Design, Synthesis and Biological Investigation of Flavone Derivatives as Potential Multi-Receptor Atypical Antipsychotics
Abstract
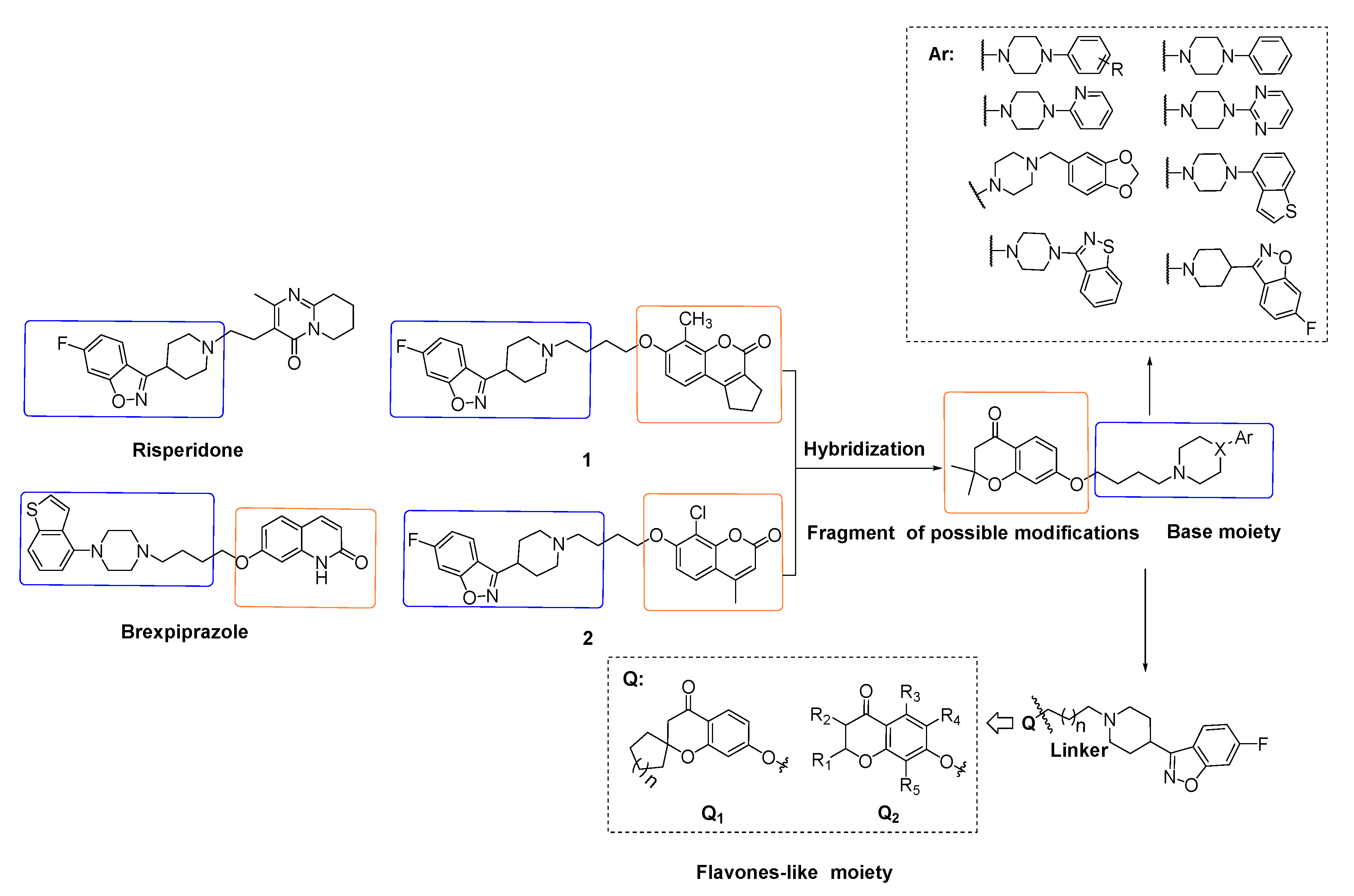
:1. Introduction
2. Results and Discussion
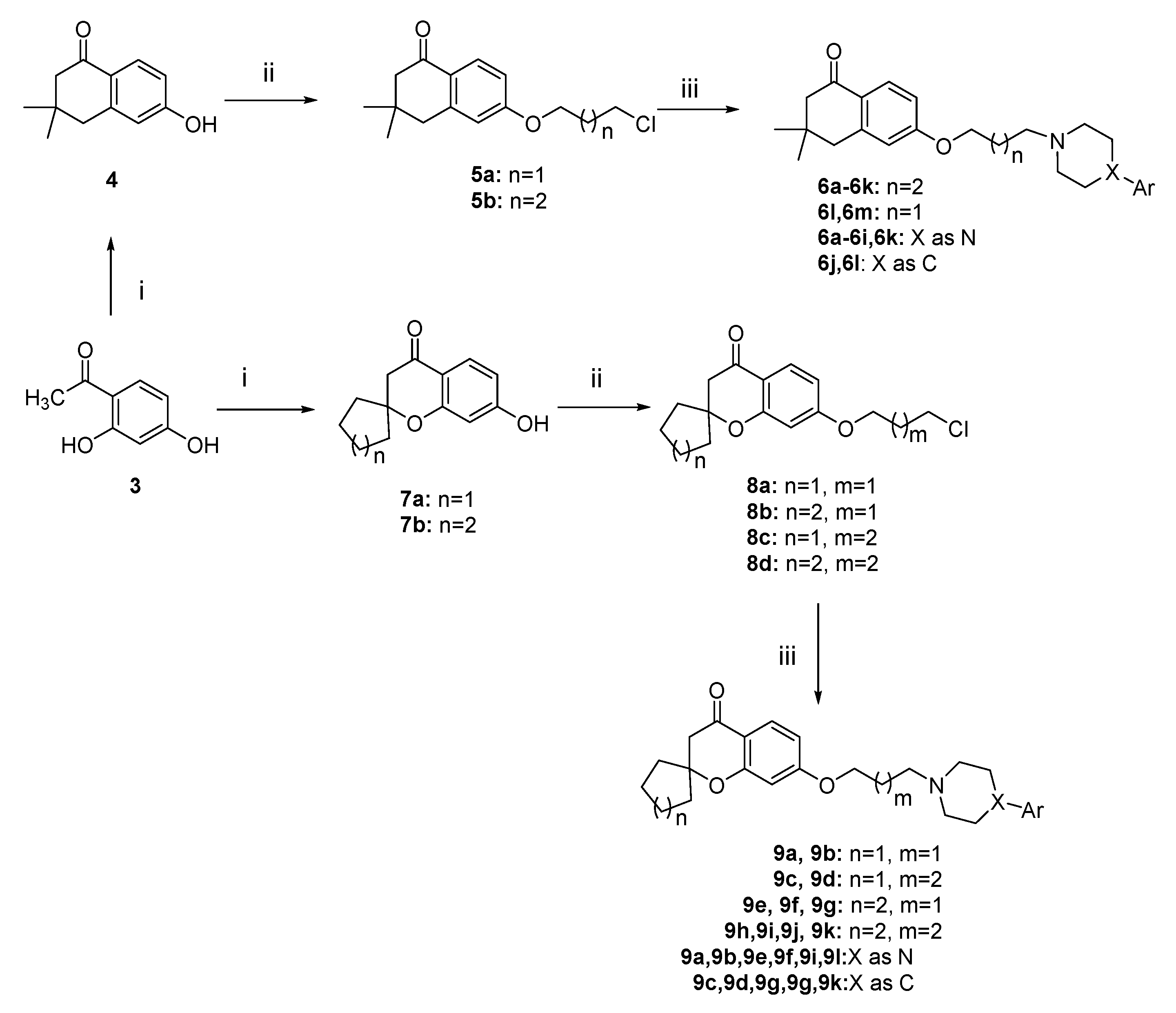
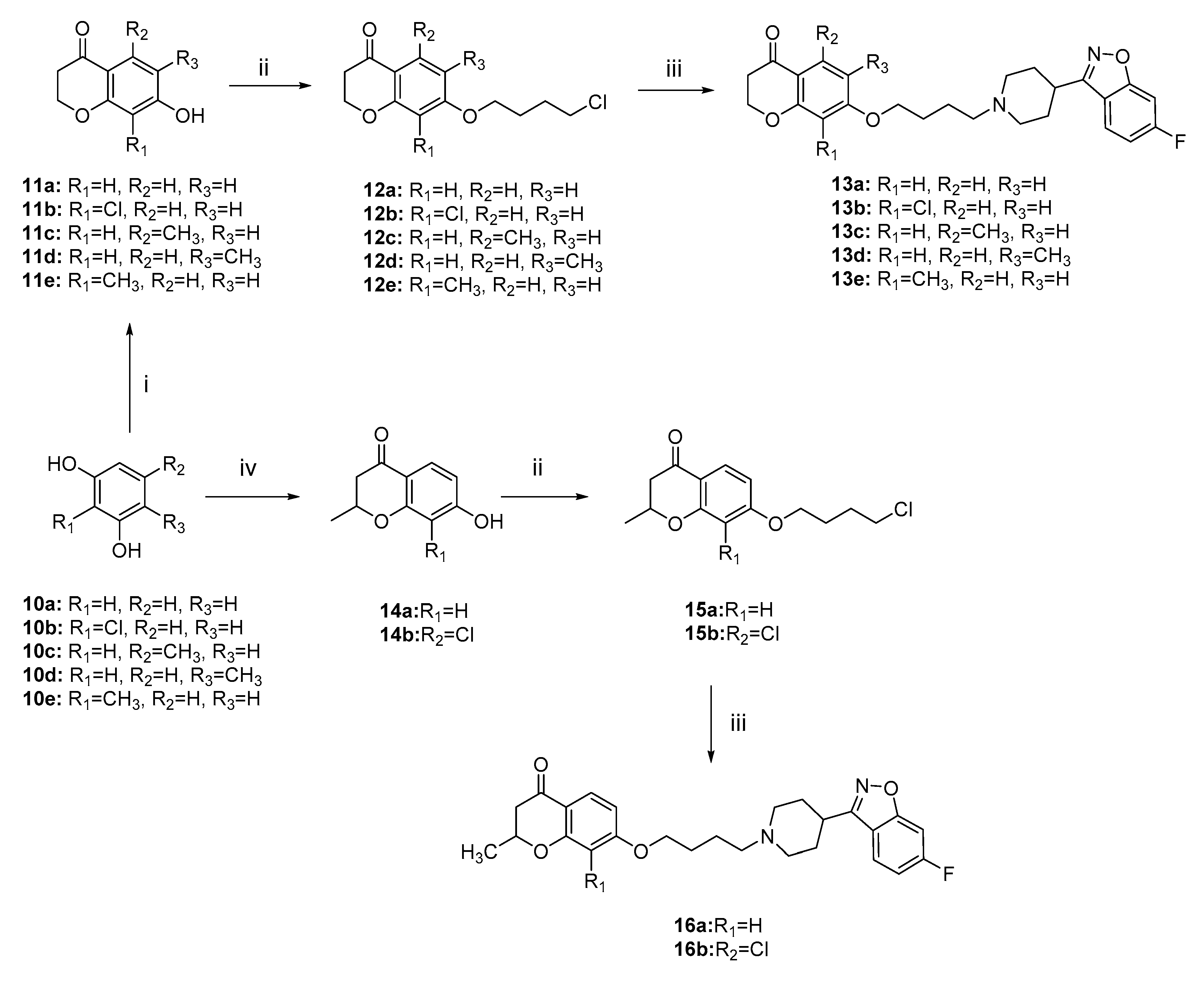
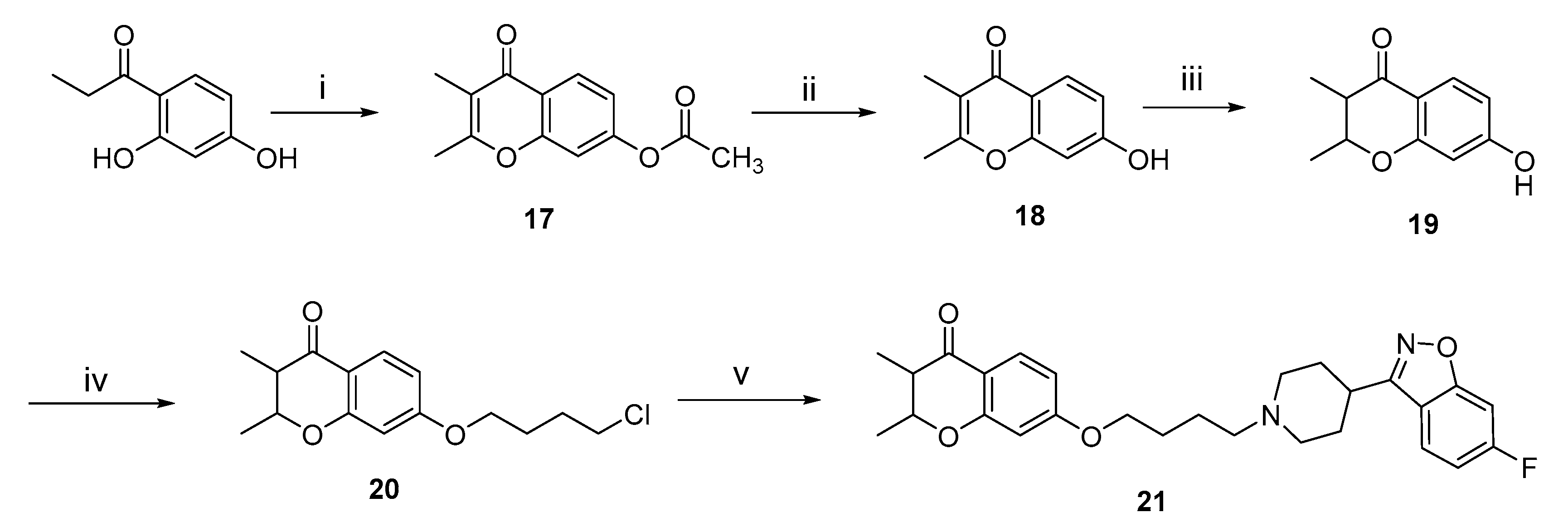
2.1. Chemistry
2.2. In Vitro Evaluation and Structure-Activity Relationship (SAR)
2.3. Acute Toxicity
2.4. Intrinsic Activity of Compound 6j
2.5. Behavioral Studies
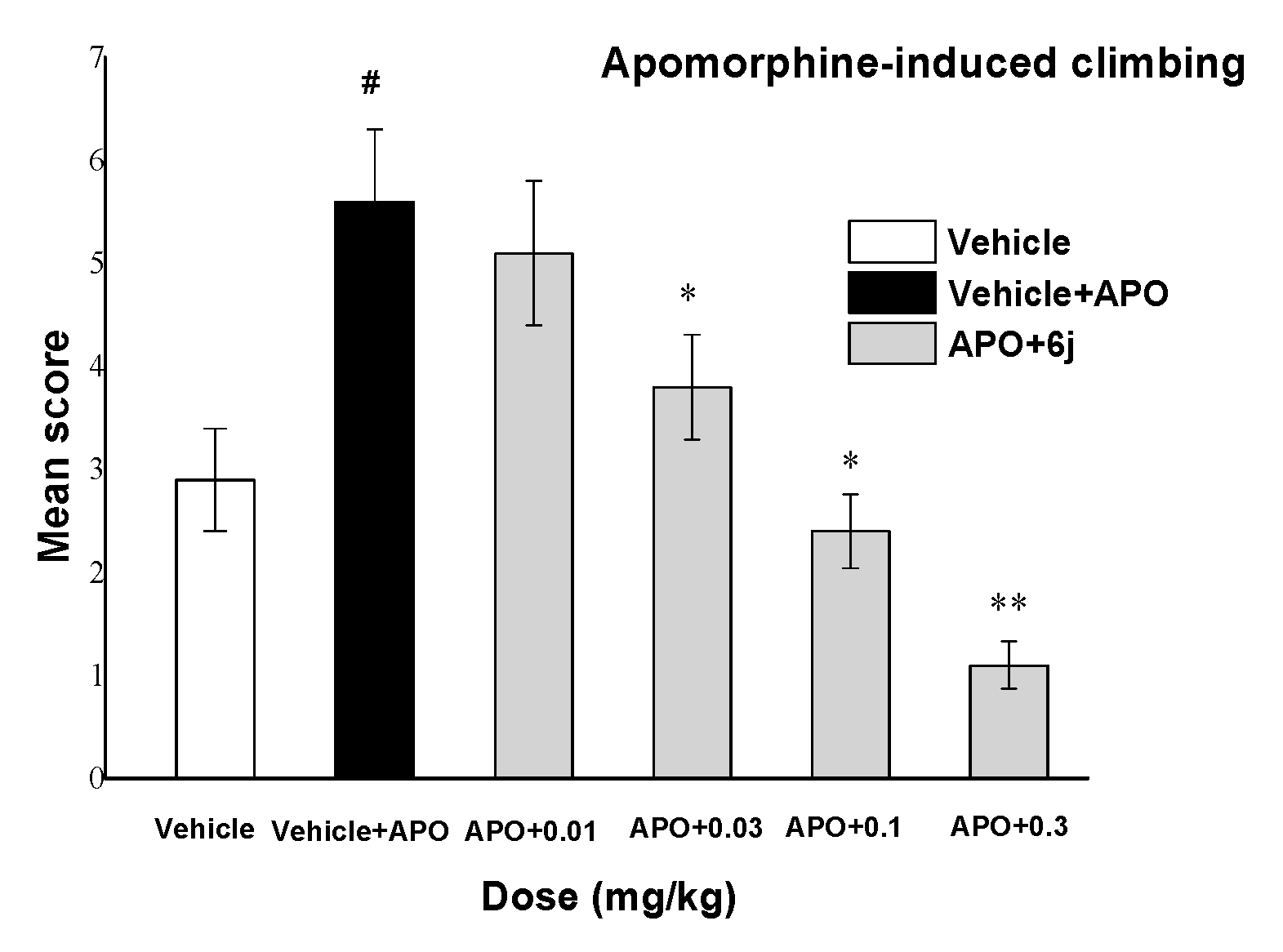
2.5.1. Apomorphine-Induced Hyperlocomotion
2.5.2. MK-801-Induced Hyperactivity
2.5.3. Catalepsy
3. Materials and Methods
3.1. General Information
3.2. Instrumentation
3.3. Synthesis
3.3.1. General Procedures for the Preparation of Intermediates 4 and 7
3.3.2. General Procedures for the Preparation of Intermediates 5 and 8
3.3.3. General Procedures for the Preparation of Target Compounds 6 and 9
3.3.4. General Procedures for the Preparation of Intermediates 11, 12, 14, and 15
3.3.5. General Procedures for the Preparation of Target Intermediates 13 and 16
3.3.6. General Procedures for the Preparation of Intermediates 17, 18 and 19
3.3.7. The Preparation of Intermediate 20 and Target Compound 21
3.3.8. The Characteristics and Spectroscopic Data of the Target Compounds
3.4. Biological Studies
Ethics Statement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- David, S. Global Mental Health: Principles and Practice. Occup. Med. 2014, 64, 649–650. [Google Scholar]
