Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms
Abstract
:1. Introduction
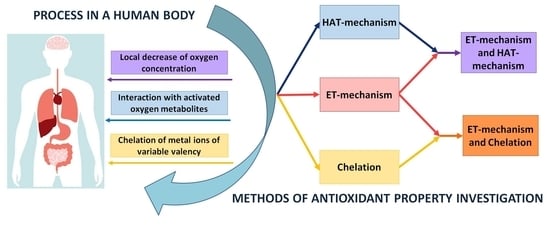
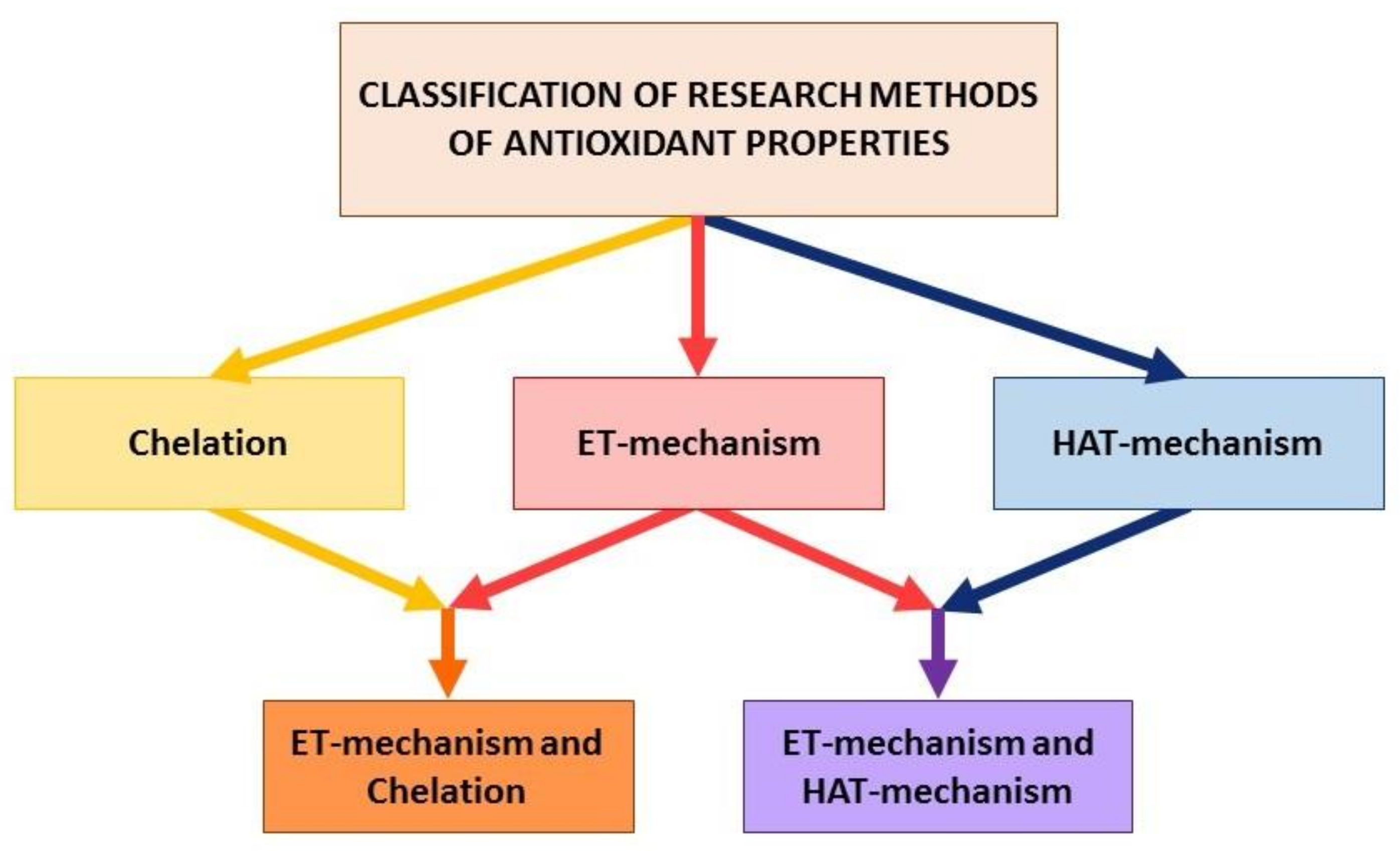
2. Basic Principles of Classification of Analytical Methods
- (i)
- electron transfer reactions from AO to the substrate (AO oxidation reaction), i.e., ET-mechanism;
- (ii)
- transfer reactions of a hydrogen atom from AO to a substrate which, in aqueous media, can be considered as proton transfer accompanied by electron transfer (AO oxidation reaction), i.e., HAT-mechanism;
- (iii)
- transfer reactions of one or more electron pairs with the formation of the covalent bond by the donor-acceptor mechanism (the reaction of the complexation of AO with metal ions of variable valency), i.e., chelating-mechanism.
3. Methods to Evaluate Integrated Antioxidant Properties
3.1. Electron-Transfer-Based Assays
3.1.1. Spectroscopic Methods
Folin-Ciocalteu Assay
ABTS/TEAC Assay
DPPH Assay
FRAP Assay
CUPRAC Assay
- Reagent CUPRAC quickly enough reacts with a wide range of AOs including thiol compounds;
- CUPRAC reagent is sufficiently stable and can be immobilized on the surface of the cation exchange polymer to create an inexpensive sensor;
- The redox reaction is carried out at a pH close to physiological (pH 7 ammonium acetate buffer) in contrast to conditions of other assays (pH 3.6 for the FRAP or pH 10 in the Folin assay). The reducing ability of AO decreases under more acidic conditions than physiological pH due to the AO protonation. In alkaline media, the AO oxidation mechanisms differ from their in vivo transformations.
