Instant Controlled Pressure Drop as Blanching and Texturing Pre-Treatment to Preserve the Antioxidant Compounds of Red Dried Beetroot (Beta vulgaris L.)
Abstract
1. Introduction
2. Results
2.1. Proximal Analysis
2.2. Bioactive Compounds Quantification
2.2.1. Total Phenol Content
2.2.2. Total Flavonoid Content
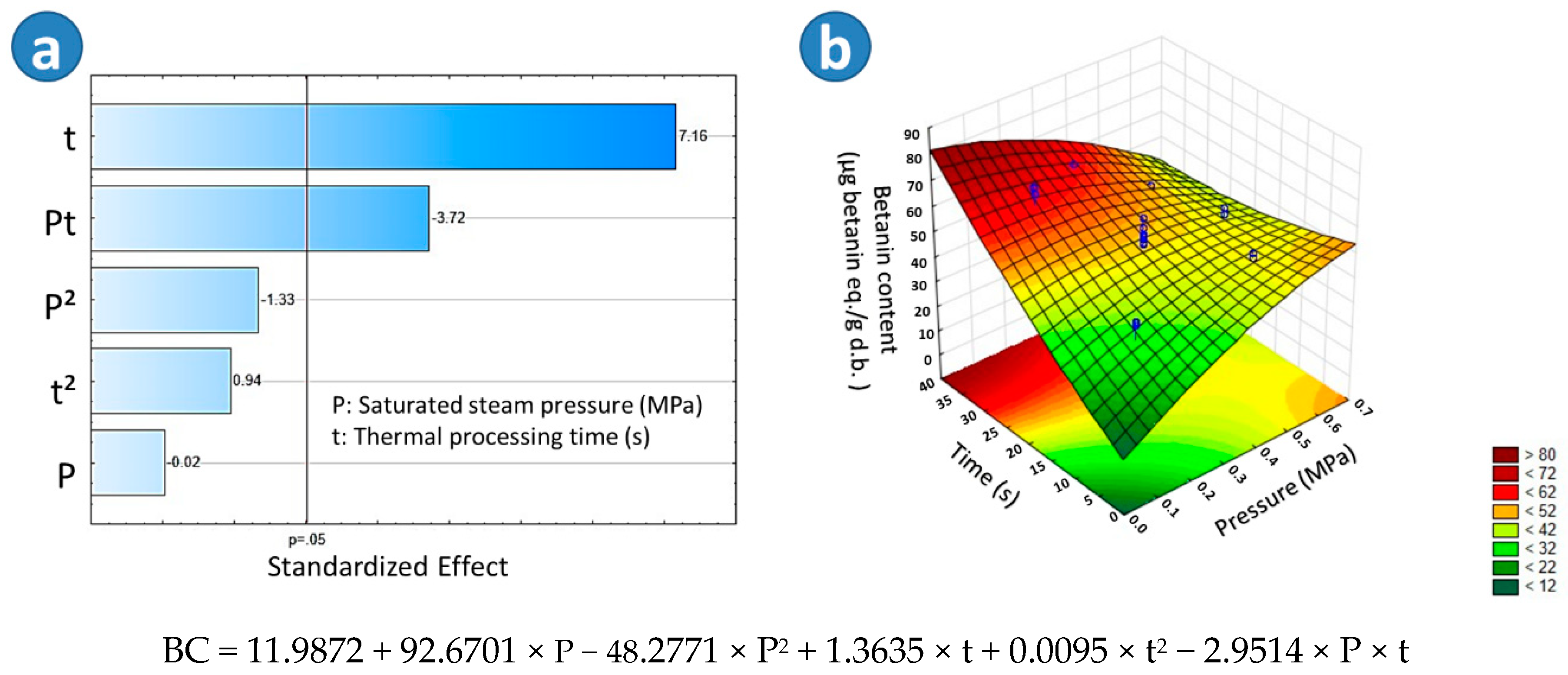
2.2.3. Betanin Concentration
2.3. Antioxidant Activity
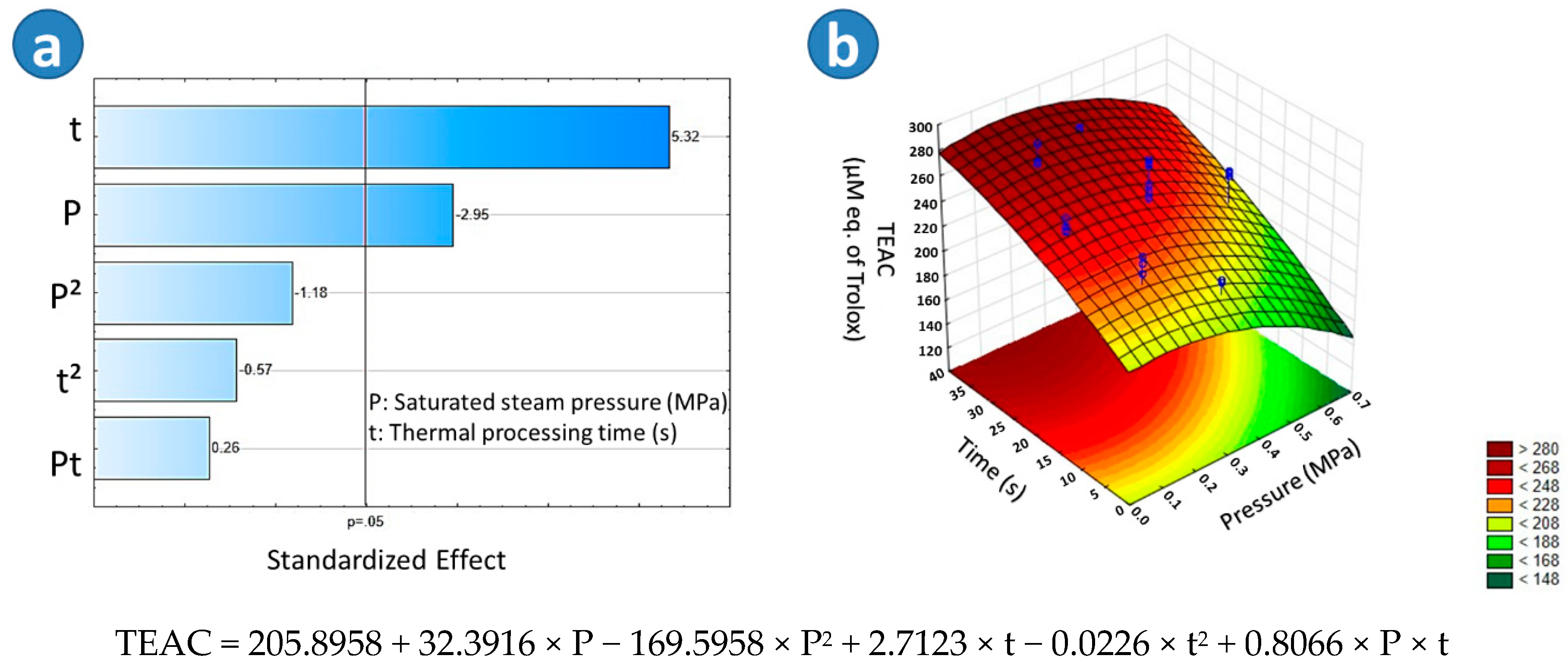
2.3.1. Trolox Equivalent Antioxidant Capacity (TEAC)
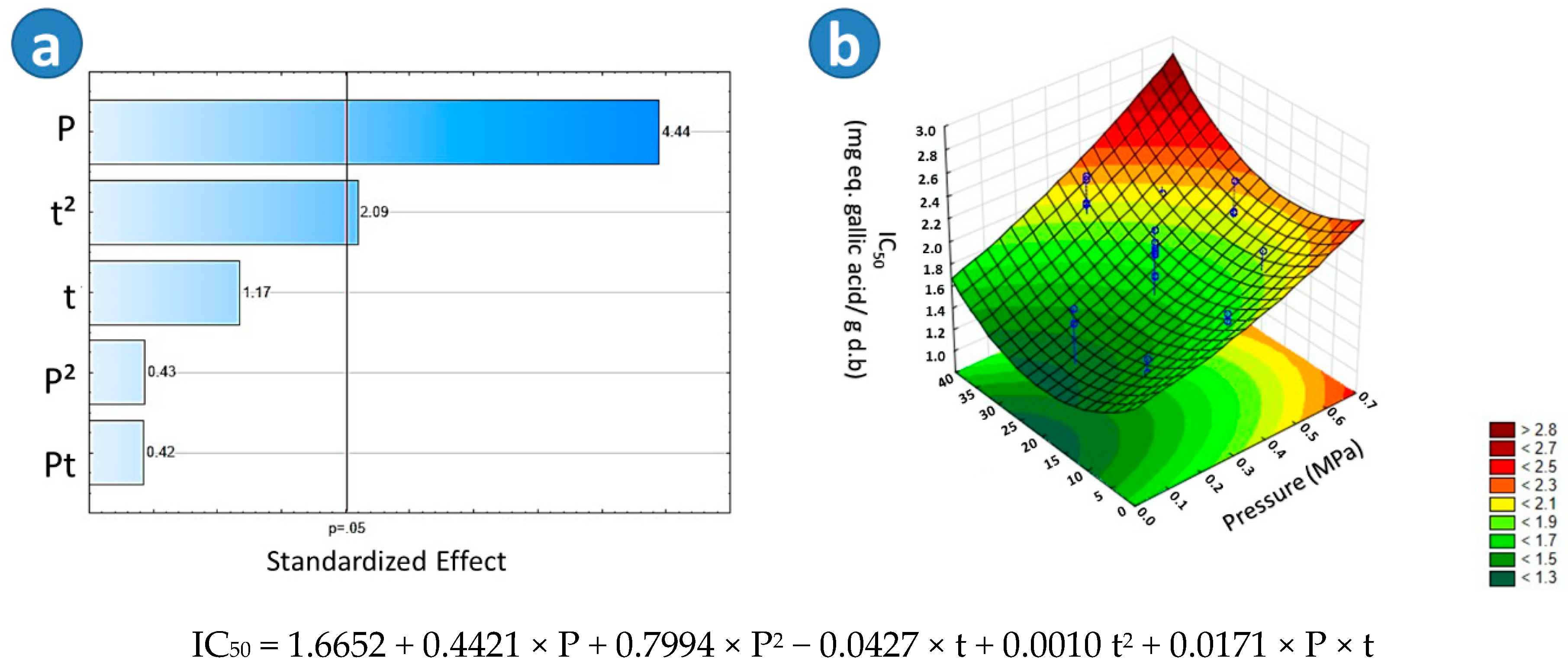
2.3.2. Free Radical Scavenging Activity by DPPH (IC50)
3. Discussion
3.1. Proximal Analysis
3.2. Bioactive Compounds Quantification
3.3. Antioxidant Activity
4. Materials and Methods
4.1. Materials
4.1.1. Biological Material
4.1.2. Reagents and Solvents
4.2. Methods
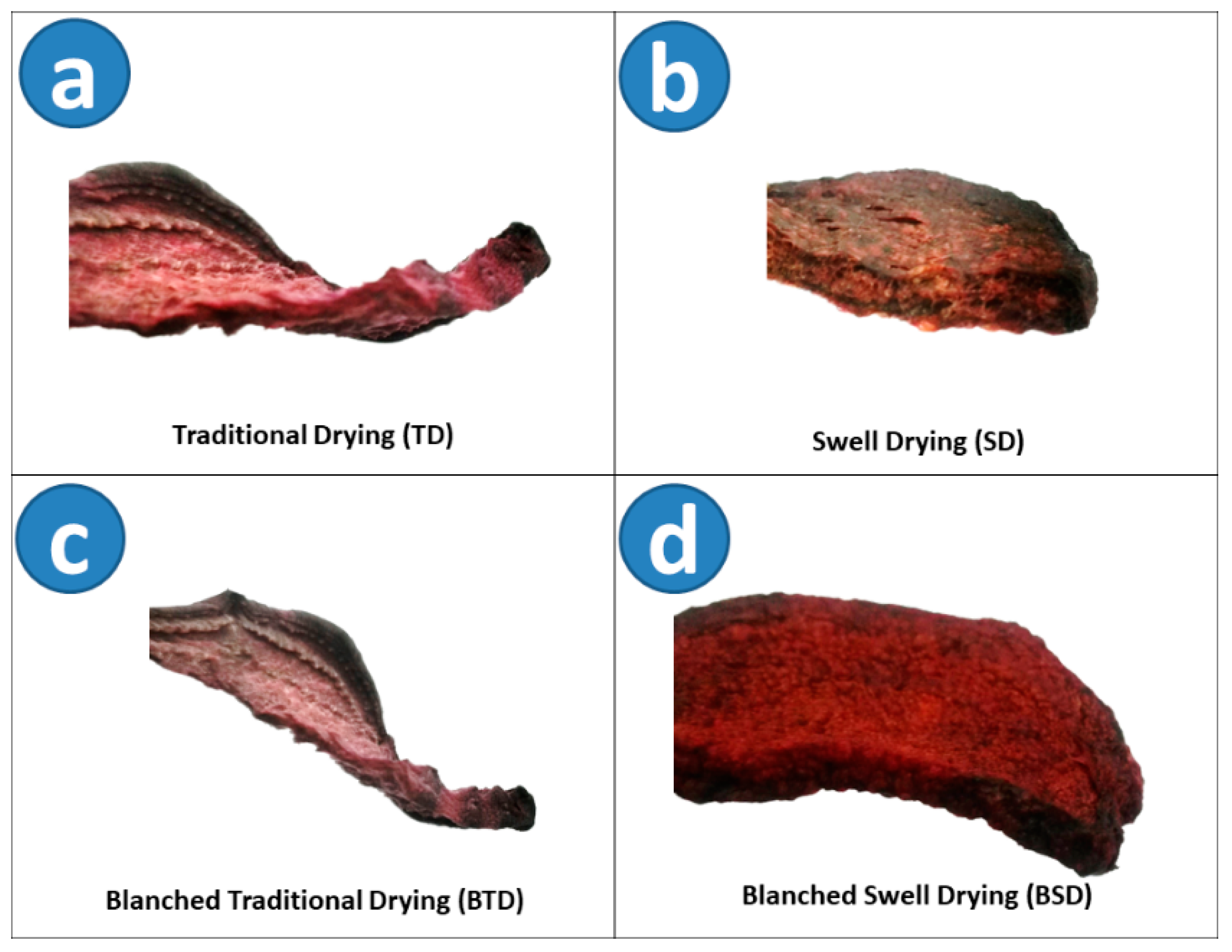
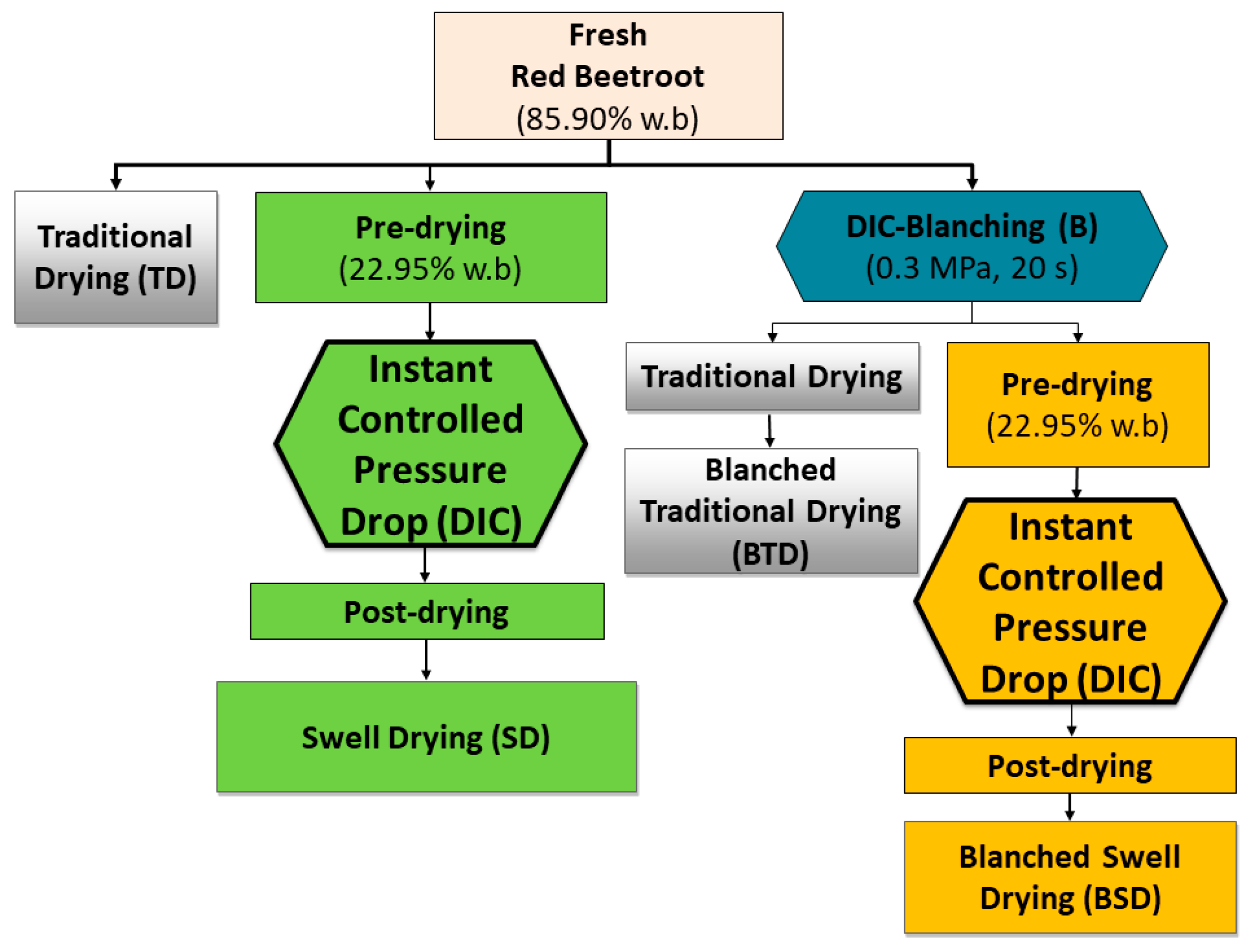
4.2.1. Blanching and Drying Procedures
- (1)
- The raw material for Traditional Drying (TD)
- (2)
- The raw material for Swell Drying (SD)
- (3)
- The raw material for DIC Blanching (B)
- (4)
- DIC Blanching + Traditional Drying (BTD)
- (5)
- DIC Blanching + Swell Drying (BSD)
- (a)
- A pre-drying of red beetroots via convective air drying until reaching a moisture content of 22.95% w.b.
- (b)
- A DIC treatment (saturated steam pressure “P” of 0.35 MPa and thermal treatment time “t” of 20 s)
- (c)
- A post-drying via convective air drying until reaching a ~4% w.b. moisture content.
4.2.2. Experimental Design
4.2.3. Proximal Analysis
