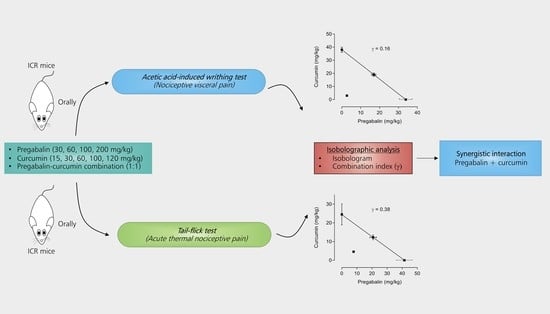
Co-administration of Pregabalin and Curcumin Synergistically Decreases Pain-Like Behaviors in Acute Nociceptive Pain Murine Models
Abstract
:1. Introduction
2. Results
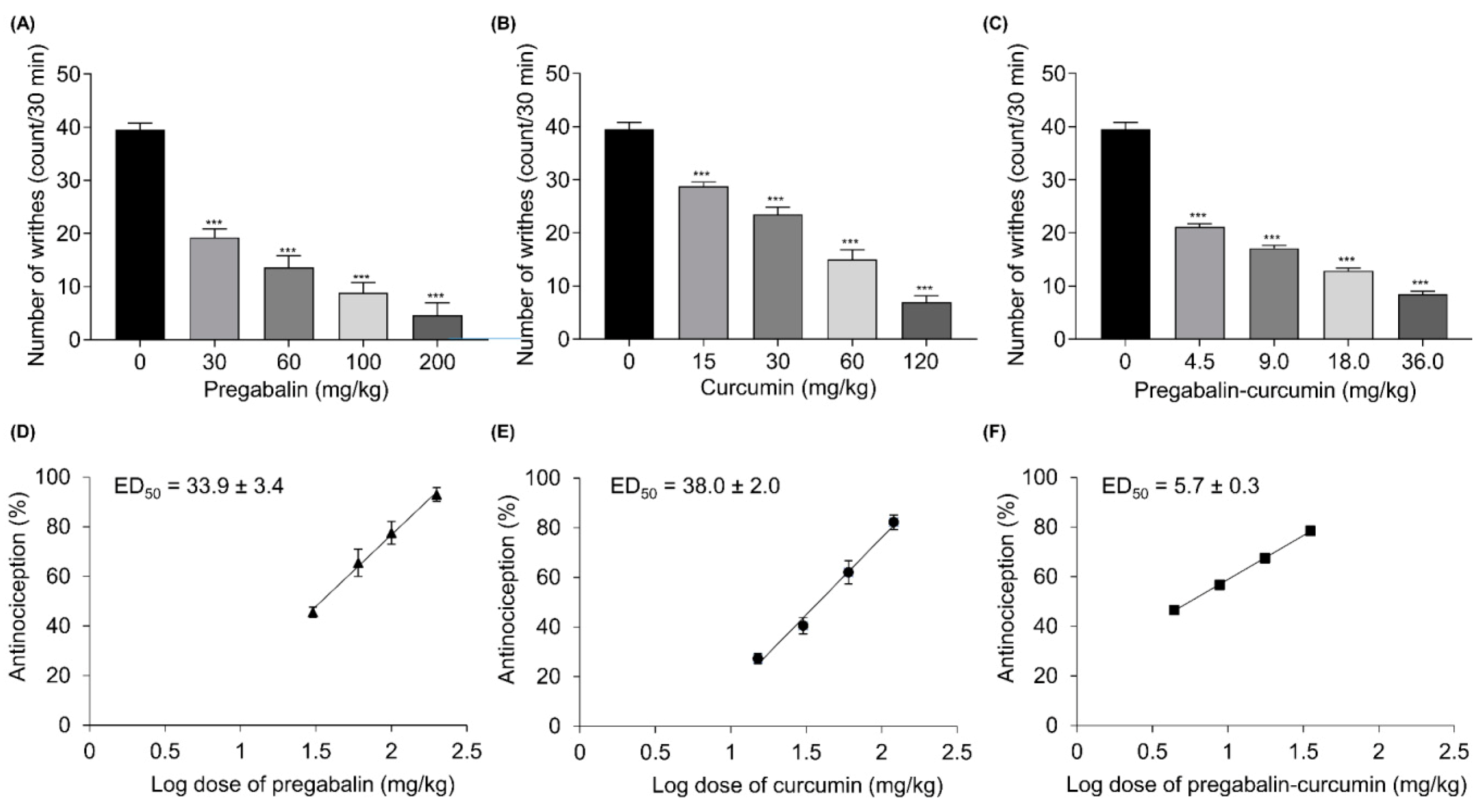
2.1. Pregabalin and Curcumin Alone Attenuate Nociceptive Visceral Pain
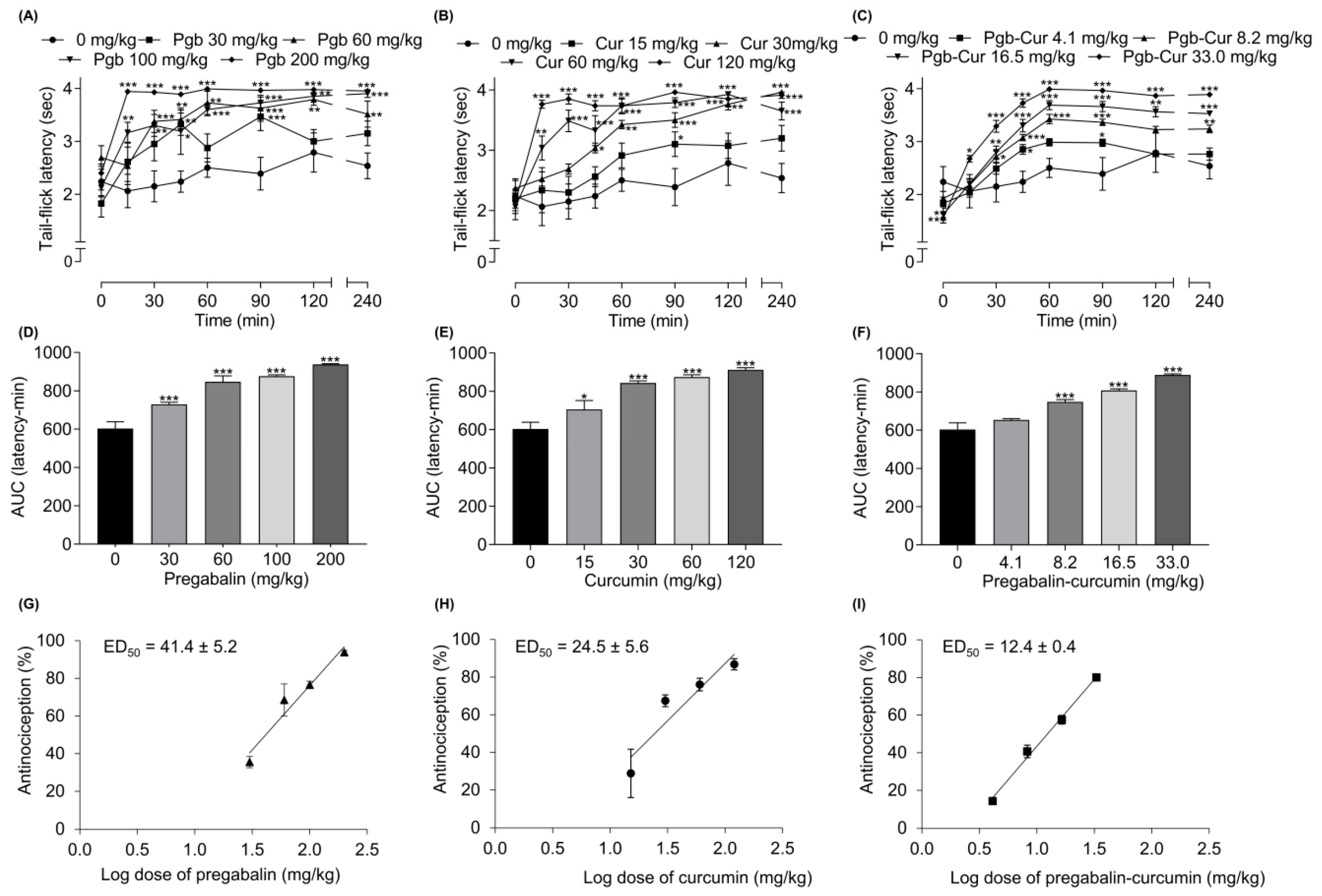
2.2. Pregabalin and Curcumin alone Attenuate Thermal Nociception
2.3. Synergistic Effects between Pregabalin and Curcumin Combination on Nociceptive Visceral Pain
2.4. Synergistic Effects between Pregabalin and Curcumin Combination on Thermal Nociception
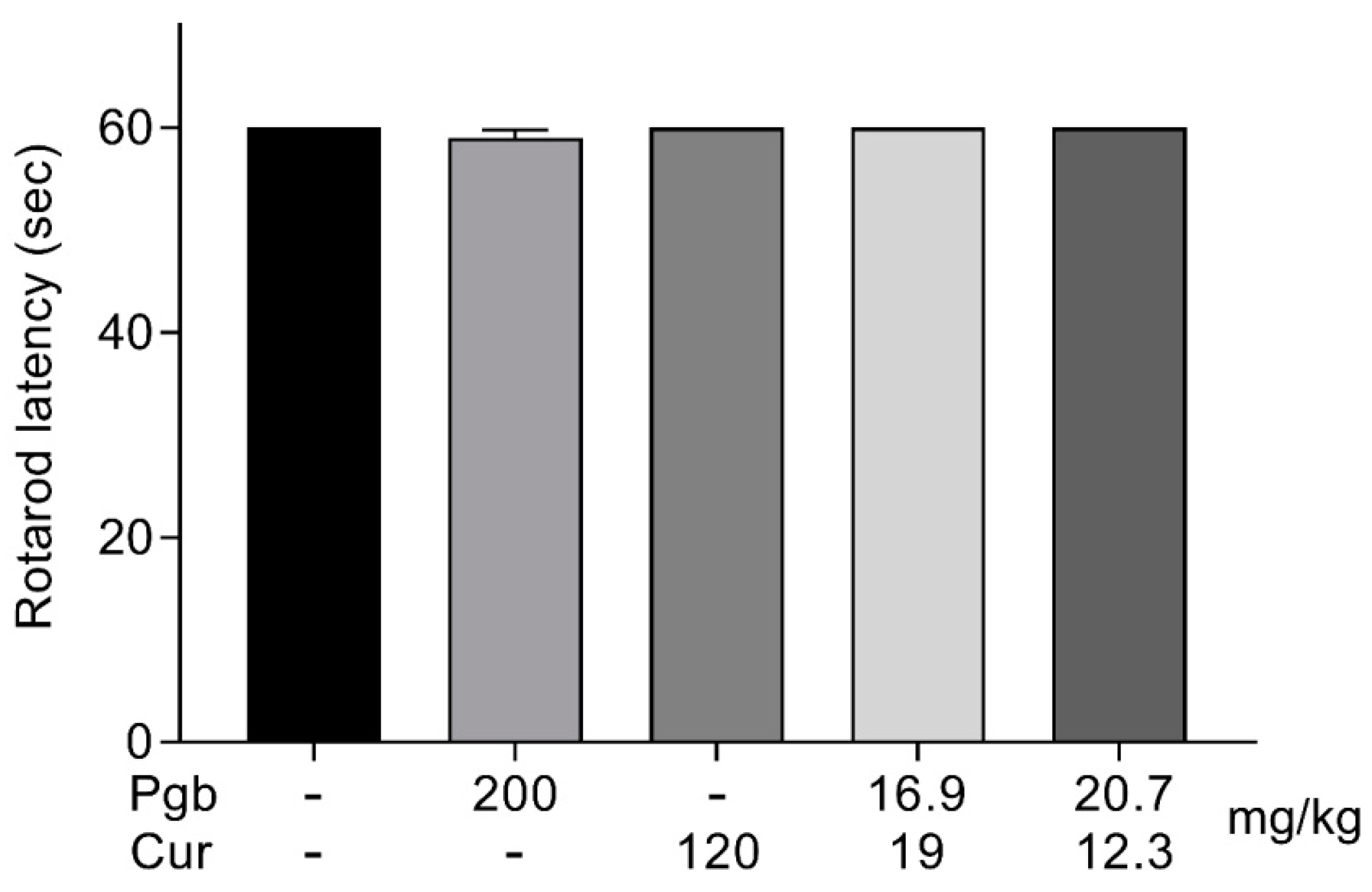
2.5. Co-Administration of Pregabalin-Curcumin Does not Affect Motor Coordination
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Reagents
4.3. Experimental Design
4.3.1. Acetic Acid-Evoked Writhing Test
4.3.2. Tail-Flick Test
4.4. Rotarod Test
4.5. Data Analysis
4.5.1. Anti-Nociceptive Effects
4.5.2. ED50 Calculation
4.5.3. Isobolographic Analysis
4.5.4. Calculation of the Combination Index (γ)
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| VGCCs | Voltage-gated calcium channels |
| CNS | Central nervous system |
| TRPV1 | Transient receptor potential vanilloid 1 |
| GARS | Generally recognized as safe |
| 5US FDA | United State Food and Drug Administration |
| NO | Nitrite oxide |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| LPS | Lipopolysaccharide |
| TNF-α | Tumor necrosis factor α |
| IL-6 | Interleukin 6 |
| IL1-β | Interleukin 1β |
