Molecules Isolated from Mexican Hypoglycemic Plants: A Review
Abstract
:1. Introduction
2. Results
2.1. Glucosidases Inhibitors
2.2. Effect over Insulin Secretion
2.3. Insulin Sensitizers
2.4. Inhibitors of Hepatic Glucose Output (HGO)
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2019, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- Carracher, A.M.; Marathe, P.H.; Close, K.L. International Diabetes Federation 2017. J. Diabetes 2018, 10, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade-Cetto, A.; Becerra-Jiménez, J.; Martínez-Zurita, E.; Ortega-Larrocea, M.P.; Heinrich, M. Disease-Consensus Index as a tool of selecting potential hypoglycemic plants in Chikindzonot, Yucatán, México. J. Ethnopharmacol. 2006, 107, 199–204. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Cruz, E.C.; Andrade-Cetto, A. Ethnopharmacological field study of the plants used to treat type 2 diabetes among the Cakchiquels in Guatemala. J. Ethnopharmacol. 2015, 159, 238–244. [Google Scholar] [CrossRef]
- Andrade-Cetto, A. Ethnobotanical study of the medicinal plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 2009, 122, 163–171. [Google Scholar] [CrossRef]
- Pěsíc, M. Development of Natural Product Drugs in A Sustainable Manner. GDSR. 2015. Available online: https://sustainabledevelopment.un.org/content/documents/6544118_Pesic_Developmentofnaturalproductdrugsinasustainablemanner.pdf (accessed on 10 June 2020).
- Bauer, A.; Broenstrup, M. Industrial natural product chemistry for drug discovery and development. Nat. Prod. Rep. 2014, 31, 35–60. [Google Scholar] [CrossRef]
- World Health Organisation (WHO) Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_3 (accessed on 10 June 2020).
- American Diabetes Association 5. Lifestyle management: Standards of medical care in diabetes—2019. Diabetes Care 2018, 42, S46–S60. [Google Scholar] [CrossRef] [Green Version]
- White, J.R. A Brief history of the development of diabetes medications. Diabetes Spectr. 2014, 27, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Méndez, A.J.; Carmen-Sandoval, W.; Lomas-Soria, C.; Guevara-Gonzalez, R.G.; Reynoso-Camacho, R.; Villagran-Herrera, M.E.; Salazar-Olivo, L.A.; Torres-Pacheco, I.; Feregrino-Perez, A.A. Timbe (Acaciella angustissima) pods extracts reduce the levels of glucose, insulin and improved physiological parameters, hypolipidemic effect, oxidative stress and renal damage in streptozotocin-induced diabetic rats. Molecules 2018, 23, 2812. [Google Scholar] [CrossRef] [Green Version]
- Martínez, A.L.; Madariaga-Mazón, A.; Rivero-Cruz, I.; Bye, R.; Mata, R. Antidiabetic and antihyperalgesic effects of a decoction and compounds from Acourtia thurberi. Planta Med. 2016, 83, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Wiedenfeld, H.; Andrade-Cetto, A. Pyrone glycosides from Acosmium panamense (Benth.) Yacovlev. Zeitschrift für Naturforschung C 2003, 58, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Wiedenfeld, H. Hypoglycemic effect of Acosmium panamense bark on streptozotocin diabetic rats. J. Ethnopharmacol. 2004, 90, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.R.M.; Perez, G.S.; Zavala, M.A.; Perez, G.S.C. Effect of Agarista mexicana and Verbesina persicifolia on blood glucose level of normoglycaemic and alloxan-diabetic mice and rats. Phytother. Res. 1996, 10, 351–353. [Google Scholar] [CrossRef]
- Perez, G.R.M.; Solis, R.V. Triterpenes from Agarista mexicana as potential antidiabetic agents. Phytother. Res. 2002, 16, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Brito, C.; Andrade-Cetto, A.; Giraldo-Aguirre, J.D.; Moreno-Vargas, A.D.; Quijano, L. Acute hypoglycemic effect and phytochemical composition of Ageratina petiolaris. J. Ethnopharmacol. 2016, 185, 341–346. [Google Scholar] [CrossRef]
- Mata-Torres, G.; Andrade-Cetto, A.; Espinoza-Hernández, F.A.; Cárdenas-Vázquez, R. Hepatic glucose output inhibition by Mexican plants used in the treatment of Type 2 Diabetes. Front. Pharmacol. 2020, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Calzada, F.; Solares-Pascasio, J.I.; Ordoñez-Razo, R.M.; Velázquez, C.; Barbosa, E.; García-Hernández, N.; Mendez-Luna, D.; Correa-Basurto, J. Antihyperglycemic Activity of the Leaves from Annona cherimola Miller and Rutin on Alloxan-induced Diabetic Rats. Pharmacogn. Res. 2017, 9, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez-Reyes, K.; Brindis, F.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Bye, R.; Linares, E.; Mata, R. Hypoglycemic, antihyperglycemic, and antioxidant effects of the edible plant Anoda cristata. J. Ethnopharmacol. 2015, 161, 36–45. [Google Scholar] [CrossRef]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Rivera-Chávez, J.; Mata, R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana Nutt. J. Ethnopharmacol. 2014, 155, 416–425. [Google Scholar] [CrossRef]
- García-Galicia, M.C.; Burgueño-Tapia, E.; Romero-Rojas, A.; García-Zebadúa, J.C.; Cornejo-Garrido, J.; Ordaz-Pichardo, C. Anti-hyperglycemic effect, inhibition of inflammatory cytokines expression, and histopathology profile in streptozotocin-induced diabetic rats treated with Arracacia tolucensis aerial-parts extracts. J. Ethnopharmacol. 2014, 152, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Espinosa, F.; Déciga-Campos, M.; Mata, R. Antinociceptive, hypoglycemic and spasmolytic effects of Brickellia veronicifolia. J. Ethnopharmacol. 2008, 118, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.R.M.; Cervantes, C.H.; Zavala, S.M.A.; Sanchez, A.J.; Perez, G.S.; Perez, G.C. Isolation and hypoglycemic activity of 5, 7,3′-trihydroxy-3,6,4′-trimethoxyflavone from Brickellia veronicaefolia. Phytomedicine 2000, 7, 25–29. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Medina-Hernández, A.E.B. Hypoglycemic effect of Bromelia plumieri (E. Morren) L.B. Sm., leaves in STZ-NA-induced diabetic rats. Front. Pharmacol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Escandón-Rivera, S.M.; Andrade-Cetto, A.; Sánchez-Villaseñor, G. Phytochemical composition and chronic hypoglycemic effect of bromelia karatas on STZ-NA-induced diabetic rats. Evid. Based Complementary Altern. Med. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Escandón-Rivera, S.M.; González-Andrade, M.; Bye, R.; Linares, E.; Navarrete, A.; Mata, R. α-Glucosidase inhibitors from Brickellia cavanillesii. J. Nat. Prod. 2012, 75, 968–974, reprint in J. Nat. Prod. 2017, 80, 233. [Google Scholar] [CrossRef]
- Escandón-Rivera, S.M.; Pérez-Vásquez, A.; Navarrete, A.; Hernández, M.; Linares, E.; Bye, R.; Mata, R. Anti-hyperglycemic activity of major compounds from Calea ternifolia. Molecules 2017, 22, 289. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Cetto, A.; Wiedenfeld, H. Hypoglycemic effect of Cecropia obtusifolia on streptozotocin diabetic rats. J. Ethnopharmacol. 2001, 78, 145–149. [Google Scholar] [CrossRef]
- Revilla-Monsalve, M.C.; Andrade-Cetto, A.; Palomino-Garibay, M.A.; Wiedenfeld, H.; Islas-Andrade, S. Hypoglycemic effect of Cecropia obtusifolia Bertol aqueous extracts on type 2 diabetic patients. J. Ethnopharmacol. 2007, 111, 636–640. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Becerra-Jiménez, J.; Cárdenas-Vázquez, R. Alfa-glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J. Ethnopharmacol. 2008, 116, 27–32. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Miranda-Torres, A.C.; González-Chávez, M.M.; Salazar-Olivo, L.A. Cecropia obtusifolia Bertol and its active compound, chlorogenic acid, stimulate 2-NBDglucose uptake in both insulin-sensitive and insulin-resistant 3T3 adipocytes. J. Ethnopharmacol. 2008, 120, 458–464. [Google Scholar] [CrossRef]
- Sánchez-Salgado, J.; Ortiz-Andrade, R.; Aguirre-Crespo, F.; Vergara-Galicia, J.; León-Rivera, I.; Montes, S.; Villalobos-Molina, R.; Estrada-Soto, S. Hypoglycemic, vasorelaxant and hepatoprotective effects of Cochlospermum vitifolium (Willd.) Sprengel: A potential agent for the treatment of metabolic syndrome. J. Ethnopharmacol. 2007, 109, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Andrade, R.; Torres-Piedra, M.; Sánchez-Salgado, J.C.; García-Jiménez, S.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Gallardo-Ortiz, I.; Estrada-Soto, S. Acute and sub-chronic effects of Cochlospermum vitifolium in blood glucose levels in normoglycemic and STZ-nicotinamide-induced diabetic rats. Rev. Latinoam. Quim. 2009, 37, 122–132. [Google Scholar]
- Brindis, F.; González-Andrade, M.; González-Trujano, M.; Estrada-Soto, S.; Villalobos-Molina, R. Postprandial glycaemia and inhibition of α-glucosidase activity by aqueous extract from Coriandrum sativum. Nat. Prod. Res. 2014, 28, 2021–2025. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Patiño, J.; Jiménez-Balderas, E.; Juárez-Oropeza, M.; Dıaz-Zagoya, J. Hypoglycemic action of Cucurbita ficifolia on Type 2 diabetic patients with moderately high blood glucose levels. J. Ethnopharmacol. 2001, 77, 99–101. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.; Hernandez-Galicia, E.; Campos-Sepulveda, A.E.; Xolalpa-Molina, S.; Rivas-Vilchis, J.F.; Vazquez-Carrillo, L.I.; Roman-Ramos, R. Evaluation of the hypoglycemic effect of Cucurbita ficifolia Bouché (Cucurbitaceae) in different experimental models. J. Ethnopharmacol. 2002, 82, 185–189. [Google Scholar] [CrossRef]
- Díaz-Flores, M.; Angeles-Mejia, S.; Baiza-Gutman, L.; Medina-Navarro, R.; Hernandez-Saavedra, D.; Ortega-Camarillo, C.; Roman-Ramos, R.; Cruz, M.; Alarcon-Aguilar, F. Effect of an aqueous extract of Cucurbita ficifolia Bouché on the glutathione redox cycle in mice with STZ-induced diabetes. J. Ethnopharmacol. 2012, 144, 101–108. [Google Scholar] [CrossRef]
- Pérez, M.E.M.; Ortega-Camarillo, C.; Escobar-Villanueva, M.D.C.; Blancas-Flores, G.; Alarcon-Aguilar, F.; Flores-Blancas, G. Cucurbita ficifolia Bouché increases insulin secretion in RINm5F cells through an influx of Ca2+ from the endoplasmic reticulum. J. Ethnopharmacol. 2016, 188, 159–166. [Google Scholar] [CrossRef]
- Jessica, G.G.; Mario, G.L.; Alejandro, Z.; Cesar, A.P.J.; Ivan, J.V.E.; Ruben, R.R.; Javier, A.-A.F. Chemical characterization of a hypoglycemic extract from Cucurbita ficifolia bouche that induces liver glycogen accumulation in diabetic mice. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Wiedenfeld, H.; Andrade-Cetto, A.; Amador, C.P. Flavonol glycosides from Equisetum myriochaetum. Biochem. Syst. Ecol. 2000, 28, 395–397. [Google Scholar] [CrossRef] [Green Version]
- Revilla, M.C.; Andrade-Cetto, A.; Islas, S.; Wiedenfeld, H. Hypoglycemic effect of Equisetum myriochaetum aerial parts on type 2 diabetic patients. J. Ethnopharmacol. 2002, 81, 117–120. [Google Scholar] [CrossRef]