4.2.4. Bioactive Compounds Quantification and Antioxidant Activity
4.3. Methanolic Extracts Preparation
4.4. Total Phenolics Content
4.5. Total Flavonoids Content
4.6. Betanin Content by HPLC
4.7. Trolox Equivalent Antioxidant Capacity (TEAC)
4.8. Free Radical Scavenging Activity by DPPH (IC50)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wootton-Beard, P.C.; Ryan, L. A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. In Proceedings of the Proceedings of the Nutrition Society, Oxford, UK, 4–6 July 2011. [Google Scholar]
- Bhupinder, S.; Bahadur, S.H. Chemical composition, functional properties and processing of beetroot-a review. Int. J. Sci. Eng. Res. 2014, 5, 679–684. [Google Scholar]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Tokuda, H.; Konoshima, T.; Nishino, H. Chemoprevention of lung and skin cancer by Beta vulgaris (beet) root extract. Cancer Lett. 1996, 100, 211–214. [Google Scholar] [CrossRef]
- Shynkaryk, M.V.; Lebovka, N.I.; Vorobiev, E. Pulsed electric fields and temperature effects on drying and rehydration of red beetroots. Dry. Technol. 2008, 26, 695–704. [Google Scholar] [CrossRef]
- Paciulli, M.; Medina-Meza, I.G.; Chiavaro, E.; Barbosa-Cánovas, G.V. Impact of thermal and high pressure processing on quality parameters of beetroot (Beta vulgaris L.). LWT 2016, 68, 98–104. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C.; Santos, A.N.; Souza, A.C.; Mendes, K.C.; Andrade, M.I.R. Betacyanin stability during processing and storage of a microencapsulated red beetroot extract. Am. J. Food Technol. 2007, 2, 307–312. [Google Scholar] [CrossRef]
