Synthesis, Characterization, Catalytic Activity, and DFT Calculations of Zn(II) Hydrazone Complexes
Abstract
1. Introduction
2. Results and Discussion
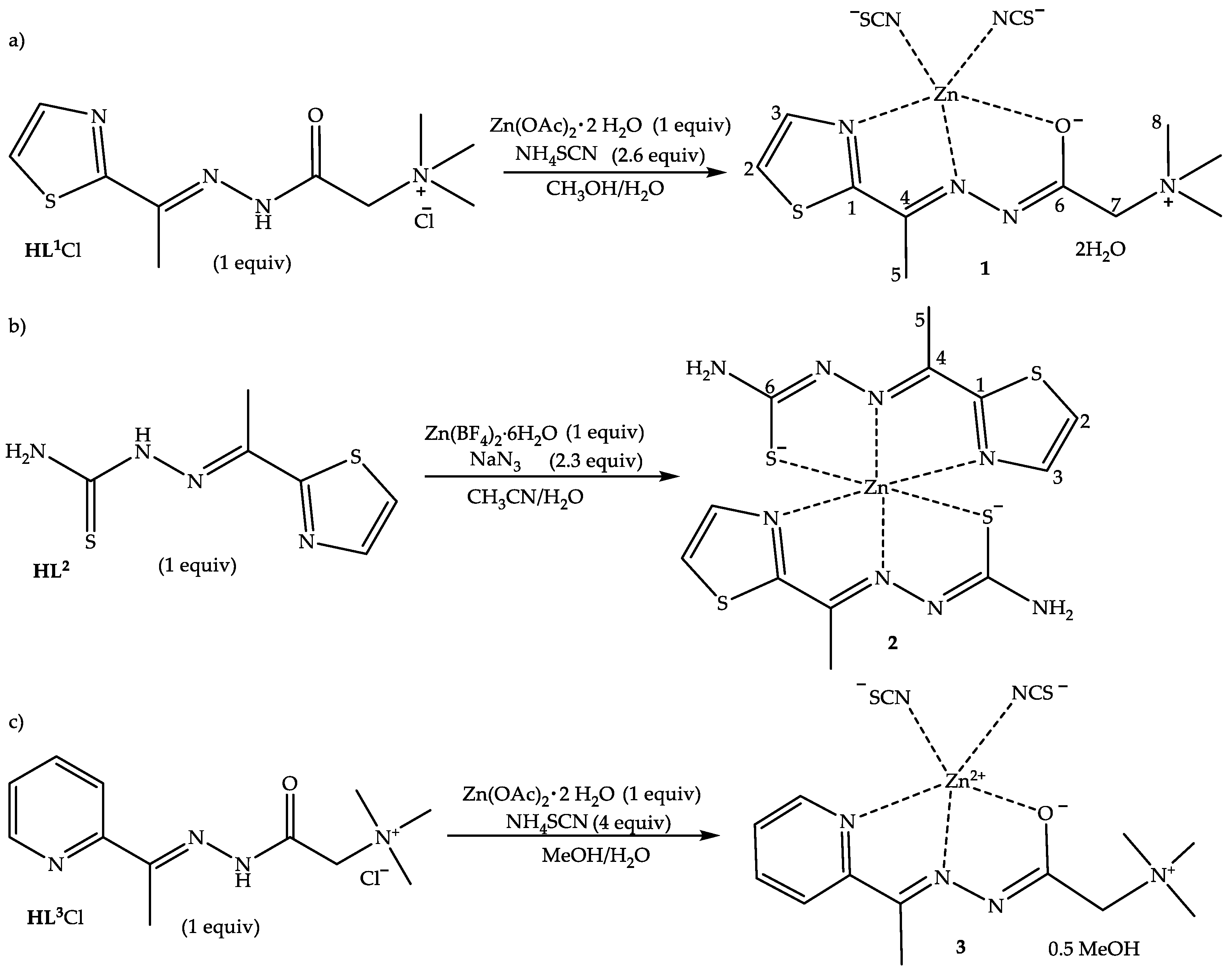
2.1. Synthesis
2.2. Crystal Structures of [ZnL1(NCS)2]⋅2H2O (1) and [Zn(L2)2] (2) Complexes
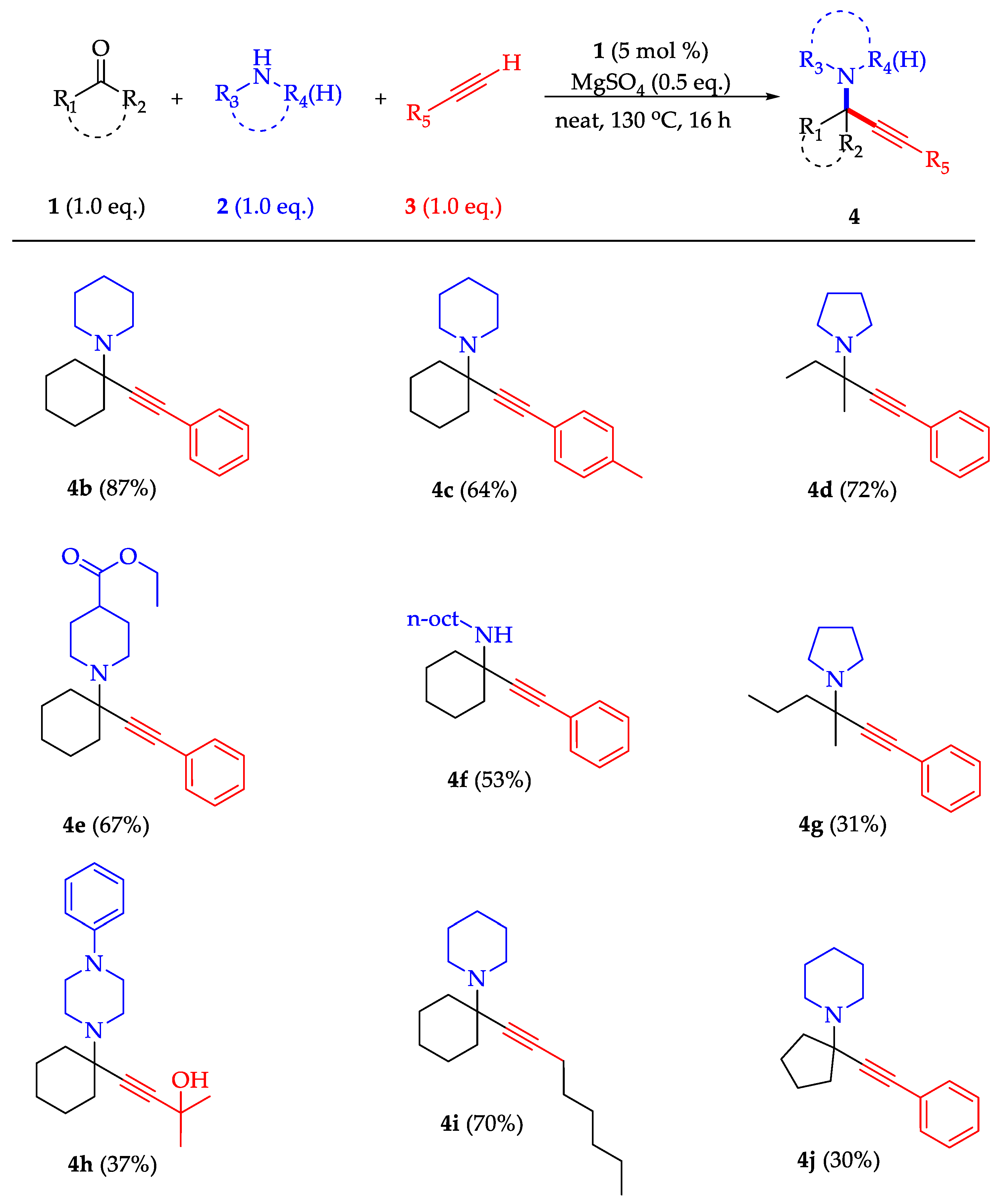
2.3. Evaluation of the Zinc Complexes’ Catalytic Activity in the KA2 Coupling Reaction
2.4. Density Functional Theory (DFT) Optimized Structures and Highest Occupied Molecular Orbital-Lowest Unoccupied Molecular Orbital (HOMO-LUMO) Analysis
3. Materials and Methods
3.1. Synthesis of (E)-N,N,N-trimethyl-2-oxo-2-(2-(1-(thiazol-2-yl)ethylidene)hydrazinyl)ethan-1-aminium Chloride (HL1Cl)
3.2. Synthesis of [ZnL1(NCS)2]⋅2H2O (1)
3.3. Synthesis of Ligand HL2 (E)-2-(1-(thiazol-2-yl)ethylidene)hydrazine-1-carbothioamide
3.4. Synthesis of [Zn(L2)2] (2)
3.5. Synthesis of Ligand HL3Cl (E)-N,N,N-trimethyl-2-oxo-2-(2-(1-(pyridin-2-yl)ethylidene)hydrazinyl) ethan-1-aminium Chloride
3.6. Synthesis of [ZnL3(NCS)2]⋅0.5CH3OH Complex (3)
3.7. X-Ray Crystallography
3.8. Catalysis General Procedure
Characterization Data for the Synthesized Propargylamines
3.9. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afkhami, F.A.; Khandar, A.A.; Mahmoudi, G.; Maniukiewicz, W.; Lipkowski, J.; White, J.M.; Waterman, R.; García-Granda, S.; Zangrando, E.; Bauzái, A.; et al. Synthesis, X-ray characterization, DFT calculations and Hirshfeld surface analysis of Zn(II) and Cd(II) complexes based on isonicotinoylhydrazone ligand. Cryst. Eng. Comm. 2016, 18, 4587–4596. [Google Scholar] [CrossRef]
- Abedi, M.; Yeșilel, O.Z.; Mahmoudi, G.; Bauzá, A.; Lofland, S.E.; Yerli, Y.; Kaminsky, W.; Garczarek, P.; Zaręba, J.K.; Ienco, A.; et al. Tetranuclear manganese(II) complexes of hydrazone and carbohydrazone ligands: Synthesis, crystal structures, magnetic properties, Hirshfeld surface analysis and DFT calculations. Inorg. Chim. Acta 2016, 443, 101–109. [Google Scholar] [CrossRef]
- Romanović, M.Č.; Čobeljić, B.R.; Pevec, A.; Turel, I.; Spasojević, V.; Tsaturyan, A.A.; Shcherbakov, I.N.; Anđelković, K.K.; Milenković, M.; Radanović, D.; et al. Synthesis, crystal structure, magnetic properties and DFT study of dinuclear Ni(II) complex with the condensation product of 2-quinolinecarboxaldehyde and Girard’s T reagent. Polyhedron 2017, 128, 30–37. [Google Scholar] [CrossRef]