- Cetto, A.A.; Wiedenfeld, H.; Revilla, M.C.; Sergio, I.A. Hypoglycemic effect of Equisetum myriochaetum aerial parts on streptozotocin diabetic rats. J. Ethnopharmacol. 2000, 72, 129–133. [Google Scholar] [CrossRef]
- Narváez-Mastache, J.M.; Garduño-Ramírez, M.L.; Alvarez, L.; Delgado, G. Antihyperglycemic Activity and Chemical Constituents of Eysenhardtia platycarpa. J. Nat. Prod. 2006, 69, 1687–1691. [Google Scholar] [CrossRef]
- Narváez-Mastache, J.M.; Soto, C.; Delgado, G. Antioxidant evaluation of eysenhardtia species (fabaceae): Relay synthesis of 3-O-acetyl-11α,12α-epoxy-oleanan-28,13β-olide isolated from E. platycarpa and Its protective effect in experimental diabetes. Biol. Pharm. Bull. 2007, 30, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.M.; Ocegueda, A.Z.; Muñoz, L.J.L.; Avila, A.J.G.; Morrow, W.W. A study of the hypoglucemic effect of some Mexican plants. J. Ethnopharmacol. 1984, 12, 253–262. [Google Scholar] [CrossRef]
- Pérez, G.R.M.; Baez, E.G. Evaluation of antidiabetic, antioxidant and antiglycating activities of the Eysenhardtia polystachya. Pharmacogn. Mag. 2014, 10, S404–S418. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Gutiérrez, R.M.; Garcia, C.A.H.; Muñiz-Ramírez, A. Properties of flavonoids isolated from the bark of eysenhardtia polystachya and their effect on oxidative stress in streptozotocin-induced diabetes mellitus in mice. Oxid. Med. Cell. Longev. 2016, 2016, 9156510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, G.R.M.; Garcia, C.A.H.; Mota, F.J.M.; Grcia, G.A.H. Dihydrochalcones from the bark of Eysenhardtia polystachya inhibits formation of advanced glycation end products at multiple stages in vitro studies. J. Pharm. Pharmacol. 2017, 1, 1–24. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, R.M.; Garcia, C.A.H.; Carrera, S.P.P.; Muñiz-Ramírez, A.; Flores, J.M.M.; Flores-Valle, S.O. 3′-O-β-d-glucopyranosyl-α,4,2′,4′,6′-pentahydroxy-dihydrochalcone, from bark of Eysenhardtia polystachya prevents diabetic nephropathy via inhibiting protein glycation in STZ-nicotinamide induced diabetic mice. Molecules 2019, 24, 1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Vásquez, A.; Castillejos-Ramírez, E.; Cristians, S.; Mata, R. Development of a UHPLC-PDA method for the simultaneous quantification of 4-phenylcoumarins and chlorogenic acid in Exostema caribaeum Stem Bark. J. Nat. Prod. 2014, 77, 516–520. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Escandón-Rivera, S.; Garcia-Luna, V. Hypoglycemic effect of Hamelia patens Jacq., aerial part in STZ-NA-induced diabetic rats. Pharmacologyonline 2015, 3, 6569. Available online: https://pharmacologyonline.silae.it/files/archives/2015/vol3/PhOL_2015_3_A010_10_21_Cetto_3_65_69.pdf (accessed on 10 June 2020).
- Rugerio-Escalona, C.; Ordaz-Pichardo, C.; Becerra-Martinez, E.; Cruz-López, M.D.C.; López, V.E.L.Y.; Mendieta, A.; Maldonado-Mendoza, I.E.; Montejo, F.E.J. “diabetes and metabolism disorders medicinal plants: A glance at the past and a look to the future 2018”: Antihyperglycemic activity of Hamelia patens Jacq. extracts. Evid. Based Complementary Altern. Med. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Analco, J.; Medina-Campos, O.; Brindis, F.; Bye, R.; Pedraza-Chaverri, J.; Navarrete, A.; Mata, R. Antidiabetic properties of selected Mexican copalchis of the Rubiaceae family. Phytochemistry 2007, 68, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Cristians, S.; Guerrero-Analco, J.A.; Pérez-Vásquez, A.; Palacios-Espinosa, F.; Ciangherotti, C.; Bye, R.; Mata, R. Hypoglycemic activity of extracts and compounds from the leaves of Hintonia standleyana and H. latiflora: Potential alternatives to the use of the stem bark of these species (1). J. Nat. Prod. 2009, 72, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chávez, J.; González-Andrade, M.; González, M.D.C.; Glenn, A.E.; Mata, R. Thielavins A, J and K: α-Glucosidase inhibitors from MEXU 27095, an endophytic fungus from Hintonia latiflora. Phytochemistry 2013, 94, 198–205. [Google Scholar] [CrossRef]
- Cristians, S.; Mata, R.; Bye, R. Phenological and geographical influence in the concentration of selected bioactive 4-phenylcoumarins and chlorogenic acid in Hintonia latiflora leaves. J. Ethnopharmacol. 2014, 152, 308–313. [Google Scholar] [CrossRef]
- Guerrero-Analco, J.A.; Hersch-MartInez, P.; Pedraza-Chaverri, J.; Navarrete, A.; Mata, R. Antihyperglycemic effect of constituents from Hintonia standleyana in streptozotocin-induced diabetic rats. Planta Med. 2005, 71, 1099–1105. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.; Campos-Sepulveda, A.; Xolalpa-Molina, S.; Hernandez-Galicia, E.; Roman-Ramos, R. Hypoglycaemic activity of Ibervillea sonorae roots in healthy and diabetic mice and rats. Pharm. Biol. 2002, 40, 570–575. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.; Calzada-Bermejo, F.; Hernandez-Galicia, E.; Ruiz-Angeles, C.; Roman-Ramos, R. Acute and chronic hypoglycemic effect of Ibervillea sonorae root extracts-II. J. Ethnopharmacol. 2005, 97, 447–452. [Google Scholar] [CrossRef]
- Hernández-Galicia, E.; Calzada, F.; Roman-Ramos, R.; Alarcón-Aguilar, F. Monoglycerides and fatty acids from Ibervillea sonorae root: Isolation and hypoglycemic activity. Planta Med. 2007, 73, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Ramírez, F.; Escalona-Cardoso, G.N.; Garduño-Siciliano, L.; Galaviz-Hernández, C.; Paniagua-Castro, N. Antiobesity and hypoglycaemic effects of aqueous extract of Ibervillea sonorae in mice fed a high-fat diet with fructose. J. Biomed. Biotechnol. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapata-Bustos, R.; Alonso-Castro, A.J.; Gomez-Sanchez, M.; Salazar-Olivo, L.A. Ibervillea sonorae (Cucurbitaceae) induces the glucose uptake in human adipocytes by activating a PI3K-independent pathway. J. Ethnopharmacol. 2014, 152, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ramirez, D.; Escandón-Rivera, S.M.; Pereda-Miranda, R. Morning glory resin glycosides as α-glucosidase inhibitors: In vitro and in silico analysis. Phytochemistry 2018, 148, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Andrade, R.; Cabañas-Wuan, A.; Arana-Argáez, V.E.; Alonso-Castro, A.J.; Zapata-Bustos, R.; Salazar-Olivo, L.A.; Domínguez, F.; Chávez, M.; Carranza-Álvarez, C.; García-Carrancá, A. Antidiabetic effects of Justicia spicigera Schltdl (Acanthaceae). J. Ethnopharmacol. 2012, 143, 455–462. [Google Scholar] [CrossRef]
- Brindis, F.; Rodríguez, R.; Bye, R.; González-Andrade, M.; Mata, R.; Rodrıguez, R. (Z)-3-Butylidenephthalide from Ligusticum porteri, an α-glucosidase Inhibitor. J. Nat. Prod. 2011, 74, 314–320. [Google Scholar] [CrossRef]
- Flores-Bocanegra, L.; Torres-Colín, R.; González-Andrade, M.; Calderón, J.S.; Mata, R. In vivo and in vitro α-glucosidase inhibitory activity of perfoliatin a from Melampodium Perfoliatum. Nat. Prod. Commun. 2019, 14, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Cetto, A.; Martínez-Zurita, E.; Wiedenfeld, H. Hypoglycemic effect of Malmea depressa root on streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 100, 319–322. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Martínez-Zurita, E.; Soto-Constantino, A.; Revilla-Monsalve, C.; Wiedenfeld, H. Chronic hypoglycemic effect of Malmea depressa root on n5-streptozotocin diabetic rats. J. Ethnopharmacol. 2008, 116, 358–362. [Google Scholar] [CrossRef]
- Andrade-Cetto, A. Inhibition of gluconeogenesis by Malmea depressa root. J. Ethnopharmacol. 2011, 137, 930–933. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Wiedenfeld, H. Anti-hyperglycemic effect of Opuntia streptacantha Lem. J. Ethnopharmacol. 2011, 133, 940–943. [Google Scholar] [CrossRef]
- Becerra-Jiménez, J.; Andrade-Cetto, A. Effect of Opuntia streptacantha Lem. on alpha-glucosidase activity. J. Ethnopharmacol. 2012, 139, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilar, F.; Roman-Ramos, R.; Jiménez-Estrada, M.; Reyes-Chilpa, R.; Gonzalez-Paredes, B.; Flores-Saenz, J. Effects of three Mexican medicinal plants (Asteraceae) on blood glucose levels in healthy mice and rabbits. J. Ethnopharmacol. 1997, 55, 171–177. [Google Scholar] [CrossRef]
- Alarcon-Aguilar, F.; Jiménez-Estrada, M.; Reyes-Chilpa, R.; Gonzalez-Paredes, B.; Contreras-Weber, C.; Roman-Ramos, R. Hypoglycemic activity of root water decoction, sesquiterpenoids, and one polysaccharide fraction from Psacalium decompositum in mice. J. Ethnopharmacol. 2000, 69, 207–215. [Google Scholar] [CrossRef]
- Estrada, M.J.; Merino-Aguilar, H.; Lopez-Fernandez, A.; Rojano-Vilchis, N.A.; Roman-Ramos, R.; Alarcon-Aguilar, F. Chemical characterization and evaluation of the hypoglycemic effect of fructooligosaccharides from Psacalium decompositum. J. Complement. Integr. Med. 2011, 8, 8. [Google Scholar] [CrossRef]
- Merino-Aguilar, H.; Arrieta-Baez, D.; Estrada, M.J.; Magos-Guerrero, G.; Hernández-Bautista, R.J.; Susunaga-Notario, A.D.C.; Almanza-Pérez, J.C.; Blancas-Flores, G.; Roman-Ramos, R.; Alarcon-Aguilar, F. Effect of fructooligosaccharides fraction from Psacalium decompositum on inflammation and dyslipidemia in rats with fructose-induced obesity. Nutrients 2014, 6, 591–604. [Google Scholar] [CrossRef]
- De Rodríguez, D.J.; García-Hernández, L.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.; Rodríguez-García, R.; Díaz-Jiménez, M.; Flores-López, M.L.; Villarreal-Quintanilla, J.; Peña-Ramos, F.; Carrillo-Lomelí, D. Hypoglycemic and anti-inflammatory effects of Psacalium paucicapitatum corms infusions. Ind. Crop. Prod. 2017, 107, 482–488. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; Rios, M.Y.; Martinez, S.L.; López-Vallejo, F.; Medina-Franco, J.L.; Paoli, P.; Camici, G.; Navarrete-Vázquez, G.; Ortiz-Andrade, R.; Estrada-Soto, S. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP–1B: In vitro, in silico, and in vivo approaches. Eur. J. Med. Chem. 2011, 46, 2243–2251. [Google Scholar] [CrossRef]
- López-Martinez, S.; Navarrete-Vázquez, G.; Estrada-Soto, S.; Leon, I.; Rios, M.Y. Chemical constituents of the hemiparasitic plant Phoradendron brachystachyum DC Nutt (Viscaceae). Nat. Prod. Res. 2013, 27, 130–136. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; García-Jimenez, S.; Rios, M.Y.; Medina-Franco, J.L.; López-Vallejo, F.; Webster, S.P.; Binnie, M.; Ibarra-Barajas, M.; Ortiz-Andrade, R.; Estrada-Soto, S. Antihyperglycemic and sub-chronic antidiabetic actions of morolic and moronic acids, in vitro and in silico inhibition of 11β-HSD 1. Phytomedicine 2013, 20, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Mares, M.L.R. Hypoglycemic effect of the Rhizophora mangle cortex on STZ-NA-induced diabetic rats. Pharmacologyonline 2012, 3, 1–5. Available online: https://pharmacologyonline.silae.it/files/archives/2012/vol3/PhOL_2012_3_A001_004_Cetto.pdf (accessed on 10 June 2020).