- Martínez-Parra, J.; Muñoz, R. Characterization of betacyanin oxidation catalyzed by a peroxidase from Beta vulgaris L. roots. J. Agric. Food Chem. 2001, 49, 4064–4068. [Google Scholar] [CrossRef]
- Guldiken, B.; Toydemir, G.; Memis, K.N.; Okur, S.; Boyacioglu, D.; Capanoglu, E. Home-processed red beetroot (Beta vulgaris L.) products: Changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 2016, 17, 858. [Google Scholar] [CrossRef]
- Allaf, T.; Allaf, K. Instant Controlled Pressure Drop (D.I.C.) in Food Processing: From Fundamental to Industrial Applications; Springer: New York, NY, USA, 2013. [Google Scholar]
- Alonzo-Macías, M.; Cardador-Martínez, A.; Mounir, S.; Montejano-Gaitán, G.; Allaf, K. comparative study of the effects of drying methods on antioxidant activity of dried strawberry (Fragaria Var. Camarosa). J. Food Res. 2013, 2, 92–107. [Google Scholar] [CrossRef]
- Alonzo-Macías, M.; Montejano-Gaitán, G.; Allaf, K. Impact of drying processes on strawberry (F ragaria var. Camarosa) texture: Identification of crispy and crunchy features by instrumental measurement. J. Texture Stud. 2014, 45, 246–259. [Google Scholar] [CrossRef]
- Setyopratomo, P.; Fatmawati, A.; Sutrisna, P.D.; Savitri, E.; Allaf, K. The dehydration kinetics, physical properties and nutritional content of banana textured by instantaneous controlled pressure drop. Asia-Pacific J. Chem. Eng. 2011, 7, 726–732. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Sabah, M.; Montejano-Gaitan, J.; Sobolik, V.; Cardador-Martínez, A.; Allaf, K. Impact of Instant Controlled Pressure Drop Treatment on Dehydration and Rehydration Kinetics of Green Moroccan Pepper (Capsicum Annuum). Procedia Eng. 2012, 42, 978–1003. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Sobolik, V.; Montejano-Gaitán, J.G.; Abdulla, G.; Allaf, K. Impact of Swell-Drying Process on Water Activity and Drying Kinetics of Moroccan Pepper (Capsicum annum). Dry. Technol. 2014, 33, 131–142. [Google Scholar] [CrossRef]
- Santiago-Mora, P.D.; Cardador-Martínez, A.; Tellez-Perez, C.; Montejano-Gaitan, J.G.; Del Campo, S.T.M. In-Vitro Antioxidant Capacity and Bioactive Compounds Preservation Post-Drying on Berrycacti (Myrtillocactus geometrizans). J. Food Res. 2017, 6, 121. [Google Scholar] [CrossRef]
- Mounir, S.; Téllez-Pérez, C.; Sunooj, K.V.; Allaf, K. Texture and color characteristics of swell-dried ready-to-eat Zaghloul date snacks: Effect of operative parameters of instant controlled pressure drop process. J. Texture Stud. 2020, 51, 276–289. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Cardador-Martínez, A.; Tejada-Ortigoza, V.; Soria-Mejía, M.C.; Balderas-León, I.; Alonzo-Macías, M. Antioxidant Content of Frozen, Convective Air-Dried, Freeze-Dried, and Swell-Dried Chokecherries (Prunus virginiana L.). Molecules 2020, 25, 1190. [Google Scholar] [CrossRef]
- Albitar, N.; Mounir, S.; Besombes, C.; Allaf, K. Improving the Drying of Onion Using the Instant Controlled Pressure Drop Technology. Dry. Technol. 2011, 29, 993–1001. [Google Scholar] [CrossRef]
