The Effect of Cultivation Method of Strawberry (Fragaria x ananassa Duch.) cv. Honeoye on Structure and Degradation Dynamics of Pectin during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Conditions
2.2. Cold Storage
2.3. Sample Preparation
Pectin Extraction with Ammonium Oxalate
2.4. Fruit Quality
2.4.1. Fresh Weight
2.4.2. Length and Width
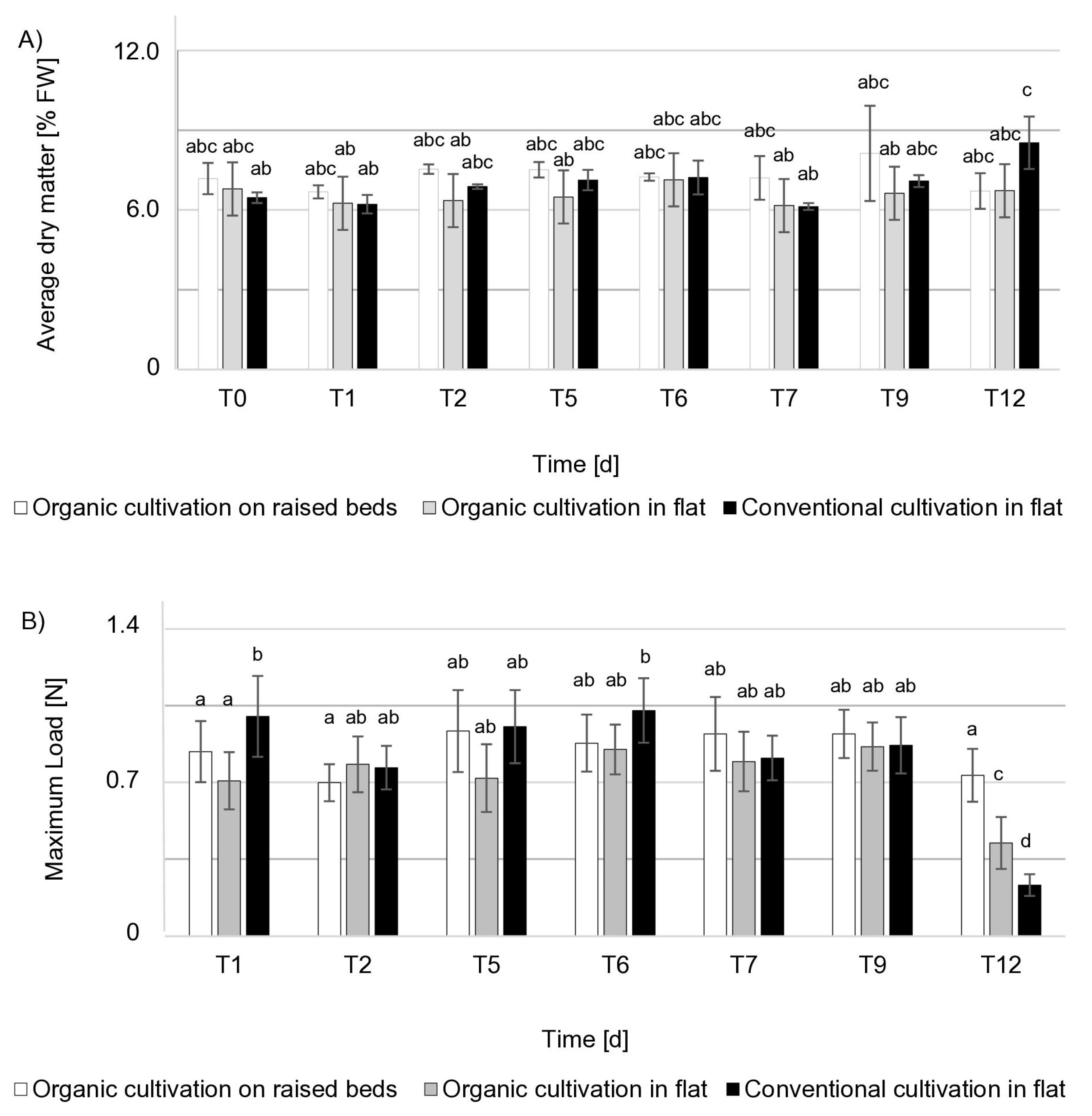
2.4.3. Dry Matter Content
2.4.4. Firmness
2.4.5. Acidity
2.4.6. Soluble Solid Content
2.4.7. Total Anthocyanin Content
2.4.8. Total Soluble Phenolic Content
2.4.9. The Content of Vitamin C
2.4.10. Galacturonic Acid Content
2.5. FT-IR Spectra of Pectin
2.6. AFM Imaging
2.7. Rheological Properties
2.8. Statistical Analysis
3. Results and Discussion
3.1. Fruit Quality
3.2. FT-IR Spectra
3.3. AFM Imaging
3.4. Rheological Properties of Pectin Extracted from Strawberries
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Faostat. Food and Agriculture. Available online: http://www.fao.org/faostat/en/ (accessed on 22 May 2020).
