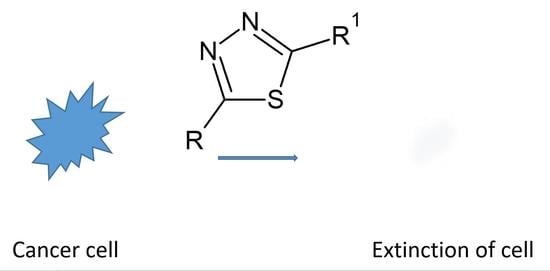
Cytotoxic Properties of 1,3,4-Thiadiazole Derivatives—A Review
Abstract
:1. Introduction
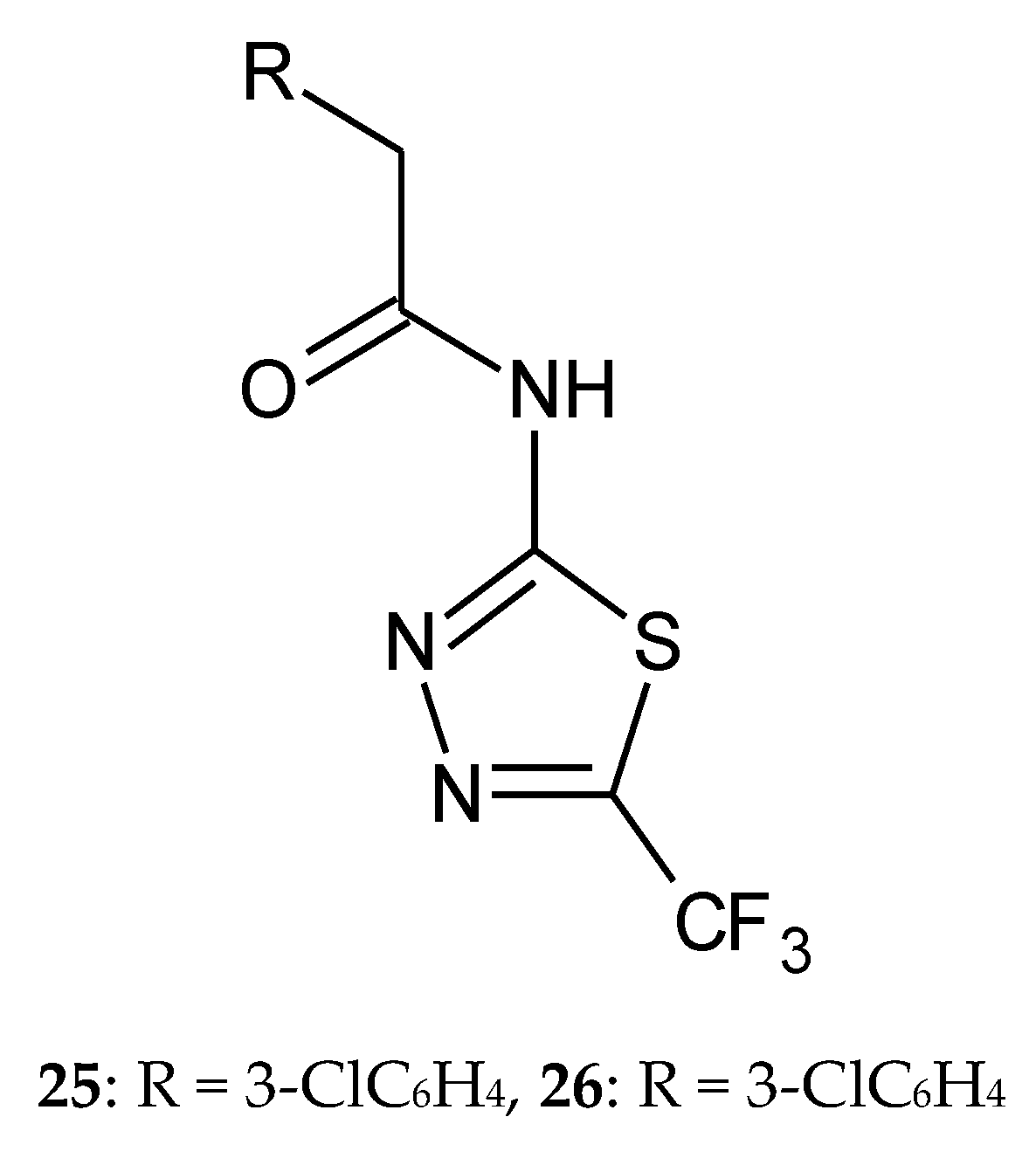
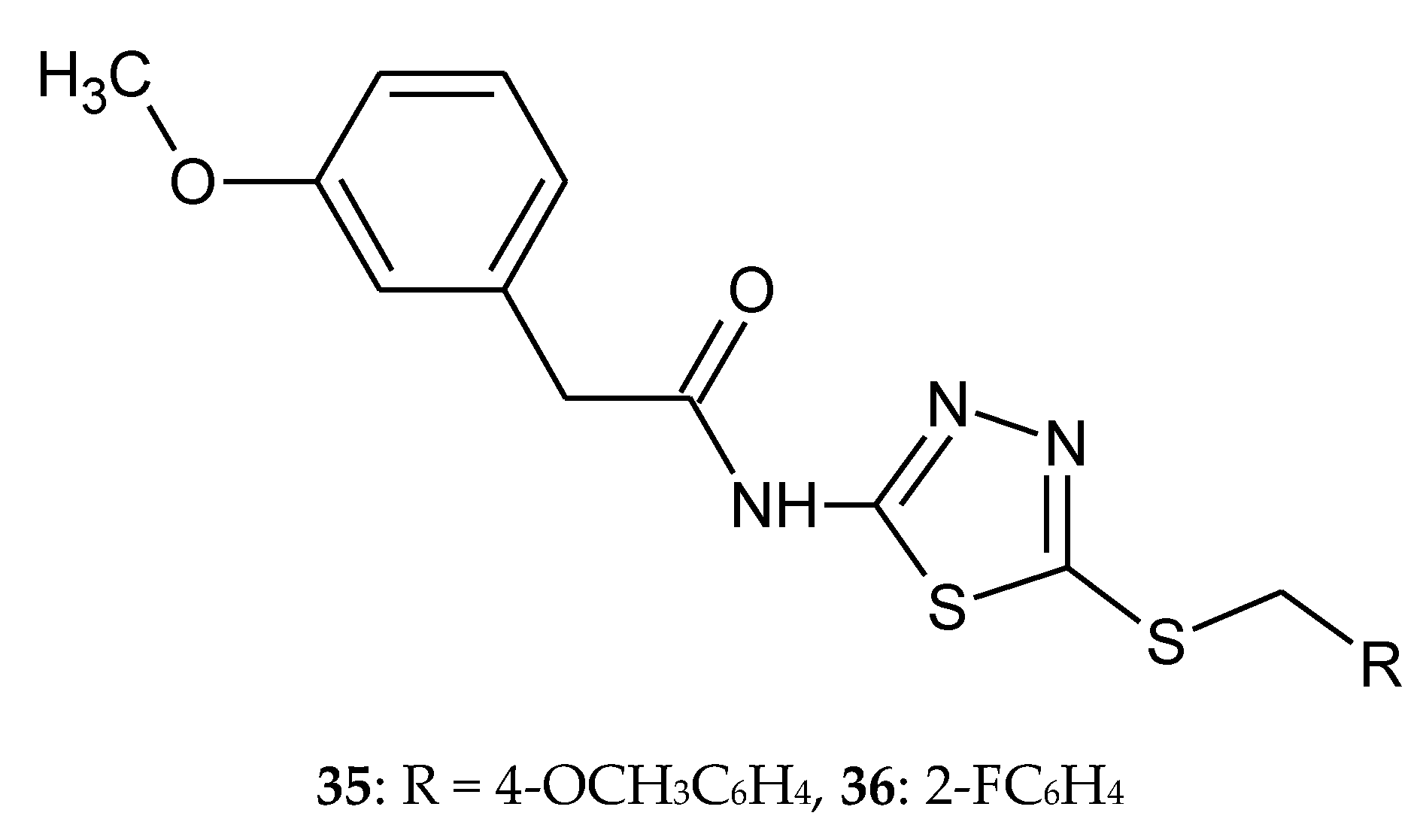
2. Derivatives of 2,5-Disubstituted-1,3,4-thiadiazole
3. 1,3,4-Thiadiazole Condensed with Other Heterocyclic Rings
3.1. Triazole–Thiadiazole Hydrids
3.2. Imidazo-Thiadiazole Hydrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kalidhar, U.; Kau, A. 1, 3, 4-Thiadiazole derivatives and their biological activities: A Review. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 1091–1106. [Google Scholar]
- Foroumadi, A.; Solani, F.; Moshafi, M.H.; Ashraf-Askari, R. Synthesis and in vitro antibacterial activity of some N-(5-aryl-1, 3, 4-thiadiazole-2-yl) piperazinyl quinolone derivatives. Farmaco 2003, 58, 1023–1028. [Google Scholar] [CrossRef]
- Chen, C.J.; Song, B.A.; Yang, S.; Xu, G.F.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Li, Z.Q.; Liu, F.; Xue, W.; et al. Synthesis and antifungal activities of 5-(3, 4, 5-trimethoxyphenyl)-2-sulfonyl-1, 3, 4-thiadiazole and 5-(3, 4, 5-trimethoxyphenyl)-2-sulfonyl-1, 3, 4-oxadiazole derivatives. Bioorg. Med. Chem. 2007, 15, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Kolavi, G.; Hegde, V.; Khazi, I.A.; Gadad, P. Synthesis and evaluation of antitubercular activity of imidazo [2, 1-b] [1,3,4] thiadiazole derivatives. Bioorg. Med. Chem. 2006, 14, 3069–3080. [Google Scholar] [CrossRef]
- Hafez, H.N.; Hegab, M.I.; Ahmed-Farag, I.S.; El-Gazzar, A.B.A. A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-9′, 2-[1,3,4] thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2008, 18, 4538–4543. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, S.; Vishwakarma, P.; Sharma, M.; Saxena, K.K.; Kumar, A. Synthesis and antipsychotic and anticonvulsant activity of some new substituted oxa/thiadiazolylazetidinonyl/thiazolidinonylcarbazoles. Eur. J. Med. Chem. 2010, 45, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, R.A.; Ahmed, B. Syntheses and anti-depressant activity of 5-amino-1, 3, 4-thiadiazole-2-thiol imines and thiobenzyl derivatives. Bioorg. Med. Chem. 2008, 16, 8029–8034. [Google Scholar] [CrossRef] [PubMed]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1, 3, 4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 1945–1954. [Google Scholar] [CrossRef]
- Yar, M.S.; Akhter, M.W. Synthesis and anticonvulsant activity of substituted oxadiazole and thiadiazole derivatives. Acta Polon. Pharm. 2009, 66, 393–397. [Google Scholar]
- Poorrajab, F.; Ardestani, S.K.; Emani, S.; Behrouzi-Fardmoghadam, M.; Shafiee, A.; Foroumadi, A. Nitroimidazolyl-1, 3, 4-thiadiazole-based anti-leishmanial agents: Synthesis and in vitro biological evaluation. Eur. J. Med. Chem. 2009, 44, 1758–1762. [Google Scholar] [CrossRef]