| IL-8 | Interleukin 8 |
| DLM | Deltamethrin |
| AUC | Area under the curve |
| ED50 | 50% Effective Dose |
| ED50 add | Theoretical ED50 |
| ED50 exp | Experimental ED50 |
| f | Fraction |
| γ | Combination index |
| ANOVA | Analysis of variance |
| Cur | Curcumin |
| Pgb | Pregabalin |
| Pgb-Cur | Pregabalin and curcumin combination |
| CGRP | Calcitonin-gene-related peptide |
| TRPV3 | Transient receptor potential vanilloid 3 |
| i.p. | Intraperitoneal injection |
| p.o. | Oral administration |
| % MPE | Percentage of maximum possible effect |
| BDNF | Brain-derived neurotrophic factor |
| PGE-2 | Prostaglandin E2 |
| MCP-1 | Monocyte chemo-attractant protein 1 |
| BBB | Blood-brain barrier |
| CMC | Carboxymethyl cellulose |
References
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Prescott, S.A.; Ratté, S. Somatosensation and pain. In Conn’s Translational Neuroscience; Conn, P.M., Ed.; Academic Press: San Diego, CA, USA, 2017; Chapter 23; pp. 517–539. [Google Scholar]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altier, C.; Zamponi, G.W. Targeting Ca channels to treat pain: T-type versus N-type. Trends Pharmacol. Sci. 2004, 25, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.D.; Chaplan, S.; Higuera, E.S.; Sorkin, L.S.; Stauderman, K.A.; Williams, M.E.; Yaksh, T.L. Upregulation of Dorsal Root Ganglion α2δ Calcium Channel Subunit and Its Correlation with Allodynia in Spinal Nerve-Injured Rats. J. Neurosci. 