CRAC Assay
3.1.2. Electrochemical Methods
Coulometry
Biamperometry
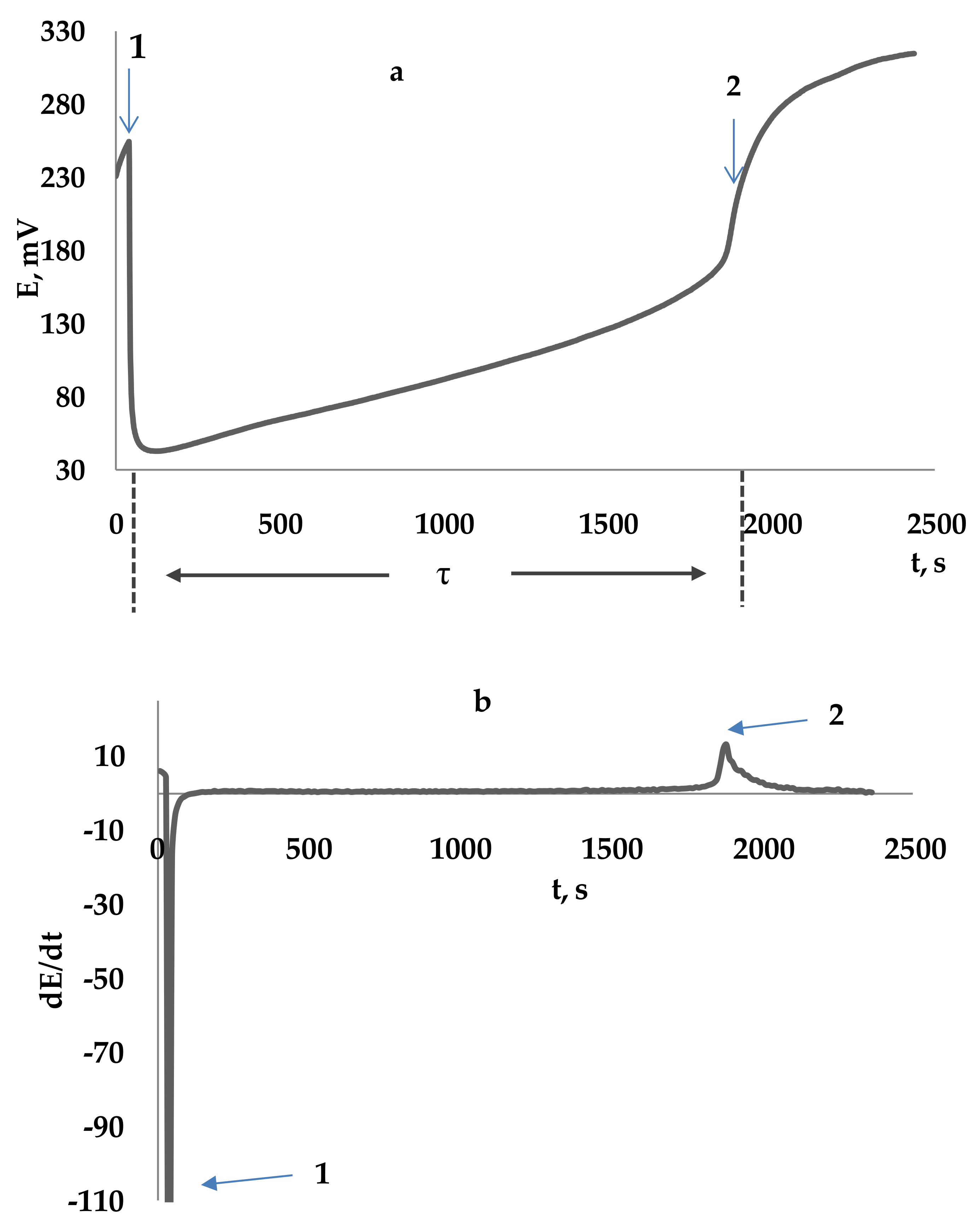
Potentiometry
- the potassium hexacyanoferrate (III) is an oxidizing agent of medium strength (E° = 0.36 V), i.e., there is a thermodynamic possibility of the reaction of the oxidizing agent with most antioxidants having, by definition, low oxidation potentials. In this case, there is no possibility of interaction of the potassium hexacyanoferrate (III) with compounds having weak reducing properties and not related to antioxidants;
- the reaction of the potassium hexacyanoferrate (III) with AOs can be realized under conditions close to physiological, i.e., at pH close to 7 (values of the conditional stability constants of potassium hexacyanoferrate (III) and its reduced form, potassium hexacyanoferrate (II), at pH = 7 significantly exceed 108);
- potassium hexacyanoferrate (III) is a one-electron electron acceptor, its reaction with AOs proceeds stoichiometrically in accordance with the number of functional groups exhibiting antioxidant properties, and the results are expressed in universal units of measurement—mol-eq/l (M-eq);
- potentiometric measurements suggest the possibility of investigating any samples including turbid and colored ones.
- the TEAC and the DPPH assays are based on the transformation of a stable radical into an inactivated form according by the antioxidant mechanisms of ET or HAT;
- methods based on the use of metals and their complexes as oxidizing agents the (FRAP, CUPRAC, CRAC, the Potentiometry assay with K3[Fe(CN)6]) are implemented by the mixed mechanism (ET + Chelating), since many antioxidants are capable of forming sufficiently strong complexes with metals of variable valency [37,38].
