Thermal-Oxidation Stability of Soybean Germ Phytosterols in Different Lipid Matrixes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Oxidation of SGPs in the Non-Oil System
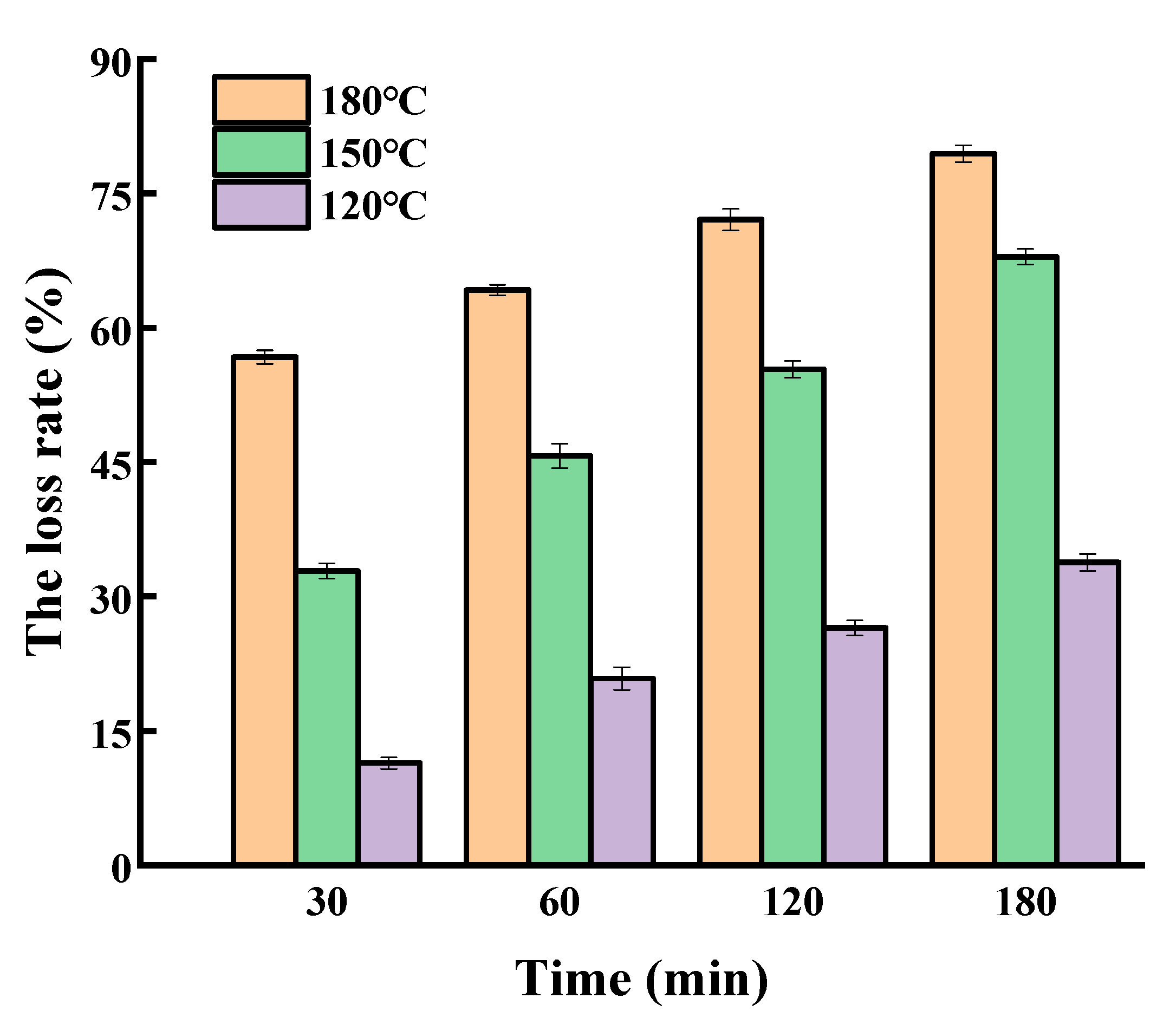
2.2. Oxidation of SGPs in Oil Systems
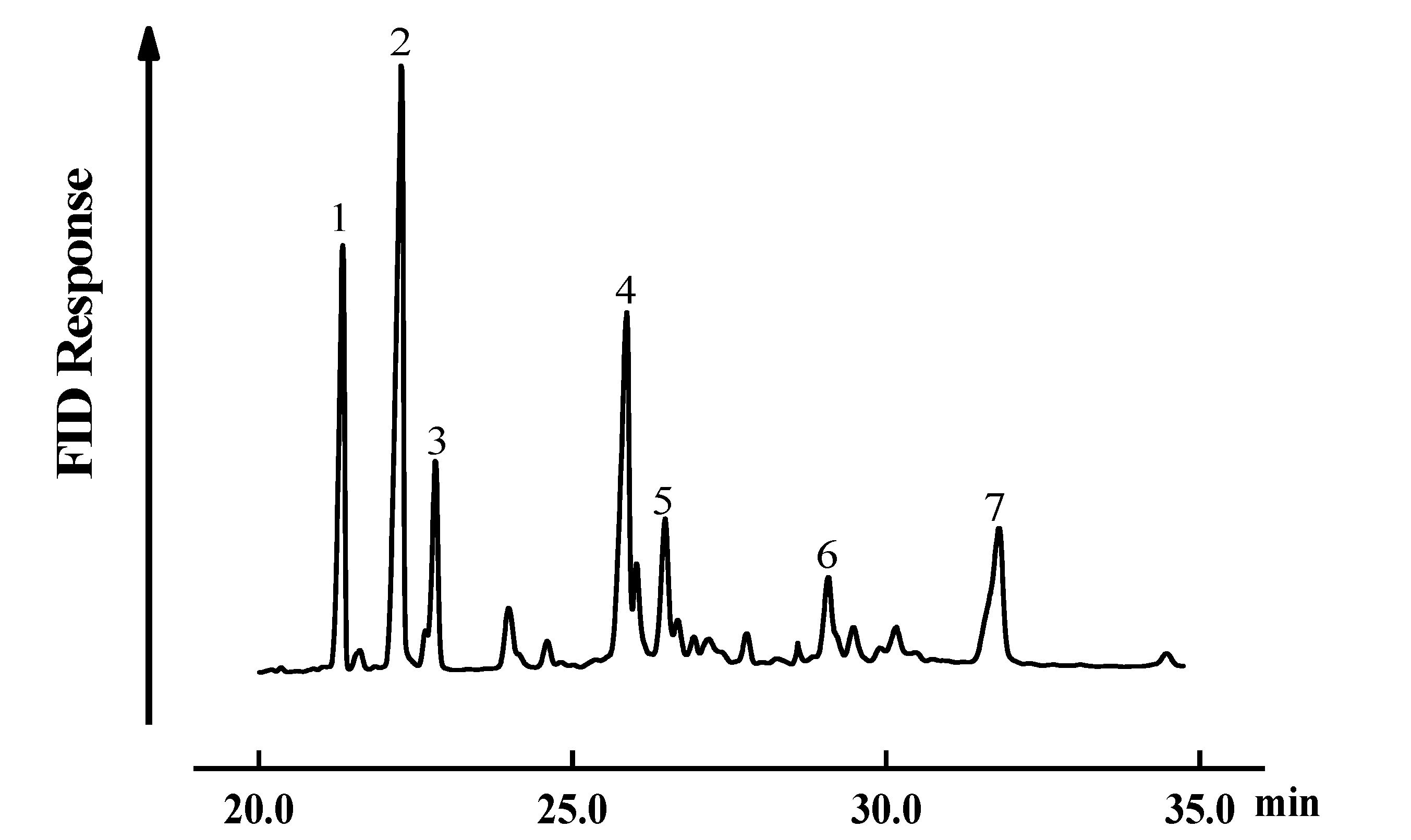
2.3. GC-MS Analysis of SGPs Oxidation Products
2.4. Composition of SGPs Oxidation Products in the Non-Oil System
2.5. The Composition of Soybean Germ Phytosterols’ Oxidation Products in the Oil System
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Soybean Germ Oil and SGPs
3.3. Quantification of SGPs
3.4. Oxidation of SGPs in a Non-Oil System
3.5. Oxidation of SGPs in Three Oil Systems
3.6. Measurement of Soybean Germ Phytosterols’ Oxidation Products
3.7. Analysis of Oxidation Products of SGPs
3.8. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jogchum, P.; Ronald, P.; Mensink. Plant stanol and sterol esters in the control of blood cholesterol levels: Mechanism and safety aspects. Am. J. Cardiol. 2005, 96, 15–22. [Google Scholar]
- Fernandes, P.; Cabral, J.M.S. Phytosterols: Applications and recovery methods. Bioresour. Technol. 2007, 98, 2335–2350. [Google Scholar] [CrossRef] [PubMed]
- García-Llatas, G.; Rodriguez-Estrada, M.T. Current and new insights on phytosterol oxides in plant sterol-enriched food. Chem. Phys. Lipids 2011, 164, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharm. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Alemany, C.L.; González, L.M.; García-Llatas, G.; Alegría, A.; Barberá, R.; Sánchez, S.L.M.; Lagarda, M.J. Sterol stability in functional fruit beverages enriched with different plant sterol sources. Food Res. Int. 2012, 48, 265–270. [Google Scholar] [CrossRef]
