Understanding the Medical Chemistry of the Cannabis Plant is Critical to Guiding Real World Clinical Evidence
Abstract
:1. Introduction
2. Current State of Affairs
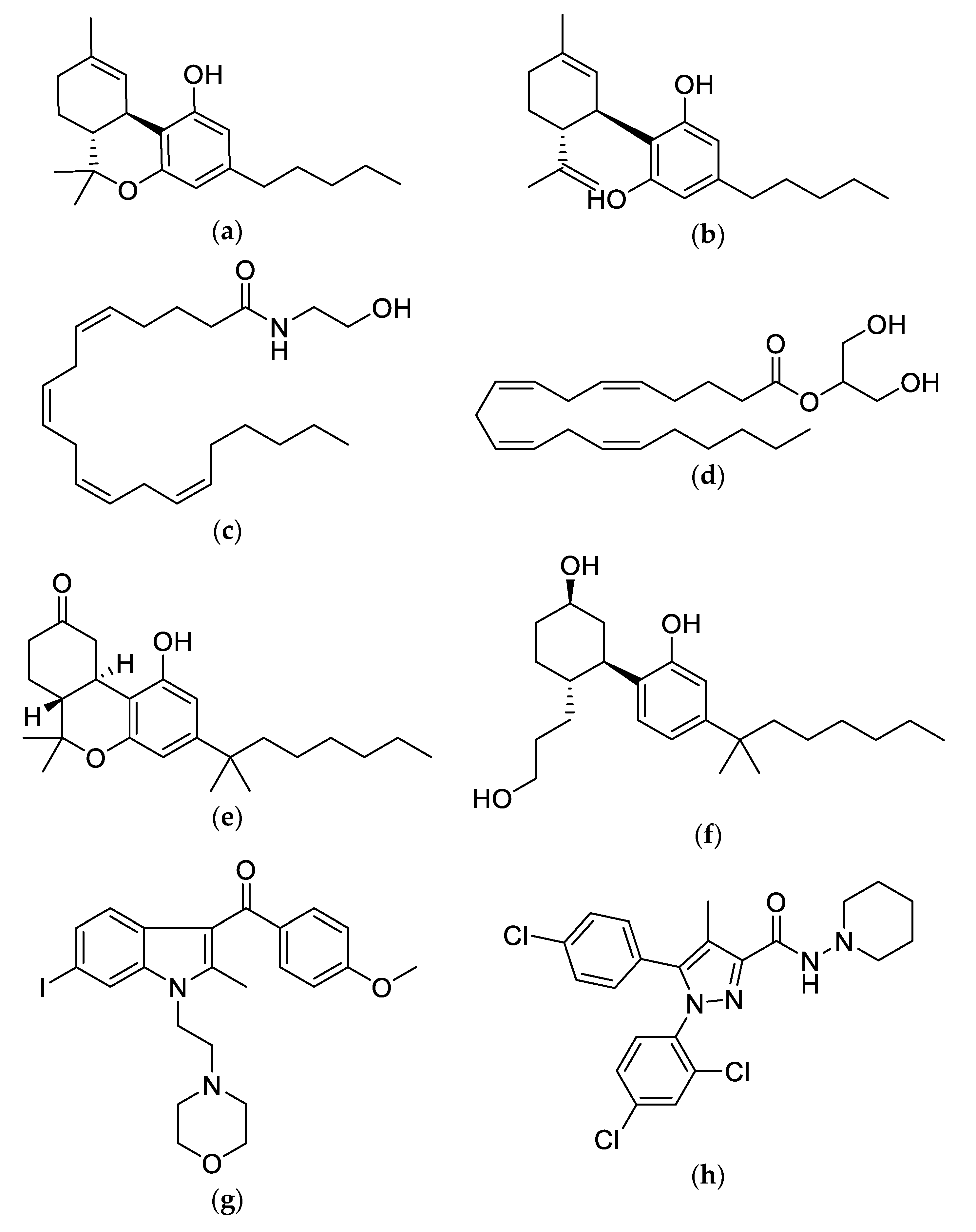
3. The Chemistry of Cannabis
4. More Research is Needed
5. Moving Forward
6. The Present and Next Steps
Author Contributions
Funding
Conflicts of Interest
References
- Mead, A. Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States. Front. Plant Sci. 2019, 10, 697. [Google Scholar] [CrossRef]
- Fischer, B.; Kuganesan, S.; Room, R. Medical Marijuana programs: Implications for cannabis control policy–Observations from Canada. Int. J. Drug Policy 2015, 26, 15–19. [Google Scholar] [CrossRef]
- Allan, G.M.; Finley, C.R.; Ton, J.; Perry, D.; Ramji, J.; Crawford, K.; Lindblad, A.J.; Korownyk, C.; Kolber, M.R. Systematic review of systematic reviews for medical cannabinoids: Pain, nausea and vomiting, spasticity and harms. Can. Fam. Physician 2018, 64, e78–e94. [Google Scholar]
- Deshpande, A.; Mailis-Gagnon, A.; Zoheiry, N.; Lakha, S.F. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: Systematic review of randomized controlled trials. Can. Fam. Physician 2015, 61, e372–e381. [Google Scholar]
- Meng, H.; Dai, T.; Hanlon, J.G.; Downar, J.; Alibhai, S.M.H.; Clarke, H. Cannabis and cannabinoids in cancer pain management. Curr. Opin. Support. Palliat. Care 2020, 14, 87–93. [Google Scholar] [CrossRef]
- Sarris, J.; Sinclair, J.; Karamacoska, D.; Davidson, M.; Firth, J. Medicinal cannabis for psychiatric disorders: A clinically-focused systematic review. BMC Psychiatry 2020, 20, 24. [Google Scholar] [CrossRef] [Green Version]
- Docter, S.; Khan, M.; Gohal, C.; Ravi, B.; Bhandari, M.; Gandhi, R.; Leroux, T. Cannabis Use and Sport: A Systematic Review. Sports Heal 2020, 12, 189–199. [Google Scholar] [CrossRef]
- Montané, E.; Duran, M.; Capellà, D.; Figueras, A. Scientific drug information in newspapers: Sensationalism and low quality. The example of therapeutic use of cannabinoids. Eur. J. Clin. Pharmacol. 2005, 61, 475–477. [Google Scholar] [CrossRef]
- Hajek, T.; Kopecek, M.; Alda, M.; Uher, R.; Höschl, C. Why negative meta-analyses may be false? Eur. Neuropsychopharmcol. 2013, 23, 1307–1309. [Google Scholar] [CrossRef]
- Yanes, J.A.; McKinnell, Z.E.; Reid, M.A.; Busler, J.N.; Michel, J.S.; Pangelinan, M.M.; Sutherland, M.T.; Younger, J.W.; Gonzalez, R.; Robinson, J.L. Effects of cannabinoid administration for pain: A meta-analysis and meta-regression. Exp. Clin. Psychopharmcol. 2019, 27, 370–382. [Google Scholar] [CrossRef]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of Medical Cannabis Cultivars and the Role of Decarboxylation in Optimal Receptor Responses. Cannabis Cannabinoid Res. 2019, 4, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G.; Cascio, M.G. Handbook of Cannabis; Pertwee, R., Ed.; Oxford Scholarship Online: Oxford, UK, 2014; pp. 115–136. [Google Scholar]