- Andrade-Cetto, A.; Escandón-Rivera, S.M.; Torres-Valle, G.M.; Quijano, L. Phytochemical composition and chronic hypoglycemic effect of Rhizophora mangle cortex on STZ-NA-induced diabetic rats. Rev. Bras. Farm. 2017, 27, 744–750. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Cabello-Hernández, C.A.; Cárdenas-Vázquez, R. Alpha-glucosidase inhibiting activity of five Mexican plants used in the treatment of type 2 diabetes. Pharmacologyonline 2015, 1, 67–71. Available online: https://pharmacologyonline.silae.it (accessed on 10 June 2020).
- Flores-Bocanegra, L.; González-Andrade, M.; Bye, R.; Linares, E.; Mata, R. α-Glucosidase Inhibitors from Salvia circinata. J. Nat. Prod. 2017, 80, 1584–1593. [Google Scholar] [CrossRef]
- Amaro, C.A.B.; González-Cortazar, M.; Herrera-Ruiz, M.; Roman-Ramos, R.; Aguilar-Santamaría, L.; Tortoriello-Garcıa, J.; Jimenez-Ferrer, I. Hypoglycemic and hypotensive activity of a root extract of Smilax aristolochiifolia, standardized on N-trans-feruloyl-tyramine. Molecules 2014, 19, 11366–11384. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Nájera, V.C.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Hidalgo-Figueroa, S.; Del-Toro-Sánchez, C.L.; Salazar-Olivo, L.A.; Cervantes, E.L. Smilax aristolochiifolia root extract and its compounds chlorogenic acid and astilbin inhibit the activity of α-amylase and α-glucosidase enzymes. Evid. Based Complementary Altern. Med. 2018, 2018, 6247306. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Cetto, A. Hypoglycemic effect of Smilax moranensis root on N5-STZ diabetic rats. Pharmacologyonline 2011, 1, 111–115. Available online: https://pharmacologyonline.silae.it/files/newsletter/2011/vol1/013.cettorev.pdf (accessed on 10 June 2020).
- Romo-Pérez, A.; Escandón-Rivera, S.M.; Andrade-Cetto, A. Chronic hypoglycemic effect and phytochemical composition of Smilax moranensis roots. Rev. Bras. Farm. 2019, 29, 246–253. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Mata, R. Hypoglycemic and antihyperglycemic effects of phytopreparations and limonoids from Swietenia humilis. Phytochemistry 2015, 110, 111–119. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Déciga-Campos, M.; Mata, R. Antihyperalgesic activity of a mexicanolide isolated from Swietenia humilis extract in nicotinamide-streptozotocin hyperglycemic mice. Biomed. Pharmacother. 2017, 92, 324–330. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Navarrete, A.; Haddad, P.S.; Tovar, A.R.; Noriega, L.G.; Tovar-Palacio, C.; Mata, R. Multi-target antidiabetic mechanisms of mexicanolides from Swietenia humilis. Phytomedicine 2019, 58, 152891. [Google Scholar] [CrossRef]
- Aguilar-Santamaría, L.; Ramirez, G.; Nicasio, P.; Alegría-Reyes, C.; Herrera-Arellano, A. Antidiabetic activities of Tecoma stans (L.) Juss. ex Kunth. J. Ethnopharmacol. 2009, 124, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.; Zamilpa, A.; Zavala, M.; Perez, J.; Morales, D.; Tortoriello-Garcıa, J.; Guillermo, R.; Alejandro, Z.; Miguel, Z.; Julia, P.; et al. Chrysoeriol and other polyphenols from Tecoma stans with lipase inhibitory activity. J. Ethnopharmacol. 2016, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parra-Naranjo, A.; Delgado-Montemayor, C.; Fraga-López, A.; Castañeda-Corral, G.; Salazar-Aranda, R.; Acevedo-Fernández, J.J.; De Torres, N.W. Acute hypoglycemic and antidiabetic effect of teuhetenone a isolated from Turnera diffusa. Molecules 2017, 22, 599. [Google Scholar] [CrossRef]
- Semaming, Y.; Kukongviriyapan, U.; Kongyingyoes, B.; Thukhammee, W.; Pannangpetch, P. Protocatechuic acid restores vascular responses in rats with chronic diabetes induced by streptozotocin. Phytother. Res. 2015, 30, 227–233. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Volti, G.L.; Galvano, F. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.-L.; Han, L.; Yang, S.-Y.; Meng, X.-M.; Ma, W.-F.; Wang, M. The mechanism of interactions between flavan-3-ols against a-glucosidase and their in vivo antihyperglycemic effects. Bioorganic Chem. 2019, 85, 364–372. [Google Scholar] [CrossRef]
- Pitchai, D.; Balasubramanian, K.; Rajalakshmi, M.; Eliza, J.; Selvaraj, J. Insulin mimetic impact of catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine 2010, 17, 28–36. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef]
- Matin, A.; Doddareddy, M.R.; Gavande, N.; Nammi, S.; Groundwater, P.W.; Roubin, R.H.; Hibbs, D.E. The discovery of novel isoflavone pan peroxisome proliferator-activated receptor agonists. Bioorg. Med. Chem. 2013, 21, 766–778. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Jung, U.J.; Lee, M.-K.; Kim, H.-J.; Jeon, S.-M.; Park, Y.B.; Chung, H.-G.; Baek, N.-I.; Lee, K.-T.; Jeong, T.-S.; et al. Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabolism and pancreatic β-cell function in type 2 diabetic mice. Diabetes Res. Clin. Pract. 2008, 82, 25–32. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Jung, U.-J.; Jeon, S.-M.; Yeo, J.-Y.; Kim, H.-J.; Kim, D.-J.; Lee, M.-K.; Baek, N.-I.; Chung, H.-G.; Choi, M.-S. Antihyperglycemic and antioxidant properties of jaceosidin, a flavonoid isolated from Artemisia Princeps, in type 2 diabetic mice. Diabetes 2007, 56, pA667. [Google Scholar]
- Chen, J.; Mangelinckx, S.; Ma, L.; Wang, Z.; Li, W.; De Kimpe, N. Caffeoylquinic acid derivatives isolated from the aerial parts of Gynura divaricata and their yeast α-glucosidase and PTP1B inhibitory activity. Fitoterapia 2014, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Somsak, N.; Peerawit, P.; Chusri, T. Hypoglycemic activity in diabetic rats of stigmasterol and sitosterol-3-O-β-d-glucopyranoside isolated from Pseuderanthemum palatiferum (Nees) Radlk. leaf extract. J. Med. Plants Res. 2015, 9, 629–635. [Google Scholar] [CrossRef]
- Varshney, R.; Varshney, R.; Mishra, R.; Gupta, S.; Sircar, D.; Roy, P. Kaempferol alleviates palmitic acid-induced lipid stores, endoplasmic reticulum stress and pancreatic β-cell dysfunction through AMPK/mTOR-mediated lipophagy. J. Nutr. Biochem. 2018, 57, 212–227. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.D.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Song, F.; Xing, J.; Tsao, R.; Liu, Z.; Liu, S. Screening and structural characterization of α-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MS n and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009, 20, 1496–1503. [Google Scholar] [CrossRef] [Green Version]
- Roghani, M.; Fallahi, F.; Moghadami, S. Citrus flavonoid naringenin improves aortic reactivity in streptozotocin-diabetic rats. Indian J. Pharmacol. 2012, 44, 382–386. [Google Scholar] [CrossRef]
- Silva, F.S.G.; Oliveira, P.J.; Duarte, M. Oleanolic, ursolic, and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: Promise or illusion? J. Agric. Food Chem. 2016, 64, 2991–3008. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.-K.; Park, Y.B.; Jeon, S.-M.; Choi, M.-S. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef] [Green Version]