- Nistor, O.-V.; Ceclu, L.S.; Andronoiu, D.G.; Rudi, L.; Botez, E. Influence of different drying methods on the physicochemical properties of red beetroot (Beta vulgaris L. var. Cylindra). Food Chem. 2017, 236, 59–67. [Google Scholar] [CrossRef]
- Latorre, M.E.; Plá, M.F.D.E.; Rojas, A.M.; Gerschenson, L.N. Blanching of red beet (Beta vulgaris L. var. conditiva) root. Effect of hot water or microwave radiation on cell wall characteristics. LWT 2013, 50, 193–203. [Google Scholar] [CrossRef]
- Huang, D.; Ou, A.B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Górnaś, P.; Dwiecki, K.; Siger, A.; Gras, J.; Michalak, M.; Polewski, K. Contribution of phenolic acids isolated from green and roasted boiled-type coffee brews to total coffee antioxidant capacity. Eur. Food Res. Technol. 2015, 242, 641–653. [Google Scholar] [CrossRef]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef]
- Koubaier, H.B.H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and Phenolic Compositions, Antioxidant Activity of Tunisian Red Beet (Beta vulgaris L.conditiva) Roots and Stems Extracts. Int. J. Food Prop. 2014, 17, 1934–1945. [Google Scholar] [CrossRef]
- Váli, L.; Stefanovits-Bányai, É.; Szentmihályi, K.; Fébel, H.; Sárdi, É.; Lugasi, A.; Kocsis, I.; Blázovics, A. Liver-protecting effects of table beet (Beta vulgaris var. rubra) during ischemia-reperfusion. Nutrients 2007, 23, 172–178. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Ulrih, N.P.; Cigić, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Kamal, I.M.; Sobolik, V.; Kristiawan, M.; Mounir, S.; Allaf, K. Structure expansion of green coffee beans using instantaneous controlled pressure drop process. Innov. Food Sci. Emerg. Technol. 2008, 9, 534–541. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC (Association of Official Analytical Chemists) International, 20th ed.; Latimer, G.W., Ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Oomah, B.D.; Cardador-Martínez, A.; Loarca-Piña, G. Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L.). J. Sci. Food Agric. 2005, 85, 935–942. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Boil. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of red dried beetroots are available from the authors. |




| Treatment | Total Phenolics Content (TPC) 1 | Total Flavonoids Content (TFC) 2 | Betanin Concentration (BC) 3 |
|---|---|---|---|
| TD | 6.95 ± 0.52 a,b | 2.04 ± 0.11 f,g | 49.36 ± 0.90 c,d |
| SD | 6.59 ± 0.74 a,b,c | 2.42 ± 0.10 a,b | 30.56 ± 0.18 h |
| BTD | 6.62 ± 1.20 a,b,c | 2.13 ± 0.14 c,d,e | 40.92 ± 2.86 f |
| BSD1 | 6.21 ± 0.52 b,c,d,e | 2.38 ± 0.18 a,b,c | 48.71 ± 2.46 d,e |
| BSD2 | 6.01 ± 0.63 c,d,e | 2.54 ± 0.15 a | 48.67 ± 1.39 d,e |
| BSD3 | 5.48 ± 0.18 d,e,f | 2.15 ± 0.18 c,d,e | 59.40 ± 3.92 b |
| BSD4 | 5.31 ± 0.11 e,f,g | 2.25 ± 0.18 c,d,e | 51.12 ± 0.21 c |
| BSD5 | 5.95 ± 0.27 c,d,e,f | 2.30 ± 0.56 b,c,d | 49.27 ± 0.82 c,d |
| BSD6 | 5.17 ± 0.23 e,f,g | 2.22 ± 0.25 c,d,e | 45.70 ± 1.72 e |
| BSD7 | 5.73 ± 0.45 d,e,f | 2.11 ± 0.19 d,e,f | 51.09 ± 1.47 c |