- Morsy, M.; Salwa, I.; Badawi, M.; Abdelfattah Cecchi, A.; Scozzafava, A.; Supuran, T.C. Carbonic anhydrase inhibitors. Biphenylsulfonamides with inhibitory action towards the transmembrane, tumor-associated isozymes IX possess cytotoxic activity against human colon, lung and breast cancer cell lines. J. Enzyme Inhib. Med. Chem. 2009, 24, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Wang, X.; Wu, X.; Zhang, Z.; Xie, S.; Hu, G. Synthesis and antitumor activity of thiadiazolylthioether dihydrazones. Huaxue Shiji 2009, 31, 682–684. [Google Scholar]
- Zhao, Y.; Ouyang, G.; Xu, W.; Jin, L.; Yuan, K. Synthesis, X-ray structure and antitumor activity of 4-(1, 3, 4-thiadiazole-2-ylthio) benzo [4,5] furo [3,2-d] pyrimidine derivatives. Youji Huaxue 2010, 30, 1093–1097. [Google Scholar]
- El-Ashmawy, M.B.; El-Sherbeny, M.A.; El-Sayed, N.S. Synthesis, in vitro antitumor activity and DNA binding affinity of novel thiadiazolopyrimidine and thiadiazoloquinazoline derivatives. Mansoura J. Pharm. Sci. 2010, 26, 60–68. [Google Scholar]
- Song, X.J.; Shao, Y.; Dong, X.G. Microwave-assisted synthesis of some novel fluorinated pyrazolo [3,4-d]pyrimidine derivatives containing 1, 3, 4-thiadiazole as potential antitumor agents. Chin. Chem. Lett. 2011, 22, 1036–1038. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Y.; Li, W.; Zhang, Y.; Wang, X.; Tang, J.; Zhu, H. Synthesis, biological evaluation and molecular docking studies of 1, 3, 4-thiadiazole derivatives containing 1, 4-benzodioxan as potential antitumor agents. Bioorg. Med. Chem. Lett. 2011, 21, 6116–6121. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; Sabry, E.; Ahmed, A.; Merveet, A.; Abdel-Shafy, F.; Talat, S. Anti-tumor activity of some 1, 3, 4-thiadiazoles and 1, 2, 4-triazine derivatives against ehrlichs ascites carcinoma. Int. J. Cancer Res. 2011, 7, 278–288. [Google Scholar] [CrossRef]
- Alam, M.; Liu, L.; Lee, D. Cytotoxicity of new 5-phenyl-4, 5-dihydro-1, 3, 4-thiadiazole analogues. Chem. Pharm. Bull. 2011, 59, 1413–1416. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wen, Q.; Zhao, T.; Sun, J.; Li, X.; Xing, M.; Lu, X.; Zhu, H. Synthesis, biological evaluation, and molecular docking studies of cinnamic acyl 1, 3, 4-thiadiazole amide derivatives as novel antitubulin agents. Bioorg. Med. Chem. 2012, 20, 1181–1187. [Google Scholar] [CrossRef]
- Juszczak, M.; Matysiak, J.; Niewiadomy, A.; Rzeski, W. The activity of a new 2-amino-1, 3, 4-thiadiazole derivative 4C1ABT in cancer and normal cells. Folia Histochem. Cytobiol. 2011, 49, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Juszczak, M.; Matysiak, J.; Szeliga, M.; Pozarowski, P.; Niewiadomy, A.; Albrecht, J.; Rzeski, W. 2-Amino-1, 3, 4-thiadiazole derivative (FABT) inhibits the extracellular signal-regulated kinase pathway and induces cell cycle arrest in human non-small lung carcinoma cells. Bioorg. Med. Chem. Lett. 2012, 22, 5466–5469. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, L.; Khorand, A.; Aliabadi, A. Discovery of 2-phenyl-N-(5-(trifluoromethyl)-1, 3, 4-thiadiazol-2-yl) acetamide derivatives as apoptosis inducers via the caspase pathway with potential anticancer activity. Arch. Pharm. 2013, 346, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Dawood, K.M.; Eldebss, T.M.A.; El-Zahabi, H.S.A.; Yousef, M.H.; Metz, P. Synthesis of some new pyrazole-based 1, 3-thiazoles and 1, 3, 4-thiadiazoles as anticancer agents. Eur. J. Med. Chem. 2013, 70, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, M.; Shrivastava, A.; Taile, V. Synthesis of 5-arylideneamino-1, 3, 4-thiadiazole-2-[(N-substituted-benzyol)] sulphonamides endowed with potent antioxidants and anticancer activity induces growth inhibition in HEK293, BT474 and NCI-H226 cells. Med. Chem. Res. 2014, 23, 3049–3064. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Song, X.; Li, X.; Ye, T.; Xiong, Y.; Yu, L. Synthesis and biological evaluation of 1, 2, 4-triazole and 1, 3, 4-thiadiazole derivatives as potential cytotoxic agents. Chem. Pharm. Bull. 2013, 61, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliabadi, A.; Hasanvand, Z.; Kiani, A.; Mirabdali, S. Synthesis and in vitro cytotoxicity assessment of N-(5-(benzylthio)-1, 3, 4-thiadiazol-2-yl)-2-(4-(trifluoromethyl) phenyl) acetamide with potential anticancer activity. Iran. J. Pharm. Res. 2013, 12, 687–693. [Google Scholar]
- Gomha, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization, and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as anticancer agents. Mon. Chem. 2015, 146, 149–158. [Google Scholar] [CrossRef]
- Mohammadi-Farani, A.; Bahrami, T.; Aliabadi, A. Synthesis, docking and cytotoxicity evaluation of N-(5-(benzylthio)-1, 3, 4-thiadiazol-2-yl)-2-(3-methoxyphenyl) acetamide derivatives as tyrosine kinase inhibitors with potential anticancer activity. J. Rep. Pharm. Sci. 2014, 3, 159–168. [Google Scholar]
- Polkam, N.; Rayam, P.; Anireddy, J.; Yennam, S.; Anantaraju, H.; Dharmarajan, S.; Perumal, Y.; Kotapalli, S.; Ummanni, R.; Balasubramanian, S. Synthesis, in vitro anticancer and antimycobacterial evaluation of new 5-(2, 5-dimethoxyphenyl)-1, 3, 4-thiadiazole-2-amino derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 1398–1402. [Google Scholar] [CrossRef]
- Yadagiri, B.; Gurrala, S.; Bantu, R.; Nagarapu, L.; Polepalli, S.; Srujana, G.; Jain, N. Synthesis and evaluation of benzosuberone embedded with 1, 3, 4-oxadiazole, 1, 3, 4-thiadiazole and 1, 2, 4-triazole moieties as new potential antiproliferative agents. Bioorg. Med. Chem. Lett. 2015, 25, 2220–2224. [Google Scholar] [CrossRef]