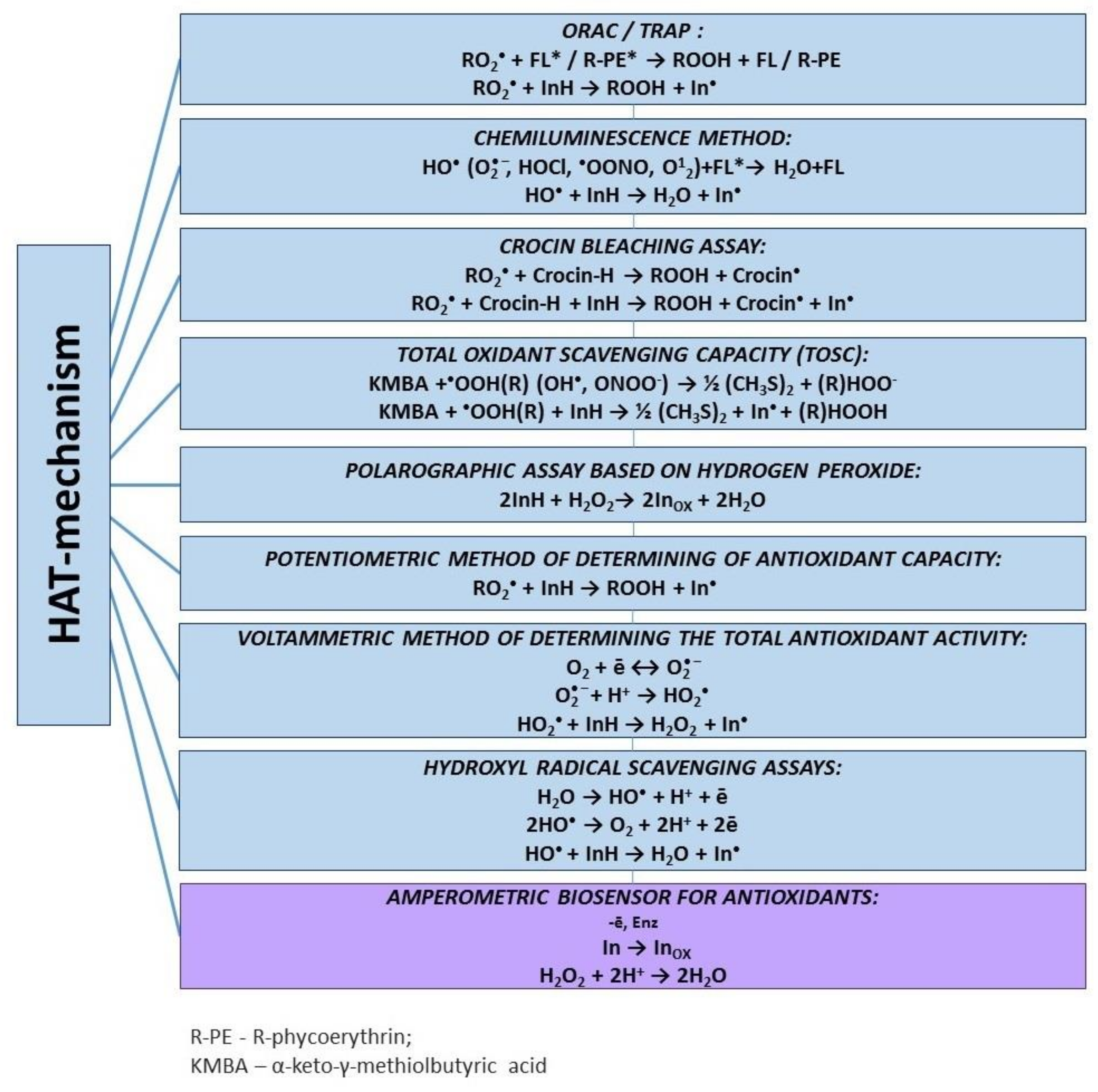
3.2. Hydrogen Atom Transfer-Based Assays
3.2.1. Spectroscopic Methods
ORAC Assay
TRAP Assay
Chemiluminescence Assay
Crocin Bleaching Assay
3.2.2. Electrochemical Methods
Voltammetric Assay
Potentiometric Assay
- (1) initiating a radical reaction by thermostating the initiator’s solution AAPH. A potential growth is observed in the system (the portion of the curve up to point t1 due to the generation of an oxidizing agent (peroxyl radicals));
- (2) the addition of oxidation processes inhibitors InH (t1) reacting with peroxyl radicals, increases the chain break in reactions (23)–(25):
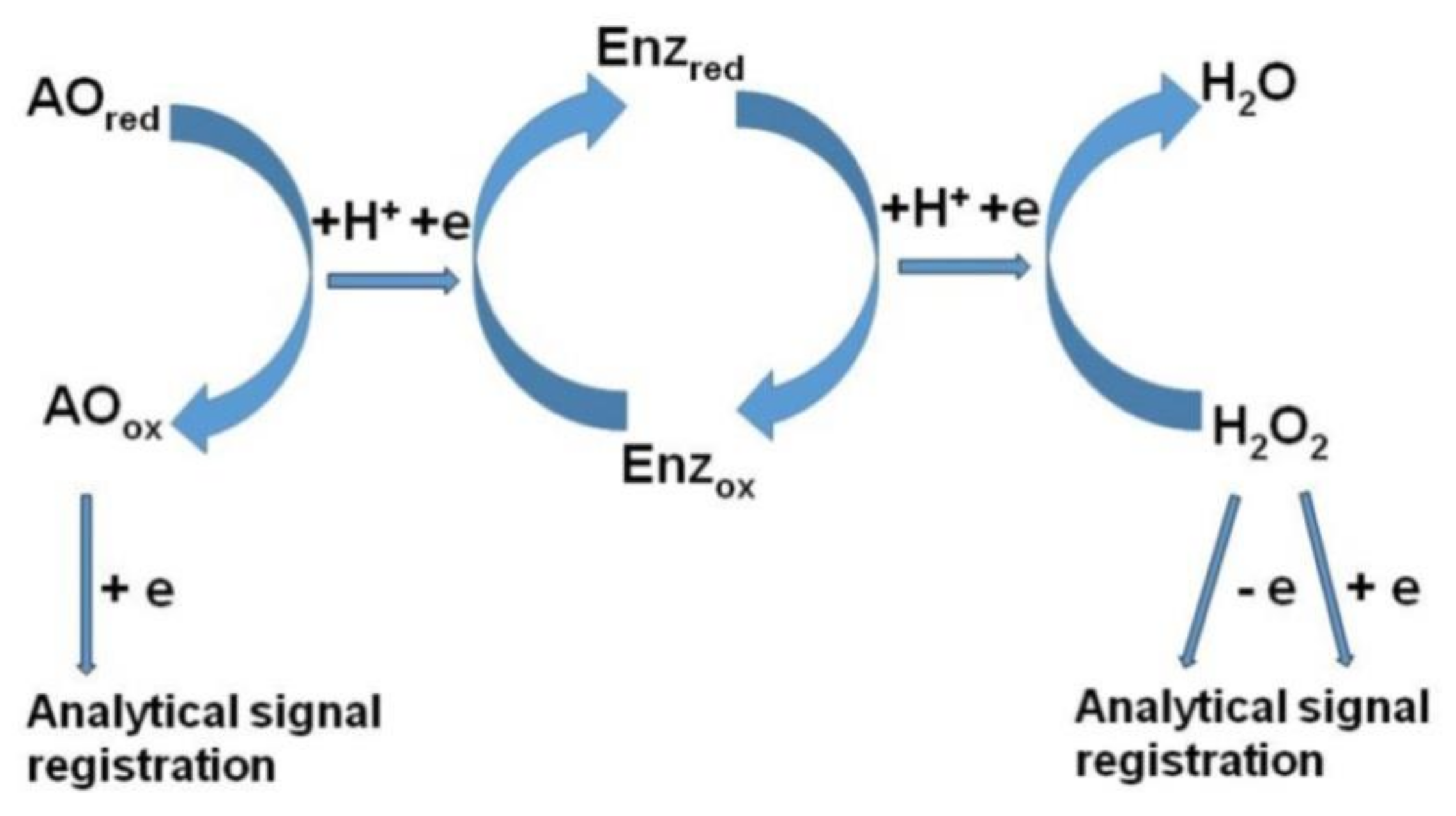
Amperometric Biosensors
Hydroxyl Radical Scavenging Assays
Polarographic Assay of Hydrogen Peroxide Scavenging
3.3. Chelating-Based Assays
3.3.1. Chelating Properties Assay with Use Ferrozine
3.3.2. Antioxidant Assay by DNA Protective Method
4. Some Comparisons of Results Obtained Using Methods by Various Mechanisms
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Menshchikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufakin, V.A. Oxidative Stress. Pathologic Statesand Diseases; Sibirskoe Univ. Izd.: Novosibirsk, Russia, 2008; p. 534. [Google Scholar]
- Menschikova, E.B.; Zenkov, N.K.; Lankin, V.Z.; Bondar, I.A.; Trufakin, V.A. Oxidative Stress. Pathological Conditions and Diseases; ARTA: Novosibirsk, Russia, 2008; p. 435. [Google Scholar]
- Galaris, D.; Barbouti, A.; Korantzopoulos, P. Oxidative Stress in Hepatic Ischemia-Reperfusion Injury: The Role of Antioxidants and Iron Chelating Compounds. Curr. Pharm. Des. 2006, 12, 2875–2890. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef] [PubMed]
- Wusigale; Hu, L.; Cheng, H.; Gao, Y.; Liang, L. Mechanism for Inhibition of Folic Acid Photodecomposition by Various Antioxidants. J. Agr. Food Chem. 2020, 68, 340. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Danet, A.F.; Fleschin, Ş.; Aboul-Enein, H.Y. Recent Applications for in Vitro Antioxidant Activity Assay. Crit. Rev. Anal. Chem. 2015, 46, 389. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2011, 1, 106. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D. Methods for determination of antioxidant capacity: A review. IJPSR 2015, 6, 546. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. Electrochemical Methods for Total Antioxidant Capacity and its Main Contributors Determination: A review. Open Chem. 2015, 13, 824. [Google Scholar] [CrossRef] [Green Version]
- Duplancic, D.; Kukoc-Modun, L.; Modun, D.; Radic, N. Simple and Rapid Method for the Determination of Uric Acid-Independent Antioxidant Capacity. Molecules 2011, 16, 7058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4390. [Google Scholar]
- Lewoyehu, M.; Amare, M. Comparative evaluation of analytical methods for determining the antioxidant activities of honey: A review. Cog. Food Agric. 2019, 5, UNSP1685059. [Google Scholar] [CrossRef]
- Hoyos-Arbelaez, J.; Vazquez, M.; Contreras-Calderon, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2018; p. 337. [Google Scholar]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–989. [Google Scholar] [CrossRef] [Green Version]
- Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. AJFS 2014, 11, 31. [Google Scholar] [CrossRef]
- Jones, A.; Pravadali-Cekic, S.; Dennis, G.R.; Bashir, R.; Mahon, P.J.; Shalliker, R.A. Ferric reducing antioxidant potential (FRAP) of antioxidants using reaction flow chromatography. Anal. Chim. Acta 2017, 967, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bonilla, P.; Gandia-Herrero, F.; Matencio, A.; Garcia-Carmona, F.; Lopez-Nicolas, J. Comparative Study of the Antioxidant Capacity of Four Stilbenes Using ORAC, ABTS(+), and FRAP Techniques. Food Anal. Meth. 2017, 10, 2994–3000. [Google Scholar] [CrossRef]
- Kandi, S.; Charles, A.L. Statistical comparative study between the conventional DPPH center dot spectrophotometric and dropping DPPH center dot analytical method without spectrophotometer: Evaluation for the advancement of antioxidant activity analysis. Food Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total antioxidant capacity-Relevance, methods and clinical implications. Andrologia 2020, e13624. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Biol. Med. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Xu, S.; Xu, Y.; Guo, L.; Liu, H.; Yang, L.; Wang, Z.; Yang, L. Chemiluminescence quenching microarrays for high throughput screening of antioxidants and its application in evaluating herbal extracts and pure compounds. Anal. Chim. Acta 2019, 1046, 148–153. [Google Scholar] [CrossRef]
- Blasco, A.J.; González Crevillén, A.; González, M.C.; Escarpa, A. Direct Electrochemical Sensing and Detection of Natural Antioxidants and Antioxidant Capacity in Vitro Systems. Electroanalysis 2007, 19, 2275. [Google Scholar] [CrossRef]
- Juarez-Luna, P.J.; Mendoza, S.; Cardenas, A. Comparison of electrochemical methods using CUPRAC, DPPH, and carbon paste electrodes for the quantification of antioxidants in food oils. Anal. Methods 2019, 11, 5755. [Google Scholar] [CrossRef]