- Romanović, M.Č.; Milenković, M.R.; Pevec, A.; Turel, I.; Spasojević, V.; Grubišić, S.; Radanović, D.; Anđelković, K.; Čobeljić, B. Crystal structures, magnetic properties and DFT study of cobalt(II) azido complexes with the condensation product of 2-quinolinecarboxaldehyde and Girard’s T reagent. Polyhedron 2018, 139, 142–147. [Google Scholar] [CrossRef]
- Pouralimardan, O.; Chamayou, A.-C.; Janiak, C.; Hosseini-Monfared, H. Hydrazone Schiff base-manganese(II) complexes: Synthesis, crystal structure and catalytic reactivity. Inorg. Chim. Acta 2007, 360, 1599–1608. [Google Scholar] [CrossRef]
- Milenković, M.R.; Papastavrou, A.T.; Radanović, D.; Pevec, A.; Jagličić, Z.; Zlatar, M.; Gruden, M.; Vougioukalakis, G.C.; Turel, I.; Anđelković, K.; et al. Highly-efficient N-arylation of imidazole catalyzed by Cu(II) complexes with quaternary ammonium-functionalized 2-acetylpyridine acylhydrazone. Polyhedron 2019, 165, 22–30. [Google Scholar] [CrossRef]
- Darmanović, D.; Shcherbakov, I.N.; Duboc, C.; Spasojević, V.; Hanžel, D.; Anđelković, K.; Radanović, D.; Turel, I.; Milenković, M.; Gruden, M.; et al. Combined experimental and theoretical investigation of the origin of magnetic anisotropy in pentagonal bipyramidal isothiocyanato Co(II), Ni(II), and Fe(III) complexes with quaternary-ammonium-functionalized 2,6-diacetylpyridine bisacylhydrazone. J. Phys. Chem. C 2019, 123, 31142–31155. [Google Scholar] [CrossRef]
- Čobeljić, B.; Pevec, A.; Stepanović, S.; Milenković, M.R.; Turel, I.; Gruden, M.; Radanović, D.; Anđelković, K. Structural diversity of isothiocyanato Cd(II) and Zn(II) Girard’s T hydrazone complexes in solution and solid state: Effect of H-bonding on coordination number and supramolecular assembly of Cd(II) complex in solid state. Struct. Chem. 2018, 29, 1797–1806. [Google Scholar] [CrossRef]
- Romanović, M.Č.; Čobeljić, B.; Pevec, A.; Turel, I.; Anđelković, K.; Milenković, M.; Radanović, D.; Belošević, S.; Milenković, M.R. Synthesis, crystal structures and antimicrobial activity of azido and isocyanato Zn(II)complexes with the condensation product of 2-quinolinecarboxaldehyde and Girard’s T reagent. J. Coord. Chem. 2017, 70, 2425–2435. [Google Scholar] [CrossRef]