- Dutta, P.C. Chemistry, analysis, and occurrence of phytosterol oxidation products in foods. In Phytosterols as Functional Food Components and Nutraceuticals; Dutta, P.C., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 397–418. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Lengyel, J.; Rimarčík, J.; Vagánek, A.; Fedor, J.; Lukeš, V.; Klein, E. Oxidation of sterols: Energetics of C-H and O-H bond cleavage. Food Chem. 2012, 133, 1435–1440. [Google Scholar] [CrossRef]
- O’Callaghan, Y.; McCarthy, F.O.; O’Brien, N.M. Recent advances in phytosterol oxidation products. Biochem. Biophys. Res. Commun. 2014, 446, 786–791. [Google Scholar] [CrossRef]
- Otaegui-Arrazola, A.; Menéndez-Carreño, M.; Ansorena, D.; Astiasarán, I. Oxysterols: A world to explore. Food Chem. Toxicol. 2010, 48, 3289–3303. [Google Scholar] [CrossRef]
- Alemany, L.; Barbera, R.; Alegría, A.; Laparra, J.M. Plant sterols from foods in inflammation and risk of cardiovascular disease: A real threat? Food Chem. Toxicol. 2014, 69, 140–149. [Google Scholar] [CrossRef]
- Kenny, O.; O’Callaghan, Y.; O’Connell, N.M.; McCarthy, F.O.; Maguire, A.R.; O’Brien, N.M. Oxidized derivatives of dihydrobrassicasterol: Cytotoxic and apoptotic potential in U937 and HepG2 cells. J. Agric. Food Chem. 2012, 60, 5952–5961. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, Y.; Kenny, O.; O’Connell, N.M.; Maguire, A.R.; McCarthy, F.O.; O’Brien, N.M. Synthesis and assessment of the relative toxicity of the oxidized derivatives of campesterol and dihydrobrassicasterol in U937 and HepG2 cells. Biochimie 2013, 95, 496–503. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, Y.C.; Foley, D.A.; O’Connell, N.M.; McCarthy, F.O.; Maguire, A.R.; O’Brien, N.M. Cytotoxic and apoptotic effects of the oxidized derivatives of stigmasterol in the U937 human monocytic cell line. J. Agric. Food Chem. 2010, 58, 10793–10798. