Anti-Inflammatory Effects of a Cordyceps sinensis Mycelium Culture Extract (Cs-4) on Rodent Models of Allergic Rhinitis and Asthma
Abstract
1. Introduction
2. Results
2.1. Marker Compound Contents in Cs-4
2.2. Cs-4 Treatment Suppressed the OVA Challenge Produced Nasal Symptoms in OVA-Sensitized Mice
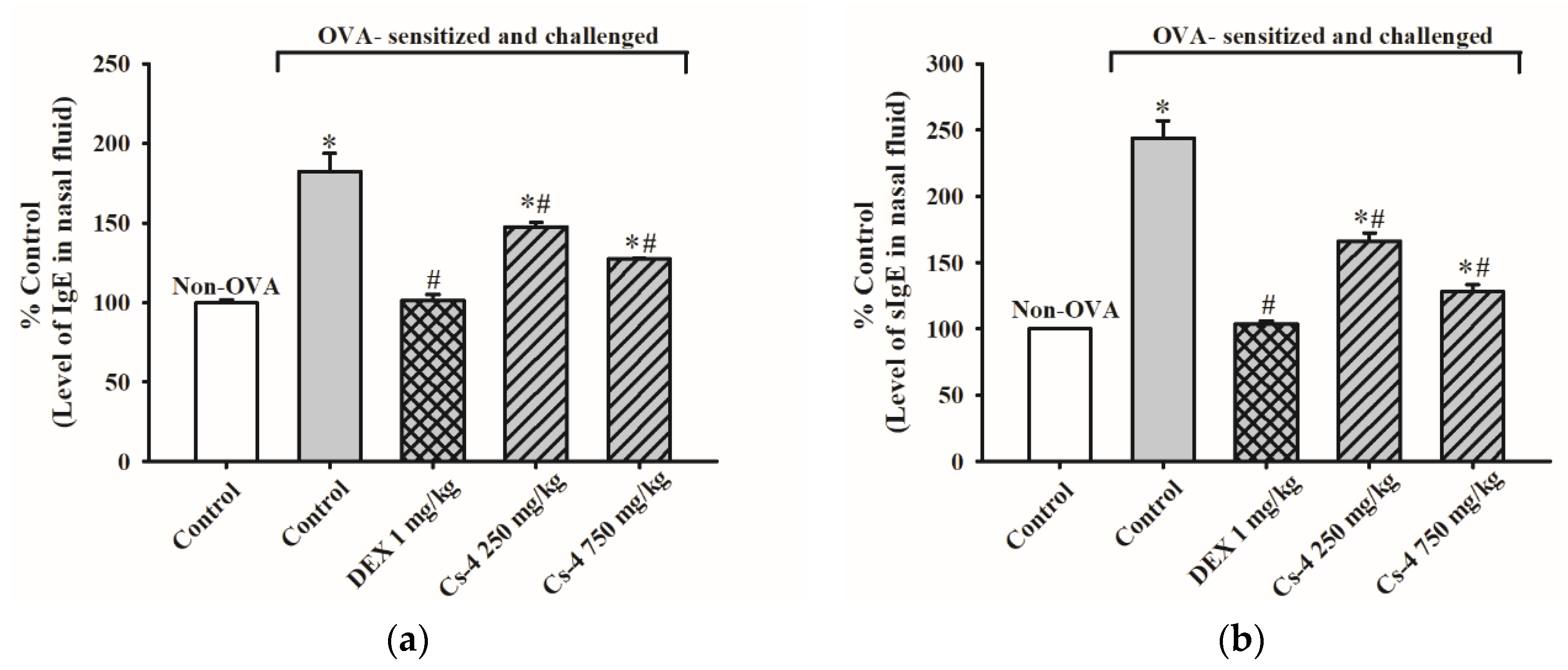
2.3. Cs-4 Treatment Reduced the Levels of IgE and OVA-specific IgE in the Lavage Fluid (NFL) of OVA-Sensitized and Challenged Mice
2.4. Cs-4 Treatment Significantly Decreased both IL-4 and IL-13 Levels in the NLF of OVA-Sensitized and Challenged Mice
2.5. Preincubation with Cs-4 Suppressed C48/80-Activated β-Hexosaminidase Release
2.6. Preincubation with Cs-4 Suppressed C48/80-Activated Histamine Release
2.7. Cs-4 Treatment Did Not Produce any Detectable Change in the Body Weight of Capsaicin-Challenged Rats, but the Splenic Index Was Reduced When Compared with the Challenged and Untreated Controls
2.8. Cs-4 Treatment Lowered the Cutaneous Lesion Induced by the Capsaicin Challenge on Rats
2.9. Cs-4 Treatment Decreased the EC50 of Methacholine (Mch) on the Induction of Contractions on Tracheal Rings and Bronchial Rings
2.10. Cs-4 Treatment Reduced the Number of Scratching Events
2.11. Cs-4 Treatment Reduced the Plasma IgE Levels in Capsaicin-Challenged Rats
2.12. Cs-4 Treatment Decreased the IgE and Eosinophil Peroxidase (EPO) Levels in the Bronchoalveolar (BAL) Fluid of Capsaicin-Challenged Rats
2.13. Cs-4 Treatment Suppressed the Capsaicin-Induced Increases in the IL-4, IL-5, and IL-13 Levels in Rat Lung Tissue
3. Discussion
4. Materials and Methods
4.1. Herbal Drug Preparation and UPLC-UV Analysis
4.2. The Mouse Model of Allergic Rhinitis
4.2.1. Challenge and Treatment
4.2.2. Measurement of Nasal Symptoms
4.2.3. Evaluation of IgE and Cytokines in NLF
4.3. RPMC Histamine Release
4.3.1. RPMC Preparation
4.3.2. RPMC Degranulation
4.3.3. RPMC Histamine Release
4.4. The Rat Model of Asthma
4.4.1. Experimental Design
4.4.2. Neonatal Capsaicin Treatment
4.4.3. Scratching Behavior
4.4.4. Evaluation of Cutaneous Lesions
4.4.5. Body and Spleen Weight
4.4.6. Evaluation of AHR in Vitro
4.4.7. Biochemical Analyses
Sampling of Lung Tissues and Bronchoalveolar Lavage
IgE Levels
Th1/Th2 Cytokine Levels
Eosinophil Infiltration in the BAL Fluid
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHR | Airway responsiveness |
| AR | Allergic rhinitis |
| BAL | Bronchoalveolar lavage |
| DEX | Dexamethasone |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EPO | Eosinophil peroxidase |
| HEPES | 4-(2-hyroxyethyl)-1-piperazineethanesulfonic acid |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| IFN | Interferon |