- Tandon, R.; Nasrallah, H.A.; Keshavan, M.S. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr. Res. 2009, 110, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, N.W., Jr. Chlorpromazine in the treatment of neuropsychiatric disorders. J. Am. Med. Assoc. 1954, 155, 18–21. [Google Scholar] [CrossRef]
- Tyler, M.W.; Zaldivar-Diez, J.; Haggarty, S.J. Classics in Chemical Neuroscience: Haloperidol. ACS Chem. Neurosci. 2017, 8, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.A.; Moore, H.; Stott, L.; Holliday, N.; Javitch, J.A.; Lane, J.R.; Charlton, S.J. Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Remington, G.; Lee, J.; Agid, O.; Takeuchi, H.; Foussias, G.; Hahn, M.; Fervaha, G.; Burton, L.; Powell, V. Clozapine’s critical role in treatment resistant schizophrenia: Ensuring both safety and use. Expert Opin. Drug Saf. 2016, 15, 1193–1203. [Google Scholar] [CrossRef]
- Chopko, T.C.; Lindsley, C.W. Classics in Chemical Neuroscience: Risperidone. ACS Chem. Neurosci. 2018, 9, 1520–1529. [Google Scholar] [CrossRef]
- Meltzer, H.Y. New Trends in the Treatment of Schizophrenia. CNS Neurol. Disord. Drug Targets 2017, 16, 900–906. [Google Scholar] [CrossRef]
- Gareri, P.; De Fazio, P.; De Fazio, S.; Marigliano, N.; Ferreri, I.; Ibbadu, G.; De Sarro, G. Adverse effects of atypical antipsychotics in the elderly: A review. Drugs Aging 2006, 23, 937–956. [Google Scholar] [CrossRef]
- Muench, J.; Hamer, A.M. Adverse effects of antipsychotic medications. Am. Fam. Physician 2010, 81, 617–622. [Google Scholar]
- Yang, F.P.; He, Y.; Wang, Z.; Wang, Y.; Shen, J.S. Research progress of antipsychotics. Acta Pharm. Sin. 2016, 51, 1809–1821. [Google Scholar]