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.; Chopra, J.; McCarthy, F.O.; Maguire, A.R.; O’Brien, N.M. Qualitative and quantitative comparison of the cytotoxic and apoptotic potential of phytosterol oxidation products with their corresponding cholesterol oxidation products. Br. J. Nutr. 2005, 94, 443–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.L. Cholesterol Autoxidation; Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- Soupas, L.; Juntunen, L.; Lampi, A.M.; Piironen, V. Effects of sterol structure, temperature, and lipid medium on phytosterol oxidation. J. Agric. Food Chem. 2004, 52, 6485–6491. [Google Scholar] [CrossRef]
- Rudzinska, M.; Przybylski, R.; Wasowicz, E. Products formed during thermo-oxidative degradation of phytosterols. J. Am. Oil Chem. Soc. 2009, 86, 651–662. [Google Scholar] [CrossRef]
- Xu, G.H.; Sun, J.L.; Liang, Y.T.; Yang, Y.; Chen, Z.Y. Interaction of fatty acids with oxidation of cholesterol and β-sitosterol. Food Chem. 2011, 124, 162–170. [Google Scholar] [CrossRef]
- Johnsson, L.; Dutta, P.C. Determination of phytosterol oxides in some food products by using an optimized transesterification method. Food Chem. 2006, 97, 606–613. [Google Scholar] [CrossRef]
- Chen, J.N.; Tang, G.Y.; Zhou, J.F.; Liu, W.; Bi, Y.L. The characterization of soybean germ oil and the antioxidative activity of its phytosterols. RSC Adv. 2019, 9, 40109–40117. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.L. Review of progress in sterol oxidations: 1987–1995. Lipids 1996, 31, 453–487. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Gawrysiak-Witulska, M.; Rudzińska, M. Dynamics of phytosterol degradation in a bulk of rapeseed stored under different temperature and humidity conditions. J. Stored Prod. Res. 2019, 83, 292–304. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. Unsaturated lipid matrices protect plant sterols from degradation during heating treatment. Food Chem. 2016, 196, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Kemmo, S.; Ollilainen, V.; Lampi, A. Determination of stigmasterol and cholesterol oxides using atmospheric pressure chemical ionization liquid chromatography-Mass spectrometry. Food Chem. 2007, 101, 1438–1445. [Google Scholar] [CrossRef]
- Hu, P.C.; Chen, B.H. Effects of riboflavin and fatty acid methyl esters on cholesterol oxidation during illumination. J. Agric. Food Chem. 2002, 50, 3572–3578. [Google Scholar] [CrossRef]
- Menendez-Carreno, M.; Ansoreena, D.; Astiasaran, I. Stability of sterols in phytosterol-enriched milk under different heating conditions. J. Agric. Food Chem. 2008, 56, 9997–10002. [Google Scholar] [CrossRef] [Green Version]
- Iuliano, L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids 2011, 164, 457–4682. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, W.O.; Schultz, H.W.; Day, E.A.; Sinnhuber, R.O. (Eds.) Mechanisms in Lipids and Their Oxidation; Avi Publishing Co. Inc.: Westport, CT, USA, 1962; pp. 31–50. [Google Scholar]
- Porter, N.A.; Caldwell, S.E.; Mills, K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 1995, 30, 277–290. [Google Scholar] [CrossRef]
- Rudzińska, M.; Przybylski, R.; Wasowicz, E. Degradation of phytosterols during storage of enriched margarines. Food Chem. 2014, 142, 294–298. [Google Scholar] [CrossRef]
- Chien, J.T.; Hsu, D.J.; Chen, B.H. Kinetic model for studying the effect of quercetin on cholesterol oxidation during heating. J. Agric. Food Chem. 2006, 54, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Ansorena, D.; Barriuso, B.; Cardenia, V. Thermo-oxidation of cholesterol: Effect of the unsaturation degree of the lipid matrix. Food Chem. 2013, 141, 