2001, 21, 1868–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, H. Molecular biology of neuronal voltage-gated calcium channels. Exp. Mol. Med. 1998, 30, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Chincholkar, M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: A narrative review. Br. J. Anaesth. 2018, 120, 1315–1334. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.P. Mechanisms of analgesia by gabapentin and pregabalin-calcium channel α2-δ [Cavα2-δ] ligands. Pain 2009, 142, 13–16. [Google Scholar] [CrossRef]
- Bockbrader, H.N.; Wesche, D.; Miller, R.; Chapel, S.; Janiczek, N.; Burger, P.; Bockbrader, H.N. A Comparison of the Pharmacokinetics and Pharmacodynamics of Pregabalin and Gabapentin. Clin. Pharmacokinet. 2010, 49, 661–669. [Google Scholar] [CrossRef]
- Kumar, N.; Laferrière, A.; Yu, J.S.C.; Leavitt, A.; Coderre, T.J. Evidence that pregabalin reduces neuropathic pain by inhibiting the spinal release of glutamate. J. Neurochem. 2010, 113, 552–561. [Google Scholar] [CrossRef] [Green Version]
- Alimian, M.; Imani, F.; Hassani, V.; Rahimzadeh, P.; Sharifian, M.; Safari, S. Effects of Single-Dose Pregabalin on Postoperative Pain in Dacryocystorhinostomy Surgery. Anesth. Pain. Med. 2012, 2, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Meymandi, M.S.; Keyhanfar, F. Pregabalin antinociception and its interaction with tramadol in acute model of pain. Pharmacol. Rep. 2012, 64, 576–585. [Google Scholar] [CrossRef]