- Kondej, M.; Stępnicki, P.; Kaczor, A.A. Multi-target approach for drug discovery against schizophrenia. Int. J. Mol. Sci. 2018, 19, 3105. [Google Scholar] [CrossRef] [Green Version]
- Milelli, A.; Turrini, E.; Catanzaro, E.; Maffei, F.; Fimognari, C. Perspectives in designing multifunctional molecules in antipsychotic drug discovery. Drug Dev. Res. 2016, 77, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Xu, X.; Liu, X.; Yu, M.; Zhao, S.; Liu, S.; Qiu, Y.; Zhang, T.; Liu, B.F.; et al. Synthesis and biological investigation of coumarin piperazine (piperidine) derivatives as potential multireceptor atypical antipsychotics. J. Med. Chem. 2013, 56, 4671–4690. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lan, Y.; Wang, S.; Zhang, H.; Xu, X.; Liu, X.; Yu, M.; Liu, B.F.; Zhang, G. Synthesis and evaluation of new coumarin derivatives as potential atypical antipsychotics. Eur. J. Med. Chem. 2014, 74, 427–439. [Google Scholar] [CrossRef]
- Yee, A. Brexpiprazole for the treatment of schizophrenia. Expert Rev. Neurother. 2016, 16, 109–122. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Chen, Y.; Qiu, Y.; Yu, M.; Xu, X.; Liu, X.; Liu, B.F.; Zhang, G. Synthesis and biological evaluation of fused tricyclic heterocycle piperazine (piperidine) derivatives as potential multireceptor atypical antipsychotics. J. Med. Chem. 2018, 61, 10017–10039. [Google Scholar] [CrossRef]
- Reynolds, G.P.; Kirk, S.L. Metabolic side effects of antipsychotic drug treatment–pharmacological mechanisms. Pharmacol. Ther. 2010, 125, 169–179. [Google Scholar] [CrossRef]
- Olten, B.; Bloch, M.H. Meta regression: Relationship between antipsychotic receptor binding profiles and side-effects. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 84, 272–281. [Google Scholar] [CrossRef]