- Plech, T.; Kapron, B.; Paneth, A.; Wujec, M.; Czarnomysy, R.; Bielawska, A.; Bielawski, K.; Trotsko, N.; Kusmierz, E.; Paneth, P. Search for human DNA topoisomerase II poisons in the group of 2, 5-disubstituted-1, 3, 4-thiadiazoles. J. Enzym. Inhib. Med. Chem. 2015, 30, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jing, F.; Fu, X.; Xiaoyun, Z.; Zhao, J.; Jijun, W.; Xuefeng, L.; Baolin, L.; Yuming, C. Synthesis and antitumor activities of disulfide derivatives containing 1, 3, 4-thiadiazole moiety. Youji Huaxue 2015, 35, 2624–2628. [Google Scholar] [CrossRef]
- Almasirad, A.; Firoozpour, L.; Nejati, M.; Edraki, N.; Firuzi, O.; Khoshneviszadeh, M.; Mahdavi, M.; Moghimi, S.; Safavi, M.; Shafiee, A. Design, synthesis and biological evaluation of new series of 2-amido-1, 3, 4-thiadiazole derivatives as cytotoxic agents. Z. Nat. B J. Chem. Sci. 2016, 71, 205–210. [Google Scholar] [CrossRef]
- Ali, K.A.; Elsayed, M.A.; Elhallouty, S.; Mahmoud, K.; Farag, A.M. Synthesis and antitumor screening of some new 2,6-bis pyridines functionalized with pyrazole-based heterocycles. Acta Pol. Pharm. 2015, 72, 1193–1200. [Google Scholar]
- Gomha, S.M.; Kheder, N.A.; Abdelhamid, A.O.; Kheder, N.A.; Mabkhot, Y.N. One pot single step synthesis and biological evaluation of some novel bis (1, 3, 4-thiadiazole) derivatives as potential cytotoxic agents. Molecules 2016, 21, 1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomha, S.M.; Abdel-Aziz, H.M.; Khalil, K.D. Synthesis and SAR study of the novel thiadiazole-imidazole derivatives as a new anticancer agents. Chem. Pharm. Bull. 2016, 64, 1356–1363. [Google Scholar] [CrossRef] [Green Version]
- Abdelriheem, A.N.; Kandeel, M.S.; Abdou, A.O.; Abdelhamid, S.; Gomha, M. Synthesis of New 3-Heteroarylindoles as Potential Anticancer. Molecules 2016, 21, 929–944. [Google Scholar]
- Flefel, E.M.; El-Sayed, W.A.; Mohamed, A.M.; El-Sofany, W.I.; Awad, H.M. Synthesis and anticancer activity of new 1-thia-4-azaspiro[4,5] decane, their derived thiazolopyrimidine and 1, 3, 4-thiadiazole thioglycosides. Molecules 2017, 22, 170. [Google Scholar] [CrossRef]
- Vudhgiri, S.; Koude, D.; Veeragoni, D.; Misra, S.; Sunil, P.; Jala, R. Synthesis and biological evaluation of 5-fatty-acylamido-1, 3, 4-thiadiazole-2-thioglycosides. Bioorg. Med. Chem. Lett. 2017, 27, 3370–3373. [Google Scholar] [CrossRef]
- Jakovljevic, K.; Matic, I.; Stanojkovic, T.; Krivokuca, A.; Markovic, V.; Joksovic, M.; Mihailovic, N.; Niciforovic, M.; Joksovic, L. Synthesis, antioxidant and antiproliferative activities of 1, 3, 4-thiadiazoles derived from phenolic acids. Bioorg. Med. Chem. Lett. 2017, 27, 3709–3715. [Google Scholar] [CrossRef]
- Rezaei, Z.; Moghimi, S.; Javaheri, R.; Asadi, M.; Mahdavi, M.; Shabani, S.; Edraki, N.; Firuzi, O.; Safavi, M.; Amini, M. Synthesis and biological evaluation of 1, 3, 4-thiadiazole linked phthalimide derivatives as anticancer agents. Lett. Drug Des. Discov. 2017, 14, 1138–1144. [Google Scholar] [CrossRef]