- World Heatlh Organization. Guidelines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerlad, 2004; Volume 1, pp. 467–486. [Google Scholar]
- Abbott, J.A. Quality Measurement of Fruits and Vegetables. Postharvest Biol. Technol. 1999, 15, 207–225. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.D.J.; Yahia, E.M.; Ramírez-Bustamante, N.; Pérez-Martínez, J.D.; Escalante-Minakata, M.D.P.; Ibarra-Junquera, V.; Acosta-Muñiz, C.; Guerrero-Prieto, V.; Ochoa-Reyes, E. Physical attributes and chemical composition of organic strawberry fruit (Fragaria x Ananassa Duch, Cv. Albion) at six stages of ripening. Food Chem. 2013, 138, 372–381. [Google Scholar] [CrossRef]
- Lobos, G.A.; Bravo, C.; Valdés, M.; Graell, J.; Lara Ayala, I.; Beaudry, R.M.; Moggia, C. Within-plant variability in blueberry (Vaccinium Corymbosum, L.): Maturity at harvest and position within the canopy influence fruit firmness at harvest and postharvest. Postharvest Biol. Technol. 2018, 146, 26–35. [Google Scholar] [CrossRef]
- Watkins, C.B.; Manzano-Mendez, J.E.; Nock, J.F.; Zhang, J.; Maloney, K.E. Cultivar variation in response of strawberry fruit to high carbon dioxide treatments. J. Sci. Food Agric. 1999, 79, 886–890. [Google Scholar] [CrossRef]
- Joshi, K.; Tiwari, B.; Cullen, P.J.; Frias, J.M. Predicting quality attributes of strawberry packed under modified atmosphere throughout the cold chain. Food Packag. Shelf Life 2019, 21, 100354. [Google Scholar] [CrossRef]
- Hannum, S.M. Potential impact of strawberries on human health: A review of the science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodríguez, M.A.; Vázquez-Odériz, M.L. Color, anthocyanin pigment, ascorbic acid and total phenolic compound determination in organic versus conventional strawberries (Fragaria × ananassa Duch, cv Selva). J. Food Compos. Anal. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Kozioł, A.; Cybulska, J.; Pieczywek, P.M.; Zdunek, A. Changes of pectin nanostructure and cell wall stiffness induced in vitro by pectinase. Carbohydr. Polym. 2017, 161, 197–207. [Google Scholar] [CrossRef]
- Cosgrove, D.J.; Jarvis, M.C. Comparative structure and biomechanics of plant primary and secondary cell walls. Front. Plant. Sci. 2012, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, M.C. Plant cell walls: Supramolecular assemblies. Food Hydrocoll. 2011, 25, 257–262. [Google Scholar] [CrossRef]
- Mierczyńska, J.; Cybulska, J.; Pieczywek, P.M.; Zdunek, A. Effect of storage on rheology of water-soluble, chelate-soluble and diluted alkali-soluble pectin in carrot cell walls. Food Bioprocess. Technol. 2014, 8, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Chylińska, M.; Szymańska-Chargot, M.; Deryło, K.; Tchórzewska, D.; Zdunek, A. Changing of biochemical parameters and cell wall polysaccharides distribution during physiological development of tomato fruit. Plant. Physiol. Biochem. 2017, 119, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Kar, F.; Arslan, N. Characterization of orange peel pectin and effect of sugars, L-ascorbic acid, ammonium persulfate, salts on viscosity of orange peel pectin solutions. Carbohydr. Polym. 1999, 40, 285–291. [Google Scholar] [CrossRef]
- Jayani, R.S.; Saxena, S.; Gupta, R. Microbial pectinolytic enzymes: A review. Process. Biochem. 2005, 40, 2931–2944. [Google Scholar] [CrossRef]