2757–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennisi-Forell, S.C.P.; Ranalli, N.; Zaritzky, N.E.; Andres, S.C.; Califano, A.N. Effect of type of emulsifiers and antioxidants on oxidative stability, colour and fatty acid profile of low-fat beef burgers enriched with unsaturated fatty acids and phytosterols. Meat Sci. 2010, 86, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Botelho, P.B.; Galasso, M.; Dias, V.; Mandrioli, M.; Lobato, L.P.; Rodriguez-Estrada, M.T.; Castro, I.A. Oxidative stability of functional phytosterol-enriched dark chocolate. LWT Food Sci. Technol. 2014, 55, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Tabee, E.; Azadmard-Damirchi, S.; Jägerstad, M.; Dutta, P.C. Effects of a-tocopherol on oxidative stability and phytosterol oxidation during heating in some regular and high-oleic vegetable oils. J. Am. Oil Chem. Soc. 2008, 85, 857–867. [Google Scholar] [CrossRef]
- Dutta, P.C.; Helmersson, S.; Kebedu, E. Variation in lipid composition of niger seed (Guizotia abyssinica, Cass.) samples collected from different regions in ethiopia. J. Am. Oil Chem. Soc. 1994, 71, 839–843. [Google Scholar] [CrossRef]
- Menendez-Carreno, M.; Garcia-Herreros, C.; Astiasaran, I.; Ansorena, D. Validation of a gas chromatography-mass spectrometry method for the analysis of sterol oxidation products in serum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 864, 61–68. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds soybean germ phytosterols are available from the authors. |

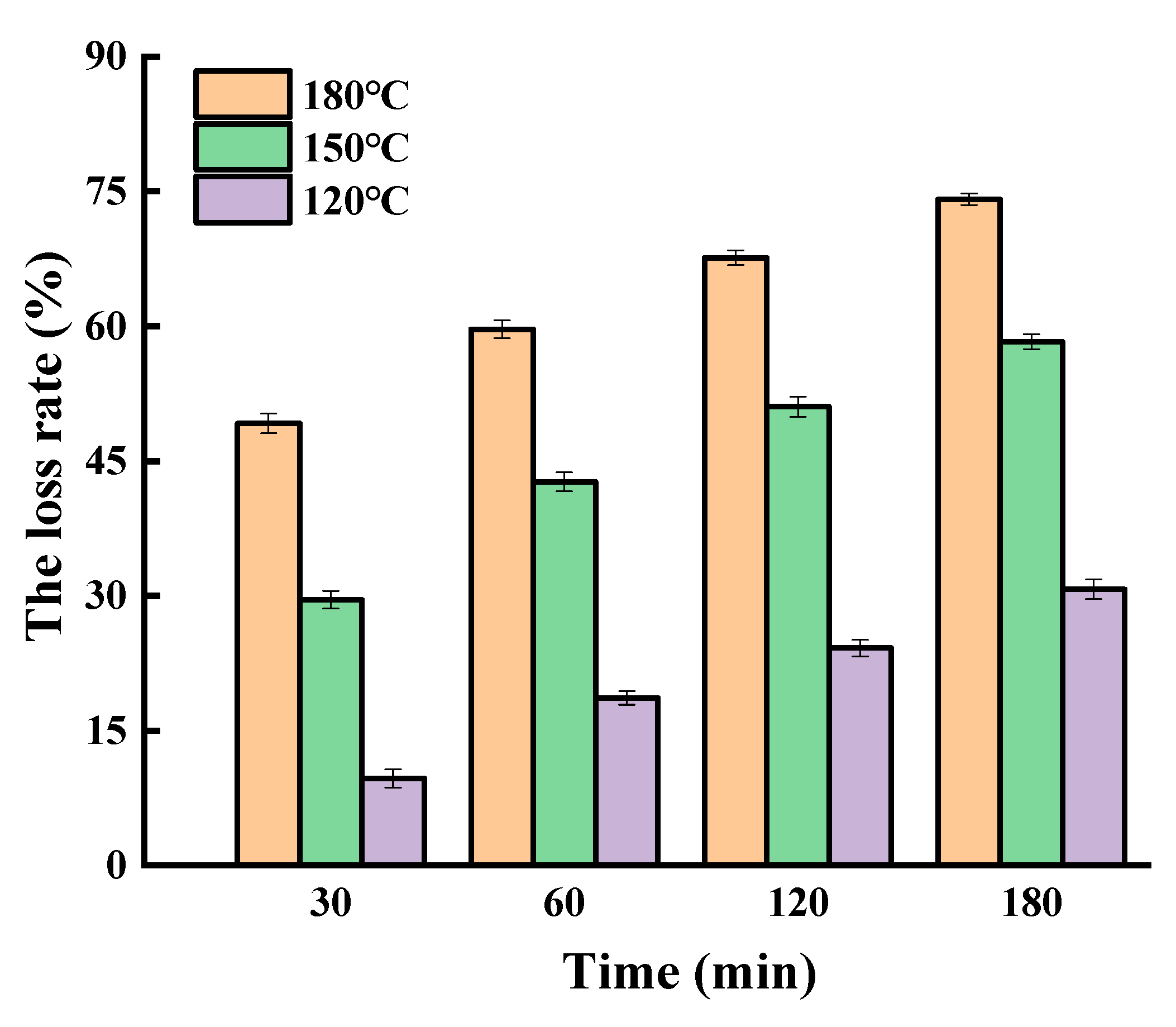

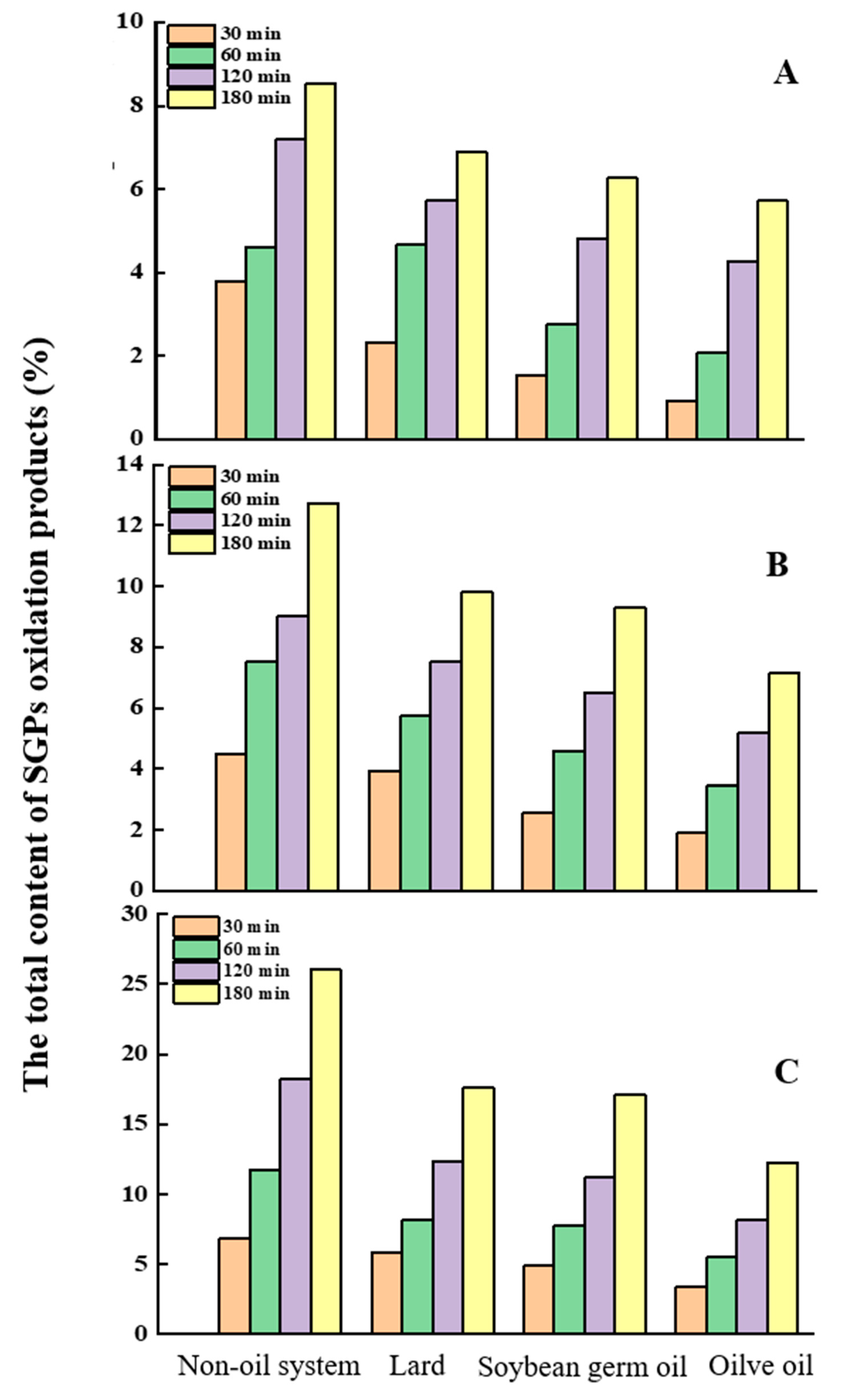
| Temperature and Time | The Composition and Content of Oxidation Products/% | |||||
|---|---|---|---|---|---|---|
| 7α(β)-Hydroxy Sterols | 5α(β), 6α(β)- Epoxy Sterols | 7-Ketosterols | Triols | Total Content | ||
