The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars Content of Red Bell Pepper
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Traditional and Non-Thermal Treatment on the Total Carotenoid Content of Red Bell Peppers
2.2. Effect of Traditional and Non-Thermal Treatment on the Vitamin C of Red Bell Peppers
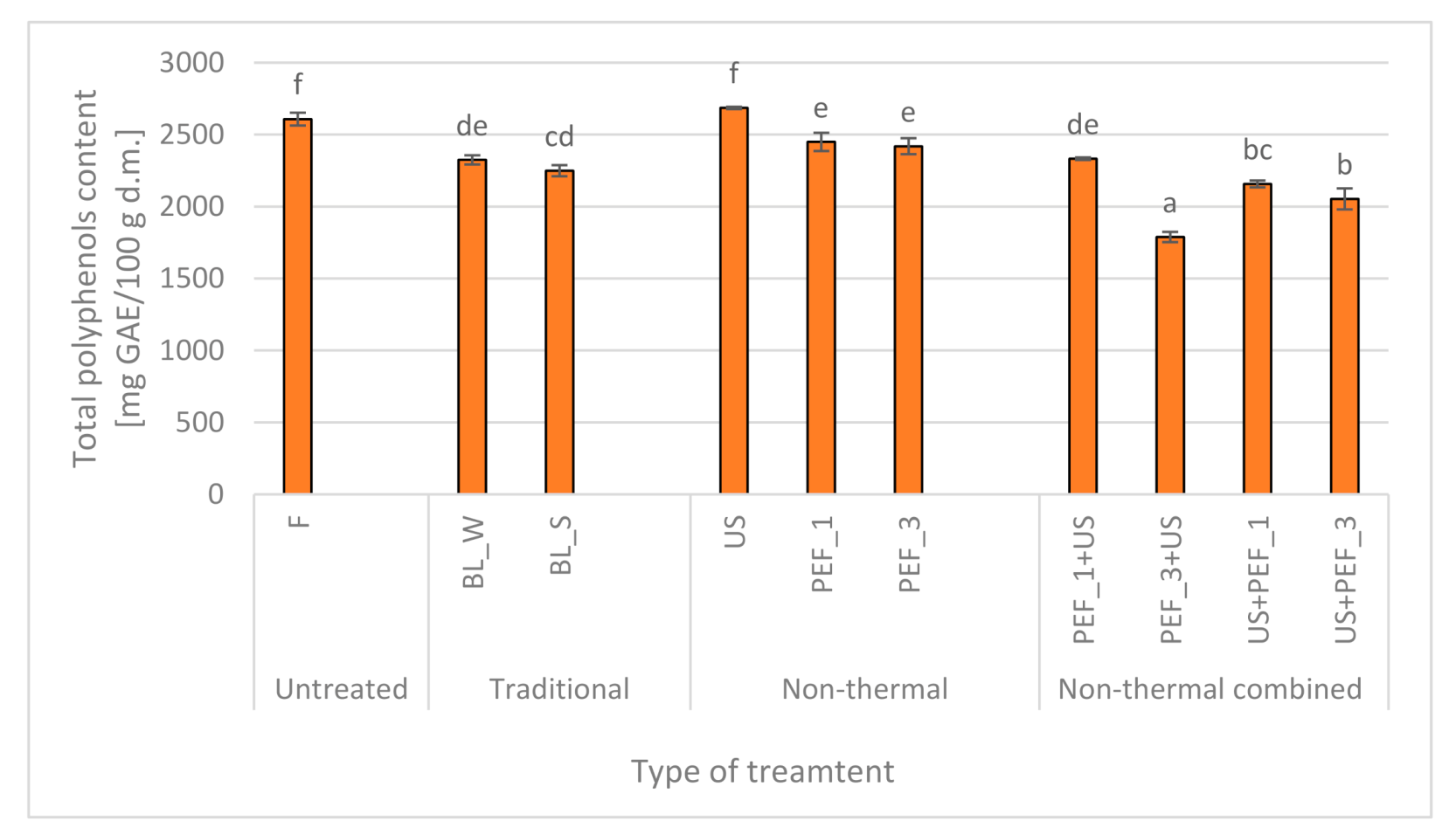
2.3. Effect of Traditional and Non-Thermal Treatment on the Polyphenols Content of Red Bell Peppers
2.4. Effect of Traditional and Non-Thermal Treatment on the Antioxidant Activity of Red Bell Peppers
2.5. Effect of Traditional and Non-Thermal Treatment on the Sugars Content of Red Bell Peppers
2.6. Cluster Analysis (CA)
3. Materials and Methods
3.1. Materials
3.2. Processing Procedure
3.2.1. Blanching with Water (BL_W) and Steam (BL_S)
3.2.2. Ultrasonic Treatment (US)
3.2.3. Pulsed Electric Field Treatment (PEF)
3.2.4. Combined Methods
3.3. Chemical Analysis
3.3.1. Total Carotenoids Content (TCC)
- ρ (C40H56)–total carotenoids, in mg/100 g
- A450–absorbance of the petroleum ether extract
- 4.00–average in the conversion factor determined on the basis of the ring test, taking into account the average β-carotene absorption coefficient in petroleum ether and dilutions made during the analysis.