- Cremonini, E.; Bettaieb, A.; Haj, F.G.; Fraga, C.G.; Oteiza, P. (-)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch. Biochem. Biophys. 2016, 599, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, M.; Hara, Y. Inhibition of rat small intestinal sucrase andα-glucosidase activities by tea polyphenols. Biosci. Biotechnol. Biochem. 1993, 57, 123–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata, R.; Cristians, S.; Escandón-Rivera, S.M.; Juárez-Reyes, K.; Rivero-Cruz, I. Mexican antidiabetic herbs: Valuable sources of inhibitors of α-glucosidases. J. Nat. Prod. 2013, 76, 468–483. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.; Zanatta, L.; Seifriz, I.; Creczynski-Pasa, T.B.; Pizzolatti, M.G.; Szpoganicz, B.; Silva, F.R.M.B. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-o-(α)-dirhamnoside from Bauhinia forficate leaves. J. Nat. Prod. 2004, 67, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.N.V.; Suma, S.M.; Banerji, A.; Gopalakrishnapillai, A. Kaempferitrin inhibits GLUT4 translocation and glucose uptake in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2009, 380, 39–43. [Google Scholar] [CrossRef]
- Babu, S.; Krishnan, M.; Rajagopal, P.; Periyasamy, V.; Veeraraghavan, V.; Govindan, R.; Jayaraman, S. Beta-sitosterol attenuates insulin resistance in adipose tissue via IRS-1/Akt mediated insulin signaling in high fat diet and sucrose induced type-2 diabetic rats. Eur. J. Pharmacol. 2020, 873, 173004. [Google Scholar] [CrossRef] [PubMed]
- Qadan, F.; Verspohl, E.J.; Nahrstedt, A.; Petereit, F.; Matalka, K. Cinchonain Ib isolated from Eriobotrya japonica induces insulin secretion in vitro and in vivo. J. Ethnopharmacol. 2009, 124, 224–227. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Anti-diabetic effects of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 34–39. [Google Scholar] [CrossRef]
- Timmers, S.; De Ligt, M.; Phielix, E.; Van De Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.C.; Schrauwen-Hinderling, V.B. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: A randomized controlled trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef] [Green Version]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Kapoor, B.; Gulati, M.; Kumar, R.; Ramanunny, A.K.; Awasthi, A.; Dua, K. Treatment strategies against diabetes: Success so far and challenges ahead. Eur. J. Pharmacol. 2019, 862, 172625. [Google Scholar] [CrossRef]
- Nenquin, M.; Henquin, J.C. Sulphonylurea receptor-1, sulphonylureas and amplification of insulin secretion by Epac activation in β cells. Diabetes Obes. Metab. 2016, 18, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.S.; Graven, L.J. Progressing from metformin to sulfonylureas or meglitinides. Workplace Health Saf. 2016, 64, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.S.; Deacon, C.F.; Holst, J.J. Do we know the true mechanism of action of the DPP-4 inhibitors? Diabetes Obes. Metab. 2018, 20, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Rivero-Cruz, I.; Cristians, S.; Ovalle-Magallanes, B.; Mata, R. Mexican copalchis of the Rubiaceae family: More than a century of pharmacological and chemical investigations. Phytochem. Rev. 2019, 18, 1435–1455. [Google Scholar] [CrossRef]
- Wang, B.; Li, N.; Liu, T.; Sun, J.; Wang, X. Synthesis and biological evaluation of novel neoflavonoid derivatives as potential antidiabetic agents. RSC Adv. 2017, 7, 34448–34460. [Google Scholar] [CrossRef] [Green Version]
- Yap, A.; Nishiumi, S.; Yoshida, K.-I.; Ashida, H. Rat L6 myotubes as an in vitro model system to study GLUT4-dependent glucose uptake stimulated by inositol derivatives. Cytotechnology 2007, 55, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ma, H.; Zhu, D.; Zhao, G.; Wang, L.; Fu, X.; Chen, W. Discovery of novel insulin sensitizers: Promising approaches and targets. PPAR Res. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Nanjan, M.; Mohammed, M.; Kumar, B.R.P.; Chandrasekar, M. Thiazolidinediones as antidiabetic agents: A critical review. Bioorganic Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef]
- Davidson, M.A.; Mattison, D.R.; Azoulay, L.; Krewski, D. Thiazolidinedione drugs in the treatment of type 2 diabetes mellitus: Past, present and future. Crit. Rev. Toxicol. 2017, 48, 52–108. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 11th ed.; Elsevier: Philadelphia, PA, USA, 2006. [Google Scholar]
- DeFronzo, R.A.; Mandarino, L.; Ferrannini, E. Metabolic and Molecular Pathogenesis of Type 2 Diabetes Mellitus. In International Textbook of Diabetes Mellitus; DeFronzo, R.A., Ferrannini, E., Keen, H., Zimet, P., Eds.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Hunter, R.W.; Hughey, C.C.; Lantier, L.; Sundelin, E.I.O.; Peggie, M.; Zeqiraj, E.; Sicheri, F.; Jessen, N.; Wasserman, D.H.; Sakamoto, K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018, 24, 1395–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, U.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
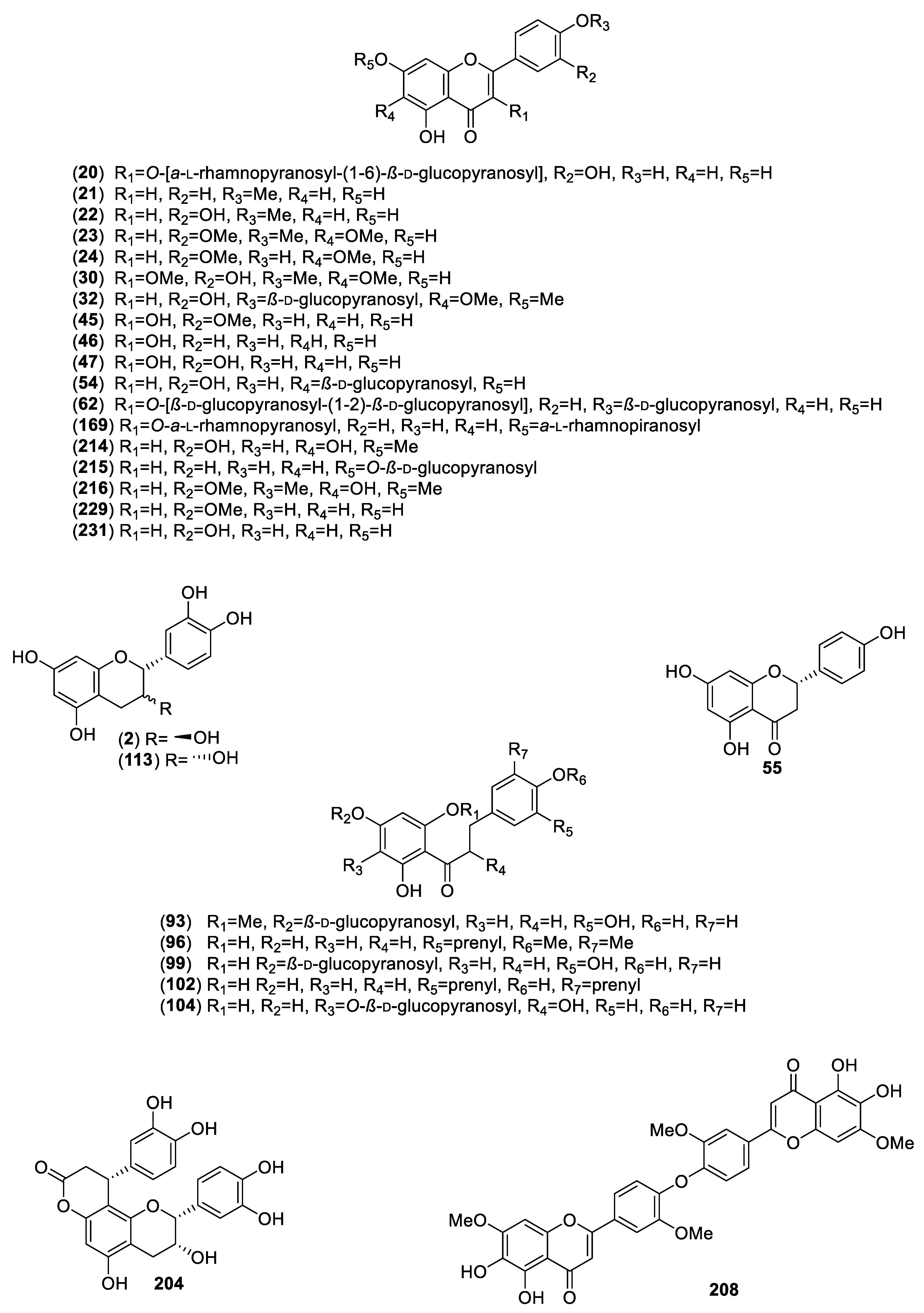
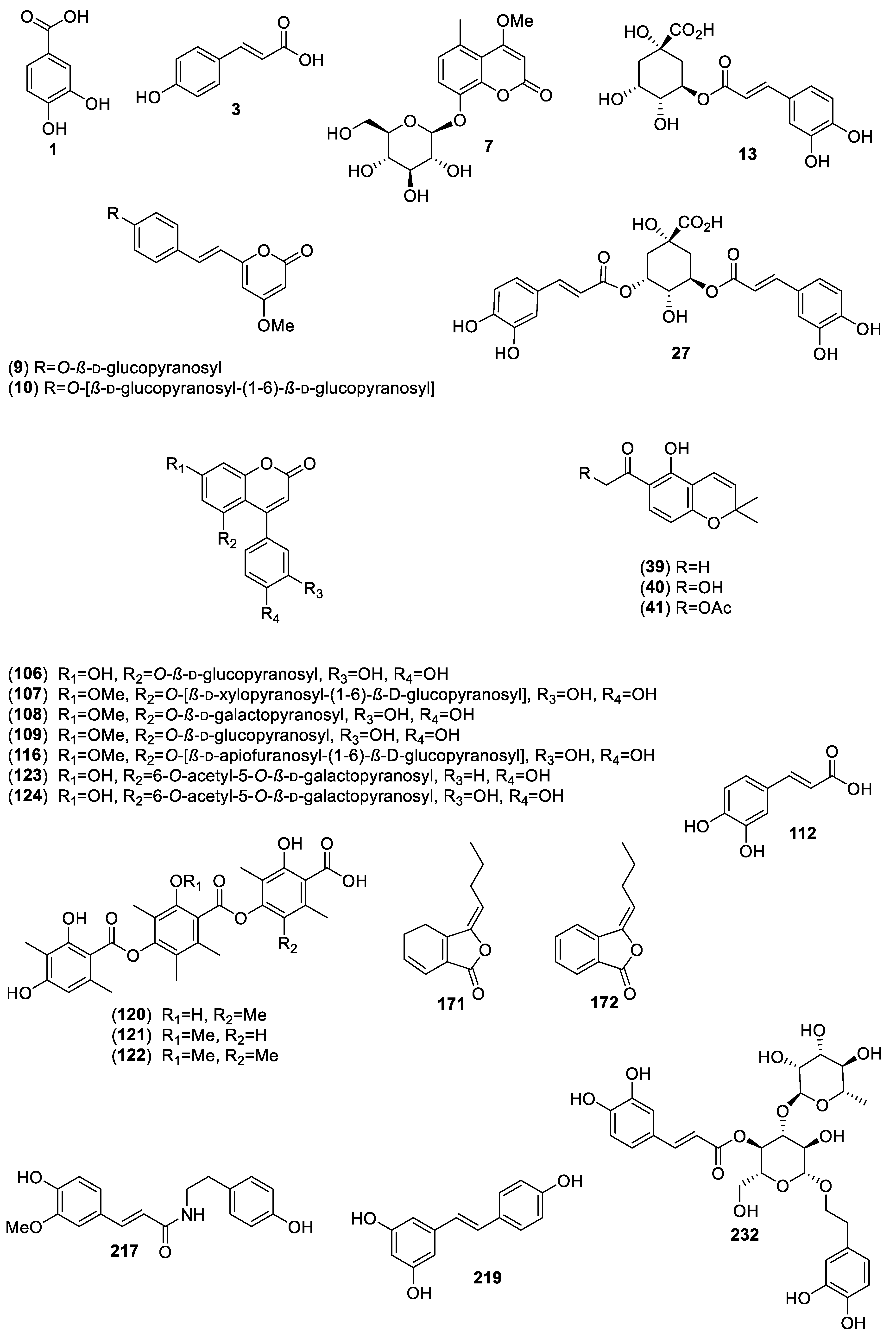

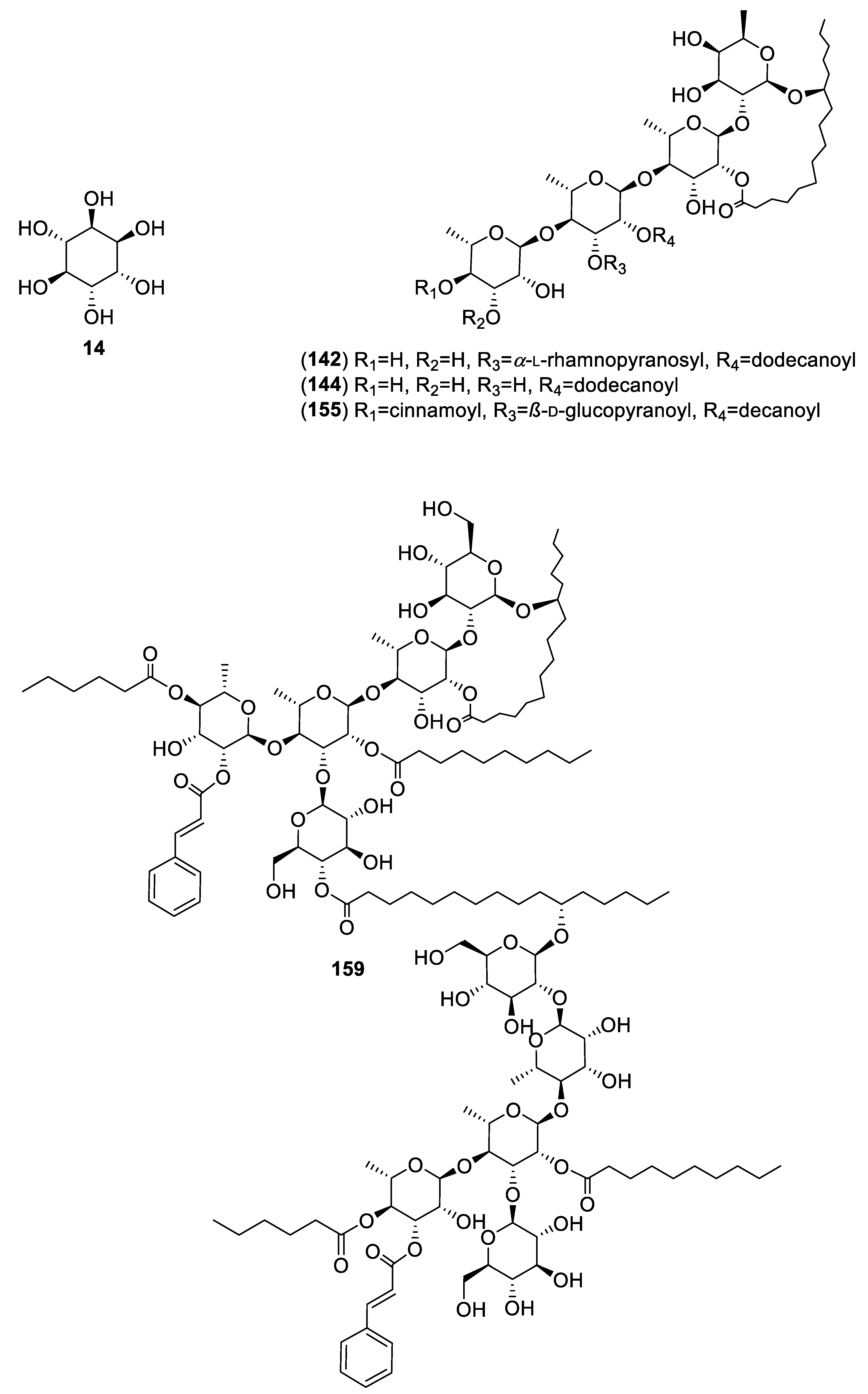
| Plant/Family/Part | Isolated Compounds | Ref. |
|---|---|---|
| Acacia angustissima (Mill.) Kuntze (Fabaceae)/Pods. | Protocatechuic acid (1); catechin (2); ρ-coumaric acid (3). | [12] |
| Acourtia thurberi (A.Gray) Reveal & R.M.King (Asteraceae)/Roots. | Perezone (4); α-pipitzol (5); β-pipitzol (6); 8-β-d-glucopyranosyloxy-4-methoxy-5-methyl-coumarin (7). | [13] |
| Acosmium panamense (Benth.) Yacolev (Fabaceae)/Roots. | Desmethylyangonine (8); desmethylangonine-O-β-d-glucopyranoside (9); desmethylangonine-O-β-d-glucopyranosyl-(1→6)-O-β-d-glucopyranoside (10). | [14,15] |
| Agarista mexicana (Hemsl.) Judd. (Ericaceae)/Bark. | 12-Ursene (11); 23,24-dimethyl-24-ethyl-stigmast-25-ene (12). | [16,17] |
| Ageratina petiolaris (Moc. & Sessé ex DC.) R. M. King & H. Rob (Asteraceae)/Aerial parts. | Chlorogenic acid (13); L-chiro-inositol (14); 2α-iso-valeroyloxyeperuic acid (15); benzyl-2-hydroxy-6-methoxybenzoate (16); benzyl-2,6-dimethoxybenzoate (17); 3-methoxybenzyl 2,6-dimethoxybenzoate (18); benzyl-2-hydroxy-3,6-dimethoxybenzoate (19). | [18,19] |
| Annona cherimola Mill (Annonaceae)/Leaves. | Rutin (20). | [20] |
| Anoda cristata (L.) Schltdl. (Malvaceae)/Aerial. | Acacetin (21); diosmetin (22). | [21] |
| Artemisia ludoviciana Nutt (Asteraceae)/Aerial. | Eupatilin (23); jaceosidin (24); arglanin (25); salvinine (26); 3,5-di-O-caffeoylquinic acid (27). | [22] |
| Arracacia tolucensis (Kunth) Hemsl. (Apiaceae)/Aerial. | (S)-(+)-4′-O-angeloylvisamminol (28); praeruptorin A (29). | [23] |
| Brickellia veronicaefolia (Khunt) Gray (Asteraceae)/Aerial. | 5,7,3′-Trihydroxy-3,6,4′-trimethoxyflavone (30). | [24,25] |
| Bromelia karatas (L) (Bromeliaceae)/Aerial. | β-Sitosterol-3-O-β-d-glucopyranoside (31); ρ-coumaric acid (3); cirsiliol-4′-O-β-d-glucopyranoside (32); stigmasterol (33); β-sitosterol (34); 1-O-feruloyl-3-O-ρ-coumaroylglycerol (35); β-d-(1-O-acetyl-3,6-O-trans-diferuloyl)-fructofuranosyl-α-d-2′,4′,6′-O-triacetyl-glucopyranoside (36); 1-O-ρ-coumaroyl-3-O-caffeoylglycerol (37); 2-propyl-β-d-glucopyranoside (38). | [19,26,27] |
| Calea oliveri B.L.Rob. & Greenm (Asteraceae)/Aerial. | 6-Acetyl-5-hydroxy-2,2-dimethyl-2H-chromene (39); 6-hydroxyacetyl-5-hydroxy-2,2-dimethyl-2H-chromene (40); 6-acetyl-5-hydroxy-2-methyl-2-hydroxymethyl-2H-chromene (41); caleins A (42) and C (43); genkwanin (44); isorhamnetin (45); kaempferol (46); quercetin (47); herniarin (48); scoparone (49); 4′,7-dimethylapigenin (50); curcumene (51); spathulenol (52); caryophyllene oxide (53). acacetin (21); 3,5-di-O-caffeoylquinic acid (27). | [28,29] |
| Cecropia obtusifolia Bertol. (Urticaceae)/Leaves. | Chlorogenic acid (13); isoorientin (54). | [30,31,32,33] |
| Cochlospermum vitifolium (Willd) (Bixaceae)/Bark. | (±)-Naringenin (55). | [34,35] |
| Coriandrum sativum L. (Apiaceae)/Aerial. | Rutin (20). | [36] |