| BSD8 | 6.34 ± 1.12 b,c,d | 1.91 ± 0.24 g | 42.13 ± 0.50 f |
| BSD9 | 5.66 ± 0.56 d,e,f | 2.11 ± 0.15 c,d,e | 67.50 ± 1.28 a |
| BSD10 | 4.22 ± 0.65 h | 2.06 ± 0.53 e,f | 56.79 ± 3.13 b |
| BSD11 | 5.05 ± 0.30 g | 1.97 ± 0.47 f,g | 38.21 ± 1.96 g |
| BSD12 | 6.41 ± 1.33 b,c,d | 2.07 ± 0.27 e,f | 37.42 ± 1.29 g |
| BSD13 | 7.03 ± 0.98 a | 2.17 ± 0.10 c,d,e | 37.46 ± 0.61 g |
| Treatment | TEAC 1 | IC50 2 |
|---|---|---|
| TD | 278.03 ± 2.81 b | 2.69 ± 0.27 a |
| SD | 202.06 ± 13.49 h,i | 1.38 ± 0.03 h,i |
| BTD | 182.19 ± 9.30 j | 1.40 ± 0.06 g,h |
| BSD1 | 225.11 ± 3.44 f,g | 1.90 ± 0.13 c,d |
| BSD2 | 243.17 ± 1.49 c,d | 2.17 ± 0.14 b |
| BSD3 | 298.72 ± 12.45 a | 2.02 ± 0.04 b,c |
| BSD4 | 305.67 ± 5.75 a | 1.90 ± 0.17 c,d |
| BSD5 | 222.61 ± 2.97 f,g | 1.52 ± 0.04 g,h |
| BSD6 | 190.11 ± 4.52 i,j | 1.32 ± 0.07 h,i |
| BSD7 | 229.97 ± 4.49 e,f | 1.20 ± 0.01 i |
| BSD8 | 234.56 ± 13.31 d,e | 1.76 ± 0.09 e,f |
| BSD9 | 260.67 ± 19.32c | 1.32 ± 0.43 h,i |
| BSD10 | 267.06 ± 12.72 b,c | 0.84 ± 0.01 j |
| BSD11 | 255.25 ± 4.17 c,d | 0.99 ± 0.02 j |
| BSD12 | 211.64 ± 11.99 g,h | 1.35 ± 0.06 h,i |
| BSD13 | 205.94 ± 4.68 h | 1.62 ± 0.16 f,g |
| Blanched Swell Drying Red Beetroots (BSD) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Pressure, P (MPa) | 0.35 | 0.60 | 0.35 | 0.35 | 0.53 | 0.53 | 0.35 | 0.17 | 0.17 | 0.35 | 0.10 | 0.35 | 0.35 |