| 180 °C | 30 min | 1.82 ± 0.02 a | 1.33 ± 0.02 a | 3.61 ± 0.04 a | 0.11 ± 0.01 a | 6.87 a |
| 60 min | 1.98 ± 0.03 b | 3.01 ± 0.03 b | 6.62 ± 0.03 b | 0.16 ± 0.01 ab | 11.77 b | |
| 120 min | 2.12 ± 0.02 c | 4.92 ± 0.02 c | 11.02 ± 0.05 c | 0.21 ± 0.02 b | 18.27 c | |
| 180 min | 2.18 ± 0.04 c | 7.42 ± 0.03 d | 16.23 ± 0.03 d | 0.22 ± 0.02 b | 26.05 d | |
| 150 °C | 30min | 1.52 ± 0.03 a | 0.52 ± 0.01 a | 2.16 ± 0.05 a | 0.28 ± 0.01 a | 4.48 a |
| 60 min | 1.73 ± 0.06 a | 0.82 ± 0.02 b | 4.64 ± 0.04 b | 0.34 ± 0.01 a | 7.53 b | |
| 120 min | 1.92 ± 0.03 a | 1.30 ± 0.04 c | 5.51 ± 0.03 c | 0.30 ± 0.02 a | 9.03 c | |
| 180 min | 2.06 ± 0.41 a | 2.65 ± 0.05 d | 7.74 ± 0.06 d | 0.28 ± 0.03 a | 12.73 d | |
| 120 °C | 30 min | 1.28 ± 0.02 a | 0.28 ± 0.02 a | 1.92 ± 0.03 a | 0.32 ± 0.03 bc | 3.80 a |
| 60 min | 1.37 ± 0.10 a | 0.43 ± 0.02 a | 2.63 ± 0.07 b | 0.18 ± 0.04 a | 4.61 b | |
| 120 min | 2.06 ± 0.08 b | 0.94 ± 0.06 b | 3.99 ± 0.04 c | 0.21 ± 0.03 ab | 7.20 c | |
| 180 min | 2.05 ± 0.02 b | 0.91 ± 0.04 b | 5.18 ± 0.03 d | 0.38 ± 0.03 c | 8.52 d | |
| Temperature and Time | Composition and Content of Oxidation Products/% | |||||
|---|---|---|---|---|---|---|
| 7α(β)-Hydroxy Sterols | 5α(β), 6α(β)- Epoxy Sterols | 7-Ketosterols | Triols | Total Content | ||
| 180 °C | 30 min | 0.84 ± 0.01 a | 0.98 ± 0.02 a | 3.01 ± 0.03 a | 0.11 ± 0.02 a | 4.94 a |
| 60 min | 1.31 ± 0.02 b | 2.23 ± 0.04 b | 4.08 ± 0.02 b | 0.15 ± 0.03 ab | 7.77 b | |
| 120 min | 1.55 ± 0.02 c | 3.45 ± 0.01 c | 6.11 ± 0.04 c | 0.12 ± 0.01 ab | 11.23 c | |
| 180 min | 1.96 ± 0.03 d | 4.76 ± 0.03 d | 10.18 ± 0.03 d | 0.20 ± 0.02 b | 17.10 d | |
| 150 °C | 30 min | 0.67 ± 0.04 a | 0.23 ± 0.02 a | 1.53 ± 0.03 a | 0.12 ± 0.02 a | 2.55 a |
| 60 min | 1.03 ± 0.03 b | 0.48 ± 0.03 b | 2.98 ± 0.06 b | 0.10 ± 0.03 a | 4.59 b | |