| Cucurbita ficifolia Bouché (Cucurbitaceae)/Fresh mature and immature fruits. | ρ-Coumaric acid (3); stigmast-7,22-dien-3-ol (56); salicin (57); stigmast-7-en-3-ol (58); ρ-hydroxybenzoic acid (59). | [37,38,39,40,41] |
| Equisetum myriochaetum Schlecht. & Cham. (Equisetaceae)/Aerial. | Kaempferol-3-O-sophoroside (60); kaempferol-3,7-di-O-β-d-glucopyranoside (61); kaempferol-3-O-sophoroside-4′-O-β-d-glucopyranoside (62); caffeoyl-methylate-4-β-d-glucopyranoside (63). | [19,32,42,43,44] |
| Eysenhardtia platycarpa Pennell & Saff. (Fabaceae)/Leaves, branches and bark. | (1″R)-5,4′,1″-Trihydroxy- 6,7-(3″,3″-dimethyl chroman) flavone (64); 5,7-dihydroxy-6-methyl-8-prenyl flavanone (65); 5,7-dihydroxy-8-methyl-6-prenyl flavanone (66); 5,7-dihydroxy-6-prenylflavanone (67); 5,7-dihydroxy-8-prenylflavanone (68); 3-O-acetyloleanolic acid (69); oleanolic acid (70); 3β-acetoxy-11α,12α-epoxy-oleanan-28,13β-olide (71); lupeol (72); betulinic acid (73); β-sitosterol (34); β-sitosterol-3-O-β-d-glucopyranoside (31); β-sitosteryl palmitate (74); 3-O-methyl-myo-inositol (75); (+)-catechin (2); (2S)-4′-O-methyl-6-methyl-8-prenyl-naringenin (76); 3,4,6,4′-O-methyl-8-prenylnaringenin (77); 5-hydroxy-7-methoxy-8-prenyl-flavanone (78); (+)-catechin 3-O-β-d-galactopyranoside (79). | [45,46] |
| Eysenhardtia polystachya (Ortega) Sarg. (Fabaceae)/Leaves and bark. | 2′,4′-Dihydroxychalcone-6′-O-β-d-glucopyranoside (80); α,3,2′,4′-tetrahydroxy-4-methoxydihydrochalcone-3′-C-β-d-glucopyranosyl-6′-O-β-d-glucopyranoside (81); 7-hydroxy-5,8′-dimethoxy-6′α-l-rhamnopyranosyl-8-(3-phenyl-trans-acryloyl)-1-benzopyran-2-one (82); 6′,7-dihydroxy-5,8-dimethoxy-8(3-phenyl-trans-acryloyl)-1-benzopyran-2-one (83); 9-hydroxy-3,8-dimethoxy-4-prenyl-pterocarpan (84); α,4,4′-trihydroxydihydro-chalcone-2′-O-β-d-glucopyranoside (85); 5,4′-dihydroxy-7,2′-dimethoxylisoflavone (86); (3R)-5,7-2′,4′-tetrahydroxyl-3′-methoxylisoflavanone (87); flemichapparin C (88); neo-hesperidin dihydrochalcone (89); hesperetin dihydrochalcone-glucoside (90); aspalathin (91); sandwicensis (92); 6′-methoxy-sieboldin (93); 2′-O-α-l-rhamnopyranosyl-α,6′-dihydroxy-4′-acetyl-4-methoxydihydro-chalcone (94); 2′-O-β-d-glucopyranosyl-4′-methoxy-4-hydroxy-3-isoprenyldihydrochalcone (95); 2′,4′,6′-trihydroxy-4,5-dimethoxy-3-isoprenyldihydrochalcone (96); 3′-C-β-glucopyranosyl-α,2′,4′,6′-trihydroxy-4-methoxy-dihydrochalcone (97); 3′-C-β-glucopyranosyl-α,2′,4-trihydroxy-4′,6′-dimethoxy-dihydrochalcone (98); 3-hydroxyphloretin-4′-O-β-d-glucopyranoside (99); 3,4′-dihydroxy-2,4,6-trimethoxy-dihydrochalcone (100); 2′,4′,4- trihydroxy-3′-methoxy-dihydrochalcone (101); 2′,4′,6′,4-tetrahydroxy-3,5-diisoprenyldihydrochalcone (102); 3′-C-β-glucopyranosyl-α,2′,4′,3,4-pentahydroxydihydroxychalcone (103); 3′-O-β-d-glucopyranosyl-α,4,2′,4,6′-pentahydroxy-dihydrochalcone (104). | [47,48,49,50,51] |
| Exostema caribaeum (Jacq.) Schult. (Rubiaceae)/Stem bark. | Chlorogenic acid (13); 5-O-[β-d-xylopyranosyl-(1→6)-β-d-glucopyranosyl]-7,3′,4′-trihydroxy-4-phenylcoumarin (105); 5-O-β-d-glucopyranosyl-7,3′,4′-trihydroxy-4-phenylcoumarin (106); 5-O-[β-d-xylopyranosyl-(1→6)-β-d-glucopyranosyl]-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (107); 5-O-β-d-galactopyranosyl-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (108); 5-O-β-d-glucopyranosyl-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (109); 5-O-(6′-acetyl-β-d-glucopyranosyl)-7,3′,4′-trihydroxy-4-phenylcoumarin (110); 5-O-(6′-acetyl-β-d-galactopyranosyl)-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (111). | [52] |
| Hamelia patens Jacq. (Rubiaceae)/Aerial parts and leaves. | Not isolated but identified chlorogenic acid (13); quercetin (47); caffeic acid (112); epicatechin (113); catechin (2). | [53,54] |
| Hintonia latiflora (Sessé & Moc. ex DC.) Bullock (Rubiaceae)/Stem bark and leaves/endophytic fungus | 5-O-β-d-Glucopyranosyl-7,3′,4′-trihydroxy-4-phenylcoumarin (106); 5-O-[β-d-xylopyranosyl-(1→6)-β-d-glucopyranosyl]-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (107); 5-O-β-d-galactopyranosyl-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (108); 5-O-β-d-glucopyranosyl-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (109); 25-O-acetyl-3-O-β-d-glucopyranosyl-23,24-dihydrocucurbitacin F (114); 3-O-β-d-glucopyranosyl-23,24-dihydrocucurbitacin F (115); 5-O-[β-d-apiofuranosyl-(1→6)-β-d-glucopyranosyl]-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (116); desoxycordifolinic acid (117); ursolic acid (118); 5-O-[β-d-xylopyranosyl-(1→6)-β-d-glucopyranosyl]-7,4′-dimethoxy-4-phenylcoumarin (119); chlorogenic acid (13); thielavins A (120), J (121) and K (122). | [55,56,57,58] |
| Hintonia standleyana Bullock (Rubiaceae)/Stem bark and leaves. This taxon is a synonym of; Hintonia latiflora (Sessé & Moc. ex DC.) | 25-O-Acetyl-3-O-β-d-glucopyranosyl-23,24-dihydrocucurbitacin F (114); 3-O-β-d-glucopyranosyl-23,24-dihydrocucurbitacin F (115); 5-O-[β-d-apiofuranosyl-(1→6)-β-d-glucopyranosyl]-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (116); 5-O-β-d-glucopyranosyl-7-methoxy-3′,4′-dihydroxy-4-phenylcoumarin (109); 6″-O-acetyl-5-O-β-d-galactopyranosyl-7,4′-dihydroxy-4-phenylcoumarin (123); 6″-O-acetyl-5-O-β-d-galactopyranosyl-7,3′,4′-trihydroxy-4-phenylcoumarin (124). | [56,59] |
| Ibervillea sonorae (S. Watson) Greene (Cucurbitaceae)/Roots. | 1-Monopalmitin (125); glyceryl-1-monomargarate (126); 1-monostearin (127); glyceryl-1-monononadecylate (128); glyceryl-1-monoarachidate (129); glyceryl-1-monobehenate (130); glyceryl-1-monotricosanoate (131); glyceryl-1-monotetracosanoate (132); glyceryl-1-monopentacosanoate (133); glyceryl-1-monohexacosanoate (134); glyceryl-1-monooctacosanoate (135); lauric acid (136); myristic acid (137); pentadecanoic acid (138); palmitic acid (139); stearic acid (140); gallic acid (141). | [60,61,62,63,64] |
| Ipomoea pes-caprae (L.) R. Br and Ipomoea purga (Wender.) Hayne (Convolvulaceae)/Roots. | Pescapreins I (142), III (143), V (144) and IX (145); stolonoferins I (146) and III (147); murucoidins IV (148), V (149), XIV (150), XVIII (151), XIX (152) and XX (153); purginosides I (154), II (155) and IV (156); purgins I (157), II (158) and III (159); tricolorins A (160), E (161) and I (162); wolcottines I (163), I (164), II (165), III (166) and IV (167); intrapilosin VII (168). | [65] |
| Justicia spicigera Schltdl (Acanthaceae)/Leaves. | Kaempferitrin (169) | [66] |
| Ligusticum porteri J.M. Coult. & Rose (Apiaceae)/Roots. | (Z)-6,6′,7,3′α-Diligustilide (170); (Z)-ligustilide (171); 3-(Z)-butylidenephthalide (172); myristicin (173), Ferulic acid (174). | [67] |
| Melampodium perfoliatum (Cav.) Kunth (Asteraceae)/Aerial. | Perfoliatin A (175). | [68] |
| Mosannona depressa (Baill.) Chatrou (Annonaceae)/Roots. | 2-Hydroxy-3,4,5-trimethoxy-1-(2′,4′-hydroxy-3′-dihydroxy) butylbenzene (176); 2-hydroxy-3,4,5-trimethoxy-1-(2′,3′,4′-hydroxy) butyl-benzene (177); 3-(3-hydroxy-2,4,5-trimethoxyphenyl) propane-1,2 diol (178). | [32,69,70,71] |
| Opuntia streptacantha Lem. (Cactaceae)/Cladodes. | 4-Hydroxyphenylacetic acid (179). | [72,73] |
| Psacalium decompositum (A.Gray) H.Rob. & Brettell (Asteraceae)/Roots. | Cacalol (180); cacalol acetate (181); cacalone (182); maturin (183); maturinone (184). | [74,75,76,77] |
| Psacalium paucicapitatum (B.L.Rob. & Greenm.) H.Rob. & Brettell (Asteraceae)/Corms. | Kestose (185); nystose (186); fructofuranosyl-nystose (187). | [78] |