- Brown, K.L.; Christenson, K.; Karlsson, A.; Dahlgren, C.; Bylund, J. Divergent Effects on Phagocytosis by Macrophage-Derived Oxygen Radicals. J. Innate Immun. 2009, 1, 592. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Yamanushi, T.; Seto, H.; Sagai, M. Oxygen radicals in lung carcinogenesis accompanying phagocytosis of diesel exhaust particles. Int. J. Oncol. 1997, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Amirtharaj, G.J.; Natarajan, S.K.; Pulimood, A.; Balasubramanian, K.A.; Venkatraman, A.; Ramachandran, A. Role of Oxygen Free Radicals, Nitric Oxide and Mitochondria in Mediating Cardiac Alterations During Liver Cirrhosis Induced by Thioacetamide. Cardiovasc. Toxicol. 2017, 17, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Menshchikova, E.B.; Zenkov, N.K.; Kandalintseva, N.V. Phenolic Antioxidants in Biology and Medicine; Lap Lambert: Saarbrücken, Germany, 2012; p. 324. [Google Scholar]
- Zulueta, A.; Esteve, M.J.; Frigola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Ulrih, N.P.; Cigić, B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin-Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Pellegrini, R.R.N.; Colombi, B.; Bianchi, M.; Brighenti, F. Application of the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation assay to a flow injection system for the evaluation of antioxidant activity of some pure compounds and beverages. J. Agric. Food Chem. 2003, 51, 260–264. [Google Scholar] [CrossRef]
- Pellegrini, R.R.N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Teslić, N.; Kilmartin, P.A.; Versari, A. Suitability of the cyclic voltammetry measurements and DPPH• spectrophotometric assay to determine the antioxidant capacity of food-grade oenological tannins. Molecules 2019, 24, 2925. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Morales, F.; Alonso-Castro, A.J.; Zapata-Morales, J.R.; Carranza-Alvarez, C.; Aragon-Martinez, O.H. Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem. Pap. 2020, 74, 3325–3334. [Google Scholar]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Izzet Tor, K.G.; Apak, R. Comparative Evaluation of Fe(III) Reducing Power-Based Antioxidant Capacity Assays in the Presence of Phenanthroline, Batho-Phenanthroline, Tripyridyltriazine (FRAP), and Ferricyanide Reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total Antioxidant Capacity as a Tool to Assess Redox Status: Critical View and Experimental Data. Free Rad. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Frohlich, K.; Bohm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (aTEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Sözgen-Başkan, K.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yıldız, L.; Karaman, Ş.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Ozyurt, D.; Demirata, B.; Apak, R. Determination of total antioxidant capacity by a new spectrophotometric method based on Ce(IV) reducing capacity measurement. Talanta 2007, 71, 1155–1165. [Google Scholar] [CrossRef]
- Stevanato, R.; Fabris, S.; Momo, F. Enzymatic method for the determination of total phenolic content in tea and wine. J. Agric. Food Chem. 2004, 52, 6287–6293. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.P. Electroanalysis of Biologically Important Compounds; Ellis Harwood: Billingham, UK, 1990; p. 256. [Google Scholar]
- Wang, J. Analytical Electrochemistry; Wiley-VCH: Hoboken, NJ, USA, 2006; p. 272. [Google Scholar]
- Bagotsky, V.S. (Ed.) Fundamentals of Electrochemistry; Wiley Interscience, John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 722. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, Fundamentals and Applications, 2nd ed.; John Wiley& Sons Inc.: Hoboken, NJ, USA, 2001; p. 850. [Google Scholar]
- Budnikov, G.K.; Maistrenko, V.N.; Vyaselyov, M.R. Fundamentals of Modern Electrochemical Analysis; Binom, Laboratory of Knowledge: Moscow, Russia, 2003; p. 592. [Google Scholar]
- Brainina, K.Z. Electroanalysis: From laboratory to field versions (Review). J. Anal. Chem. 2001, 56, 303–312. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Unal, M.A.; Ozkan, S.A. Recent developments on electrochemical flow injection in pharmaceuticals and biologically important compounds (Review). Electrochim. Acta 2018, 287, 135–148. [Google Scholar] [CrossRef]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; et al. Electrochemistry as a tool for studying antioxidant properties (Review). Int. J. Electrochem. Sci. 2013, 8, 8464–8489. [Google Scholar]
- Abrha, T.; Pal, R.; Saini, R.C. A Study on Voltametric Electro-Kinetic Mechanism of Catechol at l-Glutamic Acid-Carbon Paste Sensor. J. Surf. Sci. Technol. 2017, 33, 1. [Google Scholar] [CrossRef]
- Ge, L.; Li, S.P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Šeruga, M.; Komorsky-Lovrić, Š. Electrochemical characterization of epigallocatechin gallate using square-wave voltammetry. Electroanalysis 2009, 21, 1019. [Google Scholar] [CrossRef]
- Magarelli, G.; da Silva, J.G.; de Sousa, I.A.; Lopes, I.S.D.; SouzaDe, J.R.; Hoffmann, L.V.; de Castro, C.S.P. Development and validation of a voltammetric method for determination of total phenolic acids in cotton cultivars. Microchem. J. 2013, 109, 23–28. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, G.K. Coulometry in antioxidant analysis. Ukr. Chem. J. 2005, 71, 45. [Google Scholar]