| Mch | Methacholine |
| NLF | Nasal lavage fluid |
| OVA | Ovalbumin |
| PBS-A | Phosphate-buffer saline-A |
| RPMC | Rat peritoneal mast cell |
| Th1 | Type I T helper cell |
| Th2 | Type II T helper cell |
| TNF | Tumor necrosis factor |
| UPLC-UV | Ultra-Performance Liquid Chromatography coupled with Ultraviolet detection |
References
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.; Bunyavanich, S. Allergic rhinitis: The “Ghost Diagnosis” in patients with asthma. Asthma Res. Pract. 2015, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Aun, M.V.; Bonamichi-Santos, R.; Arantes-Costa, F.M.; Kalil, J.; Giavina-Bianchi, P. Animal models of asthma: Utility and limitations. J. Asthma Allergy 2017, 10, 293–301. [Google Scholar] [CrossRef]
- Price, D. Asthma and allergic rhinitis: Linked in treatment and outcomes. Ann. Thorac. Med. 2010, 5, 63–64. [Google Scholar] [CrossRef]
- Khan, D.A. Allergic rhinitis and asthma: Epidemiology and common pathophysiology. Allergy Asthma Proc. 2014, 35, 357–361. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signaling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef]
- Kay, A.B. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Koh, J.H.; Suh, H.J.; Ahn, T.S. Hot-water extract from mycelia of Cordyceps sinensis as a substitute for antibiotic growth promoters. Biotechnol. Lett. 2003, 25, 585–590. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Evaluation of the anti-inflammatory and anti-proliferation tumoral cells activities of Antrodia camphorata, Cordyceps sinensis, and Cinnamomum osmophloeum bark extracts. J. Ethnopharmacol. 2007, 114, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.L.; Lin, C.Y. The extract of Cordyceps sinensis inhibited airway inflammation by blocking NF-kappaB activity. Inflammation 2012, 35, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.C.; Nam, S.H.; Nam, D.Y.; Kim, J.G.; Lee, K.G.; Yeo, J.H.; Yoon, C.K.; Park, C.H.; Lee, S.H. Anti-asthmatic activities in mycelial extract and culture filtrate of Cordyceps sphecocephala J201. Int. J. Mol. Med. 2010, 26, 351–356. [Google Scholar] [PubMed]
- Amin, K.; Janson, C.; Bystrom, J. Role of Eosinophil Granulocytes in Allergic Airway Inflammation Endotypes. Scand. J. Immunol. 2016, 84, 75–85. [Google Scholar] [CrossRef]
- Kudo, M.; Melton, A.C.; Chen, C.; Engler, M.B.; Huang, K.E.; Ren, X.; Wang, Y.; Bernstein, X.; Li, J.T.; Atabai, K.; et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 2012, 18, 547–554. [Google Scholar] [CrossRef]