- Anđelković, K.; Pevec, A.; Grubišić, S.; Turel, I.; Čobeljić, B.; Milenković, M.R.; Keškić, T.; Radanović, D. Crystal structures and DFT calculations of mixed chloride-azide zinc(II)and chloride-isocyanate cadmium(II) complexes with thecondensation product of 2-quinolinecarboxaldehyde and Girard’s Treagent. J. Mol. Struct. 2018, 1162, 63–70. [Google Scholar] [CrossRef]
- Keškić, T.; Čobeljić, B.; Gruden, M.; Anđelković, K.; Pevec, A.; Turel, I.; Radanović, D.; Zlatar, M. What is the nature of interactions of BF4−, NO3−, and ClO4− to Cu(II) complexes with Girard’s T hydrazine? When can binuclear complexes be formed? Cryst. Growth Des. 2019, 19, 4810–4821. [Google Scholar] [CrossRef]
- Čobeljić, B.; Turel, I.; Pevec, A.; Jagličić, Z.; Radanović, D.; Anđelković, K.; Milenković, M.R. Synthesis, structures and magnetic properties of octahedral Co(III) complexes of heteroaromatic hydrazones with tetraisothiocyanato Co(II) anions. Polyhedron 2018, 155, 425–432. [Google Scholar] [CrossRef]
- Venkatraman, R.; Fronczek, F.R. CCDC 1058780: Experimental Crystal Structure Determination. 2015, Cambridge Crystallographic Data Centre. Available online: https://doi.org/10.5517/CC14JR6M (accessed on 25 May 2020).
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 2017, 117, 14091–14200. [Google Scholar] [CrossRef] [PubMed]
- Peshkov, V.A.; Pereshivko, O.P.; Van der Eycken, E.V. A walk around the A3-coupling. Chem. Soc. Rev. 2012, 41, 3790–3807. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W.; Irwin, I.; Langston, E.B.; Forno, L.S. Pargyline prevents MPTP-induced parkinsonism in primates. Science 1984, 225, 1480–1482. [Google Scholar] [CrossRef]
- Chen, J.J.; Swope, D.M. Clinical pharmacology of rasagiline: A novel, second-generation propargylamine for the treatment of Parkinson disease. J. Clin. Pharmacol. 2005, 45, 878–894. [Google Scholar] [CrossRef]
- Aliaga, M.J.; Ramón, D.J.; Yus, M. Impregnated copper on magnetite: An efficient and green catalyst for the multicomponent preparation of propargylamines under solvent free conditions. Org. Biomol. Chem. 2010, 8, 43–46. [Google Scholar] [CrossRef]