3.3.2. Vitamin C
3.3.3. Total Polyphenols Content (TPC)
3.3.4. Antioxidant Activity (AA)
DPPH assay
ABTS assay
3.3.5. Sugars Content (SC)
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Yang, X.H.; Mujumdar, A.S.; Fang, X.M.; Zhang, Q.; Zheng, Z.A.; Gao, Z.J.; Xiao, H.W. Effects of high-humidity hot air impingement blanching (HHAIB) pretreatment on the change of antioxidant capacity, the degradation kinetics of red pigment, ascorbic acid in dehydrated red peppers during storage. Food Chem. 2018, 259, 65–72. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, E.M.; Somerfield, S.D.; Chen, R.K.Y.; Tiffin, H.R.; Hunter, D.A.; Brummell, D.A. Cell wall composition during expansion, ripening and postharvest water loss of red bell peppers (Capsicum annuum L.). Postharvest Biol. Technol. 2020, 168, 111225. [Google Scholar] [CrossRef]
- Vengaiah, P.C.; Pandey, J.P. Dehydration kinetics of sweet pepper (Capsicum annum L.). J. Food Eng. 2007, 81, 282–286. [Google Scholar] [CrossRef]
- Hallmann, E.; Marszałek, K.; Lipowski, J.; Jasińska, U.; Kazimierczak, R.; Średnicka-Tober, D.; Rembiałkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Dorantes-Alvarez, L.; Jaramillo-Flores, E.; González, K.; Martinez, R.; Parada, L. Blanching peppers using microwaves. Procedia Food Sci. 2011, 1, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Howard, L.R.; Smith, R.T.; Wagner, A.B.; Villalon, B.; Burns, E.E. Provitamin A and Ascorbic Acid Content of Fresh Pepper Cultivars (Capsicum annuum) and Processed Jalapeños. J. Food Sci. 1994, 59, 362–365. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Brandão, T.R.S.; Silva, C.L.M. Impact of non-thermal technologies and sanitizer solutions on microbial load reduction and quality factor retention of frozen red bell peppers. Innov. Food Sci. Emerg. Technol. 2013, 17, 99–105. [Google Scholar] [CrossRef]
- Salas-Tovar, J.A.; Flores-Gallegos, A.C.; Contreras-Esquivel, J.C.; Escobedo-García, S.; González-Herrera, S.M.; Morales-Castro, J.; Rodríguez-Herrera, R. Ultrasound-vacuum infusion effect on jalapeño pepper (Capsicum annuum L.) blanching and thermal behavior of its pectin methylesterase. LWT Food Sci. Technol. 2018, 95, 150–156. [Google Scholar] [CrossRef]
- Ade-Omowaye, B.I.O.; Talens, P.; Angersbach, A.; Knorr, D. Kinetics of osmotic dehydration of red bell peppers as influenced by pulsed electric field pretreatment. Food Res. Int. 2003, 36, 475–483. [Google Scholar] [CrossRef]
- Chenlo, F.; Chaguri, L.; Santos, F.; Moreira, R. Osmotic dehydration/impregnation kinetics of padrón pepper (Capsicum annuum L. Longum) with sodium chloride solutions: Process modelling and colour analysis. Food Sci. Technol. Int. 2006, 12, 221–227. [Google Scholar] [CrossRef]
- Vega, A.; Fito, P.; Andrés, A.; Lemus, R. Mathematical modeling of hot-air drying kinetics of red bell pepper (var. Lamuyo). J. Food Eng. 2007, 79, 1460–1466. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Lemus-Mondaca, R.; Bilbao-Sáinz, C.; Fito, P.; Andrés, A. Effect of air drying temperature on the quality of rehydrated dried red bell pepper (var. Lamuyo). J. Food Eng. 2008, 85, 42–50. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Dehydration of red bell-pepper (Capsicum annuum L.): Change in drying behavior, colour and antioxidant content. Food Bioprod. Process. 2011, 89, 504–513. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.M.; Mujumdar, A.S.; Qian, J.Y.; Zhang, Q.; Yang, X.H.; Liu, Y.H.; Gao, Z.J.; Xiao, H.W. Effect of high-humidity hot air impingement blanching (HHAIB) on drying and quality of red pepper (Capsicum annuum L.). Food Chem. 2017, 220, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.; Saraiva, J.A.; Domingues, F.M.J.; Delgadillo, I. Effect of mild pressure treatments and thermal blanching on yellow bell peppers (Capsicum annuum L.). LWT Food Sci. Technol. 2011, 44, 363–369. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.H.; Mujumdar, A.S.; Wang, D.; Zhao, J.H.; Fang, X.M.; Zhang, Q.; Xie, L.; Gao, Z.J.; Xiao, H.W. Effects of various blanching methods on weight loss, enzymes inactivation, phytochemical contents, antioxidant capacity, ultrastructure and drying kinetics of red bell pepper (Capsicum annuum L.). LWT Food Sci. Technol. 2017, 77, 337–347. [Google Scholar] [CrossRef]
- Dadan, M.; Rybak, K.; Wiktor, A.; Nowacka, M.; Zubernik, J.; Witrowa-Rajchert, D. Selected chemical composition changes in microwave-convective dried parsley leaves affected by ultrasound and steaming pre-treatments—An optimization approach. Food Chem. 2018, 239, 242–251. [Google Scholar] [CrossRef]
- Wiktor, A.; Nowacka, M.; Anuszewska, A.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D. Drying kinetics and quality of dehydrated cranberries pretreated by traditional and innovative techniques. J. Food Sci. 2019, 84, 1820–1828. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Krešić, G. Ultrasonic effect on physicochemical and functional properties of α-lactalbumin. LWT Food Sci. Technol. 2010, 43, 254–262. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The influence of immersion and contact ultrasound treatment on selected properties of the apple tissue. Appl. Acoust. 2016, 103, 136–142. [Google Scholar] [CrossRef]
- Miano, A.C.; Ibarz, A.; Augusto, P.E.D. Mechanisms for improving mass transfer in food with ultrasound technology: Describing the phenomena in two model cases. Ultrason. Sonochem. 2016, 29, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Ashokkumar, M. The physical and chemical effect of ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barosa-Canovas, G.V., Weiss, J., Eds.; LLC: New York, NY, USA, 2011; pp. 1–12. ISBN 9781441974716. [Google Scholar]
- Sledz, M.; Wiktor, A.; Rybak, K.; Nowacka, M.; Witrowa-Rajchert, D. The impact of ultrasound and steam blanching pre-treatments on the drying kinetics, energy consumption and selected properties of parsley leaves. Appl. Acoust. 2016, 103, 148–156. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Gallão, M.I.; Rodrigues, S. Effect of osmosis and ultrasound on pineapple cell tissue structure during dehydration. J. Food Eng. 2009, 90, 186–190. [Google Scholar] [CrossRef]
- Pieczywek, P.M.; Kozioł, A.; Konopacka, D.; Cybulska, J.; Zdunek, A. Changes in cell wall stiffness and microstructure in ultrasonically treated apple. J. Food Eng. 2017, 197, 1–8. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Wiktor, A.; Sledz, M.; Nowacka, M. Selected emerging technologies to enhance the drying process: A review. Dry. Technol. 2014. [Google Scholar] [CrossRef]
