Influence of the Nucleophilic Ligand on the Reactivity of Carbonyl Rhenium(I) Complexes towards Methyl Propiolate: A Computational Chemistry Perspective
Abstract
1. Introduction
2. Results
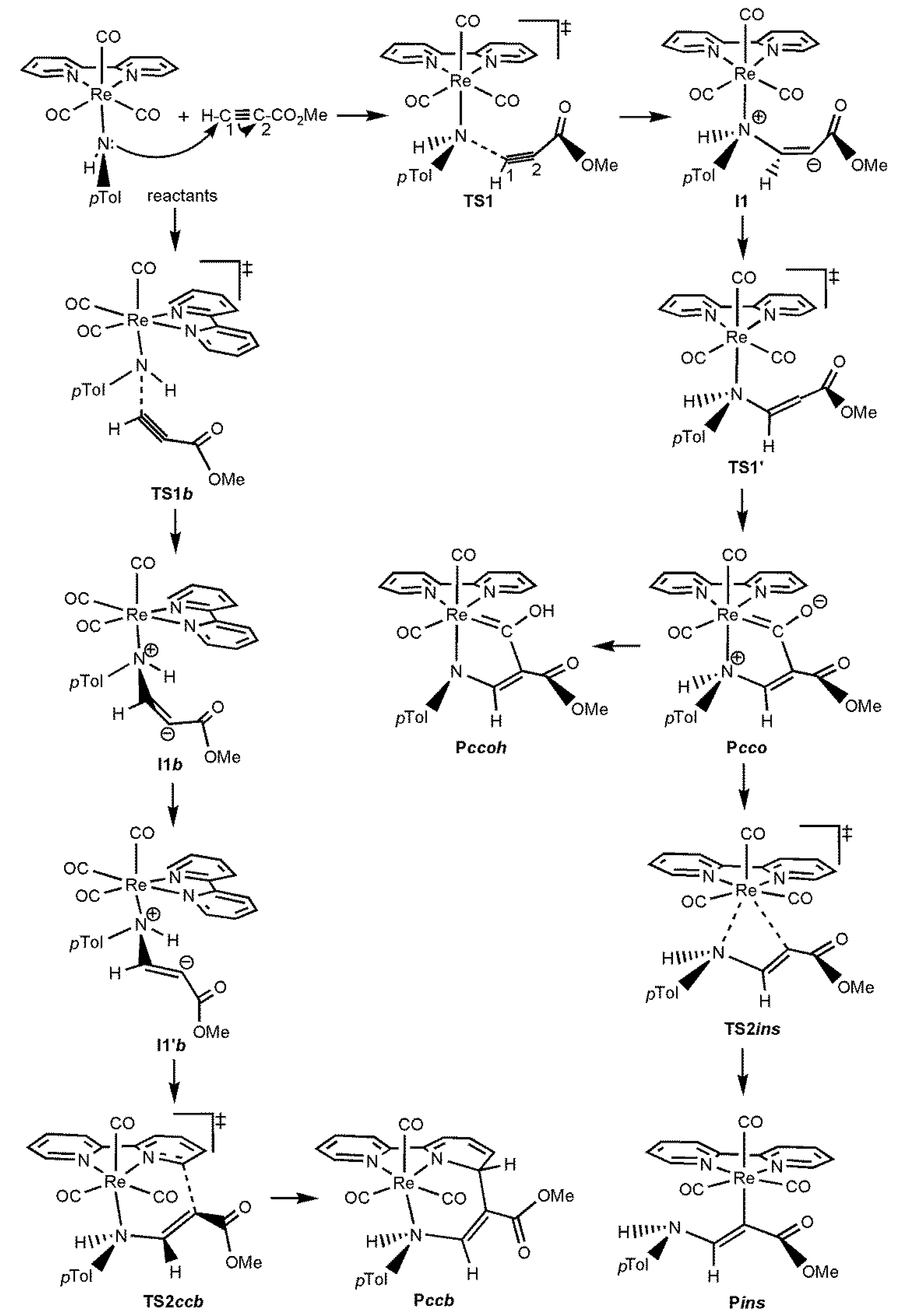
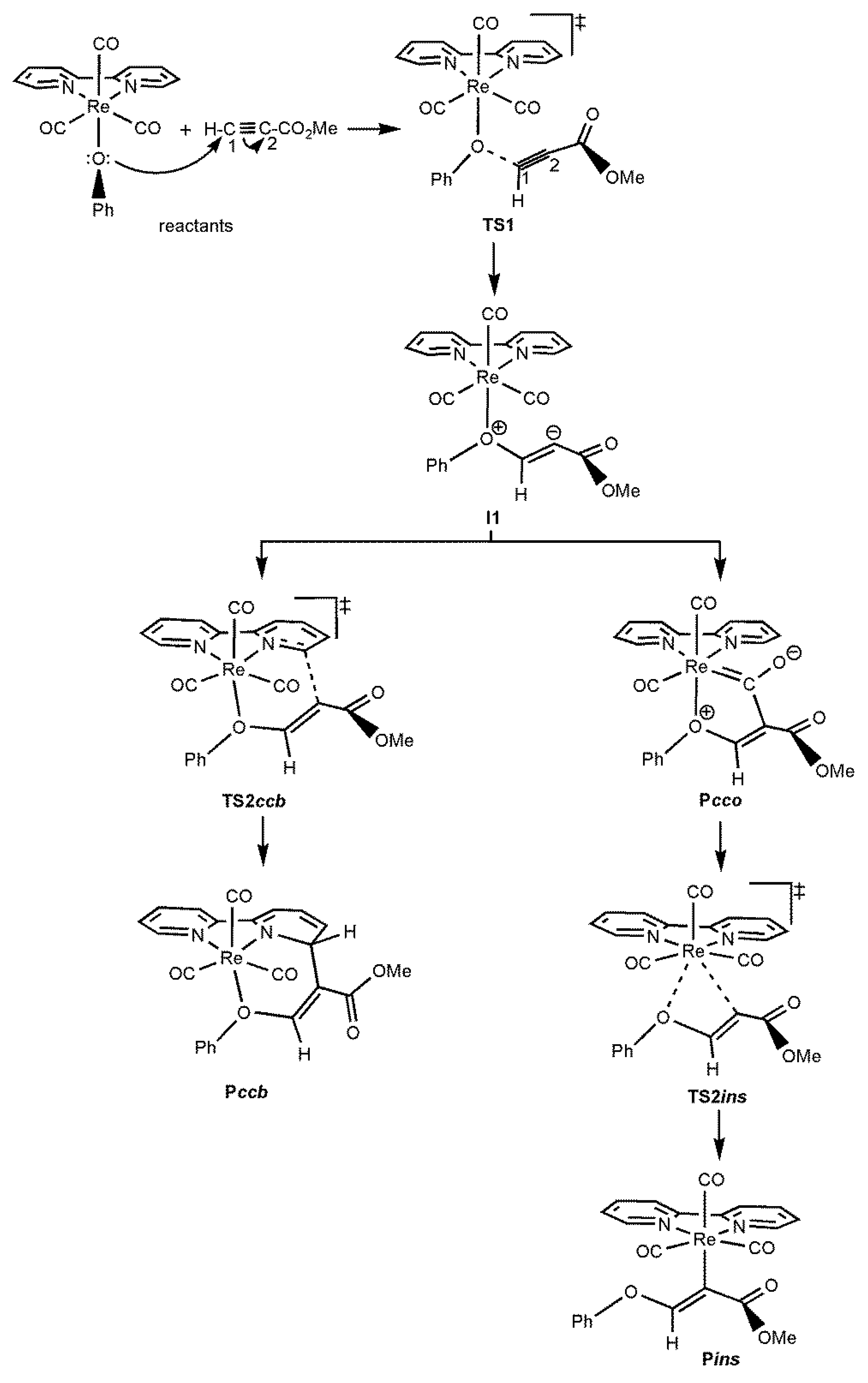
2.1. General Description of the Potential Energy Surfaces
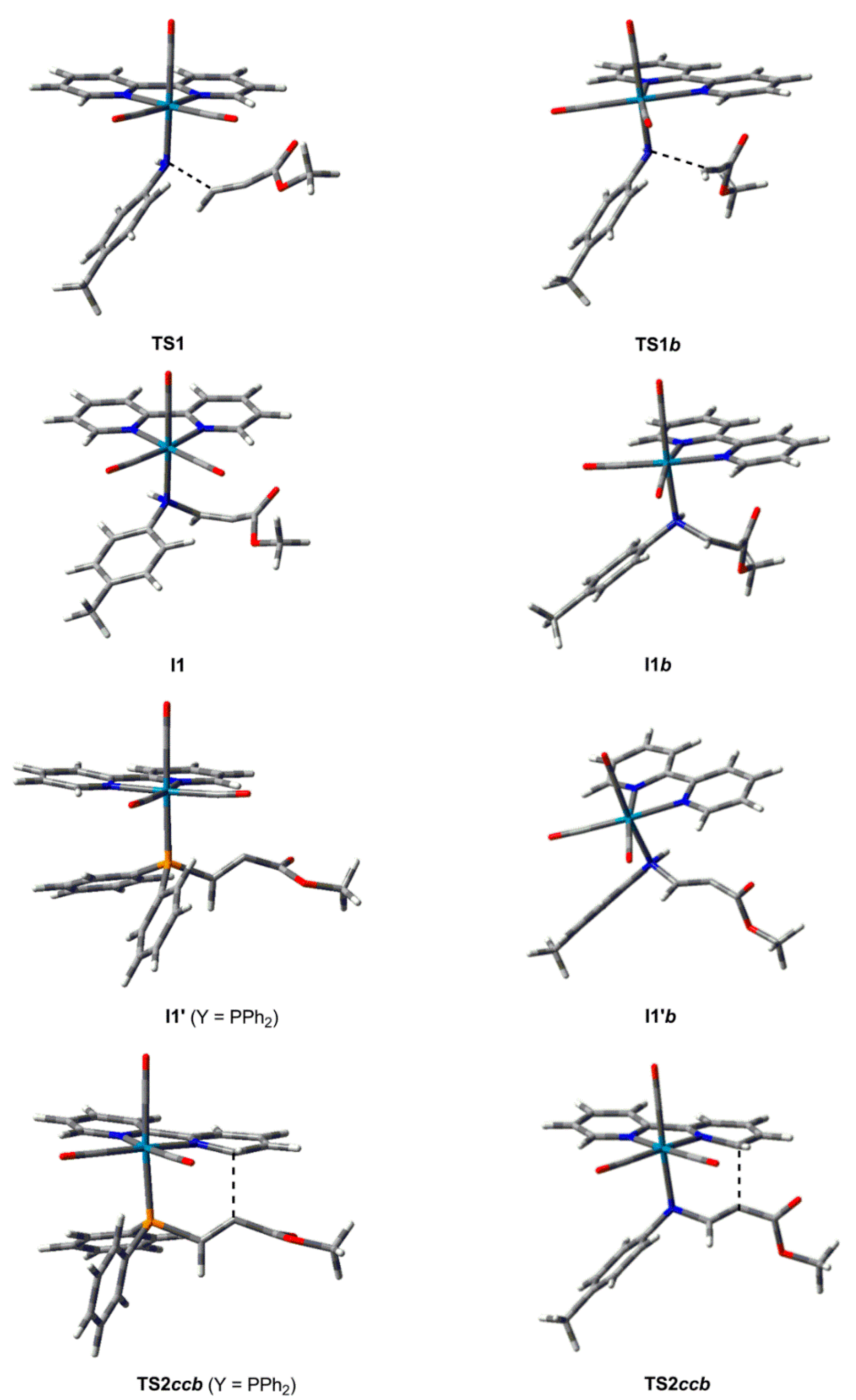
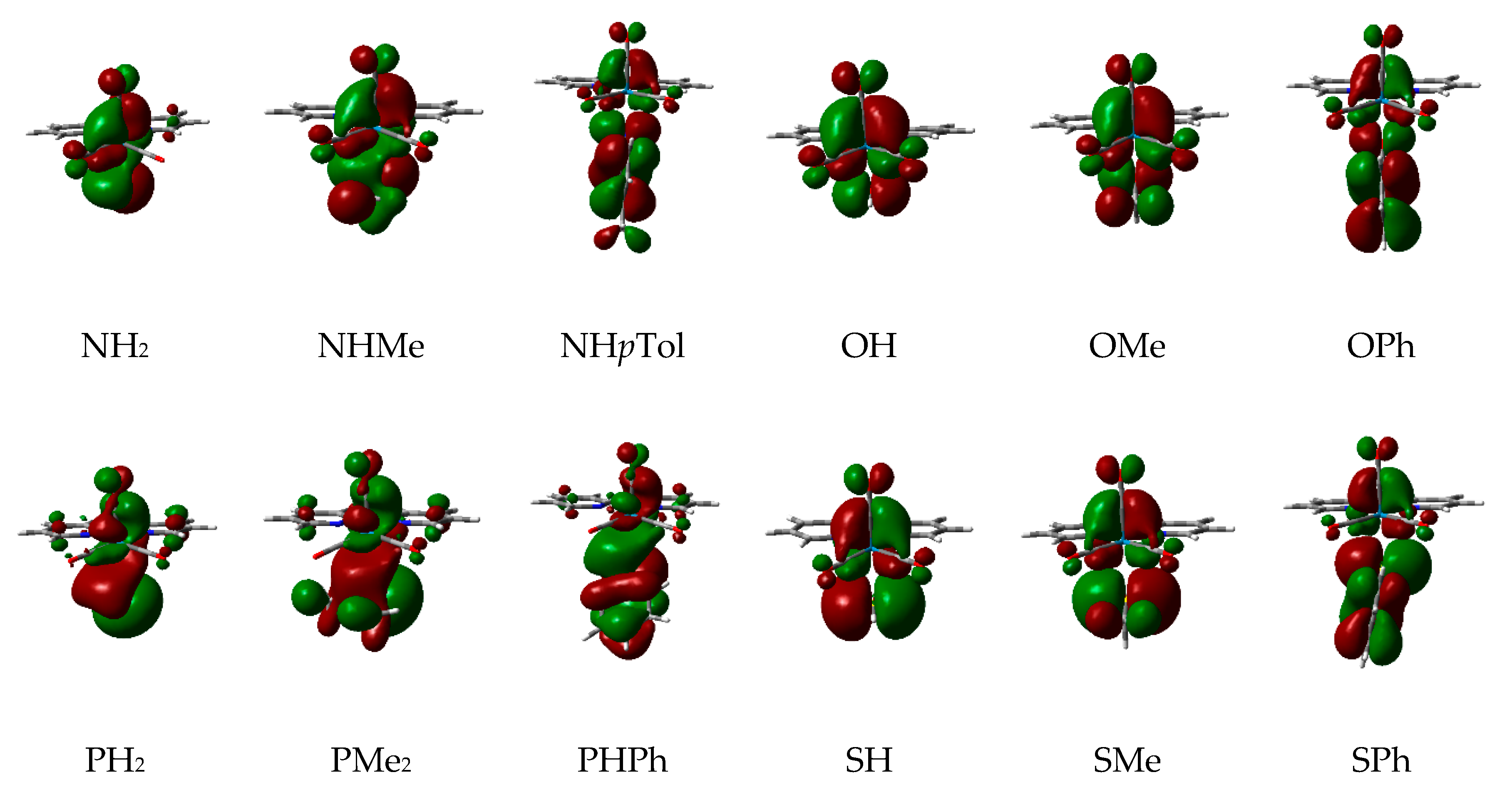
2.2. Effect of the Alkyl and Aryl Substituents of the Nucleophilic Ligand
2.3. Effect of the Heteroatom of the Nucleophilic Ligand
2.4. General Discussion
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takeada, H.; Koike, K.; Inoue, H.; Ishitani, O. Development of an efficient photocatalytic system for CO2 reduction using Rhenium(I) complexes based on mechanistic studies. J. Am. Chem. Soc. 2008, 130, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.J.; Meyer, G.J.; Fujita, E. Molecular approaches to the photocatalytic reduction of carbon dioxide for solar fuels. Acc. Chem. Res. 2009, 42, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Ishitani, O. Development of efficient photocatalytic systems for CO2 reduction using mononuclear and multinuclear metal complexes based on mechanistic studies. Coord. Chem. Rev. 2010, 254, 346–354. [Google Scholar] [CrossRef]
- Kou, Y.; Nabetani, Y.; Masui, D.; Shimada, T.; Takagi, S.; Tachibana, H.; Inoue, H. Direct detection of key reaction intermediates in photochemical CO2 reduction sensitized by a rhenium bipyridine complex. J. Am. Chem. Soc. 2014, 136, 6021–6030. [Google Scholar] [CrossRef]
- Machan, C.W.; Chabolla, S.A.; Yin, J.; Gilson, M.K.; Tezcan, F.A.; Kubiak, C.P. Supramolecular assembly promotes the electrocatalytic reduction of carbon dioxide by Re(I) bipyridine catalysts at a lower overpotential. J. Am. Chem. Soc. 2014, 136, 14598–14607. [Google Scholar] [CrossRef]
- Teesdale, J.J.; Pistner, A.J.; Yap, G.P.; Ma, Y.Z.; Lutterman, D.A.; Rosenthal, J. Reduction of CO2 using a rhenium bipyridine complex containing ancillary BODIPY moieties. Catal. Today 2014, 225, 149–157. [Google Scholar] [CrossRef]
- Windle, C.D.; George, M.W.; Perutz, R.N.; Summers, P.A.; Sun, X.Z.; Whitwood, A.C. Comparison of rhenium–porphyrin dyads for CO2 photoreduction: Photocatalytic studies and charge separation dynamics studied by time-resolved IR spectroscopy. Chem. Sci. 2015, 6, 6847–6864. [Google Scholar] [CrossRef]
- Oh, S.; Gallagher, J.R.; Miller, J.T.; Surendranath, Y. Graphite-conjugated rhenium catalysts for carbon dioxide reduction. J. Am. Chem. Soc. 2016, 138, 1820–1823. [Google Scholar] [CrossRef]
- Ci, C.; Carbó, J.J.; Neumann, R.; de Graaf, C.; Poblet, J.M. Photoreduction mechanism of CO2 to CO catalyzed by a rhenium(I)–polyoxometalate hybrid compound. ACS Catal. 2016, 6, 6422–6428. [Google Scholar] [CrossRef]