- Pereshivko, O.P.; Peshkov, V.A.; Van der Eycken, E.V. Unprecedented Cu(I)-catalyzed microwave-assisted three-component coupling of a ketone, an alkyne, and a primary amine. Org. Lett. 2010, 12, 2638–2641. [Google Scholar] [CrossRef]
- Vachhani, D.D.; Sharma, A.; Van der Eycken, E.V. Copper-catalyzed direct secondary and tertiary C-H alkylation of azoles through a heteroarene-amine-aldehyde/ketone coupling reaction. Angew. Chem. Int. Ed. 2013, 52, 2547–2550. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, Q.; Hu, X.; Li, B.; Ji, J.; Chan, A. Gold-catalyzed direct intermolecular coupling of ketones, secondary amines, and alkynes: A facile and versatile access to propargylic amines containing a quaternary carbon center. Adv. Synth. Catal. 2011, 353, 1274–1278. [Google Scholar] [CrossRef]
- Suzuki, Y.; Naoe, S.; Oishi, S.; Fujii, N.; Ohno, H. Gold-catalyzed three-component annulation: Efficient synthesis of highly functionalized dihydropyrazoles from alkynes, hydrazines, and aldehydes or ketones. Org. Lett. 2011, 14, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Meyet, C.E.; Pierce, C.J.; Larsen, C.H. A single Cu(II) catalyst for the three-component coupling of diverse nitrogen sources with aldehydes and alkynes. Org. Lett. 2012, 14, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.J.; Larsen, C.H. Copper(II) catalysis provides cyclohexanone-derived propargylamines free of solvent or excess starting materials: Sole by-product is water. Green Chem. 2012, 14, 2672–2676. [Google Scholar] [CrossRef]
- Palchak, Z.L.; Lussier, D.J.; Pierce, C.J.; Larsen, C.H. Synthesis of tetrasubstituted propargylamines from cyclohexanone by solvent-free copper(II) catalysis. Green Chem. 2015, 17, 1802–1810. [Google Scholar] [CrossRef]
- Pierce, C.J.; Nguyen, M.; Larsen, C.H. Copper/titanium catalysis forms fully substituted carbon centers from the direct coupling of acyclic ketones, amines, and alkynes. Angew. Chem. Int. Ed. 2012, 51, 12289–12292. [Google Scholar] [CrossRef]
- Tang, X.; Kuang, J.; Ma, S. CuBr for KA2 reaction: En route to propargylic amines bearing a quaternary carbon center. Chem. Commun. 2013, 49, 8976–8978. [Google Scholar] [CrossRef]
- Cai, Y.; Tang, X.; Ma, S. Identifying a highly active copper catalyst for KA2 reaction of aromatic ketones. Chem. Eur. J. 2016, 22, 2266–2269. [Google Scholar] [CrossRef]