- Grahl, T.; Märkl, H. Killing of micro-organisms by pulsed electric fields. Appl. Microbiol. Biotechnol. 1996, 45, 148–157. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Tappi, S.; Siroli, L.; Lanciotti, R.; Romani, S.; Witrowa-Rajchert, D. Ultrasound assisted osmotic dehydration of organic cranberries (Vaccinium oxycoccus): Study on quality parameters evolution during storage. Food Control 2018, 93, 40–47. [Google Scholar] [CrossRef]
- Wiktor, A.; Dadan, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The impact of combination of pulsed electric field and ultrasound treatment on air drying kinetics and quality of carrot tissue. LWT 2019, 110, 71–79. [Google Scholar] [CrossRef]
- Tao, Y.; Han, M.; Gao, X.; Han, Y.; Show, P.L.; Liu, C.; Ye, X.; Xie, G. Applications of water blanching, surface contacting ultrasound-assisted air drying, and their combination for dehydration of white cabbage: Drying mechanism, bioactive profile, color and rehydration property. Ultrason. Sonochem. 2019, 53, 192–201. [Google Scholar] [CrossRef]
- Ahmed, J.; Shivhare, U.S.; Sandhu, K.S. Thermal degradation kinetics of carotenoids and visual color of Papaya Puree. J. Food Sci. 2002, 67, 2692–2695. [Google Scholar] [CrossRef]
- Fernandes, A.S.; do Nascimento, T.C.; Jacob-Lopes, E.; Rosso, V.V. De; Zepka, L.Q. Carotenoids—A brief overview on its structure, biosynthesis, synthesis, and applications. In Progress in Carotenoid Research; Zepka, L., Jacob-Lopes, E., De Rosso, V.V., Eds.; BoD–Books on Demand: Norderstedt, Germany, 2018; pp. 1–15. [Google Scholar]
- Bhosale, P.; Bernstein, P.S. Vertebrate and invertebrate carotenoid-binding proteins. Arch. Biochem. Biophys. 2007, 458, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The impact of pulsed electric field treatment on selected bioactive compound content and color of plant tissue. Innov. Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Galindo, F.G. Responses of plant cells and tissues to pulsed electric field treatments. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: New York, NY, USA, 2017; pp. 2621–2635. [Google Scholar]
- Castro, S.M.; Saraiva, J.A.; Lopes-da-Silva, J.A.; Delgadillo, I.; Van Loey, A.; Smout, C.; Hendrickx, M. Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chem. 2008, 107, 1436–1449. [Google Scholar] [CrossRef]
- Sokhansanj, S.; Jayas, D.S. Drying of Foodstaffs. In Handbook of Industrial Drying; Mujumdar, A.S., Ed.; CRC: New York, NY, USA, 2006. [Google Scholar]
- Nowacka, M.; Fijalkowska, A.; Dadan, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D. Effect of ultrasound treatment during osmotic dehydration on bioactive compounds of cranberries. Ultrasonics 2018, 83, 18–25. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Hernández-Jover, T.; Martín-Belloso, O. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chem. 2009, 112, 258–266. [Google Scholar] [CrossRef]
- Gustavo, V.; Barbosa-Cánovas, M.; Góngora-Nieto, M.; Pothakamury, U.R.; Swanson, B.G. PEF inactivation of vegetative cells, spores, and enzymes in foods. In Preservation of Foods with Pulsed Electric Fields; Gustavo, V., Barbosa-Cánovas, M., Góngora-Nieto, M., Pothakamury, U.R., Swanson, B.G., Eds.; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Deng, J.; Yang, H.; Capanoglu, E.; Cao, H.; Xiao, J. Technological aspects and stability of polyphenols. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Tylewicz, U.; Nowacka, M.; Matin-Garcia, B.; Wiktor, A.; Gomez Caravaca, A.M. Target sources of polyphenols in different food products and their processing by-products. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Cárcel, J.A.; García-Pérez, J.V.; Benedito, J.; Mulet, A. Food process innovation through new technologies: Use of ultrasound. J. Food Eng. 2012, 110, 200–207. [Google Scholar] [CrossRef]