- Arancibia, R.A.; Motsenbocker, C.E. Pectin methylesterase activity in vivo differs from activity in vitro and enhances polygalacturonase-mediated pectin degradation in tabasco pepper. J. Plant. Physiol. 2006, 163, 488–496. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Rheology for the food industry. J. Food Eng. 2005, 67, 147–156. [Google Scholar] [CrossRef]
- Kobi, H.B.; Martins, M.C.; Silva, P.I.; Souza, J.L.; Carneiro, J.C.S.; Heleno, F.; Queiroz, M.E.L.R.; Costa, N.M. Organic and conventional strawberries: Nutritional quality, antioxidant characteristics and pesticide residues. Fruits 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Uselis, N.; Lanauskas, J.; Zalatorius, V.; Duchovskis, P.; Brazaitytė, A.; Urbonavičiūtė, A. Evaluation of the methods of soil cultivation growing dessert strawberries in beds. Sodinink. Daržininkyste 2008, 27, 295–305. [Google Scholar]
- Treftz, C.; Omaye, S.T. Comparision between hydroponic and soil systems for growing strawberries in a greenhouse. Int. J. Agric. Ext. 2015, 03, 195–200. [Google Scholar]
- Renard, C.M.G.C. Variability in cell wall preparations: Quantification and comparison of common methods. Carbohydr. Polym. 2005, 60, 515–522. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Koubala, B.B.; Mbome, L.I.; Kansci, G.; Mbiapo, F.T.; Crepeau, M. Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chem. 2008, 106, 1202–1207. [Google Scholar] [CrossRef]
- Min, B.; Lim, J.; Ko, S.; Lee, K.; Ho, S.; Lee, S. Environmentally friendly preparation of pectins from agricultural by products and their structural/rheological characterization. Bioresour. Technol. 2011, 102, 3855–3860. [Google Scholar] [CrossRef]
- PN ISO 1026:2000. Fruit and Vegetable Products-Determination of Dry Matter Content by Drying under Reduced Pressure and of Water Content by Azeotropic Distillation; Standard endorsed on 8 May; International Organization for Standardization: Warsaw, Poland, 2000. [Google Scholar]
- Shin, Y.; Getchonis, K.A.; Holliday, D.L.; Polar-cabrera, K.; Nock, J.F.; Watkins, C.B. Getting the best out of strawberry storage—What temperature and relative humidity should be used? N. Y. Fruit Q. 2005, 13, 19–21. [Google Scholar]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.M.; Sargent, S.A. Physical and chemical quality characteristics of strawberries after storage are reduced by a short delay to cooling. Postrharvest Biol. Technol. 1995, 5214, 17–28. [Google Scholar] [CrossRef]
- da Silva, F.L.; Escribano-Bailón, M.T.; Pérez Alonso, J.J.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT-Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Nunes, C.M.N.; Brecht, J.K.; Morais, A.M.M.B.; Sargent, S.A. Possible influences of water loss and polyphenol oxidase activity on anthocyanin content and discoloration in fresh ripe strawberry (cv. Oso Grande) during storage at 1 °C. J. Food Sci. 2001, 70, 79–84. [Google Scholar] [CrossRef]
- Spayd, S.E.; Morris, J.R. Influence of immature fruits on strawberry jam quality and storage stability. J. Food Sci. 1981, 46, 414–418. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungistic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–157. [Google Scholar]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 50–627. [Google Scholar]
- Polish Committee for Standardization. Produkty Spożywcze. In Oznaczanie Zawartości Witaminy C; PN-A-04019:1998; Polish Committee for Standardization: Gdansk, Poland, 1998; pp. 1–6. [Google Scholar]