- Liang, K.L.; Jiang, R.S.; Wang, R.C.; Koo, M.; Chen, S.C.; Huang, W.C.; Yeh, Y.C. Upper airway inflammation exacerbates bronchial hyperreactivity in mouse models of rhinosinusitis and allergic asthma. Int. Forum Allergy Rhinol. 2013, 3, 532–542. [Google Scholar] [CrossRef]
- Jeong, K.T.; Kim, S.G.; Lee, J.; Park, Y.N.; Park, H.H.; Park, N.Y.; Kim, K.J.; Lee, H.; Lee, Y.J.; Lee, E. Anti-allergic effect of a Korean traditional medicine, Biyeom-Tang on mast cells and allergic rhinitis. BMC Complement. Altern. Med. 2014, 14, 54. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Radinger, M.; Gilfillan, A.M. Measuring mast cell mediator release. Curr. Protoc. Immunol. 2010, 91, 7.38.1–7.38.9. [Google Scholar] [CrossRef]
- Chong, W.; Feng, X.Y.; Zhen, G.Z.; Dan, L.; Yue, D. Inhibition of mast cell degranulation by saponins from Gleditsia sinensis--structure-activity relationships. Nat. Prod. Commun. 2009, 4, 777–782. [Google Scholar] [CrossRef]
- Huang, L.; Pi, J.; Wu, J.; Zhou, H.; Cai, J.; Li, T.; Liu, L. A rapid and sensitive assay based on particle analysis for cell degranulation detection in basophils and mast cells. Pharmacol. Res. 2016, 111, 374–383. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yan, G.H.; Chai, O.H.; Song, C.H. Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation. Anat. Cell Biol. 2010, 43, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Georas, S.N.; Guo, J.; De Fanis, U.; Casolaro, V. T-helper cell type-2 regulation in allergic disease. Eur. Respir. J. 2005, 26, 1119–1137. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.; Tormey, V.; Burke, C.; Poulter, L.W. Allergen-induced cytokine production in atopic disease and its relationship to disease severity. Am. J. Respir. Cell Mol. Biol. 1997, 17, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Koning, H.; Neijens, H.J.; Baert, M.R.; Oranje, A.P.; Savelkoul, H.E. T cell subsets and cytokines in allergic and non-allergic children. I. Analysis of IL-4, IFN-gamma and IL-13 mRNA expression and protein production. Cytokine 1997, 9, 416–426. [Google Scholar] [CrossRef]
- Keeney, G.E.; Gray, M.P.; Morrison, A.K.; Levas, M.N.; Kessler, E.A.; Hill, G.D.; Gorelick, M.H.; Jackson, J.L. Dexamethason for acute asthma exacerations in children: A meta-analysis. Pediatrics 2014, 133, 433–439. [Google Scholar] [CrossRef]
- Hopkins, R.L.; Leinung, M. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol. Metab. Clin. N. Am. 2005, 34, 371–384. [Google Scholar] [CrossRef]
- Malkawi, A.K.; Alzoubi, K.H.; Jacob, M.; Matic, G.; Ali, A.; Faraj, A.; Almuhanna, F.; Dasouki, M.; Rahman, A.M.A. Metabolomics based profiling of dexamethasone side effects in rats. Front. Parmacol. 2018, 9, 46. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jee, H.; Yeom, J.H.; Jeong, H.J.; Kim, H.M. The ameliorative effect of AST2017-01 in an ovalbumin-induced allergic rhinitis animal model. Inflamm. Res. 2019, 68, 387–395. [Google Scholar] [CrossRef]