- Krebs, M.; Leopold, K.; Hinzpeter, A.; Schaefer, M. Current schizophrenia drugs: Efficacy and side effects. Expert Opin. Pharmacother. 2006, 7, 1005–1016. [Google Scholar] [CrossRef]
- Montastruc, F.; Palmaro, A.; Bagheri, H.; Schmitt, L.; Montastruc, J.L.; Lapeyre-Mestre, M. Role of serotonin 5-HT2C and histamine H1 receptors in antipsychotic-induced diabetes: A pharmacoepidemiological-pharmacodynamic study in VigiBase. Eur. Neuropsychopharmacol. 2015, 25, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Svensson, T.H. α-Adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2003, 27, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.C.; Ainslie, P.N.; Atkinson, G.; Jones, H.; Grant, E.J.; Lucas, S.J. Initial orthostatic hypotension and cerebral blood flow regulation: Effect of α1-adrenoreceptor activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R147–R154. [Google Scholar] [CrossRef] [PubMed]
- Laszy, J.; Laszlovszky, I.; Gyertyán, I. Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology 2005, 179, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Banasikowski, T.J.; Beninger, R.J. Reduced expression of haloperidol conditioned catalepsy in rats by the dopamine D3 receptor antagonists nafadotride and NGB 2904. Eur. Neuropsychopharmacol. 2012, 22, 761–768. [Google Scholar] [CrossRef]
- Moorthy, N.S.N.; Ramos, M.J.; Fernandes, P.A. Human ether-a-go-go-related gene channel blockers and its structural analysis for drug design. Curr. Drug Targets 2013, 14, 102–113. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Barakat, K.H. Development of Safe Drugs: The hERG Challenge. Med. Res. Rev. 2018, 38, 525–555. [Google Scholar] [CrossRef]