- Devane, W.; Hanus, L.; Breuer, A.; Pertwee, R.; Stevenson, L.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Bioph. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Marzo, V.D.; Bifulco, M.; Petrocellis, L.D. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Hillard, C.J. The Endocannabinoid Signaling System in the CNS: A Primer. Int. Rev. Neurobiol. 2015, 125, 1–47. [Google Scholar]
- Vaughn, L.K.; Denning, G.; Stuhr, K.L.; Wit, H.D.; Hill, M.N.; Hillard, C.J. Endocannabinoid signalling: Has it got rhythm?: Endocannabinoid signalling and biological rhythms. Br. J. Pharmacol. 2010, 160, 530–543. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-Y.; Chen, C. Endocannabinoids in Synaptic Plasticity and Neuroprotection. Neuroscientist 2014, 21, 152–168. [Google Scholar] [CrossRef] [Green Version]
- Drumond, A.; Madeira, N.; Fonseca, R. Endocannabinoid signaling and memory dynamics: A synaptic perspective. Neurobiol. Learn. Mem. 2016, 138, 62–77. [Google Scholar] [CrossRef]
- Watkins, B.A. Diet, endocannabinoids and health. Nutr. Res. 2019, 70, 32–39. [Google Scholar] [CrossRef]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharmacol. 2019, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Meccariello, R. Endocannabinoid System in Health and Disease: Current Situation and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 3549. [Google Scholar] [CrossRef]
- Meccariello, R.; Battista, N.; Bradshaw, H.B.; Wang, H. Updates in reproduction coming from the endocannabinoid system. Int. J. Endocrinol. 2014, 2014. [Google Scholar] [CrossRef]
- Fulmer, M.L.; Thewke, D.P. The Endocannabinoid System and Heart Disease: The Role of Cannabinoid Receptor Type 2. Cardiovasc. Hematol. Disord. Targets 2018, 18, 34–51. [Google Scholar]
- Woodhams, S.G.; Sagar, D.R.; Burston, J.J.; Chapman, V. Handbook of Experimental Pharmacology. Handb. Exp. Pharmacol. 2015, 227, 119–143. [Google Scholar]
- Meccariello, R.; Santoro, A.; D′Angelo, S.; Morrone, R.; Fasano, S.; Viggiano, A.; Pierantoni, R. The Epigenetics of the Endocannabinoid System. Int. J. Mol. Sci. 2020, 21, 1113. [Google Scholar] [CrossRef]
- Barchi, M.; Innocenzi, E.; Giannattasio, T.; Dolci, S.; Rossi, P.; Grimaldi, P. Cannabinoid Receptors Signaling in the Development, Epigenetics and Tumours of Male Germ Cells. Int. J. Mol. Sci. 2019, 21, 25. [Google Scholar] [CrossRef]
- D’Addario, C.; Francesco, A.D.; Pucci, M.; Agrò, A.F.; Maccarrone, M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013, 280, 1905–1917. [Google Scholar] [CrossRef]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.-H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anavi-Goffer, S.; Baillie, G.; Irving, A.J.; Gertsch, J.; Greig, I.R.; Pertwee, R.G.; Ross, R.A. Modulation of l-α-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J. Biol. Chem. 2011, 287, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and Pain: A Clinical Review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlett, A.C. Handbook of Experimental Pharmacology. Handb. Exp. Pharmacol. 2005, 168, 53–79. [Google Scholar]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.-P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the Presence and Functional Expression of Cannabinoid CB2 Receptors in Brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- Cabral, G.A.; Griffin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11. [Google Scholar] [CrossRef] [Green Version]