- Tartau, L.M.; Popa, E.G.; Lupusoru, R.V.; Lupusoru, C.E.; Stoleriu, I.; Ochiuz, L. Synergic Effects of Pregabalin-Acetaminophen Combination in Somatic and Visceral Nociceptive Reactivity. Pharmacology 2014, 93, 253–259. [Google Scholar] [CrossRef]
- Choi, Y.S.; Shim, J.-K.; Song, J.W.; Kim, J.C.; Yoo, Y.C.; Kwak, Y.-L. Combination of Pregabalin and Dexamethasone for Postoperative Pain and Functional Outcome in Patients Undergoing Lumbar Spinal Surgery. Clin. J. Pain 2013, 29, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, Y.S.; Almalki, A.; Alsaab, H.O.; Alsanie, W.; Gaber, A.; Alhadidi, Q.; Hardy, A.M.G.; Nasr, A.; Alzahrani, O.; Stary, C.M.; et al. Pregabalin: Potential for Addiction and a Possible Glutamatergic Mechanism. Sci. Rep. 2019, 9, 15136–15138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toth, C. Pregabalin: Latest safety evidence and clinical implications for the management of neuropathic pain. Ther. Adv. Drug Saf. 2013, 5, 38–56. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, R.H.E.; Jaarsma, T.; Broek, S.A.J.V.D.; Haaijer-Ruskamp, F.M. Decompensation of chronic heart failure associated with pregabalin in a 73-year-old patient with postherpetic neuralgia: A case report. Br. J. Clin. Pharmacol. 2008, 66, 327–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveed, S.; Faquih, A.E.; Chaudhary, A.M.D. Pregabalin-associated Discontinuation Symptoms: A Case Report. Cureus 2018, 10, e3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winterfeld, U.; Merlob, P.; Baud, D.; Rousson, V.; Panchaud, A.; Rothuizen, L.E.; Bernard, N.; Vial, T.; Yates, L.M.; Pistelli, A.; et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology 2016, 86, 2251–2257. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Zanjani, T.M.; Ameli, H.; Labibi, F.; Sedaghat, K.; Sabetkasaei, M. The Attenuation of Pain Behavior and Serum COX-2 Concentration by Curcumin in a Rat Model of Neuropathic Pain. Korean J. Pain 2014, 27, 246–252. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Y.; Zhao, Q.; Chen, C.-R.; Liu, A.-M.; Huang, Z.-L. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: Descending monoamine system and opioid receptors are differentially involved. Neuropharmacology 2012, 62, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.; Kim, S.; Kim, Y.; Lee, M.; Ahn, D.; Kim, H.; Kim, J.; Jung, S.; Oh, S. Curcumin Produces an Antihyperalgesic Effect via Antagonism of TRPV1. J. Dent. Res. 2009, 89, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Dong, L.; Kong, D.; Sun, B.; Sun, Q.; Grundy, D.; Zhang, G.; Rong, W. Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol. Motil. 2013, 25, e429–e440. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Rahimnia, A.-R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid Treatment for Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79%–85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1993, 427, 1–275. [Google Scholar]
- FDA. Notice to US Food and Drug Administration of the Conclusion That the Intended Use of Curcumin is Generally Recognized as Safe. Available online: https://www.fda.gov/media/132575/download (accessed on 30 June 2020).