- Rohacova, J.; Ishitani, O. Rhenium(I) trinuclear rings as highly efficient redox photosensitizers for photocatalytic CO2 reduction. Chem. Sci. 2016, 7, 6728–6739. [Google Scholar] [CrossRef]
- Clark, M.L.; Cheung, P.L.; Lessio, M.; Carter, E.A.; Kubik, C.P. Kinetic and mechanistic effects of bipyridine (bpy) substituent, labile ligand, and Brønsted acid on electrocatalytic CO2 reduction by Re(bpy) complexes. ACS Catal. 2018, 8, 2021–2029. [Google Scholar] [CrossRef]
- Zhanaidarova, A.; Jones, S.C.; Despagnet-Ayoub, E.; Pimentel, B.R.; Kubiak, C.P. Re(tBu-bpy)(CO)3Cl supported on multi-walled carbon nanotubes selectively reduces CO2 in water. J. Am. Chem. Soc. 2019, 141, 17270–17277. [Google Scholar] [CrossRef] [PubMed]
- Zhanaidarova, A.; Ostericher, A.L.; Miller, C.J.; Jones, S.C.; Kubiak, C.P. Selective reduction of CO2 to CO by a molecular Re(ethynyl-bpy)(CO)3Cl catalyst and attachment to carbon electrode surfaces. Organometallics 2019, 38, 1204–1207. [Google Scholar] [CrossRef]
- Willkomm, J.; Bertin, E.; Atwa, M.; Lin, J.-B.; Birss, V.; Piers, W.E. Grafting of a molecular rhenium CO2 reduction catalyst onto colloid-imprinted carbon. ACS Appl. Energy Mater. 2019, 2, 2414–2418. [Google Scholar] [CrossRef]
- Sato, S.; McNicholas, B.J.; Grubbs, R.H. Aqueous electrocatalytic CO2 reduction using metal complexes dispersed in polymer ion gels. Chem. Commun. 2020, 56, 4440–4443. [Google Scholar] [CrossRef]
- Yu, H.; Haviv, E.; Neumann, R. Visible-light photochemical reduction of CO2 to CO coupled to hydrocarbon dehydrogenation. Angew. Chem. Int. Ed. 2020, 59, 6219–6223. [Google Scholar] [CrossRef]
- Cannizzo, A.; Blanco-Rodríguez, A.M.; El Nahhas, A.; Sebera, J.; Zális, S.; Vlcek, A., Jr.; Chergui, M. Femtosecond fluorescence and intersystem crossing in rhenium(I) carbonyl−bipyridine complexes. J. Am. Chem. Soc. 2008, 130, 8967–8974. [Google Scholar] [CrossRef]
- Ng, C.-O.; Lai, S.-W.; Feng, H.; Yiu, S.-M.; Ko, C.-C. Luminescent rhenium(I) complexes with acetylamino- and trifluoroacetylamino-containing phenanthrolineligands: Anion-sensing study. Dalton Trans. 2011, 40, 10020–10028. [Google Scholar] [CrossRef]
- Yu, T.; Tsand, D.P.-K.; Au, V.K.-M.; Lam, W.H.; Chan, M.-Y.; Yam, V.W.-W. Deep red to near-infrared emitting rhenium(I) complexes: Synthesis, characterization, electrochemistry, photophysics, and electroluminescence studies. Chem. Eur. J. 2013, 19, 13418–13427. [Google Scholar] [CrossRef]
- Chu, W.-K.; Ko, C.-C.; Chan, K.-C.; Yiu, S.-M.; Wong, F.-L.; Lee, C.-S.; Roy, V.A.L. A simple design for strongly emissive sky-blue phosphorescent neutral rhenium complexes: Synthesis, photophysics, and electroluminescent devices. Chem. Mater. 2014, 26, 2544–2550. [Google Scholar] [CrossRef]