| Phoradendron reichenbachianum (Seem.) Oliv. (Santalaceae)/Leaves and stems. | Moronic acid (188); morolic acid (189); oleanolic acid (70); ursolic acid (118); 3,4-seco-olean-18-ene-3,28-dioic acid (190); α-amyrin (191); β-amyrin (192); oleanolic aldehyde (193); lupeol (72); lupenone (194); betulin aldehyde (195); betulon aldehyde (196); betulinic acid (73); acacetin (21); betulonic acid (197); squalene (198); triacontanol (199); β-sitosteryl linoleate (200); stigmasteryl linoleate (201); β-sitosterol (34); stigmasterol (33); acacetin 7-methyl ether (202). | [79,80,81] |
| Rhizophora mangle L. (Rizophoraceae)/Cortex. | Cinchonains Ia (203) and Ib (204); epicatechin (113); catechin-3-O-rhamnopyranoside (205); lyoniside (206); nudiposide (207). | [19,82,83,84] |
| Salvia circinnata Cav. (Lamiaceae)/Aerial. | 6,6″,3″-Trihydroxy-7,3′,7′-O-trimethyl-loniflavon (208); amarisolides B (209), C (210), D (211) and E (212); amarisolide (213); pedalitin (214); apigenin-7-O-β-d-glucopyranoside (215); 2-(3,4-dimethoxy-phenyl)-5,6-dihydroxy-7-methoxy-4H-chromen-4-one (216). | [85] |
| Smilax aristolochiifolia Mill. (Smilaceae)/Roots. | N-trans-Feruloyltyramine (217); astilbin (218); chlorogenic acid (13). | [86,87] |
| Smilax moranensis M. Martens & Galeotti (Smilaceae)/Roots. | trans-Resveratrol (219); 5-O-caffeoylquinic acid (220); chlorogenic acid (13). | [19,84,88,89] |
| Swietenia humilis Zucc (Melaiceae)/Seeds. | 2-Hydroxy-destigloyl-6-deoxyswietenine acetate (221); humulin B (222); methyl-2-hydroxy-3-β-isobutyroxy-1-oxomeliac-8(30)-enate (223); methyl-2-hydroxy-3-β-tigloyloxy-1-oxomeliac-8(30)-enate (224); humilinolide G (225); humilinolide C (226); methyl-2-hydroxy-3-β-isobutyloyl-8α,30α-epoxy-1-oxo-meliacate (227); humilinolide H (228). | [90,91,92] |
| Tecoma stans (L.) Juss. ex Kunth (Bignoniaceae)/Leaves. | Chrysoeriol (229); apigenin (230); luteolin (231); verbascoside (232); luteolin-7-O-glucopyranoside (233). | [93,94] |
| Turnera diffusa Willd. ex Schult. (Passifloraceae)/Aerial. | Teuhetenone A (234). | [95] |
| Plant/Extract | Experiment 1 | AC 2 | Other Activity Found |
|---|---|---|---|
| Acacia angustissima/Methanol extract (ME). | In vivo: ME: AHT (25, 50, and 100 mg/kg of bw) and OGTT (25, 50, and 100 mg/kg of bw) in healthy and STZ-treated rats. BP: TC, TG, LDL and HDL [12]. In vitro: Lipid peroxidation and protein content in kidney, glucose incorporation assay in adipocytic cells. | 1 2 3 | 1 Decreases blood glucose in diabetic rats; insulin-sensitizing [96,97]. 2 is in vitro and in vivo AG inhibitor [98], and hypoglycemic (CHT), glucose oxidizing and insulin-mimetic agent [99]. 3 lowers blood glucose level (CHT), glucose-6-phosphatase, and fructose-1,6-bisphosphatase; increases the activities of hexokinase, G6PD, and GSH by increasing level of insulin; reduces the total cholesterol and triglycerides in both plasma and tissues i.e., liver and kidney [100]. |
| Acourtia thurberi/Aque-ous extract (WE). | In vivo: WE: AHT, OGTT, and OSTT in healthy and STZ-treated mice using half-log interval doses (31.6, 100, and 316.2 mg/kg of bw of the extract and 3.2, 10, and 31.6 mg/kg of bw of compounds for all experiments) [13]. In vitro: WE: Y-AG (IC50 = 566.7 μg/mL). | 4(31.6 mg/kg) 5(3.2-31.6 mg/kg/944.9 μM) 6(3.2-31.6 mg/kg/944.9 μM) 7(3.2-31.6 mg/kg/3.98 μM) | - |
| Acosmium panamense/Aq-eous (WE) and butanol (BE) extracts. | In vivo: WE: AHT (20 and 200 mg/kg of bw) in STZ-treated rats. BE: AHT (20 and 100 mg/kg of bw) STZ-treated rats. Dose of compounds 9 and 10 for AHT: 20 mg/kg of bw [15]. | 9(20 mg/kg) 10(20 mg/kg) | - |
| Agarista mexicana/Chlo-roform extract (CHE). | In vivo: CHE: AHT in healthy (150 mg/kg of bw) and alloxan-treated mice (50, 100 and 150 mg/kg of bw) and OGTT in alloxan-treated rats (150 mg/kg of bw). Dose of compounds 11 and 12 for AHT was 50 mg/kg of bw [17]. | 11(50 mg/kg) 12(50 mg/kg) | - |
| Ageratina petiolaris/Aqu- eous (WE) and methanol (ME). | In vivo: WE: AHT (40 and 160 mg/kg of bw), OGTT (160 mg/kg bw); PTT (160 mg/kg of bw) in STZ-NA-treated rats. ME: AHT (67 and 268 mg/kg of bw) in STZ-NA-treated rats. Dose of 14 for AHT was 3.73 mg/kg of bw [18]. In vitro: WE: G6Pase Activity (IC50 = 223 μg/mL) [19]. | 13(56 μg/mL) 14(3.73 mg/kg) | - |
| Annona cherimola/Etha-nol extract (EE). | In vivo: EE: AHT, CHT in healthy and alloxan-treated rats; OGTT and OSTT in Normoglycemic rats at a dose of 300 mg/kg of bw for the extract and 30 mg/kg of bw for 20 in all experiments (AHT, CHT, OGTT and OSTT); 20 was active in all experiments [20]. | 20(30 mg/kg) | - |
| Anoda cristata/Mucil-age (M), free mucilage aqueous (FM-WE), aqueous (WE) and organic (OE) extracts. | In vivo: WE and M tested in AHT, OGTT, and OSTT in healthy and STZ-NA-treated mice (31.6, 100, and 316 mg/kg of bw). FM-WE: AHT, OGTT, and OSTT in healthy and STZ-NA-treated mice (31.6, 100, and 316 mg/kg of bw); OGTT and CHT in metabolic syndrome induced rats (100, and 316 mg/kg of bw). BP: cholesterol, TG, uric acid and glucose. (most active). OE: OSTT in healthy and STZ-NA-treated mice (31.6, 56.2, and 100 mg/kg of bw). Doses of 21 and 22 for AHT were 3, 10, and 31.6 mg/kg of bw [21]. | 21(3, and 31.6 mg/kg) 22(3-31.6 mg/kg) | 21 and 22 are PPAR agonists and antioxidants [101]. |
| Artemisia ludoviciana/Es- sential oil (EO), organic (OE) and aqueous (WE) extracts. | In vivo: EO, OE, and WE tested in AHT, OGTT, and OSTT in healthy and STZ-treated mice (31.6, 100, and 316 mg/kg of bw). Isolated compounds: Cotreatment with Ca2+ and K+ ion channels regulators (17.7 mg/kg bw); the doses of 23 and 25 for AHT were 5.6, 17.7, and 31.6 mg/kg of bw. In vitro: Y-AG for isolated compounds [22]. | 23(17.7, 31.6 mg/kg; 0.49 μM) 24 25(5.6,17.7 and 31.6 mg/kg) 26(545.2 μM) 27 | 23 and 24 lowers blood glucose levels through the up-regulation of GK activity, plasma insulin and adiponectin concentration, downregulated G6Pase and PEPCK activities, and sustained pancreatic β-cell function [102,103]. 27: inhibits AG [104]. |
| Arracacia tolucensis/Hex-ane (HE), ethyl acetate (EAE) and ethanol (EE) extracts. | In vivo: HE, EAE, EE: CHT (250 mg/kg of bw). Hematic biometry and BP: urea, creatinine, cholesterol, TG, HDL, LDL, VLDL, AST, ALT, and bilirubin; EAE was the most active) [23]. | no compounds were tested in this experiment | - |
| Brickellia veronicaefolia/ Essential oil (EO), chloroform (CHE) and organic (OE) extracts. | In vivo: OE and EO: AHT, OGTT in healthy and STZ-NA-treated mice (OE doses: 30, 100, and 300 mg/kg of bw; EO doses: 10 mg/kg of bw). CHE: isolation of compounds for testing in AHT in healthy and alloxan-treated mice. The doses of 30 for AHT were 10, 25, and 50 mg/kg of bw [24]. | 30(50 mg/kg) | - |
| Bromelia karatas/Ethanol:water (EWE), aqueous (WE) and organic (OE) extracts. | In vivo: WE tested in AHT (35 and 350 mg/kg of bw), CHT (218 mg/kg of bw) and PTT (218 mg/kg) in STZ-NA rats. EWE in AHT (30 and 350 mg/kg of bw) in STZ-NA rats. BP: HbA1c, HDL, TG and cholesterol. The doses of 31, 3 and 32 for AHT were 72, 3.63, and 1.8 mg/kg of bw, respectively [26,27]. In vitro: G6Pase Activity [19]. | 31(72 mg/kg) 32(1.8 mg/kg) 3(3.63 mg/kg) 33 | 31 and 33 have hypoglycemic effects in STZ-NA rats treated with doses of 0.25 and 0.50 mg/kg for 21 days to improve biochemical and hematological parameters [105]. |