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.G.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Medica 1998, 64, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.L.; Hsu, C.-H.; Lin, J.K.; Hsu, M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, 2895–2900. [Google Scholar]
- Muangnoi, C.; Jithavech, P.; Na Bhuket, P.R.; Supasena, W.; Wichitnithad, W.; Towiwat, P.; Niwattisaiwong, N.; Haworth, I.S.; Rojsitthisak, P. A curcumin-diglutaric acid conjugated prodrug with improved water solubility and antinociceptive properties compared to curcumin. Biosci. Biotechnol. Biochem. 2018, 82, 1301–1308. [Google Scholar] [CrossRef] [Green Version]
- Muangnoi, C.; Na Bhuket, P.R.; Jithavech, P.; Supasena, W.; Paraoan, L.; Patumraj, S.; Rojsitthisak, P. Curcumin diethyl disuccinate, a prodrug of curcumin, enhances anti-proliferative effect of curcumin against HepG2 cells via apoptosis induction. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Luckanagul, J.A.; Pitakchatwong, C.; Na Bhuket, P.R.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- De Paz-Campos, M.A.; Ortiz, M.I.; Piña, A.E.C.; Zazueta-Beltrán, L.; Castañeda-Hernández, G. Synergistic effect of the interaction between curcumin and diclofenac on the formalin test in rats. Phytomedicine 2014, 21, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.A.; Harvey, K.A.; Walker, C.; Altenburg, J.; Xu, Z.; Terry, C.L.; Camarillo, I.G.; Jones-Hall, Y.L.; Mariash, C.N. Characterization of synergistic anti-cancer effects of docosahexaenoic acid and curcumin on DMBA-induced mammary tumorigenesis in mice. BMC Cancer 2013, 13, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, A.; Kumar, M.; Jain, M.; Dhawan, D.K. Synergistic effects of piperine and curcumin in modulating benzo(a)pyrene induced redox imbalance in mice lungs. Toxicol. Mech. Methods 2011, 22, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Huang, Y.; Kong, A.-N.T. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 2010, 79, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, D.; Wang, L.; Sreeharsha, N.; Ning, Y. Curcumin synergistically potentiates the protective effect of sitagliptin against chronic deltamethrin nephrotoxicity in rats: Impact on pro-inflammatory cytokines and Nrf2/Ho-1 pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22386. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, F.; Braun, C.; Zhou, Y.-Q.; Rittner, H.L.; Tian, Y.; Cai, X.; Ye, D.-W. Role of curcumin in the management of pathological pain. Phytomedicine 2018, 48, 129–140. [Google Scholar] [CrossRef]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- Tjølsen, A.; Hole, K. Animal Models of Analgesia. In Handbook of Experimental Pharmacology; Springer Verlag: Berlin, Germany, 1997; pp. 1–20. [Google Scholar]
- Ribeiro, R.; Vale, M.; Thomazzi, S.; Paschoalato, A.; Poole, S.; Ferreira, S.; Cunha, F. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, M.; Willis, W.D. Gabapentin Markedly Reduces Acetic Acid–induced Visceral Nociception. Anesthesiology 2003, 98, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Bueno, L.; Fioramonti, J. Visceral perception: Inflammatory and non-inflammatory mediators. Gut 2002, 51, i19–i23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, H.O.J.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968, 32, 295–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marics, I.; Malapert, P.; Reynders, A.; Gaillard, S.; Moqrich, A. Acute Heat-Evoked Temperature Sensation Is Impaired but Not Abolished in Mice Lacking TRPV1 and TRPV3 Channels. PLoS ONE 2014, 9, e99828. [Google Scholar] [CrossRef] [Green Version]