- Vougioukalakis, G.C.; Grubbs, R.H. Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts. Chem. Rev. 2010, 110, 1746–1787. [Google Scholar] [CrossRef]
- Pinaka, A.; Vougioukalakis, G.C. Using sustainable metals to carry out “green” transformations: Fe- and Cu-catalyzed CO2 monetization. Coord. Chem. Rev. 2015, 288, 69–97. [Google Scholar] [CrossRef]
- Tzouras, N.V.; Stamatopoulos, I.K.; Papastavrou, A.T.; Liori, A.A.; Vougioukalakis, G.C. Sustainable metal catalysis in C-H activation. Coord. Chem. Rev. 2017, 343, 25–138. [Google Scholar] [CrossRef]
- Liori, A.; Stamatopoulos, I.K.; Papastavrou, A.T.; Pinaka, A.; Vougioukalakis, G.C. A novel, sustainable, user-friendly protocol for the Pd-free Sonogashira coupling reaction. Eur. J. Org. Chem. 2018, 2018, 6134–6139. [Google Scholar] [CrossRef]
- Papastavrou, A.T.; Pauze, M.; Gomez-Bengoa, E.; Vougioukalakis, G.C. Unprecedented multicomponent organocatalytic synthesis of propargylic esters via CO2 activation. Chem. Cat. Chem. 2019, 11, 5379–5386. [Google Scholar]
- Tzouras, N.V.; Neofotistos, S.P.; Vougioukalakis, G.C. Zn-catalyzed multicomponent KA2 coupling: One-pot assembly of propargylamines bearing tetrasubstituted carbon centers. ACS Omega 2019, 4, 10279–10292. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Moroz, Y.S.; Sliva, T.Y.; Kulon, K.; Kozłowski, H.; Fritsky, I.O. Di-chlorido{2-hy-droxy-imino-N′-[1-(2-pyrid-yl)ethylidene]propanohydrazide-κ3N,N′,O}zinc(II) hemihydrate. Acta Cryst. 2008, E64, m353–m354. [Google Scholar]
- Chaur, M.N. Di-chlorido{(E)-4-di-methyl-amino-N′-[(pyri-din-2-yl)methyl--idene-κN]benzo-hydrazide-κO}zinc. Acta Cryst. 2013, E69, m27. [Google Scholar]
- Reena, T.A.; Seena, E.B.; Prathapachandra Kurup, M.R. Zinc(II) complexes derived from di-2-pyridyl ketone N4-phenyl-3-semicarbazone: Crystal structures and spectral studies. Polyhedron 2008, 27, 3461–3466. [Google Scholar] [CrossRef]