- Rieder, S.A.; Chauhan, A.; Singh, U.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010, 215, 598–605. [Google Scholar] [CrossRef] [Green Version]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottone, E.; Pomatto, V.; Cerri, F.; Campantico, E.; Mackie, K.; Delpero, M.; Guastalla, A.; Dati, C.; Bovolin, P.; Franzoni, M.F. Cannabinoid receptors are widely expressed in goldfish: Molecular cloning of a CB2-like receptor and evaluation of CB1 and CB2 mRNA expression profiles in different organs. Fish Physiol. Biochem. 2013, 39, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [PubMed] [Green Version]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPartland, J.M.; MacDonald, C.; Young, M.; Grant, P.S.; Furkert, D.P.; Glass, M. Affinity and Efficacy Studies of Tetrahydrocannabinolic Acid A at Cannabinoid Receptor Types One and Two. Cannabis Cannabinoid Res. 2017, 2, 87–95. [Google Scholar] [CrossRef]
- Udoh, M.; Santiago, M.; Devenish, S.; McGregor, I.S.; Connor, M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br. J. Pharmacol. 2019, 176, 4537–4547. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; de Medina, V.S.; Rivas-Santisteban, R.; Callado, C.S.-C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB1 and CB2 Receptors and at CB1–CB2 Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Klauke, A.-L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Mudge, E.M.; Murch, S.J.; Brown, P.N. Chemometric Analysis of Cannabinoids: Chemotaxonomy and Domestication Syndrome. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Mammen, G.; de Freitas, L.; Rehm, J.; Rueda, S. Cannabinoid concentrations in Canada’s regulated medical cannabis industry. Addict. (Abingdon Engl.) 2017, 112, 730–732. [Google Scholar] [CrossRef]
- Yang, Y.; Vyawahare, R.; Lewis-Bakker, M.; Clarke, H.A.; Wong, A.H.C.; Kotra, L.P. Bioactive Chemical Composition of Cannabis Extracts and Cannabinoid Receptors. Molecules 2020, 25, 3466. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, M. Medicinal cannabis: A primer for nurses. Nursing 2017, 47, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.M.; Ramji, J.; Perry, D.; Ton, J.; Beahm, N.P.; Crisp, N.; Dockrill, B.; Dubin, R.E.; Findlay, T.; Kirkwood, J.; et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can. Fam. Physician 2018, 64, 111–120. [Google Scholar] [PubMed]