- Kaygisiz, B.; Kilic, F.; Senguleroglu, N.; Baydemir, C.; Erol, K. The antinociceptive effect and mechanisms of action of pregabalin in mice. Pharmacol. Rep. 2015, 67, 129–133. [Google Scholar] [CrossRef]
- Keyhanfar, F.; Meymandi, M.S.; Sepehri, G.; Rastegaryanzadeh, R.; Heravi, G. Evaluation of antinociceptive effect of pregabalin in mice and its combination with tramadol using tail flick test. Iran. J. Pharm. Res. 2013, 12, 483–493. [Google Scholar]
- Jokinen, V.; Lilius, T.O.; Laitila, J.; Niemi, M.; Rauhala, P.V.; Kalso, E. Pregabalin enhances the antinociceptive effect of oxycodone and morphine in thermal models of nociception in the rat without any pharmacokinetic interactions. Eur. J. Pain 2015, 20, 297–306. [Google Scholar] [CrossRef]
- Meymandi, M.S.; Keyhanfar, F. Assessment of the antinociceptive effects of pregabalin alone or in combination with morphine during acetic acid-induced writhing in mice. Pharmacol. Biochem. Behav. 2013, 110, 249–254. [Google Scholar] [CrossRef]
- Jacob, J.N.; Badyal, D.K.; Bala, S.; Toloue, M. Evaluation of the in vivo anti-inflammatory and analgesic and in vitro anti-cancer activities of curcumin and its derivatives. Nat. Prod. Commun. 2013, 8, 359–362. [Google Scholar] [CrossRef] [Green Version]
- Motaghinejad, M.; Bangash, M.Y.; Hosseini, P.; Karimian, S.M.; Motaghinejad, O. Attenuation of Morphine Withdrawal Syndrome by Various Dosages of Curcumin in Comparison with Clonidine in Mouse: Possible Mechanism. Iran. J. Med. Sci. 2015, 40, 125–132. [Google Scholar]
- Tajik, H.; Tamaddonfard, E.; Hamzeh-Gooshchi, N. The effect of curcumin (active substance of turmeric) on the acetic acid-induced visceral nociception in rats. Pak. J. Boil. Sci. 2008, 11, 312–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curatolo, M.; Sveticic, G. Drug combinations in pain treatment: A review of the published evidence and a method for finding the optimal combination. Best Pr. Res. Clin. Anaesthesiol. 2002, 16, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Pergolizzi, J.V.; Tallarida, R.J. The Determination and Application of Fixed-Dose Analgesic Combinations for Treating Multimodal Pain. J. Pain 2010, 11, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehrenbacher, J.C.; Taylor, C.P.; Vasko, M.R. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain 2003, 105, 133–141. [Google Scholar] [CrossRef]
- Panahi, Y.; Sahebkar, A.; Parvin, S.; Saadat, A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann. Clin. Biochem. Int. J. Lab. Med. 2012, 49, 580–588. [Google Scholar] [CrossRef]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.A.; Parizadeh, S.M.R.; Avan, A. Investigation of the Effects of Curcumin on Serum Cytokines in Obese Individuals: A Randomized Controlled Trial. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Baral, P.; Udit, S.; Chiu, I.M. Pain and immunity: Implications for host defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef]
- Raffa, R.B. Pharmacology of oral combination analgesics: Rational therapy for pain. J. Clin. Pharm. Ther. 2001, 26, 257–264. [Google Scholar] [CrossRef]
- Hendrich, J.; Bauer, C.S.; Dolphin, A.C. Chronic pregabalin inhibits synaptic transmission between rat dorsal root ganglion and dorsal horn neurons in culture. Channels 2012, 6, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Valverde, Y.; Benson, B.; Gupta, M.; Gupta, K. Spinal glial activation and oxidative stress are alleviated by treatment with curcumin or coenzyme Q in sickle mice. Haematology 2015, 101, e44–e47. [Google Scholar] [CrossRef] [Green Version]