- Mohammadi-Farani, A.; Hosseinzadeh, L.; Barazesh, P.; Ahmadi, F.; Aliabadi, A. Evaluation of cytotoxicity and apoptosis inducing effects of N-(5-mercapto-1, 3, 4-thiadiazol-2-yl)-2-phenylacetamide derivatives as caspase enzymes activators. Pharma Chem. 2017, 9, 40–45. [Google Scholar]
- Abdelhamid, A.O.; Gomha, S.M.; Abdelrehem, N.A.; ShalabyKandeel, A.M. Synthesis and biological evaluation of some novel thiadiazole-benzofuran hybrids as potential antitumor agents. Synth. Commun. 2018, 48, 677–684. [Google Scholar] [CrossRef]
- Azaam, M.M.; Kenawy, E.; Badr, E.; Ahmed, S.; Khamis, A.; El-Magd, M.A. Antioxidant and anticancer activities of α aminophosphonates containing thiadiazole moiety. J. Saudi Chem. Soc. 2018, 22, 34–41. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Mishra, P. Synthesis, antimicrobial and anticancer activities of 5-(4-substituted-phenyl)-1, 3, 4-thiadiazole-2-amines. Rasayan J. Chem. 2017, 10, 254–262. [Google Scholar]
- Altintop, M.; Ciftci, H.; Radwan, M.; Sever, B.; Kaplancikli, Z.; Ali, T.; Koga, R.; Fujita, M.; Otsuka, M. Design, synthesis, and biological evaluation of novel 1, 3, 4-thiadiazole derivatives as potential antitumor agents against chronic myelogenous leukemia: Striking effect of nitrothiazole moiety. Molecules 2018, 23, 59. [Google Scholar]
- Farooqi, S.; Arshad, N.; Channar, P.; Perveen, F.; Saeed, A.; Larik, F.; Javeed, A. Synthesis, theoretical, spectroscopic and electrochemical DNA binding investigations of 1, 3, 4-thiadiazole derivatives of ibuprofen and ciprofloxacin: Cancer cell line studies. J. Photochem. Photobiol. B Biol. 2018, 189, 104–118. [Google Scholar] [CrossRef]
- Liu, B.; Qin, S.; Yu, S.; Wu, Z.; Liu, A. Synthesis and evaluation of new tegafur derivatives containing 1, 3, 4-thiadiazole moiety. Indian J. Heterocy. Chem. 2017, 27, 355–360. [Google Scholar]
- Fathy, U.; Awad, H. Synthesis and anti-cancer activity of certain novel pyrazoline-based 1, 3-oxathioles and 1, 3, 4-thiadiazoles. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 1–13. [Google Scholar]
- Zhang, S.; Liu, X.; Tang, R.; Wang, H.; Liu, H.; Liu, Y.; Chen, B. Design, synthesis and antiproliferative evaluation of novel disulfides containing 1, 3, 4-thiadiazole moiety. Chem. Pharm. Bull. 2017, 65, 950–958. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Wu, X.; Li, Z.; Yu-Ming, L.L.; Chen, W. Synthesis, antitumor and antimicrobial evaluation of novel 1, 3, 4-thiadiazole derivatives bearing disulfide bond. Med. Chem. Res. 2018, 27, 1929–1940. [Google Scholar] [CrossRef]