- Ziyatdinova, G.K.; Budnikov, G.K.; Pogorel’tsev, V.I. Electrochemical determination of lipoic acid. J. Anal. Chem. 2004, 59, 288–290. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, G.K.; Lapin, A.A. Direct determination of hypoxen and its analogs by galvanostatic coulometry. J. Anal. Chem. 2007, 62, 260–262. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Direct determination of captopril using electrogenerated halogens for pharmaceuticals quality control. Eurasian J. Anal. Chem. 2007, 2, 84. [Google Scholar]
- Ziyatdinova, G.K.; Budnikov, G.K. Determination of some catecholamines by coulometric titration and cyclic voltammetry. J. Anal. Chem. 2005, 60, 673–677. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Grigor’eva, L.V.; Budnikov, G.K. Coulometric determination of sulfur-containing amino acids using halogens as oxidizing titrants. J. Anal. Chem. 2007, 62, 1176–1179. [Google Scholar] [CrossRef]
- Budnikov, G.K.; Ziyatdinova, G.K.; Valitova, Y.R. Electrochemical determination of glutathione. J. Anal. Chem. 2004, 59, 573–576. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, G.K.; Pogorel’Tsev, V.I. Determination of serum albumin in blood by constant-current coulometry using electrochemically generated oxidizers. J. Anal. Chem. 2004, 59, 659–661. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Khuzina, A.A.; Budnikov, H.C. Reactions of phenolic antioxidants with electrogenerated hexacyanoferrate(III) ions and their use in vegetable oils analysis. J. Anal. Chem. 2013, 68, 80–85. [Google Scholar] [CrossRef]
- Tougas, T.P.; Jannetti, J.M.; Collier, W.G. Theoretical and experimental response of biamperometric detector for flow injection analysis. Anal. Chem. 1985, 57, 1377–1381. [Google Scholar] [CrossRef]
- Moreno Gálvez, A.; García Mateo, J.V.; Martínez Calatayud, J. Study of various indicating redox systems on the indirect flow-injection biamperometric determination of pharmaceuticals. Anal. Chim. Acta 1999, 396, 161–170. [Google Scholar] [CrossRef]
- Milardovic, S.; Ivekovic, D.; Rumenjak, V.; Grabaric, B.S. Use of DPPH•/DPPH redox couple for biamperometric determination of antioxidant activity. Electroanalysis 2005, 17, 1847–1853. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cheregi, M.C.; Danet, A.F. Total antioxidant capacity of some commercial fruit juices: Electrochemical and spectrophotometrical approaches. Molecules 2009, 14, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Milardovic, S.; Kerekovic, I.; Derrico, R.; Rumenjak, V. A novel method for flow injection analysis of total antioxidant capacity using enzymatically produced ABTS•+ and biamperometric detector containing interdigitated electrode. Talanta 2007, 71, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Milardovic, S.; Kerekovic, I.; Rumenjak, V. A flow injection biamperometric method for determination of total antioxidant capacity of alcoholic beverages using bienzymatically produced ABTS∙+. Food Chem. 2007, 105, 1688–1694. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina, K.Z. Potentiometric Study of Antioxidant Activity: Development and Prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E.; Popova, K.G.; Matern, A.I. Study of the antioxidant activity and total polyphenol concentration of medicinal plants. J. Anal. Chem. 2017, 72, 415. [Google Scholar] [CrossRef]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E.; Borisova, M.; Drokin, R.; Gorbunov, E.; Ulomskiy, E.; Rusinov, V. The antioxidant screening of potential materials for drugs based on 6-nitro-1,2,4-triazoloazines containing natural polyphenol fragments. Anal. Bioanal. Chem. 2020. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activityinvestigation. Talanta 2007, 71, 13. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R.; Okulova, Y.A.; Matern, A.I.; Rusinov, V.L. Antioxidant and Antiradical Activity of Drugs Intended for Treating Ophthalmic Disorders. Pharm. Chem. J. 2018, 52, 694. [Google Scholar] [CrossRef]
- Kohri, S.; Fujii, H. 2,2′-Azobis(isobutyronitrile)-derived alkylperoxyl radical scavenging activity assay of hydrophilic antioxidants by employing EPR spin trap method. J. Clin. Biochem. Nutr. 2013, 53, 134. [Google Scholar] [CrossRef] [Green Version]
- Kasaikina, O.T.; Kartasheva, Z.S.; Kancheva, V.D.; Yanishlieva, N.V.; Totseva, I.R. Consumption of quercetin and rutin in reactions with free radicals. Bulg. Chem. Commun. 2010, 42, 153. [Google Scholar]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the Activity of Phenolic Antioxidants: Theoretical Method, Analysis of Substituent Effects, and Application to Major Families of Antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Ortega, E.; Zabalgogeazcoa, I.; de Aldana, B.R.V.; Arellano, J.B. Solutions to decrease a systematic error related to AAPH addition in the fluorescence-based ORAC assay. Anal. Biochem. 2017, 519, 27. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluoresce as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, A.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Int. J. Food Sci. Technol. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Wayner, D.D.; Burton, G.W.; Ingold, K.U.; Locke, S.J. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett. 1985, 187, 33–37. [Google Scholar] [CrossRef]
- Wayner, D.D.; Burton, G.W.; Ingold, K.U.; Barclay, L.R.C.; Locke, S.J. The Relative Contributions of Vitamin E, Urate, Ascorbate and Proteins to the Peroxyl Radical-Trapping Antioxidant Activity of Human Blood Plasma. Biochim. Biophys. Acta 1987, 924, 408–419. [Google Scholar] [CrossRef] [Green Version]