- Despaigne, A.A.R.; Da Silva, J.G.; Do Carmo, A.C.M.; Sives, F.; Piro, O.E.; Castellano, E.E.; Beraldo, H. Copper(II) and zinc(II) complexes with 2-formylpyridine-derived hydrazones. Polyhedron 2009, 28, 3797–3803. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.Z.; Liu, E.; Yang, C.; Golen, J.A.; Rheingold, A.L.; Zhang, G. Synthesis and structural characterization of zinc(II) and cobalt(II) complexes based on multidentatehydrazone ligands. J. Mol. Struct. 2016, 1110, 180–184. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Neofotistos, S.P.; Tzouras, N.V.; Pauze, M.; Gomez-Bengoa, E.; Vougioukalakis, G.C. Manganese-Catalyzed Multicomponent Synthesis of Tetrasubstituted Propargylamines: System Development and Theoretical Study. Adv. Synth. Catal. 2020, 362. [Google Scholar] [CrossRef]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino)methyl]phenol. Spectrochim. Acta A 2011, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Rauk, A. Orbital Interaction Theory of Organic Chemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; p. 34. [Google Scholar]
- Tabares-Mendoza, C.; Guadarrama, P. Predicting the catalytic efficiency by quantum-chemical descriptors: Theoretical study of pincer metallic complexes involved in the catalytic Heck reaction. J. Organomet. Chem. 2006, 691, 2978–2986. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Govindarajan, M.; Karabacak, M.; Periandy, S.; Tanuja, D. Spectroscopic (FT-IR, FT-Raman, UV and NMR) investigation and NLO, HOMO–LUMO, NBO analysis of organic 2,4,5-trichloroaniline. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.G.; Nichols, J.A.; Dixon, D.A. Ionization Potential, Electron Affinity, Electronegativity, Hardness, and Electron Excitation Energy: Molecular Properties from Density Functional Theory Orbital Energies. J. Phys. Chem. A 2003, 107, 4184–4195. [Google Scholar] [CrossRef]
- Fukui, K. Role of Frontier Orbitals in Chemical Reactions. Science 1982, 218, 747. [Google Scholar] [CrossRef]
- Otwinowsky, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar]
- CrysAlis PRO. Oxford Diffraction; Oxford Diffraction Ltd.: Yarnton, UK, 2009. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision D.01); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Hehre, J.; Ditchfield, R.; Pople, J.A. Self–Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian–Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Caricato, M.; Mennucci, B.; Tomasi, J.; Ingrosso, F.; Cammi, R.; Corni, S.; Scalmani, G. Formation and relaxation of excited states in solution: A new time dependent polarizable continuum model based on time dependent density functional theory. J. Chem. Phys. 2006, 124, 124520–124532. [Google Scholar] [CrossRef]