- Xu, G.; Lu, M.; Huang, C.; Wang, Y.; Ge, S. Study on an oxygen sensing rhenium(I) complex with enlarged sensing/active area: Fabrication, photophysical parameters and molecular oxygen sensing performance. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 123, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.W.-T.; Tso, K.K.-S.; Yim, V.M.-W.; Liu, H.-W.; Lo, K.K.-W. Modification of 1,2,4,5-tetrazine with cationic rhenium(I) polypyridine units to afford phosphorogenic bioorthogonal probes with enhanced reaction kinetics. Chem. Commun. 2015, 51, 3442–3445. [Google Scholar] [CrossRef]
- Lo, K.K.-W. Luminescent Rhenium(I) and iridium(III) polypyridine complexes as biological probes, imaging reagents, and photocytotoxic agents. Acc. Chem. Res. 2015, 48, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-K.; Wei, X.-G.; Yiu, S.-M.; Ko, C.-C.; Lau, K.-C. Strongly phosphorescent neutral rhenium(I) isocyanoborato complexes: Synthesis, characterization, and photophysical, electrochemical, and computational studies. Chem. Eur. J. 2015, 21, 2603–2612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-W.; Zhao, J.-H.; Hu, Y.-X.; Zhang, D.-Y.; Li, X. Recent advances of neutral rhenium(I) tricarbonyl complexes for application in organic light-emitting diodes. Synth. Met. 2016, 212, 131–141. [Google Scholar] [CrossRef]
- Lee, L.C.; Leung, K.K.; Lo, K.K. Recent development of luminescent rhenium(I) tricarbonyl polypyridine complexes as cellular imaging reagents, anticancer drugs, and antibacterial agents. Dalton Trans. 2017, 46, 16357–16380. [Google Scholar] [CrossRef] [PubMed]
- Raszeja, L.J.; Siegmund, D.; Cordes, A.L.; Gueldenhaupt, J.; Gerwert, K.; Hahn, S.; Metzler-Nolte, N. Asymmetric rhenium tricarbonyl complexes show superior luminescence properties in live cell imaging. Chem. Commun. 2017, 53, 905–908. [Google Scholar] [CrossRef]
- Hu, Y.-X.; Zhao, G.-W.; Dong, Y.; Lu, Y.-L.; Li, X.; Zhang, D.-Y. New rhenium(I) complex with thiadiazole-annelated 1,10-phenanthroline for highly efficient phosphorescent OLEDs. Dyes Pigments 2017, 137, 569–575. [Google Scholar] [CrossRef]
- Skiba, J.; Bernás, T.; Trzybinski, D.; Wozniak, K.; Ferraro, G.; Marasco, D.; Merlino, A.; Shafikov, M.Z.; Czerwieniec, R.; Kowalski, K. Mitochondria targeting with luminescent rhenium(I) complexes. Molecules 2017, 22, 809. [Google Scholar] [CrossRef]