- Wayner, D.D. Radical-trapping antioxidants in vitro and vivo. Bioelectrochem. Bioenerg. 1987, 18, 219–229. [Google Scholar] [CrossRef]
- Whitehead, T.P.; Thorpe, G.H.G.; Maxwell, S.R.J. Enhanced chemiluminescent assay for antioxidant capacity in biological fluids. Anal. Chim. Acta 1992, 266, 265–277. [Google Scholar] [CrossRef]
- Bastos, E.L.; Romoff, P.; Eckert, C.R.; Baader, W.J. Evaluation of Antiradical Capacity by H2O2-Hemin-Induced Luminol Chemiluminescence. J. Agric. Food Chem. 2003, 57, 7481–7488. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Hirata, Y.; Nagano, T. Fluorescent Indicators for Imaging Nitric Oxide Production. Angew. Chem. Int. Ed. 1999, 38, 3209–3212. [Google Scholar] [CrossRef]
- Tanaka, K.; Miura, T.; Umezawa, N.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Rational design of fluorescein-based fluorescent probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen. J. Am. Chem. Soc. 2001, 123, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Chignell, C.F.; Mason, R.P. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: Possible implications for oxidative stress measurements. Free Radic. Biol. Med. 1999, 27, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.J.; Glazer, R.N. Phycoerythrin fluorescence-base assay for peroxy radicals: A screen for biologically relevant protective agents. Anal. Biochem. 1989, 177, 300–308. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Rad. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G.; Janaszewska, A.; Ertel, D.; Bartosz, M. Simple determination of peroxyl radical-trapping capacity. Biochem. Mol. Biol. Int. 1998, 46, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, M.; Kuusi, T. Spectrophotometric assay for total peroxyl radical-trapping antioxidant potential in human serum. J. Lipid Res. 1997, 38, 823–833. [Google Scholar]
- Proskurnina, E.V.; Dzhatdoeva, A.A.; Lobichenko, E.N.; Shalina, R.I.; Vladimirov, Y.A. Chemiliminescence determination of lipid hydroperoxides in biological fluids. J. Anal. Chem. 2017, 72, 751. [Google Scholar] [CrossRef]
- Dzhatdoeva, A.A.; Polimova, A.M.; Proskurnina, E.V.; Vladimirov, Y.A. Tissue chemiluminescence as a method of evaluation of superoxide radical producing ability of mitochondria. Bull. Russ. State Med. Univ. 2016, 1, 49. [Google Scholar]
- Zhidkova, T.V.; Proskurnina, E.V.; Parfenov, E.A.; Vladimirov, Y.A. Determination of superoxide dismutase and SOD-mimetic activities by a chemical system: Co2/H2O2/lucigenin. Anal. Bioanal. Chem. 2011, 401, 381. [Google Scholar] [CrossRef]
- Vladimirov, Y.A.; Proskurnina, E.V.; Izmailov, D.Y. Chemiluminescence as a method for detection and study of free radicals in biological systems. Bull. Exp. Biol. Med. 2007, 144, 390. [Google Scholar] [CrossRef] [PubMed]
- Rusina, I.F.; Karpukhin, O.N.; Kasaikina, O.T. Chemiluminescent methods for studying inhibited oxidation. Russ. J. Phys. Chem. 2013, 7, 463. [Google Scholar] [CrossRef]
- Tan, H.N.; Zhao, Y.X.; Xu, X.T.; Sun, Y.; Li, Y.H.; Du, J.X. A covalent triazine framework as an oxidase mimetic in the luminol chemiluminescence system: Application to the determination of the antioxidant rutin. Microchim. Acta 2020, 187, 42. [Google Scholar] [CrossRef] [PubMed]
- Vladimirov, Y.A.; Proskurnina, E.V.; Demin, E.M.; Matveeva, N.S.; Lubitskiy, O.B.; Novikov, A.A.; Izmailov, D.Y.; Osipov, A.N.; Tikhonov, V.P.; Kagan, V.E. Dihydroquercetin (taxifolin) and other flavonoids as inhibitors of free radical formation at key stages of apoptosis. Biochem. Mosc. 2009, 74, 301. [Google Scholar] [CrossRef]
- Vladimirov, G.K.; Sergunova, E.V.; Izmaylov, D.Y.; Vladimirov, Y.A. Chemiluminescent determination of total antioxidant capacity in medicinal plant material. Bull. Russ. State Med. Univ. 2016, 2, 62. [Google Scholar]
- Malicanin, M.; Rac, V.; Antic, V.; Antic, M.; Palade, L.M.; Kefalas, P.; Rakic, V. Content of Antioxidants, Antioxidant Capacity and Oxidative Stability of Grape Seed Oil Obtained by Ultra Sound Assisted Extraction. J. Am. Oil Chem. Soc. 2014, 91, 989. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Tsimidou, M.Z. Crocin bleaching assay (CBA) in structure-radical scavenging activity studies of selected phenolic compounds. J. Agric. Food Chem. 2006, 54, 9347–9356. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Karbainov, Y.A.; Shevchuk, A.V. Study of antioxidant properties by voltammetry. J. Electroanal. Chem. 2002, 508, 56–60. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Freinbichler, W.; Linert, W.; Dorozhko, E.V.; Bukkel, M.V.; Plotnikov, E.V.; Voronova, O.A. Study of Total Antioxidant Activity of Human Serum Blood in the Pathology of Alcoholism. Molecules 2013, 18, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, E.I.; Karbainov, Y.A.; Avramchik, O.A. Investigation of antioxidant and catalytic properties of some biologically active substances by voltammetry. Anal. Bioanal. Chem. 2003, 375, 465–468. [Google Scholar] [CrossRef]
- Korotkova, E.I. Voltammetric Method for Determination of the Total Activity of Antioxidants in Objects of Synthetic and Natural Origin. Abstract of Dissertation for the Degree of Doctor of Chemical Sciences. Available online: https://www.researchgate.net/publication/331117082_Voltammetric_method_for_determination_of_glutathione_on_a_gold-carbon-containing_electrode (accessed on 15 September 2020).