- Jones, C.A.; Watson, D.J.; Fone, K.C. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Ljungberg, T. Dopamine D2 antagonists reverse apomorphine-induced decreased water intake in the rat: Prediction of antipsychotic drugs with few extrapyramidal side-effects? J. Neural Transm. 1989, 76, 79–90. [Google Scholar] [CrossRef]
- Tuplin, E.W.; Stocco, M.R.; Holahan, M.R. Attenuation of MK-801-induced behavioral perseveration by typical and atypical antipsychotic pretreatment in rats. Behav. Neurosci. 2015, 129, 399–411. [Google Scholar] [CrossRef]
- Hoffman, D.C.; Donovan, H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology 1995, 120, 128–133. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

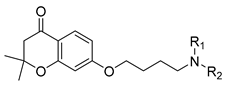
| Compound | NR1R2 | Receptor Affinity Ki ± SEM (nM) a | ||
|---|---|---|---|---|
| D2 | 5-HT1A | 5-HT2A | ||
| 6a | 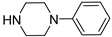 | 553.5 ± 68.7 | 118.9 ± 17.4 | 89.8 ± 11.3 |
| 6b | 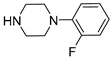 | 776.8 ± 84.7 | 155.6 ± 19.5 | 72.5 ± 8.4 |
| 6c | 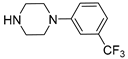 | 1128.6 ± 108.4 | 269.1 ± 30.3 | 147.4 ± 16.9 |
| 6d | 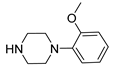 | 2351.5 ± 317.4 | 275.2 ± 25.2 | 86.8 ± 5.6 |
| 6e | 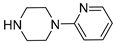 | 4587.3 ± 510.3 | 627.4 ± 75.3 | 722.1 ± 64.9 |
| 6f | 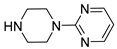 | >10,000 b | 735.6 ± 82.7 | 1135.1 ± 151.7 |
| 6g | 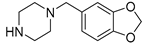 | 1135.2 ± 140.4 | 216.5 ± 20.8 | 379.5 ± 39.1 |