- Yip, A.M.-H.; Shum, J.; Liu, H.-W.; Zhou, H.; Jia, M.; Niu, N.; Li, Y.; Yu, C.; Lo, K.K.-W. Luminescent rhenium(I)–polypyridine complexes appended with a perylene diimide or benzoperylene monoimide moiety: Photophysics, intracellular sensing, and photocytotoxic activity. Chem. Eur. J. 2019, 25, 8970–8974. [Google Scholar] [CrossRef]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic anticancer compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Pierri, A.E.; Pallaoro, A.; Wien, G.; Ford, P.C. A luminescent and biocompatible PhotoCORM. J. Am. Chem. Soc. 2012, 134, 18197–18200. [Google Scholar] [CrossRef] [PubMed]
- Kastl, A.; Dieckmann, S.; Wähler, K.; Völker, T.; Kastl, L.; Merkel, A.L.; Vultur, A.; Shanna, B.; Harms, K.; Ocker, M.; et al. Rhenium Complexes with Visible-Light-Induced Anticancer Activity. ChemMedChem 2013, 8, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Leonidova, A.; Pierroz, V.; Adams, L.A.; Barlow, N.; Ferrari, S.; Graham, B.; Gasser, G. Enhanced cytotoxicity through conjugation of a “clickable” luminescent Re(I) complex to a cell-penetrating lipopeptide. ACS Med. Chem. Lett. 2014, 5, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Kaplanis, M.; Stamatakis, G.; Papakonstantinou, V.D.; Paravatou-Petsotas, M.; Demopoulos, C.A.; Mitsopoulou, C.A. Re(I) tricarbonyl complex of 1,10-phenanthroline-5,6-dione: DNA binding, cytotoxicity, anti-inflammatory and anti-coagulant effects towards platelet activating factor. J. Inorg. Biochem. 2014, 135, 1–9. [Google Scholar] [CrossRef]
- Leonidova, A.; Gasser, G. Underestimated potential of organometallic rhenium complexes as anticancer agents. ACS Chem. Biol. 2014, 9, 2180–2193. [Google Scholar] [CrossRef]
- Ye, R.R.; Tan, C.P.; Chen, M.H.; Hao, L.; Ji, L.N.; Mao, Z.W. Mono- and dinuclear phosphorescent rhenium(I) complexes: Impact of subcellular localization on anticancer mechanisms. Chem. Eur. J. 2016, 22, 7800–7809. [Google Scholar] [CrossRef]
- Knopf, K.M.; Murphy, B.L.; MacMillan, S.N.; Baskin, J.M.; Barr, M.P.; Boros, E.; Wilson, J.J. In Vitro anticancer activity and in vivo biodistribution of rhenium(I) tricarbonyl aqua complexes. J. Am. Chem. Soc. 2017, 139, 14302–14314. [Google Scholar] [CrossRef]
- Marker, S.C.; MacMillan, S.N.; Zipfel, W.R.; Li, Z.; Ford, P.C.; Wilson, J.J. Photoactivated in vitro anticancer activity of rhenium(I) tricarbonyl complexes bearing water-soluble phosphines. Inorg Chem. 2018, 57, 1311–1331. [Google Scholar] [CrossRef]
- King, A.P.; Marker, S.C.; Swanda, R.V.; Woods, J.J.; Qian, S.B.; Wilson, J.J. A rhenium isonitrile complex induces unfolded protein response-mediated apoptosis in cancer cells. Chemistry 2019, 25, 9206–9210. [Google Scholar] [CrossRef]
- Collery, P.; Desmaele, D.; Vijaykumar, V. Design of Rhenium compounds in targeted anticancer therapeutics. Curr. Pharm. Des. 2019, 25, 3306–3322. [Google Scholar] [CrossRef] [PubMed]