| Calea oliveri/Aqueo-us extract (WE) and essential oil (EO). | In vivo: WE tested in AHT, OGTT, and OSTT in healthy and STZ-NA mice (dose of 56, 100, and 316 mg/kg of bw for all experiments). EO: OSTT (31.6, 100 and 316 mg/kg of bw). The dose of 39 for OSTT were 5.6, 10, and 31.6 mg/kg of bw; the dose of both 42 and 43 for OSTT were 3.16, 7 and 10 mg/kg of bw. In vitro: Y-AG for WE (IC50 = 0.169 mg/mL) and isolated compounds [28,29]. | 21 27 39(5.6-31.6 mg/kg) 40(0.42 mM) 41 42(3.16-10 mg/kg) 43(3.16-10 mg/kg; 0.28 mM) 45(0.16 mM) 46 47(0.53 mM) | 46 Restores PA-induced loss of β-cell mass and function through AMPK/mTOR-mediated autophagy [106]; inhibits AG [107]. 45 and 47 Increase glucose uptakes in skeletal muscle by activating the JAK/STAT pathway, and by CaMKKβ/AMPK and insulin signalling pathways, respectively [108]. |
| Cecropia obtusifolia/But-anol (BE) and aqueous (WE) extracts. | In vivo: WE tested in AHT (90 and 150 mg/kg of bw) in STZ-treated rats. CHT in diagnosed type 2 diabetic patients. BP: Serum glucose, cholesterol, TG and insulin levels were determined every 15 days; HbA1c, ALT, AST, and ALKP measured every month. BE: AHT (9 and 15 mg/kg of bw), OMTT (96 mg/kg of bw) in STZ-NA-treated rats. The dose of both 13 and 54 for AHT were 10 mg/kg of bw). In vitro: Y-AG for BE (IC50 =14 μg/mL); adipogenesis and 2-NBDglucose uptake in 3T3-F442A murine adipocytes [30,31,32]. | 13(10 mg/kg) 54(10 mg/kg) | 54 Inhibits AG [109]. |
| Cochlospermum vitifolium/Hex- ane (HE), dichlorometh-ane (DE) and methanol (ME) extracts. | In vivo: HE and DE assay in AHT (120 mg/kg of bw) in healthy and STZ-NA-treated rats. ME: in AHT (100 mg/kg of bw), OGTT (100 mg/kg of bw), CHT (100 mg/kg of bw) in healthy and STZ-NA-treated rats. BP: Glucose, total cholesterol, HDL and TG. In vitro: Hepatoprotective activity assay and RI-AG for ME (IC50 = 1.9 mg/ML) [34,35]. | 55 | 55 Could prevent functional changes in vascular reactivity in diabetic rats through nitric oxide- and no prostaglandin-dependent pathways [110]. |
| Coriandrum sativum/Aque-ous extract (WE). | In vivo: WE tested in OSTT (100, 300, and 500 mg/kg of bw) in healthy rats. The dose of 20 for OSTT was 50 mg/kg of bw). In vitro: Y-AG for WE (IC50 = 1.63 mg/mL) [36]. | 20(50 mg/kg) | - |
| Cucurbita ficifolia/Juice (J) and aqueous (WE) extracts. | In vivo: J tested in AHT (4ml/kg) in Type 2 diabetic patients with moderate hyperglycemia; AHT (125, 250, 500, 594.49, 750, 1000, and 1250 mg/kg of bw) and CHT (1000 mg/kg of bw) in healthy and alloxan-treated mice. EW: CHT (200 mg/kg of bw) in STZ-treated mice [37,39,41]. In vitro: Effect on [Ca2+]i in RINm5F cells. Viability assays using DRAQ7™ probe. Participation of C. ficifolia as regulator of [Ca2+]i through K+ ATP channels [40]. | 3 | - |
| Equisetum myriochaetum/Aqueous (WE) and butanol (BE) extracts. | In vivo: WE and BE assayed in AHT (7 and 13, 8 and 16 mg/kg of bw for WE and BE, respectively) in STZ-treated rats. WE tested in AHT (330 mg/kg of bw) in type 2 diabetic patients. BP: Glucose, TG, cholesterol, and glycated hemoglobin. OMTT (96 mg/kg of bw) and PTT (330 mg/kg of bw) in STZ-treated rats. Dose not reported for 62 in AHT. In vitro: G6Pase activity and Y-AG for WE [19,32,43,44]. | 62 | - |
| Eysenhardtia platycarpa/Met-hanol extract (ME). | In vivo: ME tested in AHT (30, 100, and 300 mg/kg of bw) in STZ-treated rats. The doses of 69 for AHT were 3.1, 10, and 31 mg/kg of bw [45,46]. | 69(31 mg/kg) 2 70 34 | 70 Acts as hypoglycemic and anti-obesity agent mainly through reducing the absorption of glucose, decreasing endogenous glucose production, increasing insulin sensitivity, improving lipid homeostasis, and weight regulation [111]. |
| Eysenhardtia polystachya/A-queous (WE) and methanol: water (MWE) extracts. | In vivo: WE tested in AHT in alloxan-treated mice. MWE in AHT (100, 200, and 400 mg/kg of bw) in STZ-treated mice; CHT (400 mg/kg of bw) in STZ-treated mice; OGTT (400 mg/kg of bw) in normal and STZ-treated mice. Compound 104: Tested in experimental diabetic nephropathy model to study pathological changes in the kidney (dose: 100 mg/kg of bw) [47,48,49,50,51]. In vitro: MWE tested for determining advanced glycation end-product formation [50]. | 93 96 99 102 104(100 mg/kg) | - |
| Exostema caribaeum/Aq-ueous extract (WE). | In vivo: WE tested in AHT and OSTT in healthy and STZ-NA-treated mice. Doses of 100, 300, and 500 mg/kg of bw for all experiments [52]. | 13 106 | - |
| Hamelia patens/Ethanol:water (1:1) (EWE), aqueous (WE) and methanol (ME) extracts. | In vivo: EWE and WE tested in AHT (30 and 300 mg/kg of bw, and 60 and 600 mg/kg of bw for EWE and WE, respectively) in STZ-NA-treated rats. ME assayed in CHT (35, 75 and 150 mg/kg of bw) in healthy and STZ-treated rats [53,54]. In vitro: Y-AG for ME (IC50 = 78.3 μg/mL). | 13 47 2 112 113 | 112 Exhibits significant potential as an antidiabetic agent by suppressing the progression of type 2 diabetic states that is suggested by attenuation of hepatic glucose output and enhancement of adipocyte glucose uptake, insulin secretion, and antioxidant capacity [112]. 113 improves insulin sensitivity in high fat diet-fed mice and inhibits AG [113,114]. |
| Hintonia latiflora/Orga- nic (OE) and aqueous (WE) and endophytic fungus extracts. | In vivo: OE tested in AHT (10, 30, 100, and 300 mg/kg of bw) in healthy and STZ-treated rats. In CHT (50 and 100 mg/kg of bw) in STZ rats. The doses of compounds 106-109 and 114-117 for CHT were 15 and 30 mg/kg of bw. WE tested in AHT (100, 300 and 500 mg/kg of bw), OSTT (100, 300, and 500 mg/kg of bw) in healthy and STZ-NA-treated rats. The doses of 122 for AHT and OSTT were 3.1, 10, and 31.6 mg/kg of bw [55,56,57,58,115]. The doses of 116 for OSTT was 50 mg/kg of bw. In vitro: Determination of hepatic glycogen, Y-AG for compounds. | 106(30 mg/kg) 107(30 mg/kg) 108(30 mg/kg) 109(30 mg/kg) 115(30 mg/kg) 116(30 mg/kg) 120(23.8 μM) 121(15.8 μM) 122(AHT: 31.6mg/kg; OSTT:10 mg/kg; 22.1 μM) 13 118 | - |
| Hintonia standleyana/Or-ganic extract (OE). | In vivo: OE tested in AHT (10 and 100 mg/kg of bw) in healthy and STZ-treated rats; CHT (50 and 100 mg/kg of bw) in STZ rats and developing hyperglycemic situation in rats. The doses of compounds 115, 116, 123, and 124 for AHT were 10 mg/kg of bw. The doses of both 115 and 116 for CHT were 15 and 30 mg/kg of bw [56,59]. | 115(15 mg/kg) 116(15 mg/kg) 123(10 mg/kg) 124(10 mg/kg) 109 | - |
| Ibervillea sonorae/Aque-ous (WE), juice (J), Dichlorometh-ane (DE) and methanol (ME) extracts. | In vivo: extracts tested in AHT in healthy and alloxan-treated mice (ip administration; the doses of WE were 150, 300, 600, and 850mg/kg of bw; dose for J, DE, and ME: 300 and was 600 mg/kg of bw). WE: Tested in a murine model of obesity and hyperglycemia, induced by a high-calorie diet; the relationship of these effects with hepatic oxidation were observed. In vitro: WE was assayed for glucose uptake in insulin- sensitive, and insulin-resistant murine and human cultured adipocytes; both in the absence or the presence of insulin signaling pathway inhibitors, and on murine and human adipogenesis [60,61,62,63,64]. | - | - |
| Ipomoea pes-caprae/hexane (HE) and chloroform (CHE) extracts. | In vitro: Y-AG of isolated compounds [65]. | 142(626 μM) 144(724 μM) 155(1067 μM) 159(330 μM) | - |