| Time, t (s) | 20 | 20 | 35 | 20 | 31 | 9 | 20 | 9 | 31 | 20 | 20 | 5 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonzo-Macías, M.; Cardador-Martínez, A.; Besombes, C.; Allaf, K.; Tejada-Ortigoza, V.; Soria-Mejía, M.C.; Vázquez-García, R.; Téllez-Pérez, C. Instant Controlled Pressure Drop as Blanching and Texturing Pre-Treatment to Preserve the Antioxidant Compounds of Red Dried Beetroot (Beta vulgaris L.). Molecules 2020, 25, 4132. https://doi.org/10.3390/molecules25184132
Alonzo-Macías M, Cardador-Martínez A, Besombes C, Allaf K, Tejada-Ortigoza V, Soria-Mejía MC, Vázquez-García R, Téllez-Pérez C. Instant Controlled Pressure Drop as Blanching and Texturing Pre-Treatment to Preserve the Antioxidant Compounds of Red Dried Beetroot (Beta vulgaris L.). Molecules. 2020; 25(18):4132. https://doi.org/10.3390/molecules25184132
Chicago/Turabian StyleAlonzo-Macías, Maritza, Anaberta Cardador-Martínez, Colette Besombes, Karim Allaf, Viridiana Tejada-Ortigoza, Marla C. Soria-Mejía, Rosa Vázquez-García, and Carmen Téllez-Pérez. 2020. "Instant Controlled Pressure Drop as Blanching and Texturing Pre-Treatment to Preserve the Antioxidant Compounds of Red Dried Beetroot (Beta vulgaris L.)" Molecules 25, no. 18: 4132. https://doi.org/10.3390/molecules25184132
APA StyleAlonzo-Macías, M., Cardador-Martínez, A., Besombes, C., Allaf, K., Tejada-Ortigoza, V., Soria-Mejía, M. C., Vázquez-García, R., & Téllez-Pérez, C. (2020). Instant Controlled Pressure Drop as Blanching and Texturing Pre-Treatment to Preserve the Antioxidant Compounds of Red Dried Beetroot (Beta vulgaris L.). Molecules, 25(18), 4132. https://doi.org/10.3390/molecules25184132