- Konkankit, C.C.; Vaughn, B.A.; MacMillan, S.N.; Boros, E.; Wilson, J.J. Combinatorial synthesis to identify a potent, necrosis-inducing rhenium anticancer agent. Inorg. Chem. 2019, 58, 3895–3909. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.B.; Haase, A.A.; Reich, R.M.; Crans, D.C.; Kuehn, F.E. Organometallic and coordination rhenium compounds and their potential in cancer therapy. Coord. Chem. Rev. 2019, 393, 79–117. [Google Scholar] [CrossRef]
- Luengo, A.; Redrado, M.; Marzo, I.; Fernandez-Moreira, V.; Gimeno, M.C. Luminescent Re(I)/Au(I) species as selective anticancer agents for HeLa cells. Inorg. Chem. 2020, 59, 8960–8970. [Google Scholar] [CrossRef] [PubMed]
- Capper, M.S.; Packman, H.; Rehkaemper, M. Rhenium-based complexes and in vivo testing: A brief history. ChemBioChem 2020. [Google Scholar] [CrossRef]
- Marker, S.C.; King, A.P.; Swanda, R.V.; Vaughn, B.; Boros, E.; Qian, S.-B.; Wilson, J.J. Exploring ovarian cancer cell resistance to rhenium anticancer complexes. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Gupta, G.; Sathiyendiran, M. Rhenium-carbonyl-based supramolecular coordination complexes: Synthesis, structure and properties. ChemistrySelect 2018, 3, 7439–7458. [Google Scholar] [CrossRef]
- Hevia, E.; Pérez, J.; Riera, L.; Riera, V. Reactive alkoxide complexes of groups 6 and 7 metals. Organometallics 2002, 21, 1750–1752. [Google Scholar] [CrossRef]
- Hevia, E.; Pérez, J.; Riera, L.; Riera, V.; del Río, I.; García-Granda, S.; Miguel, D. Insertion of unsaturated organic electrophiles into molybdenum-alkoxide and rhenium-alkoxide bonds of neutral, stable carbonyl complexes. Chem. Eur. J. 2002, 8, 4510–4521. [Google Scholar] [CrossRef]
- Hevia, E.; Pérez, J.; Riera, V.; Miguel, D. New octahedral rhenium(I) tricarbonyl amido complexes. Organometallics 2002, 21, 1966–1974. [Google Scholar] [CrossRef]
- Hevia, E.; Pérez, J.; Riera, V.; Miguel, D. Reactivity of the amido complex [Re(NHpTol)(CO)3(bipy)] toward neutral organic electrophiles. Organometallics 2003, 22, 257–263. [Google Scholar] [CrossRef]
- Cuesta, L.; Gerbino, D.C.; Hevia, E.; Morales, D.; Navarro-Clemente, M.E.; Pérez, J.; Riera, L.; Riera, V.; Miguel, D.; del Río, I.; et al. Reactivity of molybdenum and rhenium hydroxo-carbonyl complexes toward organic electrophiles. Chem. Eur. J. 2004, 10, 1765–1777. [Google Scholar] [CrossRef]
- Cuesta, L.; Hevia, E.; Morales, D.; Pérez, J.; Riera, V.; Rodríguez, E.; Miguel, D. Activation of a 1,10-phenanthroline ligand on a rhenium tricarbonyl complex. Chem. Commun. 2005, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, L.; Hevia, E.; Morales, D.; Pérez, J.; Riera, V.; Seitz, M.; Miguel, D. Activation of ancillary ligands in the reactions of DMAD with phosphido and alkylideneamido rhenium complexes. Organometallics 2005, 24, 1772–1775. [Google Scholar] [CrossRef]