- Cybulska, J.; Zdunek, A.; Kozioł, A. The self-assembled network and physiological degradation of pectins in carrot cell walls. Food Hydrocoll. 2015, 43, 41–50. [Google Scholar] [CrossRef]
- Cybulska, J.; Halaj, M.; Cepák, V.; Lukavský, J.; Capek, P. Nanostructure features of microalgae biopolymer. Starch-Staerke 2015, 67, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Yang, H.; Sun, X.; Liu, H.; Gong, X.; Jiang, C.; Ding, C. Changes in firmness, pectin content and nanostructure of two crisp peach cultivars after storage. LWT-Food Sci. Technol. 2010, 43, 26–32. [Google Scholar] [CrossRef]
- Penna, A.L.B.; Sivieri, K.; Oliveira, M.N. Relation between quality and rheological properties of lactic beverages. J. Food Eng. 2001, 49, 7–13. [Google Scholar] [CrossRef]
- Farcuh, M.; Toubiana, D.; Sade, N.; Rivero, R.M.; Doron-Faigenboim, A.; Nambara, E.; Sadka, A.; Blumwald, E. Hormone balance in a climacteric plum fruit and its non-climacteric bud mutant during ripening. Plant. Sci. 2019, 280, 51–65. [Google Scholar] [CrossRef]
- Harker, F.R.; Elgar, H.J.; Watkins, C.B.; Jackson, P.J.; Hallett, I.C. Physical and mechanical changes in strawberry fruit after high carbon dioxide treatments. Postharvest Biol. Technol. 2000, 19, 139–146. [Google Scholar] [CrossRef]
- Tarantino, A.; Lops, F.; Disciglio, G.; Lopriore, G. Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of ‘Orange rubis®’ apricot (Prunus armeniaca L.) cultivar in two consecutive years. Sci. Hortic. 2018, 239, 26–34. [Google Scholar] [CrossRef]
- Liu, B.; Wang, K.; Shu, X.; Liang, J.; Fan, X.; Sun, L. Changes in fruit firmness, quality traits and cell wall constituents of two highbush blueberries (Vaccinium corymbosum L.) during postharvest cold storage. Sci. Hortic. 2019, 246, 557–562. [Google Scholar] [CrossRef]
- Posé, S.; Kirby, A.R.; Paniagua, C.; Waldron, K.W.; Morris, V.J.; Quesada, M.A.; Mercado, J.A. The nanostructural characterization of strawberry pectins in pectate lyase or polygalacturonase silenced fruits elucidates their role in softening. Carbohydr. Polym. 2015, 132, 134–145. [Google Scholar] [CrossRef]
- Bose, S.K.; Howlader, P.; Xiaochen, J.; Wenxia, W.; Heng, Y. Alginate oligosaccharide postharvest treatment preserve fruit quality and increase storage life via abscisic acid signaling in strawberry. Food Chem. 2019, 283, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008, 43, 951–957. [Google Scholar] [CrossRef]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible Coatings to Improve Storability and Enhance Nutritional Value of Fresh and Frozen Strawberries (Fragaria x ananassa) and Raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- García-Alonso, M.; Rimbach, G.; Rivas-Gonzalo, J.C.; De Pascual-Teresa, S. Antioxidant and cellular activities of anthocyanins and their corresponding vitisins a-studies in platelets, monocytes, and human endothelial cells. J. Agric. Food Chem. 2004, 52, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- López-Ortiz, A.; Méndez-Lagunas, L.L.; Delesma, C.; Longoria, A.; Escobar, J.; Muñiz, J. Understanding the drying kinetics of phenolic compounds in strawberries: An experimental and density functional theory study. Innov. Food Sci. Emerg. Technol. 2019, 60, 102283. [Google Scholar] [CrossRef]