| 6h |  | 43.1 ± 5.7 | 25.3 ± 2.8 | 55.2 ± 4.9 |
| 6i | 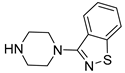 | 26.2 ± 2.7 | 81.7 ± 9.5 | 19.5 ± 2.2 |
| 6j | 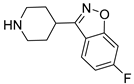 | 12.1 ± 1.5 | 9.7 ± 1.3 | 3.2 ± 0.4 |
| Compound | Structure | Receptor Affinity Ki ± SEM (nM) a | ||
|---|---|---|---|---|
| D2 | 5-HT1A | 5-HT2A | ||
| 6k | 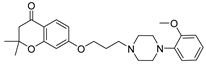 | 3157.4 ± 466.4 | 115.8 ± 15.8 | 33.1 ± 4.5 |
| 6l | 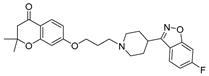 | 18.6 ± 2.3 | 11.5 ± 1.2 | 6.7 ± 0.7 |
| 9a | 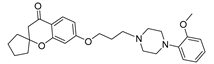 | 3853.8 ± 327.9 | 476.1 ± 57.5 | 72.0 ± 6.8 |
| 9b | 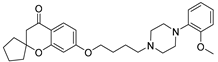 | 3793.4 ± 408.7 | 384.9 ± 49.4 | 61.7 ± 8.6 |
| 9c |  | 18.9 ± 2.1 | 10.4 ± 1.4 | 8.3 ± 0.9 |
| 9d |  | 15.7 ± 1.9 | 8.2 ± 0.8 | 8.5 ± 0.7 |
| 9e | 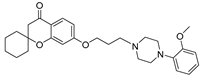 | 2054.9 ± 229.5 | 312.1 ± 41.4 | 93.5 ± 7.9 |
| 9f | 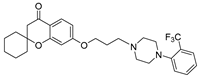 | 914.6 ± 90.3 | 48.3 ± 5.2 | 76.7 ± 9.4 |
| 9g |  | 18.4 ± 1.8 | 7.7 ± 0.9 | 9.1 ± 1.1 |
| 9h | 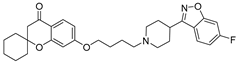 | 17.0 ± 2.3 | 5.6 ± 0.3 | 7.3 ± 0.4 |
| 9i | 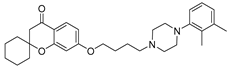 | 154.2 ± 19.6 | 92.1 ± 8.8 | 67.5 ± 7.4 |
| 9j | 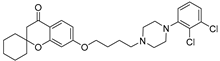 | 81.7 ± 9.7 | 76.3 ± 7.1 | 35.8 ± 5.2 |
| 9k | 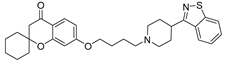 | 35.1 ± 4.7 | 213.3 ± 39.5 | 75.6 ± 8.6 |
| 13a |  | 22.5 ± 2.6 | 13.1 ± 1.7 | 9.6 ± 0.8 |
| 13b | 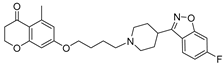 | 28.9 ± 3.4 | 24.6 ± 2.9 | 10.5 ± 1.3 |
| 13c | 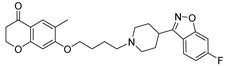 | 25.8 ± 3.9 | 18.1 ± 1.3 | 15.9 ± 2.3 |