- Cañadas, P.; Ziegler, S.; Fombona, S.; Hevia, E.; Miguel, D.; Pérez, J.; Riera, L. Molybdenum and rhenium carbonyl complexes containing thiolato ligands. J. Organomet. Chem. 2019, 896, 113–119. [Google Scholar] [CrossRef]
- Arévalo, R.; Espinal-Viguri, M.; Huertos, M.A.; Pérez, J.; Riera, L. Dearomatization of transition metal-coordinated N-heterocyclic ligands and related chemistry. Adv. Organomet. Chem. 2016, 65, 47–114. [Google Scholar]
- Álvarez, D.; Mera-Adasme, R.; Riera, L.; Cárdenas-Jirón, G.I.; Pérez, J.; Díaz, J.; Menéndez, M.I.; López, R. Insights on the reactivity of terminal phosphanido metal complexes toward activated alkynes from theoretical computations. Inorg. Chem. 2017, 56, 6652–6661. [Google Scholar] [CrossRef]
- Álvarez, D.; Díaz, J.; Menéndez, M.I.; López, R. Addition of Re-bonded nucleophilic ligands to activated alkynes: A theoretical rationalization. Eur. J. Inorg. Chem. 2020, 269–280. [Google Scholar] [CrossRef]
- Villafañe, F. ReI(CO)3 complexes with diimine ligands synthesized in situ. Coord. Chem. Rev. 2017, 339, 128–137. [Google Scholar] [CrossRef]
- Kurtz, D.A.; Brereton, K.R.; Ruoff, K.P.; Tang, H.M.; Felton, G.A.N.; Miller, A.J.M.; Dempsey, J.L. Bathochromic shifts in rhenium carbonyl dyes induced through destabilization of occupied orbitals. Inorg. Chem. 2018, 57, 5389–5399. [Google Scholar] [CrossRef]
- Lee, T.J.; Taylor, P.R. A diagnostic for determining the quality of single-reference electron correlation methods. Int. J. Quantum Chem. 1989, 36, 199–207. [Google Scholar] [CrossRef]
- McQuarrie, D.A. Statistical Mechanics; Harper and Row: New York, NY, USA, 1976. [Google Scholar]
- Ribeiro, R.F.; Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Use of solution-phase vibrational frequencies in continuum models for the free energy of solvation. J. Phys. Chem. B 2011, 115, 14556–14562. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Biegler-Konig, F.W.; Bader, R.F.W.; Hua-Tang, T. Calculation of the average properties of atoms in molecules. II. J. Comput. Chem. 1982, 3, 317–328. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. A Quantum Theory; University Press: Oxford, UK, 1990. [Google Scholar]
- Bader, R.F.W.; Popelier, P.L.A.; Keith, T.A. Theoretical definition of a functional group and the molecular orbital paradigm. Angew. Chem. Int. Ed. 1994, 33, 620–631. [Google Scholar] [CrossRef]
- Fradera, X.; Austen, M.A.; Bader, R.F.W. The Lewis model and beyond. J. Phys. Chem. A 1999, 103, 304–314. [Google Scholar] [CrossRef]