- Korotkova, E.I.; Karbainov, Y.A.; Avramchik, O.A. Voltammetric determination of antioxidant activity of plant materials and certain foods. Univ. News. Chem. Chem. Technol. 2002, 45, 110–112. [Google Scholar]
- Korotkova, E.I.; Avramchik, O.A.; Yusubov, M.S.; Belousov, M.V.; Andreeva, T.I. Determination of the antioxidant activity of plant extracts by means of cathode voltammetry. Pharm. Chem. J. 2003, 37, 511–512. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C.; Pogorel’tzev, V.I. Electrochemical determination of total antioxidant capacity of human plasma. Anal. Bioanal. Chem. 2005, 381, 1546. [Google Scholar] [CrossRef] [PubMed]
- Ulomskiy, E.N.; Ivanova, A.V.; Gorbunov, E.B.; Esaulkova, I.L.; Slita, A.V.; Sinegubova, E.O.; Voinkov, E.K.; Drokin, R.A.; Butorin, I.I.; Gazizullina, E.R.; et al. Synthesis and biological evaluation of 6-nitro-1,2,4-triazoloazines containing polyphenol fragments possessing antioxidant and antiviral activity. Bioorganic Med. Chem. Lett. 2020, 30, 127216. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. New antiradical capacity assay with the use potentiometric method. Anal. Chim. Acta 2019, 1046, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Imabayashi, S.; Kong, Y.; Watanabe, M. Amperometric biosensor for polyphenol based on horseradish peroxidase immobilized on gold electrodes. Electroanalysis 2001, 13, 408–412. [Google Scholar] [CrossRef]
- Kong, Y.T.; Imabayashi, S.; Kano, K.; Ikeda, T.; Kakiuchi, T. Peroxidase-based amperometric sensor for the determination of total phenols using two-stage peroxidase reactions. Am. J. Enol. Viticult. 2001, 52, 381–385. [Google Scholar]
- Mello, L.D.; Alves, A.A.; Macedo, D.V.; Kubota, L.T. Peroxidase-based biosensor as a tool for a fast evaluation of antioxidant capacity of tea. Food Chem. 2005, 92, 515–519. [Google Scholar] [CrossRef]
- Karyakina, E.E.; Vokhmyanina, D.V.; Sizova, N.V.; Sabitov, A.N.; Borisova, A.V.; Sazontova, T.G.; Arkhipenko, Y.V.; Tkachuk, V.A.; Zolotov, Y.A.; Karyakin, A.A. Kinetic approach for evaluation of total antioxidant activity. Talanta 2009, 80, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A. Prussian blue and its analogues: Electrochemistry and analytical applications. Electroanalysis 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Oliveira, R.; Geraldo, D.; Bento, F. Electrogenerated HO radical reactions: The role of competing reactions on the degradation kinetics of hydroxy-containing aromatic compounds. Electrochim. Acta 2014, 135, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.; Geraldo, D.; Bento, F. Radical scavenging activity of antioxidants evaluated by means of electrogenerated HO radical. Talanta 2014, 129, 320–327. [Google Scholar] [CrossRef]
- Oliveira, R.; Bento, F.; Geraldo, D. Aromatic hydroxylation reactions by electrogenerated HO radicals: A kinetic study. J. Electroanal. Chem. 2012, 682, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar]
- Gorjanović, S.Z.; Novaković, M.M.; Vukosavljević, P.V.; Pastor, F.T.; Tesević, V.V.; Suznjević, D.Z. Polarographic assay based on hydrogen peroxide scavenging in determination of antioxidant activity of strong alcohol beverages. J. Agric. Food Chem. 2010, 58, 8400–8406. [Google Scholar] [CrossRef] [PubMed]
- Gorjanovic, S.; Novakovic, M.; Potkonjak, N.; Suznjevic, D. Antioxidant activity of wines determined by a polarographic assay based on hydrogen peroxide scavenge. J. Agric. Food Chem. 2010, 58, 4626–4631. [Google Scholar] [CrossRef] [PubMed]
- Gorjanovic, S.; Novakovic, M.; Potkonjak, N.; Leskosek-Cukalovic, I.; Suznjevic, D. Application of a novel antioxidative assay in beer analysis and brewing process monitoring. J. Agric. Food Chem. 2010, 58, 744–751. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Mauricio, A.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar] [CrossRef] [Green Version]
- Hippeli, S.; Elstner, E.F. Transition metal ion-catalyzed oxygen activation during pathogenic processes. FEBS Lett. 1999, 443, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Kim, R.Y.; Brown, A.C.; Vanka, K.S.; Mayall, J.R.; Liu, G.; Pillar, A.L.; Jones-Freeman, B.; Xenaki, D.; Borghuis, T.; et al. Critical role for iron accumulation in the pathogenesis of fibrotic lung disease. J. Pathol. 2020. [Google Scholar] [CrossRef]
- Berg, D.; Gerlach, M.; Youdim, M.B.H.; Double, K.L.; Zecca, L.; Riederer, P.; Becker, G. Brain iron pathways and their relevance to Parkinson’s disease. J. Neurochem. 2001, 79, 225–236. [Google Scholar] [CrossRef]
- Siah, C.W.; Trinder, D.; Olynyk, J.K. Iron overload. Clin. Chim. Acta 2005, 358, 24–36. [Google Scholar]
- Ullen, H.; Augustsson, K.; Gustavsson, C.; Steineck, G. Supplementary iron intake and risk of cancer: Reversed causality? Cancer Lett. 1997, 114, 215–216. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncola, J.; Izakovic, M.; Mazura, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 1997, 160, 1–10. [Google Scholar] [CrossRef]