- Hsu, C.H.; Sun, H.L.; Sheu, J.M.; Ku, M.S.; Hu, C.M.; Chan, Y.; Lue, K.H. Effects of the immunomodulatory agent Cordyceps militaris on airway inflammation in a mouse asthma model. Pediatr. Neonatol. 2008, 49, 171–178. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, L.; Cai, T. Cordyceps polysaccharide ameliorates airway inflammation in an ovalbumin- induced mouse model of asthma via TGF-β1/Smad signaling pathway. Respir. Physiol. Neurobiol. 2020, 276, 103412. [Google Scholar] [CrossRef]
- Hosoi, T.; Ino, S.; Ohnishi, F.; Todoroki, K.; Yoshii, M.; Kakimoto, M.; Muller, C.E.; Ozawa, K. Mechanisms of the action of adenine on anti-allergic effects in mast cells. Immun. Inflamm. Dis. 2018, 6, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Hasko, G.; Cronstein, B. Regulation of inflammation by adenosine. Front. Immunol. 2013, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; He, Y.; Li, T.; Wang, W.; Zhang, J.; Wei, J.; Deng, Y.; Lin, R. Cordycepin alleviates airway hyperreactivity in a murine model of asthma by attenuating the inflammatory process. Int. Immunopharmacol. 2015, 26, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Carr, V.M.; Robinson, A.M. Induction of allergic rhinitis in mice. Methods Mol. Biol. 2013, 1032, 145–158. [Google Scholar] [PubMed]
- Ko, M.T.; Huang, S.C.; Kang, H.Y. Establishment and characterization of an experimental mouse model of allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2015, 272, 1149–1155. [Google Scholar] [CrossRef]
- McCusker, C.; Chicoine, M.; Hamid, Q.; Mazer, B. Site-specific sensitization in a murine model of allergic rhinitis: Role of the upper airway in lower airways disease. J. Allergy Clin. Immunol. 2002, 110, 891–898. [Google Scholar] [CrossRef]
- Hussain, I.; Randolph, D.; Brody, S.L.; Song, S.K.; Hsu, A.; Kahn, A.M.; Chaplin, D.D.; Hamilos, D.L. Induction, distribution and modulation of upper airway allergic inflammation in mice. Clin. Exp. Allergy 2001, 31, 1048–1059. [Google Scholar] [CrossRef]
- Cho, S.H.; Oh, S.Y.; Zhu, Z.; Lee, J.; Lane, A.P. Spontaneous eosinophilic nasal inflammation in a genetically-mutant mouse: Comparative study with an allergic inflammation model. PLoS ONE 2012, 7, e35114. [Google Scholar] [CrossRef]
- Jung, H.W.; Jung, J.K.; Park, Y.K. Antiallergic effect of Ostericum koreanum root extract on ovalbumin-induced allergic rhinitis mouse model and mast cells. Asian Pac J. Allergy Immunol. 2011, 29, 338–348. [Google Scholar]