| 120 min | 1.42 ± 0.02 c | 0.94 ± 0.03 c | 4.02 ± 0.05 c | 0.13 ± 0.04 a | 6.51 c | |
| 180 min | 1.74 ± 0.11 d | 1.56 ± 0.05 d | 5.87 ± 0.03 d | 0.13 ± 0.03 a | 9.30 d | |
| 120 °C | 30 min | 0.38 ± 0.33 a | 0.16 ± 0.03 a | 0.88 ± 0.06 a | 0.13 ± 0.02 a | 1.55 a |
| 60 min | 0.89 ± 0.05 ab | 0.32 ± 0.03 b | 1.47 ± 0.05 b | 0.09 ± 0.05 a | 2.77 b | |
| 120 min | 1.29 ± 0.06 b | 0.57 ± 0.02 c | 2.21 ± 0.06 c | 0.11 ± 0.04 a | 4.81 c | |
| 180 min | 1.56 ± 0.03 b | 0.63 ± 0.03 c | 3.95 ± 0.03 d | 0.13 ± 0.05 a | 6.27 d | |
| Temperature and Time | Composition and Content of Oxidation Products/% | |||||
|---|---|---|---|---|---|---|
| 7α(β)-Hydroxy Sterols | 5α(β), 6α(β)- Epoxy Sterols | 7-Ketosterols | Triols | Total Content | ||
| 180 °C | 30 min | 0.98 ± 0.07 a | 0.58 ± 0.06 a | 1.83 ± 0.05 a | ND | 3.39 a |
| 60 min | 1.35 ± 0.09 a | 0.84 ± 0.05 a | 3.29 ± 0.04 b | ND | 5.48 b | |
| 120 min | 2.03 ± 0.12 b | 1.09 ± 0.96 a | 5.08 ± 0.11 c | ND | 8.20 c | |
| 180 min | 2.11 ± 0.11 b | 1.34 ± 0.05 a | 8.79 ± 0.16 d | ND | 12.24 d | |
| 150 °C | 30 min | 0.48 ± 0.08 a | 0.20 ± 0.03 a | 1.21 ± 0.04 a | ND | 1.89 a |
| 60 min | 0.72 ± 0.05 b | 0.57 ± 0.07 b | 2.15 ± 0.04 b | ND | 3.44 b | |
| 120 min | 1.11 ± 0.03 c | 0.99 ± 0.03 c | 3.09 ± 0.06 c | ND | 5.19 c | |
| 180 min | 1.49 ± 0.05 d | 1.28 ± 0.06 d | 4.37 ± 0.05 d | ND | 7.14 d | |
| 120 °C | 30 min | 0.28 ± 0.04 a | 0.13 ± 0.02 a | 0.53 ± 0.07 a | ND | 0.94 a |
| 60 min | 0.69 ± 0.05 b | 0.37 ± 0.01 b | 1.03 ± 0.02 b | ND | 2.09 b | |
| 120 min | 0.94 ± 0.07 b | 0.86 ± 0.04 c | 2.47 ± 0.05 c | ND | 4.27 c | |
| 180 min | 1.36 ± 0.11 c | 1.05 ± 0.03 d | 3.31 ± 0.01 d | ND | 5.72 d | |
| Temperature and Time | Composition and Content of Oxidation Products/% | |||||
|---|---|---|---|---|---|---|
| 7α(β)-Hydroxy Sterols | 5α(β), 6α(β)- Epoxy sterols | 7-ketosterols | Triols | Total Content | ||