- Fradera, X.; Poater, J.; Simon, S.; Durán, M.; Solá, M. Electron-pairing analysis from localization and delocalization indices in the framework of the atoms-in-molecules theory. Theor. Chem. Acc. 2002, 108, 214–224. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Neese, F. Software Update: The ORCA Program System, Version 4.0.1. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, version 3.1; University of Wisconsin: Madison, WI, USA, 2003. [Google Scholar]
- Keith, T.A. AIMAll Program, version 10.12.11; TK Gristmill Software: Overland Park, KS, USA, 2010. [Google Scholar]
Sample Availability: Not available. |
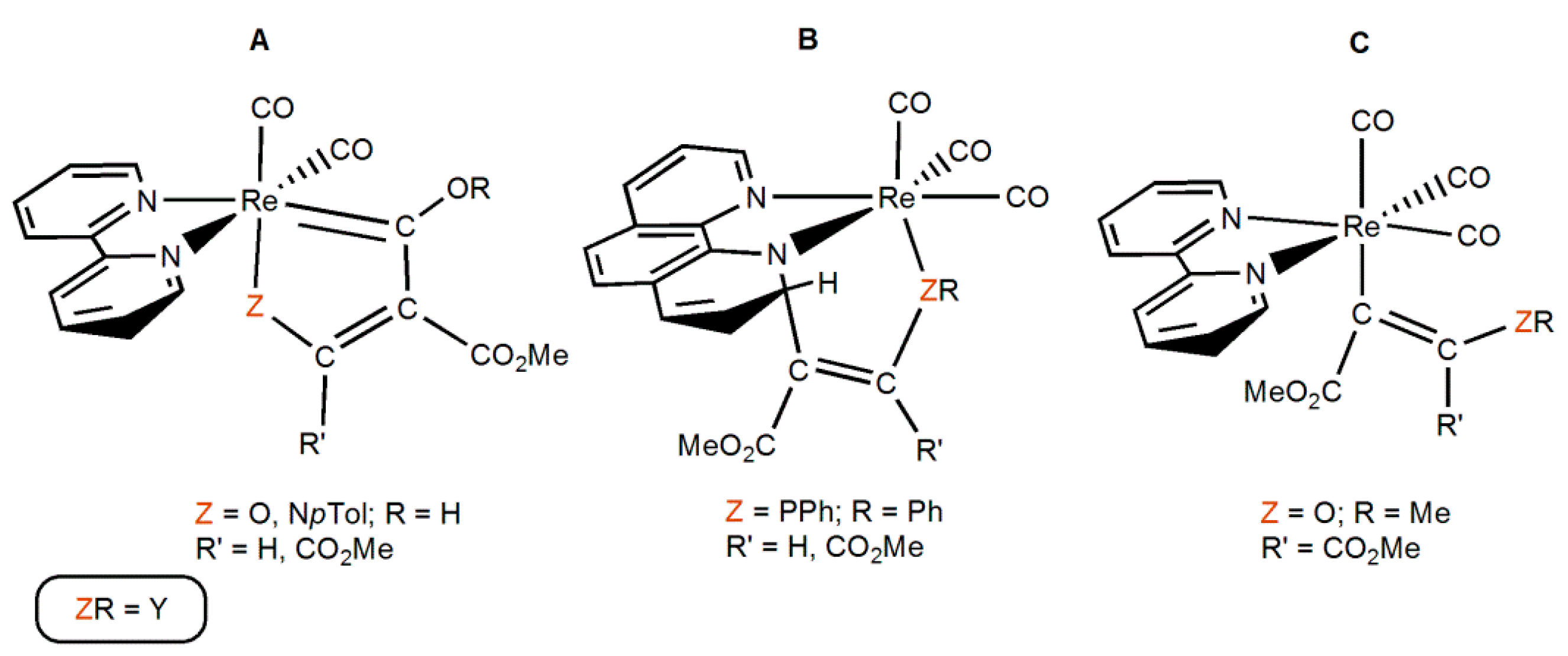



| Y | TS1 | TS1′ | TS1b | TS2cco | TS2ccb | TS2ins | Pcco (Pccoh) | Pccb | Pins | PES |
|---|---|---|---|---|---|---|---|---|---|---|
| NH2 | 18.5 | 8.5 | 19.3 | 4.3 | 9.9 | 11.2 | −8.0 (−24.9) | −15.1 | −29.1 | III |
| NHMe | 17.2 | 4.3 | 14.0 | 0.7 | 9.6 | 9.2 | −12.4 (−32.3) | −19.3 | −31.2 | III |
| NHpTol | 22.4 | 17.0 | 21.4 | 19.0 | 20.6 | −0.2 (−21.1 3) | −5.1 | −22.4 | III | |
| OH | 27.0 | 28.0 | 26.8 | 31.2 | 28.3 | 15.2 (−12.0 3) | 8.1 | −13.7 | I | |
| OMe | 25.9 | 24.8 | 29.5 | 24.0 | 9.9 | 4.9 | −16.4 3 | II | ||
| OPh | 33.5 | 38.8 | 36.5 | 24.2 | 16.2 | −5.6 | IV | |||
| PH2 | 27.6 | 14.6 | 13.5 | 13.5 | 34.2 | 0.1 (0.3) | −9.5 | −13.1 | I | |
| PHMe | 30.9 | 6.2 | 5.1 | 5.8 | 21.1 | −8.9 (−4.3) | −16.3 | −18.3 | I | |
| PMe2 | 17.4 | 4.2 | 5.6 | 4.3 | 20.3 | −17.3 | −25.5 | −24.1 | I | |
| PHPh | 17.6 | 9.7 | 8.2 | 9.4 | 17.5 | −7.4 (−4.2) | −12.8 | −17.5 | I | |
| PPh2 | 17.5 | 4.2 | 5.2 | 5.0 | 20.6 | −9.3 | −20.0 3 | −16.8 | I | |
| PMePh | 18.7 | 3.9 | 3.7 | 5.1 | 18.7 | −11.6 | −20.0 | −21.1 | I | |
| SH | 32.7 | 28.0 | 32.9 | 35.8 | 39.1 | 21.1 (−2.6) | 13.7 | −2.4 | III | |
| SMe | 23.1 | 26.1 | 21.0 | 24.7 | 26.4 | 32.1 | 11.0 | 3.1 | −9.1 | III |
| SPh | 30.8 | 25.6 | 29.0 | 31.0 | 34.8 | 14.4 | 8.9 | −5.7 | III |
| Y | d(Re-Y) | d(Y-CS) | DI(Re-Y) | DI(Y-CS) | NAC(Y) | NAC(CCO) | NAC(Cbipy) |
|---|---|---|---|---|---|---|---|
| NH2 | 2.174 | 0.6687 | −1.164 | 0.734 | 0.063 | ||
| NHMe | 2.156 | 1.461 | 0.6689 | 1.0399 | −0.799 | 0.733 | 0.066 |
| NHpTol | 2.147 | 1.372 | 0.6218 | 1.1322 | −0.790 | 0.734 | 0.068 |
| OH | 2.115 | 0.6145 | −1.016 | 0.724 | 0.069 | ||
| OMe | 2.096 | 1.400 | 0.5998 | 0.9459 | −0.752 | 0.724 | 0.067 |
| OPh | 2.134 | 1.329 | 0.5231 | 0.9925 | −0.678 | 0.728 | 0.069 |
| PH2 | 2.586 | 0.6736 | −0.128 | 0.739 | 0.056 | ||
| PHMe | 2.582 | 1.882 | 0.6803 | 0.9166 | 0.180 | 0.737 | 0.059 |
| PMe2 | 2.602 | 1.876 | 0.6661 | 0.8962 | 0.462 | 0.734 | 0.058 |
| PHPh | 2.591 | 1.844 | 0.6525 | 0.9337 | 0.232 | 0.735 | 0.062 |
| PPh2 | 2.614 | 1.864 | 0.6352 | 0.8793 | 0.519 | 0.739 | 0.060 |
| PMePh | 2.615 | 1.847 | 0.6341 | 0.9256 | 0.510 | 0.735 | 0.058 |
| SH | 2.551 | 0.6541 | −0.482 | 0.747 | 0.068 | ||