- Goula, A.M.; Tzika, A.; Adamopoulos, K.G. Kinetic models of evaporation and total phenolics degradation during pomegranate juice concentration. Int. J. Food Eng. 2014, 10, 383–392. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Cardoso, J.C.; Rubio-Senent, F.; Serrano, A.; Borja, R.; Fernández-Bolaños, J.; Fermoso, F.G. Thermally-treated strawberry extrudate: A rich source of antioxidant phenols and sugars. Innov. Food Sci. Emerg. Technol. 2019, 51, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Color and stability of anthocyanins in strawberry nectars containing various co-pigment sources and sweeteners. Food Chem. 2019, 310, 125856. [Google Scholar] [CrossRef]
- Cassani, L.; Gerbino, E.; del Moreira, M.R.; Gómez-Zavaglia, A. Influence of non-thermal processing and storage conditions on the release of health-related compounds after in vitro gastrointestinal digestion of fiber-enriched strawberry juices. J. Funct. Foods 2018, 40, 128–136. [Google Scholar] [CrossRef]
- Balasooriya, H.; Dasanayake, K.; Ajlouni, S. Bioaccessibility of micronutrients in fresh and frozen strawberry fruits grown under elevated carbon dioxide and temperature. Food Chem. 2020, 309, 125662. [Google Scholar] [CrossRef] [PubMed]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Agius, F.; González-Lamothe, R.; Caballero, J.L.; Muñoz-Blanco, J.; Botella, M.A.; Valpuesta, V. Engineering increased vitamin c levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef]
- Davey, M.W.; Van Montagu, M.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.F.F.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Boulet, J.C.; Williams, P.; Doco, T. A Fourier transform infrared spectroscopy study of wine polysaccharides. Carbohydr. Polym. 2007, 69, 79–85. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Chylinska, M.; Kruk, B.; Zdunek, A. Combining FT-IR spectroscopy and multivariate analysis for qualitative and quantitative analysis of the cell wall composition changes during apples development. Carbohydr. Polym. 2015, 115, 93–103. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Kruk, B.; Zdunek, A. Study on dietary fibre by Fourier transform-infrared spectroscopy and chemometric methods. Food Chem. 2016, 196, 114–122. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A.; Kac, M. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Jouini, M.; Abdelhamid, A.; Chaouch, M.A.; le Cerf, D.; Bouraoui, A.; Majdoub, H.; Ben Jannet, H. Physico-chemical characterization and pharmacological activities of polysaccharides from Opuntia microdasys var. rufida cladodes. Int. J. Biol. Macromol. 2018, 107, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Jiang, G.; Shen, G.; Wu, H.; Liu, Y.; Zhang, Z. Optimization, characterization and rheological behavior study of pectin extracted from chayote (Sechium edule) using ultrasound assisted method. Int. J. Biol. Macromol. 2020, 147, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic property in pharmaceutical formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Nazaruddin, R.; Noor Baiti, A.A.; Foo, S.C.; Tan, Y.N.; Ayob, M.K. Comparative chemical characteristics of hydrochloric acid- and ammonium oxalate-extracted pectin from roselle (Hibiscus sabdariffa L.) calyces. Int. Food Res. J. 2013, 20, 281–284. [Google Scholar]
- Manconi, M.; Aparicio, J.; Vila, A.O.; Pendás, J.; Figueruelo, J.; Molina, F. Viscoelastic properties of concentrated dispersions in water of soy lecithin. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 222, 141–145. [Google Scholar] [CrossRef]