- Institute of Physics Belgrade. PARADOX Cluster User Guide v2.1: PARADOX IV Cluster; Institute of Physics Belgrade: Belgrade, Serbia, 2018. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
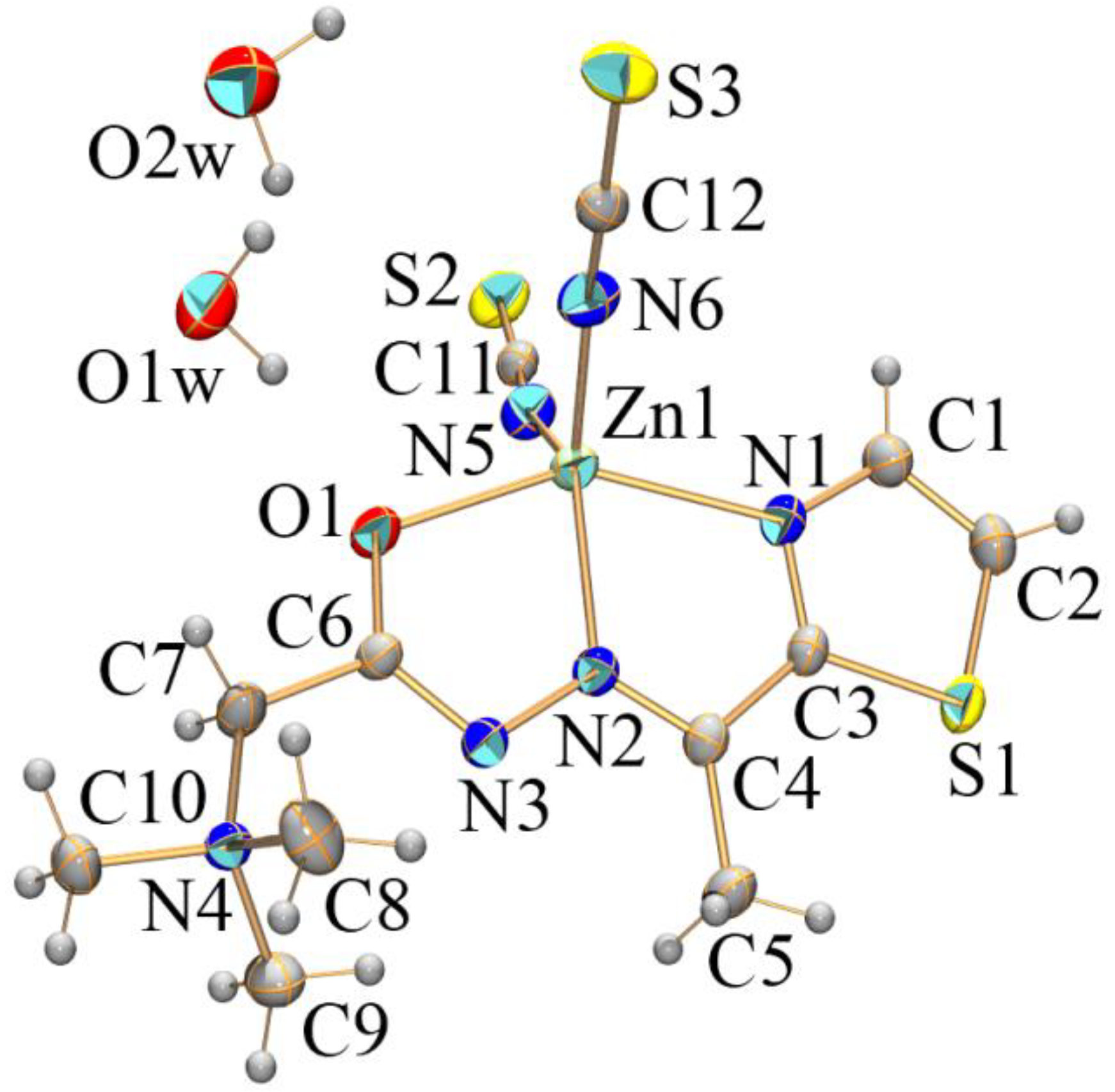



| 1 | 2 | ||
|---|---|---|---|
| Zn1–N6 | 1.955(3) | Zn1–N6 | 2.149(2) |
| Zn1–N5 | 1.959(2) | Zn1–N5 | 2.1869(19) |
| Zn1–N2 | 2.058(2) | Zn1–N2 | 2.147(2) |
| Zn1–O1 | 2.1778(19) | Zn1–N1 | 2.318(2) |
| Zn1–N1 | 2.212(2) | Zn1–S2 | 2.4516(7) |
| O1–C6 | 1.265(3) | Zn1–S4 | 2.4109(7) |
| S2–C11 | 1.626(3) | S2–C6 | 1.716(3) |
| S3–C12 | 1.624(3) | S4–C12 | 1.712(3) |
| N2–C4 | 1.285(3) | N2–C4 | 1.290(3) |
| N2–N3 | 1.389(3) | N2–N3 | 1.370(3) |
| N3–C6 | 1.317(3) | N6–N7 | 1.364(3) |
| N5–C11 | 1.146(3) | N3–C6 | 1.327(3) |
| N6–C12 | 1.149(4) | N4–C6 | 1.360(3) |
| N6–C10 | 1.301(3) | ||
| N7–C12 | 1.332(3) | ||
| N8–C12 | 1.360(3) | ||
| N6–Zn1–N5 | 110.73(11) | N2–Zn1–N6 | 166.84(8) |
| N6–Zn1–N2 | 121.74(10) | N2–Zn1–N5 | 103.08(7) |
| N5–Zn1–N2 | 127.42(10) | N6–Zn1–N5 | 75.28(7) |
| N6–Zn1–O1 | 96.93(10) | N2–Zn1–N1 | 73.63(8) |
| N5–Zn1–O1 | 97.85(9) | N6–Zn1–N1 | 93.21(8) |
| N2–Zn1–O1 | 74.02(8) | N5–Zn1–N1 | 84.54(8) |
| N6–Zn1–N1 | 101.94(10) | N2–Zn1–S4 | 101.19(5) |
| N5–Zn1–N1 | 97.92(9) | N6–Zn1–S4 | 79.42(5) |
| N2–Zn1–N1 | 75.37(8) | N5–Zn1–S4 | 154.65(6) |
| O1–Zn1–N1 | 149.20(7) | N1–Zn1–S4 | 95.42(6) |
| C6–O1–Zn1 | 109.97(16) | N2–Zn1–S2 | 79.08(6) |
| C11–N5–Zn1 | 168.5(3) | N6–Zn1–S2 | 113.88(6) |
| C12–N6–Zn1 | 171.7(2) | N5–Zn1–S2 | 91.66(6) |
| N5–C11–S2 | 179.0(3) | N1–Zn1–S2 | 150.76(6) |
| N6–C12–S3 | 179.2(3) | S4–Zn1–S2 | 100.03(3) |
| C6–S2–Zn1 | 95.20(9) | ||
| C12–S4–Zn1 | 95.96(8) |
| Entry | Catalyst | Mol% | Temp.(°C) | Solvent | Additive (0.5 eq.) | % Isolated Yield 1 |
|---|---|---|---|---|---|---|
| 1 | 1 | 10 | 120 | toluene (1 M) | - | 85 |
| 2 | HL1Cl | 10 | 120 | toluene (1 M) | - | 0 |
| 3 | 3 | 10 | 120 | toluene (1 M) | - | 67 |
| 4 | HL3Cl | 10 | 120 | toluene (1 M) | - | 0 |
| 5 | 2 | 10 | 120 | toluene (1 M) | - | 56 |