| SMe | 2.535 | 1.841 | 0.6758 | 1.1198 | −0.188 | 0.745 | 0.066 |
| SPh | 2.545 | 1.782 | 0.6427 | 1.1956 | −0.085 | 0.740 | 0.068 |
| Y | d(Re-Y) | DI(Re-Y) | NAC(Y) | NAC(CCO) | NAC(Cbipy) | NAC(C1) | NAC(C2) |
|---|---|---|---|---|---|---|---|
| NH2 | 2.271 | 0.4893 | −0.825 | 0.740 | 0.081 | −0.155 | −0.348 |
| NHMe | 2.292 | 0.4650 | −0.646 | 0.727 | 0.080 | −0.139 | −0.336 |
| NHpTol 1 | 2.314 | 0.4594 | −0.652 | 0.737 | 0.078 | −0.154 | −0.317 |
| OH | 2.253 | 0.3747 | −0.706 | 0.730 | 0.082 | −0.004 | −0.392 |
| OMe 1 | 2.256 | 0.3930 | −0.568 | 0.725 | 0.089 | −0.047 | −0.388 |
| OPh 1 | 2.316 | 0.3269 | −0.544 | 0.726 | 0.087 | 0.003 | −0.370 |
| PH2 | 2.514 | 0.6458 | 0.686 | 0.762 | 0.079 | −0.671 | −0.298 |
| PHMe | 2.518 | 0.6496 | 0.956 | 0.763 | 0.081 | −0.681 | −0.295 |
| PMe2 | 2.534 | 0.6412 | 1.199 | 0.761 | 0.080 | −0.679 | −0.290 |
| PHPh | 2.525 | 0.6389 | 0.970 | 0.762 | 0.081 | −0.669 | −0.290 |
| PPh2 | 2.561 | 0.6135 | 1.225 | 0.756 | 0.078 | −0.671 | −0.293 |
| PMePh | 2.544 | 0.6295 | 1.215 | 0.756 | 0.080 | −0.672 | −0.288 |
| SH 1 | 2.572 | 0.5528 | 0.199 | 0.756 | 0.083 | −0.456 | −0.333 |
| SMe | 2.565 | 0.5593 | 0.444 | 0.756 | 0.084 | −0.479 | −0.319 |
| SPh 1 | 2.582 | 0.5372 | 0.470 | 0.750 | 0.085 | −0.464 | −0.311 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, D.; López-Castro, E.; Guerrero, A.; Riera, L.; Pérez, J.; Díaz, J.; Menéndez, M.I.; López, R. Influence of the Nucleophilic Ligand on the Reactivity of Carbonyl Rhenium(I) Complexes towards Methyl Propiolate: A Computational Chemistry Perspective. Molecules 2020, 25, 4134. https://doi.org/10.3390/molecules25184134
Álvarez D, López-Castro E, Guerrero A, Riera L, Pérez J, Díaz J, Menéndez MI, López R. Influence of the Nucleophilic Ligand on the Reactivity of Carbonyl Rhenium(I) Complexes towards Methyl Propiolate: A Computational Chemistry Perspective. Molecules. 2020; 25(18):4134. https://doi.org/10.3390/molecules25184134
Chicago/Turabian StyleÁlvarez, Daniel, Elena López-Castro, Arturo Guerrero, Lucía Riera, Julio Pérez, Jesús Díaz, M. Isabel Menéndez, and Ramón López. 2020. "Influence of the Nucleophilic Ligand on the Reactivity of Carbonyl Rhenium(I) Complexes towards Methyl Propiolate: A Computational Chemistry Perspective" Molecules 25, no. 18: 4134. https://doi.org/10.3390/molecules25184134
APA StyleÁlvarez, D., López-Castro, E., Guerrero, A., Riera, L., Pérez, J., Díaz, J., Menéndez, M. I., & López, R. (2020). Influence of the Nucleophilic Ligand on the Reactivity of Carbonyl Rhenium(I) Complexes towards Methyl Propiolate: A Computational Chemistry Perspective. Molecules, 25(18), 4134. https://doi.org/10.3390/molecules25184134