- Manconi, M.; Aparicio, J.; Seyler, D.; Vila, A.O.; Figueruelo, J.; Molina, F. Effect of several electrolytes on the rheopectic behaviour of concentrated soy lecithin dispersions. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 270, 102–106. [Google Scholar] [CrossRef]
Sample Availability: Samples of the fruits and pectin are available from the authors. |



| Organic Cultivation on Raised Beds | Organic-Cultivation–Flat-Planting Method | Conventional-Cultivation–Flat-Planting Method |
|---|---|---|
| Leżachów Osada (N 50.12972, E 022.62469) | Rozbórz (N 50.0548563, E 022.5451595) | Jawornik Polski (N 49.95751 E 022.54519) |
| Soil preparations: Polyversum WP (Bio Agris, Poland) (150 g ha−1) Amylo-X (Certis, UK) (2.5 kg ha−1) Vaxiplant (Galvet, Poland) (1 L ha−1) Serenade ASO (Bayer, UK) (8 L ha−1) Beta-Chikol (Poli-Farm Company, Poland) (35 L ha−1) Wetcit (BICONT, Poland) (10 L ha−1) VitiSan (Biofa AG, Germany) (16 L ha−1; main component is KHCO3 in dose 19 g ha−1) Lecitec (Target S.A., Poland) (16 L ha−1) Megis (ICBpharma, Poland) (3.5 L ha−1; main component is Si in dose 0.04 g ha−1) EmFarma Plus (ProBiotics, Poland) 920 L ha−1) Humus 1 (Agrecol, Poland) (20 L ha−1) biFosfor (Agrarius, Poland) (1 kg ha−1) Biprotect (AEF Global INC, Canada) (1 kg ha−1) Pest control: Naturalis L (Fargro, UK) (2 L ha−1) Wetcit (Biocont, Poland) (3.5 L ha−1) Wetcit(Biocont, Poland) (14 L ha−1) Microcat Flic (Vitera, Suwałki, Poland) (14 L ha−1; main component is K2O in dose 0.8 g ha−1) Microcat Bon (Vitera, Suwałki, Poland) (0.15 L ha−1; main component is P2O5 in dose 0.12 g ha−1) | Soil preparations: Condit (JSC ‘JuknevičiausKompostas’, Vilnius, Lithuania) (2 t ha−1; main component is N in dose 20 kg ha−1) Potassium sulfate (AMPOL-MEROL, Poland) (300 kg ha−1) Physio Natur PKS (TimacAgro, Romania) (1 t ha−1 main components are CaO in dose 0.28 t ha−1, P2O5 in dose 0.13 t ha−1, K2O in dose 0.15 t ha−1, MgO in dose 0.02 t ha−1 and SO3 in dose 0.19 t ha−1) Foliar preparations: Mc Calcium (BiodevasLaboratoires, France) (1 L ha−1; main component is Ca in dose 0.07 g ha−1) Calcio Sprint (Martinez and Valdivieso, Chile) (6 L ha−1; main component is water-soluble CaO 0.5 g ha−1) Megafol (Valagro, USA) (2 L ha−1; main components are N ain dose 0.05 g ha−1 and K2O in dose 0.2 g ha−1) Olibio (Biodevas, Spain) (3 L ha−1; main components are Cu, Fe, Mn, Zn in dose 0.02 g ha−1) Activstart (BiodevasLaboratoires, France) (2 L ha−1) Ecovigor (TimacAgro, Spain) (5 L ha−1; main components are N in dose 0.2 g ha−1 and K2O in dose 0.3 g ha−1) | Soil preparations: PERLKA (Alz Chem, Germany) (300 kg ha−1; main components are N in dose 59 kg ha−1 and CaO in dose 150 kg ha−1) Captan (Bonide, New York, USA) (2.1 kg ha−1) Switch 62,5 WG (Syngenta, Basel, Switzerland) (0.75 kg ha−1) Signum 33 WG (Syngenta, Basel, Switzerland) (1 kg ha−1) Luna Sensation (Bayer, UK) (0.7 L ha−1) Amylo-X (Fitogest, Italy) (2.5 kg ha−1) Serenade Max (Bayer, UK) (8 L ha−1) Foliar preparations: Mc Calcium (BiodevasLaboratoires, France) (1 L ha−1; main component is Ca in dose 0.07 g ha−1) Megafol (Valagro, USA) (2 l ha−1; main components are N ain dose 0.05 g ha−1 and K2O in dose 0.17 g ha−1) Potassium phosphide (AMPOL-MEROL, Poland) (1.5 kg ha−1) Olibio (Biodevas, Spain) (2 L ha−1; main component are Cu, Fe, Mn, Zn in dose 0.01 g ha−1) Humus 1 (Agrecol, Poland) (10.5 L ha−1) Pest control: Mospilan 20 SP (SumiAgro, Durham, USA) (0.125 kg ha−1) SpinTor 240 EC (Corteva, Warsaw, Poland) (0.15 L ha) Microcat Ole (Vitera, Suwałki, Poland) (1.5 L ha−1; main component is K2O in dose 0.1 g ha−1) Microcat Flic (Vitera, Suwałki, Poland) (2 L ha−1; main component is K2O in dose 0.1 g ha−1) |