| 6 | 1 | 5 | 110 | neat | - | 51 |
| 7 | 1 | 10 | 110 | neat | - | 65 |
| 8 | 3 | 10 | 110 | neat | - | 57 |
| 9 | 1 | 5 | 130 | neat | MgSO4 | 91 |
| 10 | 3 | 5 | 130 | neat | MgSO4 | 61 |
| 11 2 | 1 | 5 | 130 | neat | MgSO4 | 33 |
| [ZnL1(NCS)2] (1) | [Zn(L2)2] (2) | [ZnL3(NCS)2] (3) | |
|---|---|---|---|
| EHOMO (eV) | −4.970 (−5.692) | −5.054 (−5.268) | −4.903 (−5.645) |
| ELUMO (eV) | −2.803 (−2.695) | −2.141 (−2.252) | −2.438 (−2.330) |
| ΔEgap(eV) | 2.167 (2.977) | 2.913 (3.016) | 2.465 (3.315) |
| μ (eV) | −3.886 (−4.193) | −3.597 (−3.76) | −3.670 (−3.987) |
| η (eV) | 1.083 (1.488) | 1.456 (1.508) | 1.232 (1.657) |
| S (eV) | 0.461 (0.336) | 0.343 (0.331) | 0.405 (0.301) |
| Χ (eV) | 3.886 (4.193) | 3.597 (3.760) | 3.670 (3.987) |
| ω (eV) | 6.971 (5.908) | 4.443 (4.687) | 5.466 (4.797) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adejumo, T.T.; Tzouras, N.V.; Zorba, L.P.; Radanović, D.; Pevec, A.; Grubišić, S.; Mitić, D.; Anđelković, K.K.; Vougioukalakis, G.C.; Čobeljić, B.; et al. Synthesis, Characterization, Catalytic Activity, and DFT Calculations of Zn(II) Hydrazone Complexes. Molecules 2020, 25, 4043. https://doi.org/10.3390/molecules25184043
Adejumo TT, Tzouras NV, Zorba LP, Radanović D, Pevec A, Grubišić S, Mitić D, Anđelković KK, Vougioukalakis GC, Čobeljić B, et al. Synthesis, Characterization, Catalytic Activity, and DFT Calculations of Zn(II) Hydrazone Complexes. Molecules. 2020; 25(18):4043. https://doi.org/10.3390/molecules25184043
Chicago/Turabian StyleAdejumo, Temiloluwa T., Nikolaos V. Tzouras, Leandros P. Zorba, Dušanka Radanović, Andrej Pevec, Sonja Grubišić, Dragana Mitić, Katarina K. Anđelković, Georgios C. Vougioukalakis, Božidar Čobeljić, and et al. 2020. "Synthesis, Characterization, Catalytic Activity, and DFT Calculations of Zn(II) Hydrazone Complexes" Molecules 25, no. 18: 4043. https://doi.org/10.3390/molecules25184043
APA StyleAdejumo, T. T., Tzouras, N. V., Zorba, L. P., Radanović, D., Pevec, A., Grubišić, S., Mitić, D., Anđelković, K. K., Vougioukalakis, G. C., Čobeljić, B., & Turel, I. (2020). Synthesis, Characterization, Catalytic Activity, and DFT Calculations of Zn(II) Hydrazone Complexes. Molecules, 25(18), 4043. https://doi.org/10.3390/molecules25184043