- Wiktor, A.; Witrowa-Rajchert, D. Drying kinetics and quality of carrots subjected to microwave-assisted drying preceded by combined pulsed electric field and ultrasound treatment. Dry. Technol. 2020, 38, 176–188. [Google Scholar] [CrossRef]
- Ohshima, T.; Tamura, T.; Sato, M. Influence of pulsed electric field on various enzyme activities. J. Electrostat. 2007, 65, 156–161. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Khorokhorina, V.A.; Bowman, A.M.; Rodaite-Riševičiene, R.; Saulis, G.; Xiao, S.; Pakhomov, A.G. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch. Biochem. Biophys. 2012, 527, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Mannozzi, C.; Rompoonpol, K.; Fauster, T.; Tylewicz, U. Influence of pulsed electric field and ohmic heating pretreatments on enzyme and antioxidant activity of fruit and vegetable juices. Foods 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Cai, C.; Wang, Y.; Zheng, X. The inactivation kinetics of polyphenol oxidase and peroxidase in bayberry juice during thermal and ultrasound treatments. Innov. Food Sci. Emerg. Technol. 2018, 45, 169–178. [Google Scholar] [CrossRef]
- Jordens, J.; Bamps, B.; Gielen, B.; Braeken, L.; Van Gerven, T. The effects of ultrasound on micromixing. Ultrason. Sonochem. 2016, 32, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Granados-Guzman, G.; Salazar-Aranda, R.; Garza-Tapia, M.; Castro-Rios, R.; Waksman de Torres, N. Optimization and validation of two high-throughput methods indicating antiradical activity. Curr. Anal. Chem. 2017, 13, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floegel, A.; Kim, D.; Chung, S.; Koo, S.I.; Chun, O.K. Journal of Food Composition and Analysis Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods §. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Wiktor, A.; Dadan, M.; Tylewicz, U.; Dalla Rosa, M.; Witrowa-Rajchert, D. Influence of power ultrasound on the main quality properties and cell viability of osmotic dehydrated cranberries. Ultrasonics 2018, 83, 33–41. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Fu, N.; Yu, S.J.; Chen, X.D.; Kennedy, J.F. Effects of pulsed electric field treatments on some properties of tapioca starch. Carbohydr. Polym. 2012, 89, 1012–1017. [Google Scholar] [CrossRef]
- Wiktor, A.; Nowacka, M.; Dadan, M.; Rybak, K.; Lojkowski, W.; Chudoba, T.; Witrowa-Rajchert, D. The effect of pulsed electric field on drying kinetics, color, and microstructure of carrot. Dry. Technol. 2016, 34, 1286–1296. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A. Beneficial effects of cranberry in the prevention of obesity and related complications: Metabolic syndrome and diabetes—A review. J. Funct. Foods 2016, 20, 171–181. [Google Scholar] [CrossRef]
- Rybak, K.; Samborska, K.; Jedlinska, A.; Parniakov, O. The impact of pulsed electric field pretreatment of bell pepper on the selected properties of spray dried juice. Innov. Food Sci. Emerg. Technol. 2020, 65, 102446. [Google Scholar] [CrossRef]