| Justicia spicigera/Etha- nol extract (EE). | In vivo: EE tested in OSTT (100 mg/kg of bw) in healthy and STZ-NA-treated rats. Effect on the glucose uptake in insulin-sensitive and insulin-resistant murine 3T3-F442A and human subcutaneous adipocytes [66]. | 169 | 169 Induces hypoglycemic effect in normal and in alloxan-induced diabetic rats; inhibits GLUT4 mediated glucose uptake in differentiated 3T3-L1 cells by interfering with the insulin signaling pathway, and by directly interacting with membrane GLUT4 [116,117]. |
| Ligusticum porteri/Organic extract (OE). | In vivo: OE tested in AHT, OGTT, and OSTT in healthy and STZ-NA mice; the doses were 56.2, 100, and 316 mg/kg of bw for all experiments. The doses of 170–171 for OGTT were 10, 31.2 and 56.2 mg/kg of bw for all compounds. The doses of 172 for OSTT were 10 and 56.2 mg/kg of bw. In vitro: Y-AG for isolated compounds [67]. | 171(10-56.2 mg/kg) 172(10 and 56.2 mg/kg; 2.5 mM) | - |
| Melampodium perfoliatum/Aq-ueous extract (WE). | In vivo: OSTT in STZ-NA-treated mice for isolated compound 175 (doses: 3.16, 10 and 31.6 mg/kg of bw). In vitro: RI-AG for extract (IC50 = 985.2 µg/mL) and isolated compound [68]. | 175(3.16-31.6 mg/kg; 6.5 mM) | - |
| Mosannona depressa/Aqu-eous (WE), butanol (BE) and ethanol (EE) extracts. | In vivo: AHT in STZ-treated rats for WE (40 and 80 mg/kg of bw), EE (113 mg/kg of bw) and BE (80 mg/kg bw); the last one was the most active. BE tested in OMTT (96 mg/kg of bw) and CHT (50 mg/kg of bw) in STZ-treated rats; and stimulation of insulin secretion in STZ-treated rats; BP measuring glucose, TG, cholesterol, and glycosylated hemoglobin were measured. EE: PTT (60 and 80 mg/kg of bw) in n5-STZ rats after an 18-h fasting period. In vitro: Effect on glucose-6-phosphatase activity for EE (IC50= 267.62 μg/mL) and Y-AG for BE (IC50= 267.62 μg/mL) [32,69,70,71]. | - | - |
| Opuntia streptacantha/Li-quefied (LE) filtrate extract (FE) and juice (J). | In vivo: LE tested in AHT (135 mg/kg of bw) and MTT (135 mg/kg of bw) in n5-STZ rats. FE: in AHT (12 and 27 mg/kg of bw) and MTT (12 and 27 mg/kg of bw) in n5-STZ rats. J in MTT (4 mL/kg) in n5-STZ rats. In vitro: RI-AG [72,73]. | - | - |
| Psacalium decompositum/Aqueous (WE), methanol (ME) and hexane (HE) extracts. | In vivo: WE tested in AHT (50, 100, 200, or 400 mg/kg of bw) in healthy and alloxan mice; in OGTT (dose not specified) in healthy rabbits; CHT (150 mg/kg of bw) in rats with 12 weeks fructose feeding. ME and HE tested in AHT (50, 100, 200, or 400 mg/kg of bw for both extracts) in healthy mice. The doses of 180-183 for AHT were 50 and 100 mg/kg of bw [74,75,76,77]. In vitro: Compounds tested in diazoxide-induced relaxation of male rat aortic rings precontracted with phenylephrine. | 180 182 | - |
| Psacalium paucicapitatum Aqueous extract (WE). | In vivo: WE tested in CHT and OGTT in mice with 12 weeks fructose feedings [78]. | - | - |
| Phoradendron reichenbachianum/Acetone extract (AE) | In vivo: AE tested in AHT (100 mg/kg of bw) in STZ-NA rats. CHT, OGTT, and OSTT for isolated compounds in STZ-NA rats (the doses of all the compounds tested were 50 mg/kg of bw) [79,80,81]. In vitro: Inhibitory activity of compounds against protein tyrosine phosphatase1B (PTP-1B). Assay for 11β-HSD1 inhibition [79]. | 188(50 mg/kg) 189(50 mg/kg) 70(50 mg/kg) 118(50 mg/kg) 21 34 | 34 Attenuates insulin resistance in adipose tissue via IRS-1/Akt mediated insulin signaling in high fat diet and sucrose induced type-2 diabetic rats [118]. |
| Rhizophora mangle/Aque-ous (WE) and ethanol:water (EW) extracts. | In vivo: WE tested in AHT (5.9 and 59 mg/kg of bw), OMTT (56 mg/kg of bw) in STZ-NA-treated rats. EW assayed in AHT (9 and 90 mg/kg of bw), CHT (90 mg/kg of bw) and PTT (90 mg/kg) in healthy and STZ-NA rats [19,82,83,84]. In vitro: G6Pase activity for EW (IC50= 99 μg/mL) and RI-AG [19,32]. | 113 204 | 204 Induces insulin secretion in vitro and in vivo [119]. |
| Salvia circinnata/Aqu-eous extract (WE). | In vivo: WE tested in AHT, OGTT, and OSTT in healthy and STZ-NA-treated mice. Doses of 31.6, 100 and 316 mg/kg of bw for all experiments. The doses for 213 and 214 for OSTT were 3.1, 10, and 31.6 mg/kg of bw, and 1, 3.1, and 10 mg/kg of bw, respectively. In vitro: RI-AG [85]. | 208(39 μM) 213(3.1-31.6 mg/kg; 500 μM) 214(1-10 mg/kg; 810 μM) 215(200 μM) 216(1800 μM) | - |
| Smilax aristolochiifolia/Acetone (AE), ethanol:water (EWE) and aqueous (WE) extracts. | In vivo: AE and 217 (25 mg/kg of bw) tested in the insulin tolerance curve in mice with a high-caloric diet. In vitro: Pancreatic α-amylase and Y-AG testing for WE, EWE, and compounds [86,87]. | 217(25 mg/kg) 13 | - |
| Smilax moranensis/Aq-ueous (WE) and ethanol (EE) extracts. | In vivo: WE tested in AHT (20 and 200 mg/kg of bw) in n5-STZ-treated rats. EE assayed in AHT (8 and 80 mg/kg of bw), CHT (80 mg/kg of bw), PTT (80 mg/kg of bw), MTT (80 mg/kg of bw) in healthy and STZ-NA rats; and BP measuring glycated hemoglobin (HbA1c) and lipid profile (HDL, TG and cholesterol). In vitro: G6Pase activity for EE (IC50 = 84 μg/mL) and Y-AG [19,84,88,89]. | 13(63 μg/mL) 219 | 219 Induces effects that might contribute to the protection of β cells in diabetes; it reduces insulin secretion in animals with hyperinsulinemia [120,121]. |
| Swietenia humilis/Aqueo-us extract (WE). | In vivo: WE tested in AHT (31.6, 100, and 316 mg/kg of bw) OGTT (31.6, 100, and 316 mg/kg of bw), OSTT (100, 177, and 316 mg/kg of bw) in healthy and STZ-NA-treated mice; OGTT (100 and 316 mg/kg of bw) in metabolic syndrome in Sprague Dawley rats (FF-MS). CHT (100 and 316 mg/kg of bw) in FF-MS-induced rats; BP measuring glucose, TG, total cholesterol and uric acid. The doses of all 221, 222, and 224 for AHT and OGTT were 3.16, 10 and 31.6 mg/kg of bw. In vitro: Measurement of hepatic glycogen content and serum insulin levels. Studies on INSE1, H4IIE and C2C12 cells to assess insulin secretion; glucose uptake and mitochondrial bioenergetics, respectively; and glucose-6-phosphatase inhibition [90,91,92]. | 221(3.16-31.6 mg/kg) 222(3.16-31.6 mg/kg) 224(3.16-31.6 mg/kg) 228(16.27 μM) | - |
| Tecoma stans/Aqueous (WE) and ethanol:water (EWE) extracts. | In vivo: WE tested in AHT (500 mg/kg of bw), CHT (125, 250, and 500 mg/kg of bw), OGTT (500 mg/kg of bw) and OSTT (125, 250, and 500 mg/kg of bw) in healthy and STZ-treated rats. In vitro: Pancreatic lipase inhibition for EWE (30% inhibition) and compounds [93,94]. | 229(85.03%) 231(32.83%) 232(36.29%) | 231 Inhibits alpha glucosidases [109]. |
| Turnera diffusa/Metha-nol extract (ME). | In vivo: ME assayed in AHT in normoglycemic and alloxan-treated mice. The doses of 234 for AHT were 1 and 5 mg/kg of bw. In vitro: Y-AG [95]. | 234(1-5mg/kg; > 330μg/mL) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escandón-Rivera, S.M.; Mata, R.; Andrade-Cetto, A. Molecules Isolated from Mexican Hypoglycemic Plants: A Review. Molecules 2020, 25, 4145. https://doi.org/10.3390/molecules25184145
Escandón-Rivera SM, Mata R, Andrade-Cetto A. Molecules Isolated from Mexican Hypoglycemic Plants: A Review. Molecules. 2020; 25(18):4145. https://doi.org/10.3390/molecules25184145
Chicago/Turabian StyleEscandón-Rivera, Sonia Marlen, Rachel Mata, and Adolfo Andrade-Cetto. 2020. "Molecules Isolated from Mexican Hypoglycemic Plants: A Review" Molecules 25, no. 18: 4145. https://doi.org/10.3390/molecules25184145
APA StyleEscandón-Rivera, S. M., Mata, R., & Andrade-Cetto, A. (2020). Molecules Isolated from Mexican Hypoglycemic Plants: A Review. Molecules, 25(18), 4145. https://doi.org/10.3390/molecules25184145