- Ponka, P. Cellular iron metabolism. Kidney Int. 1999, 55, S2–S11. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.J.; Ronnenberg, A.G. Modern nutrition in health and disease. In Book Lippincott Williams and Wilkins, 10th ed.; Shils, M.E., Ed.; Lippincott Williams & Wilkins (LWW): Philadelphia, PE, USA, 2006; p. 248. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicology, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–4. [Google Scholar] [CrossRef]
- Gulcin, I.; Sat, E.G.; Beydemir, S.; Elmastas, M.; Kufrevioglu, O.I. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Kazazica, S.P.; Butkovica, V.; Srazica, D.; Klasinc, L. Gas-phase ligation of Fe2+ and Cu+ ions with some flavonoids. J. Agric. Food Chem. 2006, 54, 8391–8396. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant and antiradical activities of L-Carnitine. Life Sci. J. 2006, 78, 803–811. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Int. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant properties of resveratrol: A structureactivity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Fiorucci, S.B.; Golebiowski, J.; Cabrol-Bass, D.; Antonczak, S. DFT study of quercetin activated forms involved in antiradical, antioxidant, and prooxidant biological processes. J. Agric. Food Chem. 2007, 55, 903–911. [Google Scholar] [CrossRef]
- Saiga, A.I.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Lopes, G.K.B.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Wang, E.M.; Schulman, H.M.; Storrey, K.B.; Ponka, P. Deoxyribose degradation catalyzed by Fe(III)EDTA: Kinetic aspects and potential usefulness for submicromolar iron measurements. Mol. Cell. Biochem. 1994, 137, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The ability of scavengers to distinguish •OH production in the iron-catalyzed Haber–Weiss reaction: Comparison of four assays for •OH. Free Rad. Biol. Med. 1987, 3, 33–39. [Google Scholar] [CrossRef]
- Damaskin, B.B.; Petriy, O.A.; Podlovchenko, B.I. Workshop on Electrochemistry; Damascene, B., Ed.; Higher School Publisher: Moscow, Russia, 1991; p. 287. [Google Scholar]
- Kravtsov, V.I. The Equilibrium and Kinetics of Electrode Reactions of Metal Complexes; Chemistry: Leningrad, Russia, 1985; p. 207. [Google Scholar]
- Varga, L.P.; Hume, D.N. Computer Analysis of Potentiometric and Thenoyltrifluoroacetone (TTA) Solvent Extraction Studies on the Fluoride Complexes of Hafnium. Inorg. Chem. 1963, 2, 201–206. [Google Scholar] [CrossRef]
- Garrett, E.R.; Weber, D.J. Metal complexes of thiouracils I: Stability constants by potentiometric titration studies and structures of complexes. J. Pharm. Sci. 1970, 59, 1383–1398. [Google Scholar] [CrossRef]
- Davis, D.G.; Martin, R.F. Electrochemical Studies of Some Iron-Protoporphyrin Complexes. J. Am. Chem. Soc. 1966, 88, 1365–1371. [Google Scholar] [CrossRef]
- Lau, A.L.Y.; Hubbard, A.T. Study of the kinetics of electrochemical reactions by thin-layer voltammetry. III. Electroreduction of the chloride complexes of platinum(II) and (IV). J. Electroanal. Chem. 1970, 24, 237–249. [Google Scholar] [CrossRef]
- Dance, I.G. A Voltammetric Study of the Coordinative Reactions of Pyridine with is(maleonitriledithiolate)cobalt Complexes. Inorg. Chem. 1973, 12, 2381–2388. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Gerasimova, E.L.; Gazizullina, E.R. An integrated approach to the investigation of antioxidant properties by potentiometry. Anal. Chim. Acta 2020. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Zhao, L.; Zhu, Y.; Hao, T. Antioxidant Activities and Major Bioactive Components Of Consecutive Extracts From Blue Honeysuckle (Lonicera Caerulea, L.) Cultivated In China. J. Food Biochem. 2015, 39, 653–662. [Google Scholar] [CrossRef]
- Gorinstein, S.; Park, Y.S.; Heo, B.G.; Namiesnik, J.; Leontowica, H.; Leontowica, M.; Ham, K.S.; Cho, J.Y.; Kang, S.G. A comparative study of phenolic compounds and antioxidant and antiproliferative activities in frequently consumed raw vegetables. Eur. Food Res. Technol. 2009, 228, 903–911. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, M.; Leontowicz, H.; Najman, K.; Namiesnik, J.; Park, Y.S.; Jung, S.T.; Kang, S.G.; Trakhtenberg, S. Supplementation of garlic lowers lipids and increases antioxidant capacity in plasma of rats. Nutr. Res. 2006, 26, 362–368. [Google Scholar] [CrossRef]
- Kusznierewics, B.; Bartoszek, A.; Wolska, L.; Drzewiwcki, J.; Gorinstein, S.; Namiesnik, J. Partial Characterization of White Cabbages (Brassica oleracea var. capitata f. alba) from Different Regions by Glucosinolates, Bioactive Compounds, Total Antioxidant Activities and Proteins. LWT Food Sci. Technol. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules 2020, 25, 4251. https://doi.org/10.3390/molecules25184251
Ivanova A, Gerasimova E, Gazizullina E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules. 2020; 25(18):4251. https://doi.org/10.3390/molecules25184251
Chicago/Turabian StyleIvanova, Alla, Elena Gerasimova, and Elena Gazizullina. 2020. "Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms" Molecules 25, no. 18: 4251. https://doi.org/10.3390/molecules25184251
APA StyleIvanova, A., Gerasimova, E., & Gazizullina, E. (2020). Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules, 25(18), 4251. https://doi.org/10.3390/molecules25184251