- Polish Standard. Fruit and Vegetable Juice—Total Carotenoids and Carotenoids Fraction Determination (PN-EN 12136); Polish Committee for Standardization: Warsaw, Poland, 2000. (In Polish) [Google Scholar]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kossori, R.L.; Villaume, C.; El Boustani, E.; Sauvaire, Y.; Méjean, L. Composition of pulp, skin and seeds of prickly pears fruit (Opuntia ficus indica sp.). Plant. Foods Hum. Nutr. 1998, 52, 263–270. [Google Scholar] [CrossRef] [PubMed]




| Type of Treatment | Sugars Content | ||
|---|---|---|---|
| Single Treatment | Sucrose [g/100 g d.m.] | Glucose [g/100 g d.m.] | Fructose [g/100 g d.m.] |
| F | 4.7 ± 0.1 d | 28.7 ± 0.5 d | 29.7 ± 0.3 d |
| BL_W | 3.1 ± 0.2 b | 17.3 ± 1.4 a | 20.7 ± 0.6 a |
| BL_S | 4.1 ± 0.1 c | 27.0 ± 0.8 cd | 26.2 ± 0.7 c |
| US | 4.2 ± 0.1 cd | 21.5 ± 0.5 b | 23.4 ± 0.7 b |
| PEF_1 | 4.6 ± 0.3 cd | 31.8 ± 0.3 e | 30.6 ± 0.9 d |
| PEF_3 | 4.2 ± 0.1 cd | 29.3 ± 0.4 d | 27.3 ± 1.3 c |
| Combined treatment | |||
| PEF_1 + US | 2.4 ± 0.3 a | 16.2 ± 1.1 a | 19.6 ± 0.7 a |
| PEF_3 + US | 2.2 ± 0.2 a | 18.4 ± 1.2 a | 20.1 ± 0.4 a |
| US + PEF_1 | 4.6 ± 0.2 cd | 24.7 ± 0.5 c | 23.6 ± 0.5 b |
| US + PEF_3 | 4.3 ± 0.1 cd | 26.2 ± 1.0 c | 25.7 ± 0.6 c |
| Treatment | Description | Parameters of Treatment |
|---|---|---|
| F | untreated sample | - |
| BL_W | blanching in water | temp: 98 °C, time: 3 min |
| BL_S | blanching in steam | temp: 98 °C, time: 3 min |
| US | ultrasound treatment | ultrasound intensity 3 W/cm2, frequency: 21 kHz, time: 30 min |
| PEF_1 | pulsed electric field treatment | pulse number: 6, electric field intensity: 1.07 kV/cm, 1 Ws: 1 kJ/kg |
| PEF_3 | pulsed electric field treatment | pulse number: 12, electric field intensity: 1.07 kV/cm, 1 Ws: 3 kJ/kg |
| Combined treatment | I step | II step |
| Parameters of treatment | ||
| PEF_1 + US | pulsed electric field pulse number: 12, electric field intensity: 1.07 kV/cm, 1 Ws: 1 kJ/kg | ultrasound treatment, ultrasound intensity 3 W/cm2, frequency: 21 kHz, time: 30 min |
| PEF_3 + US | pulsed electric field pulse number: 34, electric field intensity: 1.07 kV/cm, 1 Ws: 3 kJ/kg | ultrasound treatment, ultrasound intensity 3 W/cm2, frequency: 21 kHz, time: 30 min |
| US + PEF_1 | ultrasound treatment, ultrasound intensity 3 W/cm2, frequency: 21 kHz, time: 30 min | pulsed electric field pulse number: 12, electric field intensity: 1.07 kV/cm, 1 Ws: 1 kJ/kg |
| US + PEF_3 | ultrasound treatment, ultrasound intensity 3 W/cm2, frequency: 21 kHz, time: 30 min | pulsed electric field pulse number: 34, electric field intensity: 1.07 kV/cm, 1 Ws: 3 kJ/kg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D.; Parniakov, O.; Nowacka, M. The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars Content of Red Bell Pepper. Molecules 2020, 25, 4287. https://doi.org/10.3390/molecules25184287
Rybak K, Wiktor A, Witrowa-Rajchert D, Parniakov O, Nowacka M. The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars Content of Red Bell Pepper. Molecules. 2020; 25(18):4287. https://doi.org/10.3390/molecules25184287
Chicago/Turabian StyleRybak, Katarzyna, Artur Wiktor, Dorota Witrowa-Rajchert, Oleksii Parniakov, and Małgorzata Nowacka. 2020. "The Effect of Traditional and Non-Thermal Treatments on the Bioactive Compounds and Sugars Content of Red Bell Pepper" Molecules 25, no. 18: 4287. https://doi.org/10.3390/molecules25184287