- Muangnoi, C.; Na Bhuket, P.R.; Jithavech, P.; Wichitnithad, W.; Srikun, O.; Nerungsi, C.; Patumraj, S.; Rojsitthisak, P. Scale-Up Synthesis and In Vivo Anti-Tumor Activity of Curcumin Diethyl Disuccinate, an Ester Prodrug of Curcumin, in HepG2-Xenograft Mice. Pharmacology 2019, 11, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ness, T.J. Models of Visceral Nociception. ILAR J. 1999, 40, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayla, C.; Labuz, D.; Machelska, H.; Bader, M.; Schäfer, M.; Stein, C. Impaired Nociception and Peripheral Opioid Antinociception in Mice Lacking Both Kinin B1 and B2 Receptors. Anesthesiology 2012, 116, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Masocha, W.; Horvath, G.; Agil, A.; Ocaña, M.; Del Pozo, E.; Szikszay, M.; Baeyens, J.M.; Butelman, E.R.; Ball, J.W.; Harris, T.J.; et al. Role of Na+,K+-ATPase in Morphine-Induced Antinociception. J. Pharmacol. Exp. Ther. 2003, 306, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Freitas, K.; Negus, S.; Carroll, F.; Damaj, M.I. In vivo pharmacological interactions between a type II positive allosteric modulator of α7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br. J. Pharmacol. 2013, 169, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Tallarida, R.J. Drug Synergism and Dose-Effect Data Analysis; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Tallarida, R.J. The interaction index: A measure of drug synergism. Pain 2002, 98, 163–168. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound curcumin are available from the authors. |

| Compound | ED50 a ± SEM | |||||
|---|---|---|---|---|---|---|
| Writhing Test | Tail-Flick Test | |||||
| Pregabalin | 33.9 ± 3.4 | 41.4 ± 5.2 | ||||
| Curcumin | 38.0 ± 2.0 | 24.5 ± 5.6 | ||||
| ED50 ± SEM | ||||||
| Combination (1:1) | ED50 exp b | ED50 add c | γ d | ED50 exp e | ED50 add f | γ g |
| Pregabalin + Curcumin | 5.7 ± 0.3 *** | 35.9 ± 2.4 | 0.16 | 12.4 ± 0.4 *** | 32.9 ± 3.5 | 0.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leksiri, S.; Hasriadi; Dasuni Wasana, P.W.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Co-administration of Pregabalin and Curcumin Synergistically Decreases Pain-Like Behaviors in Acute Nociceptive Pain Murine Models. Molecules 2020, 25, 4172. https://doi.org/10.3390/molecules25184172
Leksiri S, Hasriadi, Dasuni Wasana PW, Vajragupta O, Rojsitthisak P, Towiwat P. Co-administration of Pregabalin and Curcumin Synergistically Decreases Pain-Like Behaviors in Acute Nociceptive Pain Murine Models. Molecules. 2020; 25(18):4172. https://doi.org/10.3390/molecules25184172
Chicago/Turabian StyleLeksiri, Sarinee, Hasriadi, Peththa Wadu Dasuni Wasana, Opa Vajragupta, Pornchai Rojsitthisak, and Pasarapa Towiwat. 2020. "Co-administration of Pregabalin and Curcumin Synergistically Decreases Pain-Like Behaviors in Acute Nociceptive Pain Murine Models" Molecules 25, no. 18: 4172. https://doi.org/10.3390/molecules25184172
APA StyleLeksiri, S., Hasriadi, Dasuni Wasana, P. W., Vajragupta, O., Rojsitthisak, P., & Towiwat, P. (2020). Co-administration of Pregabalin and Curcumin Synergistically Decreases Pain-Like Behaviors in Acute Nociceptive Pain Murine Models. Molecules, 25(18), 4172. https://doi.org/10.3390/molecules25184172