- Gowramma, B.; Praveen, T.K.; Gomathy, S.; Kalirajan, R.; Babu, B.; Krishnavenic, N. Synthesis of 2-(bis(2-chloroethyl)amino)-N-(5-substituted-phenyl)-1, 3, 4-thiadiazol-2-yl) acetohydrazide and evaluation of anticancer activity. Curr. Bioact. Compd. 2018, 14, 309–316. [Google Scholar] [CrossRef]
- Raj, V.; Rai, A.; Singh, A.K.; Keshari, A.K.; Trivedi, P.; Ghosh, B.; Kumar, U.; Kumar, D.; Saha, S. Discovery of novel 2-amino-5-(substituted)-1, 3, 4-thiadiazole derivatives: New utilities for colon cancer treatment. Anti-Cancer Agent Med. Chem. 2018, 18, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Nassar, I.F.; Att-Allah, S.R.; Hemdan, M.M. Utility of thiophene-2-carbonyl isothiocyanate as a precursor for the synthesis of 1, 2, 4-triazole, 1, 3, 4-oxadiazole and 1, 3, 4-thiadiazole derivatives with evaluation of their antitumor and antimicrobial activities. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 630–636. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Farag, M.M.; El-Gendey, M.S.; Mounier, M.M. Toward rational design of novel anti-cancer drugs based on targeting, solubility, and bioavailability exemplified by 1, 3, 4-thiadiazole derivatives synthesized under solvent-free conditions. Molecules 2019, 24, 2371. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.D.; Venkata, R.D.V.; Tejeswara, R.A.; Lav, K.U.; Jha, A. Design and synthesis of 1, 3, 4-thiadiazole derivatives as novel anticancer and antitubercular agents. Russ. J. Gen. Chem. 2019, 89, 770–779. [Google Scholar] [CrossRef]
- El-Naggar, M.; Sallam, H.A.; Shaban, S.S.; Abdel-Wahab, S.S.; El-Galil, E.A.; Azab, M.E.; Nossier, E.S.; Al-Omar, M.A. Design, synthesis, and molecular docking study of novel heterocycles incorporating 1, 3, 4-thiadiazole moiety as potential antimicrobial and anticancer agents. Molecules 2019, 24, 1066. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, D.K.; Dadure, K.M.; Haldar, A.G.M. Exploring the anti-breast cancer (against MCF-7 Cell Line) potentials of uracil substituted hippuric acid based 1, 3, 4-thiadiazole compound. Int. J. Pharm. Life Sci. 2019, 10, 6013–6015. [Google Scholar]
- Hu, G.; Xie, S.; Hou, L.; Sun, M.; Zhang, J.; Yang, Y.; Yi, L. Preparation of 1, 2, 4-triazolo[3, 4-b] [1,3,4] thiadiazole linked fluoroquinolone dimers for treating tumor and antimicrobial infection. CN 101648962 A, 17 February 2010. [Google Scholar]
- Hu, G.Q.; Zhang, Z.Q.; Xie, S.Q.; Huang, W. Synthesis and antitumor evaluation of C3/C3 fluoroquinolone dimers (I), tethered with a fused heterocyclic s-triazolo [2, 1-b] [1,3,4] thiadiazole. Chin. Chem. Lett. 2010, 21, 661–663. [Google Scholar] [CrossRef]
- Hu, G.; Yang, Y.; Yi, L.; Wang, G.; Duan, N.; Wen, X.; Cao, T.; Xie, S.; Huang, W. Synthesis and antitumor activity of C3/C3 bis-fluoroquinolones cross-linked with [1,2,4] triazolo [3,4-b] [1,3,4]thiadiazole. Acta Pharm. Sin. B 2011, 1, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Sunil, D.; Isloor, A.M.; Shetty, P.; Satyamoorthy, K.; Bharath, A.S. Synthesis, characterization, antioxidant, and anticancer studies of 6-[3-(4-chlorophenyl)-1H-pyrazol-4-yl]-3-[(2-naphthyloxy)methyl][1,2,4]triazolo[3,4-b][1,3,4]thiadiazole in HepG2 cell lines. Med. Chem. Res. 2011, 20, 1074–1080. [Google Scholar] [CrossRef]