| 13d | 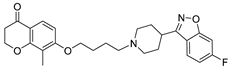 | 22.9 ± 3.1 | 17.0 ± 2.2 | 13.7 ± 1.9 |
| 16a | 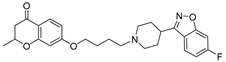 | 19.7 ± 2.7 | 10.1 ± 1.3 | 7.9 ± 0.5 |
| 16b | 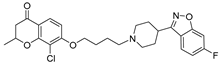 | 21.4 ± 2.4 | 78.8 ± 8.9 | 8.1 ± 1.1 |
| 21 | 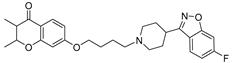 | 28.4 ± 3.2 | 16.2 ± 1.5 | 2.4 ± 1.9 |
| Clozapine | 132.5 ± 13.7 | 188.5 ± 18.3 | 12.7 ± 1.8 | |
| Risperidone | 3.5 ± 0.4 | 191.5 ± 16.9 | 0.17 ± 0.03 | |
| Compound | Receptor Affinity Ki ± SEM (nM) a | hERG IC50 (nM) | |||
|---|---|---|---|---|---|
| D3 | 5-HT2C | α1 | H1 | ||
| 6j | 25.4 ± 3.1 | 565.8 ± 75.4 | 237.2 ± 86.4 | 938.5 ± 101.3 | 3769.8 ± 297.5 |
| 9d | 42.6 ± 5.3 | 425.6 ± 47.8 | 161.7 ± 57.1 | 784.2 ± 98.3 | 3380.3 ± 352.3 |
| 9g | 35.2 ± 2.8 | 401.1 ± 55.6 | 138.2 ± 39.8 | 909.3 ± 78.5 | 3015.5 ± 290.8 |
| 9h | 38.6 ± 3.7 | 528.2 ± 68.5 | 101.5 ± 50.6 | 819.5 ± 91.8 | 3982.6 ± 411.5 |
| Risperidone | 10.9 ± 1.9 | 19.7 ± 2.7 | 2.8 ± 0.4. | 26.1 ± 2.8 | 168.5 ± 23.1 |
| Clozapine | 265.2 ± 18.5 | 16.1 ± 3.1 | 33.6 ± 3.9 | 4.3 ± 0.5 | 2750.4 ± 317.2 |
| Compound | APO a | MK-80 b | CAT c | CAT/APO | CAT/MK-801 | LD50 |
|---|---|---|---|---|---|---|
| 6j | 0.19 | 0.16 | 56.84 | 299.16 | 355.25 | >2000 |
| Clozapine | 17.92 | 2.28 | 50.0 | 21.93 | 5.58 | 150.0 |
| Risperidone | 0.046 | 0.011 | 0.92 | 20 | 83.63 | 82.1 |
| Receptor | Compd | Activation (10 μM, %) (n = 3) | EC50 (nM) | Inhibition (10 μM, %) (n = 3) | IC50 (nM) |
|---|---|---|---|---|---|
| D2L | dopamine | 101.3 ± 5.3 | 19.1 | ||
| SCH23390 | 99.1 ± 3.2 | 31.6 | |||
| 6j | 4.5 ± 0.6 | 97.4 ± 4.1 | 8.9 | ||