- Meurer, S.K.; Ness, M.; Weiskirchen, S.; Kim, P.; Tag, C.G.; Kauffmann, M.; Huber, M.; Weiskirchen, R. Isolation of Mature (Peritoneum-Derived) Mast Cells and Immature (Bone Marrow-Derived) Mast Cell Precursors from Mice. PLoS ONE 2016, 11, e0158104. [Google Scholar] [CrossRef] [PubMed]
- Gulledge, T.V.; Collette, N.M.; Mackey, E.; Johnstone, S.E.; Moazami, Y.; Todd, D.A.; Moeser, A.J.; Pierce, J.G.; Cech, N.B.; Laster, S.M. Mast cell degranulation and calcium influx are inhibited by an Echinacea purpurea extract and the alkylamide dodeca-2E,4E-dienoic acid isobutylamide. J. Ethnopharmacol. 2018, 212, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.; Kim, I.K.; Kwon, T.K.; Moon, J.Y.; Park, W.H.; Shin, T.Y. Suppression of mast cell-mediated allergic reaction by Amomum xanthiodes. Food Chem. Toxicol. 2007, 45, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Im, S.J.; Ahn, M.H.; Han, I.H.; Song, H.O.; Kim, Y.S.; Kim, H.M.; Ryu, J.S. Histamine and TNF-alpha release by rat peritoneal mast cells stimulated with Trichomonas vaginalis. Parasite 2011, 18, 49–55. [Google Scholar] [CrossRef]
- Tiligada, E.; Aslanis, D.; Delitheos, A.; Varonos, D. Changes in histamine content following pharmacologically-induced mast cell degranulation in the rat conjunctiva. Pharmacol. Res. 2000, 41, 667–670. [Google Scholar] [CrossRef]
- Han, R.T.; Kim, S.; Choi, K.; Jwa, H.; Lee, J.; Kim, H.Y.; Kim, H.J.; Kim, H.R.; Back, S.K.; Na, H.S. Asthma-like airway inflammation and responses in a rat model of atopic dermatitis induced by neonatal capsaicin treatment. J. Asthma Allergy 2017, 10, 181–189. [Google Scholar] [CrossRef]
- Thomsen, J.S.; Petersen, M.B.; Benfeldt, E.; Jensen, S.B.; Serup, J. Scratch induction in the rat by intradermal serotonin: A model for pruritus. Acta Derm. Venereol. 2001, 81, 250–254. [Google Scholar] [CrossRef]
- Sundaram, A.; Chen, C.; Khalifeh-Soltani, A.; Atakilit, A.; Ren, X.; Qiu, W.; Jo, H.; DeGrado, W.; Huang, X.; Sheppard, D. Targeting integrin alpha5beta1 ameliorates severe airway hyperresponsiveness in experimental asthma. J. Clin. Investig. 2017, 127, 365–374. [Google Scholar] [CrossRef]
- Sakae, R.S.; Leme, A.S.; Dolhnikoff, M.; Pereira, P.M.; do Patrocinio, M.; Warth, T.N.; Zin, W.A.; Saldiva, P.H.; Martins, M.A. Neonatal capsaicin treatment decreases airway and pulmonary tissue responsiveness to methacholine. Am. J. Physiol 1994, 266 Pt 1, L23–L29. [Google Scholar] [CrossRef]
- Whitehead, G.S.; Grasman, K.A.; Kimmel, E.C. Lung function and airway inflammation in rats following exposure to combustion products of carbon-graphite/epoxy composite material: Comparison to a rodent model of acute lung injury. Toxicology 2003, 183, 175–197. [Google Scholar] [CrossRef]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |










| Group | Body Weight (g) | Splenic Index | |
|---|---|---|---|
| Noncapsaicin | Control | 274 ± 4.04 | 0.00271 ± 0.00004 |
| Capsaicin | Control | 267 ± 6.39 | 0.00323 ± 0.00015 * |
| DEX (1 mg/kg) | 108 ± 2.48 *# | 0.00178 ± 0.00007 *# | |
| Cs-4 (123 mg/kg) | 269 ± 4.77 | 0.00323 ± 0.00011 * | |
| Cs-4 (375 mg/kg) | 275 ± 5.56 | 0.00282 ± 0.00008 # |
| Group | Score | |
|---|---|---|
| Noncapsaicin | Control | 0.150 ± 0.105 |
| Capsaicin | Control | 3.104 ± 0.148 * |
| DEX (1 mg/kg) | 2.167 ± 0.474 * | |
| Cs-4 (123 mg/kg) | 2.462 ± 0.798 * | |
| Cs-4 (375 mg/kg) | 0.952 ± 0.422 # |
| Group | Tracheal Ring | Bronchial Ring | |
|---|---|---|---|
| EC50 (μM) | EC50 (μM) | ||
| Noncapsaicin | Control | 52.20 ± 24.97 | 15.39 ± 6.92 |
| Capsaicin | Control | 0.68 ± 0.38 * | 0.61 ± 0.16 * |
| DEX (1 mg/kg) | 7.68 ± 7.02 | 0.90 ± 0.06 | |
| Cs-4 (123 mg/kg) | 24.84 ± 13.17# | 24.00 ± 10.54 # | |
| Cs-4 (375 mg/kg) | 46.11 ± 23.26# | 34.34 ± 17.88 # |
| Region | Score | Skin Condition |
|---|---|---|
| Face | 0 | Normal |
| 1 | Wispy fur | |
| 2 | Alopecia and flare | |
| 3 | Bleeding or ulcerative lesion | |
| Ears | 0 | Normal |
| 1 | Flare | |
| 2 | Bleeding | |
| 3 | Loss of part of the ear | |
| Body | 0 | Normal |
| 1 | Wispy fur | |
| 2 | Alopecia and flare | |
| 3 | Bleeding or ulcerative lesion |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Chan, W.M.; Leung, H.Y.; Leong, P.K.; Yan, C.T.M.; Ko, K.M. Anti-Inflammatory Effects of a Cordyceps sinensis Mycelium Culture Extract (Cs-4) on Rodent Models of Allergic Rhinitis and Asthma. Molecules 2020, 25, 4051. https://doi.org/10.3390/molecules25184051
Chen J, Chan WM, Leung HY, Leong PK, Yan CTM, Ko KM. Anti-Inflammatory Effects of a Cordyceps sinensis Mycelium Culture Extract (Cs-4) on Rodent Models of Allergic Rhinitis and Asthma. Molecules. 2020; 25(18):4051. https://doi.org/10.3390/molecules25184051
Chicago/Turabian StyleChen, Jihang, Wing Man Chan, Hoi Yan Leung, Pou Kuan Leong, Choly Tat Ming Yan, and Kam Ming Ko. 2020. "Anti-Inflammatory Effects of a Cordyceps sinensis Mycelium Culture Extract (Cs-4) on Rodent Models of Allergic Rhinitis and Asthma" Molecules 25, no. 18: 4051. https://doi.org/10.3390/molecules25184051
APA StyleChen, J., Chan, W. M., Leung, H. Y., Leong, P. K., Yan, C. T. M., & Ko, K. M. (2020). Anti-Inflammatory Effects of a Cordyceps sinensis Mycelium Culture Extract (Cs-4) on Rodent Models of Allergic Rhinitis and Asthma. Molecules, 25(18), 4051. https://doi.org/10.3390/molecules25184051