- Sunil, D.; Isloor, A.M.; Shetty, P.; Satyamoorthy, K.; Prasad, A.S. 6-[3-(4-Fluorophenyl)-1H-pyrazol-4-yl]-3-[(2-naphthyloxy)methyl][1,2,4]triazolo[3,4-b][1,3,4]-thiadiazole as a potent antioxidant and an anticancer agent induces growth inhibition followed by apoptosis in HepG2 cells. Arab. J. Chem. 2010, 3, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Lauffer, D.; Li, P. Preparation of [1,2,4] triazolo [3,4-b] [1,3,4]thiadiazole derivatives as inhibitors of c-Met protein kinase. WO2010138673A1, 2 December 2010. [Google Scholar]
- Deepak, C.; Chandrabose, K.; Lokesh, C.; Sahabjada, S.; Madhu, G.; Md, A.; Piyush, T. Synthesis, characterization and anticancer activity of some fluorinated 3,6-diaryl-[1,2,4] triazolo [3,4-b] [1,3,4] thiadiazoles. Arab. J. Chem. 2017, 10, 2424–2428. [Google Scholar]
- Ramaprasad, G.C.; Kalluraya, B.; Kumar, B.S.; Mallya, S. Microwave assisted synthesis of triazolothiadiazole analogues as anticancer and antibacterial agents. Pharma Chem. 2012, 4, 1026–1032. [Google Scholar]
- Ningegowda, R.; Priya, B.S.; Shivananju, N.S.; Achar, R.R.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Sethi, G.; Basappa, K.S.; Rangappa, K. A novel 4, 6- disubstituted-1, 2, 4-triazolo-1, 3, 4-thiadiazole derivative inhibits tumor cell invasion and potentiates the apoptotic effect of TNFα by abrogating NF-κB activation cascade. Apoptosis 2017, 22, 145–157. [Google Scholar] [CrossRef]
- Chen, B.; Liu, X.; Tang, R.; Zhang, S.; Wang, H.; Liu, H. Preparation of 1, 2, 4-triazolo[3, 4-b]-1, 3, 4-thiadiazole derivatives as antitumor agents. CN 106866708 A, 20 June 2017. [Google Scholar]
- Venepally, V.; Sirisha, K.; Kumar, C.; Krishna, E.; Misra, S.; Jala, R.C.R. Synthesis and biological evaluation of 3,6-dialkylsubstituted-[1,2,4] triazolo [3,4-b] [1,3,4]thiadiazoles. J. Chem. Sci. 2018, 130, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhao, J.; Zhang, B.; Lu, T.; Chen, Y. Discovery of [1,2,4] triazolo [3,4-b] [1,3,4]thiadiazole derivatives as novel, potent and selective c-Met kinase inhibitors: Synthesis, SAR study, and biological activity. Eur. J. Med. Chem. 2018, 150, 809–816. [Google Scholar] [CrossRef]
- Wang, L.S.; Hai-Xin, L.; Hai-Ying, L.; Fen, J.; Xiao-Yun, F.; Cai-Wen, L.; Yan-Ping, S.; Bao-Quan, C. Synthesis and biological evaluation of novel disulfides incorporating 1, 3, 4-thiadiazole scaffold as promising antitumor agents. Med. Chem. Res. 2019, 28, 1502–1508. [Google Scholar]