| D3 | dopamine | 98.4 ± 4.4 | 2.5 | ||
| spiperone | 98.8 ± 4.8 | 93.9 | |||
| 6j | 5.9 ± 0.7 | 97.5 ± 2.7 | 31.5 | ||
| 5-HT1A | 5-HT | 99.7 ± 3.8 | 2.3 | ||
| WAY-100635 | 98.4 ± 3.7 | 11.2 | |||
| 6j | 0.8 ± 0.1 | 99.1 ± 4.5 | 201.4 | ||
| 5-HT2A | 5-HT | 100.2 ± 2.6 | 20.4 | ||
| ketanserin | 98.2 ± 2.6 | 87.6 | |||
| 6j | 1.4 ± 0.2 | 99.8 ± 2.2 | 195.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Yang, Z.; Xiong, J.; Hao, C.; Ma, R.; Liu, X.; Liu, B.-F.; Jin, J.; Zhang, G.; Chen, Y. Design, Synthesis and Biological Investigation of Flavone Derivatives as Potential Multi-Receptor Atypical Antipsychotics. Molecules 2020, 25, 4107. https://doi.org/10.3390/molecules25184107
Gao L, Yang Z, Xiong J, Hao C, Ma R, Liu X, Liu B-F, Jin J, Zhang G, Chen Y. Design, Synthesis and Biological Investigation of Flavone Derivatives as Potential Multi-Receptor Atypical Antipsychotics. Molecules. 2020; 25(18):4107. https://doi.org/10.3390/molecules25184107
Chicago/Turabian StyleGao, Lanchang, Zhengge Yang, Jiaying Xiong, Chao Hao, Ru Ma, Xin Liu, Bi-Feng Liu, Jian Jin, Guisen Zhang, and Yin Chen. 2020. "Design, Synthesis and Biological Investigation of Flavone Derivatives as Potential Multi-Receptor Atypical Antipsychotics" Molecules 25, no. 18: 4107. https://doi.org/10.3390/molecules25184107
APA StyleGao, L., Yang, Z., Xiong, J., Hao, C., Ma, R., Liu, X., Liu, B. -F., Jin, J., Zhang, G., & Chen, Y. (2020). Design, Synthesis and Biological Investigation of Flavone Derivatives as Potential Multi-Receptor Atypical Antipsychotics. Molecules, 25(18), 4107. https://doi.org/10.3390/molecules25184107