| Organic Cultivation on Raised Beds | Organic-Cultivation–Flat-Planting Method | Conventional-Cultivation–Flat-Planting Method | |
|---|---|---|---|
| Fresh weight [g] | 8.1 a ± 0.4 | 16.6 b ± 0.7 | 16.6 b ± 2.3 |
| Length [mm] | 26.5 a ± 2.7 | 34.5 b ± 3.2 | 36.9 c ± 3.1 |
| Width [mm] | 26.8 a ± 4.0 | 34.5 b ± 4.5 | 36.3 b ± 3.8 |
| T0 | T1 | T2 | T5 | T6 | T7 | T9 | T12 | ||
|---|---|---|---|---|---|---|---|---|---|
| Average acidity [%] | Organic cultivation on raised beds | 1.2 a ± 0.0 | 1.1 a ± 0.0 | 1.0 bd ± 0.0 | 0.8 bcde ± 0.0 | 0.8 bde ± 0.0 | 0.8 bd ± 0.0 | 0.9 bd ± 0.0 | 0.6 ce ± 0.0 |
| Organic-cultivation–flat-planting | 1.1 ab ± 0.0 | 0.9abd ± 0.0 | 0.9 abd± 0.0 | 0.6 cde ± 0.0 | 0.7 cde ±0.0 | 0.7 bd ± 0.0 | 0.7 bd ± 0.0 | 0.5 ce ± 0.0 | |
| Conventional-cultivation–flat-planting | 1.3 a ± 0.0 | 1.0 a ± 0.1 | 1.1 bd ± 0.0 | 0.7 bd ± 0.0 | 0.8 bd ± 0.0 | 0.8 bd ± 0.0 | 0.9 bd ± 0.0 | 0.7 e ± 0.0 | |
| pH [-log H+] | Organic cultivation on raised beds | 3.4 a ± 0.0 | 3.2 ab ± 0.1 | 3.3 abc± 0.1 | 3.2 ab ± 0.0 | 3.4 ac± 0.0 | 3.2 ab± 0.0 | 3.3 ab± 0.0 | 3.6 ad± 0.0 |
| Organic-cultivation– flat-planting | 3.4 ab ± 0.0 | 3.2 ac ± 0.0 | 3.4 a± 0.0 | 3.4 ad ± 0.1 | 3.4 ab ± 0.1 | 3.4 a ± 0.0 | 3.3 acd ± 0.0 | 3.8 ae ± 0.0 | |
| Conventional-cultivation–flat-planting | 3.3 ab ± 0.0 | 3.2 ad ± 0.0 | 3.2 ad ± 0.1 | 3.2 ad ± 0.0 | 3.4 abc ± 0.0 | 3.3 acd ± 0.0 | 3.3 ac ± 0.0 | 3.2 ad ± 0.1 | |
| Average soluble solid content [%] | Organic cultivation on raised beds | 6.8 a ± 0.0 | 6.3 ab± 0.0 | 6.6 a ± 0.0 | 6.7 a ± 0.0 | 6.3 a ± 0.0 | 6.7 a ± 0.0 | 6.4 a ± 0.0 | 5.3 c ± 0.0 |
| Organic-cultivation–flat-planting | 6.4 a ± 0.0 | 5.9 bc ± 0.0 | 5.5 c ± 0.0 | 5.3 c ± 0.0 | 5.7 c ± 0.0 | 6.4 a ± 0.0 | 6.3 a ± 0.0 | 4.7 d ± 0.1 | |
| Conventional-cultivation–flat-planting | 6.3 a ± 0.0 | 6.1 bc ± 0.0 | 6.2 a ± 0.0 | 5.7 bc ± 0.1 | 5.8 cb ± 0.0 | 5.7 c ± 0.0 | 6.7 a ± 0.0 | 5.7 c ± 0.0 | |
| Average anthocyanin content [mg 100 g−1 FW] | Organic cultivation on raised beds | 33.1 ab± 2.8 | 29.4 a ± 0.4 | 29.5 a ± 1.4 | 29.6 a ± 1.1 | 36.3 a ± 1.9 | 40.2 a ± 5.3 | 15.7 c ± 0.8 | 11.2 c ± 2.6 |
| Organic-cultivation–flat-planting | 34.0 ad± 4.0 | 30.0 a ± 1.7 | 32.5 a ± 4.0 | 37.2 a ± 1.5 | 32.9 bd ± 1.0 | 31.5 a ± 2.1 | 29.2 d ± 9.0 | 15.4 cb ± 0.8 | |
| Conventional-cultivation–flat-planting | 12.2 c ± 0.9 | 14.1 c ± 0.6 | 14.7 c ± 0.7 | 11.7 c ± 1.6 | 19.9 c ± 3.3 | 15.2 c ± 0.8 | 14.8 c ± 4.0 | 12.9 cabc ± 0.6 | |
| Average soluble phenolic content [mg 100 g−1 FW] | Organic cultivation on raised beds | 124.3 a ± 0.1 | 142.8 b ± 0.1 | 127.2 a ± 1.0 | 136.9 b ± 0.2 | 143.4 b ± 0.3 | 144.9 b ± 0.4 | 111.2 c ± 0.1 | 142.3 b± 0.2 |
| Organic-cultivation–flat-planting | 109.2 c ± 0.2 | 125.4 a ± 0.2 | 114.4 ac ± 0.6 | 132.7 ab ± 0.2 | 127.5 a ± 0.3 | 131.4 a ± 0.5 | 89.4 d ± 0.3 | 130.3 a ± 0.8 | |
| Conventional-cultivation–flat-planting | 103.8 cd ± 0.2 | 119.1 ac ± 0.2 | 106.7 cd ± 0.4 | 129.2 a ± 0.6 | 95.9 d ± 0.1 | 123.1 a ± 0.2 | 104.7 cd ± 0.3 | 126.5 a ± 0.3 | |