- Nir, U.; Shpungin, S.; Yaffe, E.; Cohen, M. Preparation of phenylpiperazinylmethylpiperidinyl-imidazothiadiazole derivatives and analogs for use as antitumor agents. PCT Int. Appl. Patent WO2010097798 A1 20100902, 2010. [Google Scholar]
- Karki, S.S.; Panjamurthy, K.; Kumar, S.; Nambiar, M.; Ramareddy, S.A.; Chiruvella, K.K.; Raghavan, S.C. Synthesis and biological evaluation of novel 2-aralkyl-5-substituted-6-(4’-fluorophenyl)-imidazo[2,1-b] [1,3,4] thiadiazole derivatives as potent anticancer agents. Eur. J. Med. Chem. 2011, 46, 2109–2116. [Google Scholar] [CrossRef]
- Noolvi, M.N.; Patel, H.M.; Singh, N.; Gadad, A.K.; Cameotra, S.S.; Badiger, A. Synthesis and anticancer evaluation of novel 2-cyclopropylimidazo[2,1-b] [1,3,4]-thiadiazole derivatives. Eur. J. Med. Chem. 2011, 46, 4411–4418. [Google Scholar] [CrossRef]
- Kumar, S.; Hegde, M.; Gopalakrishnan, V.; Renuka, V.K.; Ramareddy, S.A.; De Clercq, E.; Schols, D.; Gudibabande, A.K.N.; Raghavan, S.C.; Karki, S.S. 2-(4-Chlorobenzyl)-6-arylimidazo[2,1-b][1,3,4] thiadiazoles: Synthesis, cytotoxic activity and mechanism of action. Eur. J. Med. Chem. 2014, 84, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Rahman, E.; Doaa, M.; Khaled, O. Synthesis of novel 1, 3, 4-thiadiazole analogues with expected anticancer activity. Pharma Chem. 2014, 6, 323–335. [Google Scholar]
- Romagnoli, R.; Baraldi, P.G.; Precipe, F.; Balzarini, J.; Liekens, S.; Estevez, F. Synthesis and antiproliferative activity of novel heterobivalent hybrids based on imidazo [2,1-b] [1,3,4] thiadiazole and imidazo [2,1-b] [1,3] thiazole scaffolds. Eur. J. Med. Chem. 2015, 101, 205–217. [Google Scholar] [CrossRef]
- Narasimha, R.M.P.; Nagaraju, B.; Kovvuri, J.; Polepalli, S.; Alavala, S.; Vishnuvardhan, M.; Swapna, P.; Nimbarte, V.; Lakshmi, J.K.; Jain, N. Synthesis of imidazo-thiadiazole linked indolinone conjugates and evaluated their microtubule network disrupting and apoptosis inducing ability. Bioorg. Chem. 2018, 76, 420–436. [Google Scholar] [CrossRef] [PubMed]





































































© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowska, S.; Paneth, A.; Wujec, M. Cytotoxic Properties of 1,3,4-Thiadiazole Derivatives—A Review. Molecules 2020, 25, 4309. https://doi.org/10.3390/molecules25184309
Janowska S, Paneth A, Wujec M. Cytotoxic Properties of 1,3,4-Thiadiazole Derivatives—A Review. Molecules. 2020; 25(18):4309. https://doi.org/10.3390/molecules25184309
Chicago/Turabian StyleJanowska, Sara, Agata Paneth, and Monika Wujec. 2020. "Cytotoxic Properties of 1,3,4-Thiadiazole Derivatives—A Review" Molecules 25, no. 18: 4309. https://doi.org/10.3390/molecules25184309
APA StyleJanowska, S., Paneth, A., & Wujec, M. (2020). Cytotoxic Properties of 1,3,4-Thiadiazole Derivatives—A Review. Molecules, 25(18), 4309. https://doi.org/10.3390/molecules25184309