La/Zn nanocomposite with graphene oxide for photodegradation of phenylhydrazine. J. Mol. Liq. 2019, 285, 362–374. [Google Scholar] [CrossRef]
- Kubacka, A.; Ferrer, M.; Cerrada, M.L.; Serrano, C.; Sánchez-Chaves, M.; Fernández-García, M.; De Andrés, A.; Riobóo, R.J.J.; Fernández-Martín, F. Boosting TiO2-anatase antimicrobial activity: Polymer-oxide thin films. Appl. Catal. B Environ. 2009, 89, 441–447. [Google Scholar] [CrossRef]
- Kubacka, A.; Ferrer, M.; Fernández-García, M.; Serrano, C.; Cerrada, M.L.; Fernández-García, M. Tailoring polymer-TiO2 film properties by presence of metal (Ag, Cu, Zn) spec ies: Optimization of antimicrobial properties. Appl. Catal. B Environ. 2011, 104, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bonilla, A.; Cerrada, M.L.; Fernández-García, M.; Kubacka, A.; Ferrer, M.; Fernández-García, M. Biodegradable polycaprolactone-titania nanocomposites: Preparation, characterization and antimicrobial properties. Int. J. Mol. Sci. 2013, 14, 9249–9266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Bonilla, A.; Kubacka, A.; Fernández-García, M.; Ferrer, M.; Fernández-García, M.; Cerrada, M.L. Visible and ultraviolet antibacterial behavior in PVDF-TiO2 nanocomposite films. Eur. Polym. J. 2015, 71, 412–422. [Google Scholar] [CrossRef]
- Kubacka, A.; Ferrer, M.; Lündsdorf, H.; Fernández-García, M.; Serrano-Selva, C.; Cerrada, M.L.; Fernández-García, M. High-performance, dual-action TiO2-EVOH nanocomposites prepared via by a simple melt method. Nano Lett. 2007, 7, 2529–2534. [Google Scholar] [CrossRef]
- Molinari, R.; Pirillo, F.; Falco, M.; Loddo, V.; Palmisano, L. Photocatalytic degradation of dyes by using a membrane reactor. Chem. Eng. Process. 2004, 43, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Sopajaree, K.; Qasim, S.A.; Basak, S.; Rajeshwar, K. An integrated flow reactor-membrane filtration system for heterogeneous photocatalysis. Part II: Experiments on the ultrafiltration unit and combined operation. J. Appl. Electrochem. 1999, 29, 1111–1118. [Google Scholar] [CrossRef]
- Hairom, N.H.H.; Mohammad, A.W.; Ng, L.Y.; Kadhumb, A.A.H. Utilization of self-synthesized ZnO nanoparticles in MPR for industrial dye wastewater treatment using NF and UF membrane. Desalin. Water Treat. 2015, 54, 944–955. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-J.; Zeng, X.-F.; Wang, J.-X.; Zhang, L.-L.; Chen, J.-F. Synthesis of mono-dispersed ZnO@SiO2 nanoparticles for anti-UV aging application in highly transparent polymer-based nanocomposites. J. Mater. Sci. 2019, 54, 8581–8589. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barba-Nieto, I.; Caudillo-Flores, U.; Fernández-García, M.; Kubacka, A. Sunlight-Operated TiO2-Based Photocatalysts. Molecules 2020, 25, 4008. https://doi.org/10.3390/molecules25174008
Barba-Nieto I, Caudillo-Flores U, Fernández-García M, Kubacka A. Sunlight-Operated TiO2-Based Photocatalysts. Molecules. 2020; 25(17):4008. https://doi.org/10.3390/molecules25174008
Chicago/Turabian StyleBarba-Nieto, Irene, Uriel Caudillo-Flores, Marcos Fernández-García, and Anna Kubacka. 2020. "Sunlight-Operated TiO2-Based Photocatalysts" Molecules 25, no. 17: 4008. https://doi.org/10.3390/molecules25174008