| 180 °C | 30 min | 1.03 ± 0.09 a | 1.06 ± 0.14 a | 3.37 ± 0.13 a | 0.37 ± 0.04 a | 5.83 a |
| 60 min | 1.49 ± 0.10 b | 1.23 ± 0.12 ab | 5.13 ± 0.10 b | 0.32 ± 0.07 a | 8.17 b | |
| 120 min | 1.88 ± 0.08 bc | 1.78 ± 0.09 bc | 8.38 ± 0.09 c | 0.28 ± 0.03 a | 12.32 c | |
| 180 min | 2.12 ± 0.16 c | 2.05 ± 0.23 c | 13.12 ± 0.17 d | 0.35 ± 0.04 a | 17.64 d | |
| 150 °C | 30 min | 1.01 ± 0.11 a | 0.38 ± 0.12 a | 2.28 ± 0.02 a | 0.27 ± 0.02 a | 3.94 a |
| 60 min | 1.44 ± 0.09 ab | 0.57 ± 0.07 a | 3.47 ± 0.06 b | 0.25 ± 0.03 a | 5.73 b | |
| 120 min | 1.87 ± 0.18 b | 1.27 ± 0.15 b | 4.11 ± 0.05 c | 0.28 ± 0.05 a | 7.53 c | |
| 180 min | 2.03 ± 0.22 b | 1.68 ± 0.18 b | 5.87 ± 0.03 d | 0.22 ± 0.08 a | 9.80 d | |
| 120 °C | 30 min | 0.62 ± 0.21 a | 0.23 ± 0.04 a | 1.23 ± 0.16 a | 0.24 ± 0.12 a | 2.32 a |
| 60 min | 1.03 ± 0.15 ab | 0.41 ± 0.13 ab | 2.94 ± 0.17 b | 0.29 ± 0.03 a | 4.67 b | |
| 120 min | 1.59 ± 0.07 bc | 0.68 ± 0.09 bc | 3.23 ± 0.09 b | 0.22 ± 0.02 a | 5.72 c | |
| 180 min | 1.84 ± 0.12 c | 0.82 ± 0.11 c | 4.06 ± 0.21 c | 0.18 ± 0.06 a | 6.90 d | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, D.; Tang, G.; Zhou, J.; Liu, W.; Bi, Y. Thermal-Oxidation Stability of Soybean Germ Phytosterols in Different Lipid Matrixes. Molecules 2020, 25, 4079. https://doi.org/10.3390/molecules25184079
Chen J, Li D, Tang G, Zhou J, Liu W, Bi Y. Thermal-Oxidation Stability of Soybean Germ Phytosterols in Different Lipid Matrixes. Molecules. 2020; 25(18):4079. https://doi.org/10.3390/molecules25184079
Chicago/Turabian StyleChen, Jingnan, Dami Li, Guiyun Tang, Jinfen Zhou, Wei Liu, and Yanlan Bi. 2020. "Thermal-Oxidation Stability of Soybean Germ Phytosterols in Different Lipid Matrixes" Molecules 25, no. 18: 4079. https://doi.org/10.3390/molecules25184079