- Watanabe, K.; Yamaori, S.; Funahashi, T.; Kimura, T.; Yamamoto, I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007, 80, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Barrie, N.; Manolios, N. The endocannabinoid system in pain and inflammation: Its relevance to rheumatic disease. Eur. J. Rheumatol. 2017, 4, 210–218. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Jacobs, D.S.; Kohut, S.J.; Jiang, S.; Nikas, S.P.; Makriyannis, A.; Bergman, J. Acute and chronic effects of cannabidiol on Δ9-tetrahydrocannabinol (Δ9-THC)-induced disruption in stop signal task performance. Exp. Clin. Psychopharmacol. 2016, 24, 320–330. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor: Negative allosteric modulation of CB1 by cannabidiol. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Gonçalves, E.C.D.; Baldasso, G.M.; Bicca, M.A.; Paes, R.S.; Capasso, R.; Dutra, R.C. Terpenoids, Cannabimimetic Ligands, beyond the Cannabis Plant. Molecules 2020, 25, 1567. [Google Scholar] [CrossRef] [Green Version]
- Fisar, Z. Phytocannabinoids and Endocannabinoids. Curr. Drug Abuse Rev. 2009, 2, 51–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherma, M.; Masia, P.; Satta, V.; Fratta, W.; Fadda, P.; Tanda, G. Brain activity of anandamide: A rewarding bliss? Acta Pharmacol. Sin. 2018, 40, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Spaderna, M.; Addy, P.H.; D′Souza, D.C. Spicing things up: Synthetic cannabinoids. Psychopharmacology 2013, 228, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Assessment of Dronabinol and Its Stereo-Isomers. Available online: https://www.who.int/medicines/areas/quality_safety/4.2DronabinolCritReview.pdf (accessed on 11 August 2020).
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, R.S.; Gustafson, R.A.; Barnes, A.; Nebro, W.; Moolchan, E.T.; Huestis, M.A. Δ9-Tetrahydrocannabinol, 11-Hydroxy-Δ9-Tetrahydrocannabinol and 11-Nor-9-Carboxy-Δ9-Tetrahydrocannabinol in Human Plasma after Controlled Oral Administration of Cannabinoids. Ther. Drug Monit. 2006, 28, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A.; Schulz, K.F. Bias and causal associations in observational research. Lancet 2002, 359, 248–252. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladha, K.S.; Ajrawat, P.; Yang, Y.; Clarke, H. Understanding the Medical Chemistry of the Cannabis Plant is Critical to Guiding Real World Clinical Evidence. Molecules 2020, 25, 4042. https://doi.org/10.3390/molecules25184042
Ladha KS, Ajrawat P, Yang Y, Clarke H. Understanding the Medical Chemistry of the Cannabis Plant is Critical to Guiding Real World Clinical Evidence. Molecules. 2020; 25(18):4042. https://doi.org/10.3390/molecules25184042
Chicago/Turabian StyleLadha, Karim S., Prabjit Ajrawat, Yi Yang, and Hance Clarke. 2020. "Understanding the Medical Chemistry of the Cannabis Plant is Critical to Guiding Real World Clinical Evidence" Molecules 25, no. 18: 4042. https://doi.org/10.3390/molecules25184042
APA StyleLadha, K. S., Ajrawat, P., Yang, Y., & Clarke, H. (2020). Understanding the Medical Chemistry of the Cannabis Plant is Critical to Guiding Real World Clinical Evidence. Molecules, 25(18), 4042. https://doi.org/10.3390/molecules25184042