| Average vitamin C content [mg 100 g−1 FW] | Organic cultivation on raised beds | 63.4 a ± 2.2 | nd | 62.3 ab ± 0.4 | nd | 37.4 c ± 0.5 | nd | 30.2 d ± 1.8 | nd |
| Organic-cultivation in–flat-planting | 59.9 ae± 0.6 | nd | 58.8 be ± 0.6 | nd | 38.6 c ± 0.6 | nd | 24.8 f ± 1.8 | nd | |
| Conventional-cultivation–flat-planting | 52.9 g ± 0.6 | nd | 65.3 a ± 1.2 | nd | 36.7 c ± 0.8 | nd | 25.5 f ± 2,8 | nd | |
| Galacturonic acid content [%] | Organic cultivation on raised beds | 53.6 a ± 1.3 | nd | nd | nd | nd | nd | nd | 40.4 c ± 1.3 |
| Organic-cultivation–flat-planting | 55.8 b ± 3.6 | nd | nd | nd | nd | nd | nd | 59.3 d ± 2.5 | |
| Conventional-cultivation–flat-planting | 53.3 a ± 3.2 | nd | nd | nd | nd | nd | nd | 55.2 ab ± 1.3 |
| Sample | Average Height [nm] | % of Branches in Total Skeleton Length | The Percentage of Pectin Molecules Characterized by Different Fibre Lengths (% of Total Fibre Length) | ||||
|---|---|---|---|---|---|---|---|
| Range of Fibre Length [nm] | |||||||
| 0–100 | 100–500 | 500–1000 | 1000–5000 | >5000 | |||
| Organic cultivation on raised beds T0 | 0.42 ± 0.28 c | 89.1 | 5.5 | 17.1 | 11.6 | 1.8 | 64.1 |
| Organic cultivation on raised beds T12 | 0.74 ± 0.37 d | 85.8 | 5.8 | 6.6 | 9.1 | 36.1 | 42.4 |
| Organic-cultivation–flat-planting T0 | 0.30 ± 0.16 ab | 66.4 | 10.1 | 48.0 | 21.3 | 6.7 | 13.9 |
| Organic-cultivation–flat-planting T12 | 0.47 ± 0.21 c | 55.8 | 6.5 | 32.0 | 14.1 | 39.1 | 8.3 |
| Conventional-cultivation–flat-planting T0 | 0.26 ± 0.02 a | 85.2 | 6.2 | 22.8 | 8.2 | 15.8 | 47.0 |
| Conventional-cultivation–flat-planting T12 | 0.33 ± 0.24 b | 80.8 | 5.8 | 21.6 | 13.7 | 22.5 | 36.4 |
| Sample | Viscosity [Pas] | Upward Curve | Downward Curve | ||||
|---|---|---|---|---|---|---|---|
| k | n | R2 | k | n | R2 | ||
| Organic cultivation on raised beds T0 | 0.40 a ± 0.01 | 0.75 a ± 0.04 | 0.81 a ± 0.01 | 0.997 | 0.88 a ± 0.09 | 0.79 a ± 0.01 | 0.997 |
| Organic cultivation on raised beds T12 | 0.03 b ± 0.01 | 0.29 b ± 0.09 | 0.57 b ± 0.02 | 0.979 | 0.35 ab ± 0.10 | 0.53 ±0.02 | 0.982 |
| Organic-cultivation–flat-planting T0 | 0.21 c ± 0.00 | 0.10 b ± 0.01 | 0.97 c ± 0.01 | 1.000 | 0.12 ab ± 0.01 | 0.95 c ± 0.01 | 1.000 |
| Organic-cultivation–flat-planting T12 | 0.07 b ± 0.01 | 0.05 b ± 0.00 | 0.99 c ± 0.01 | 1.000 | 0.05 b ± 0.01 | 0.98 c ± 0.01 | 1.000 |
| Conventional-cultivation–flat-planting T0 | 0.51 ad ± 0.03 | 2.67 c ± 0.26 | 0.68 d ± 0.01 | 0.995 | 2.92 c ± 0.38 | 0.67 d ± 0.01 | 0.995 |
| Conventional-cultivation–flat-planting T12 | 0.40 a ± 0.03 | 1.75 d ± 0.23 | 0.74 e ± 0.01 | 0.996 | 2.36 c ± 0.57 | 0.70 d ± 0.03 | 0.997 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drobek, M.; Frąc, M.; Zdunek, A.; Cybulska, J. The Effect of Cultivation Method of Strawberry (Fragaria x ananassa Duch.) cv. Honeoye on Structure and Degradation Dynamics of Pectin during Cold Storage. Molecules 2020, 25, 4325. https://doi.org/10.3390/molecules25184325
Drobek M, Frąc M, Zdunek A, Cybulska J. The Effect of Cultivation Method of Strawberry (Fragaria x ananassa Duch.) cv. Honeoye on Structure and Degradation Dynamics of Pectin during Cold Storage. Molecules. 2020; 25(18):4325. https://doi.org/10.3390/molecules25184325
Chicago/Turabian StyleDrobek, Magdalena, Magdalena Frąc, Artur Zdunek, and Justyna Cybulska. 2020. "The Effect of Cultivation Method of Strawberry (Fragaria x ananassa Duch.) cv. Honeoye on Structure and Degradation Dynamics of Pectin during Cold Storage" Molecules 25, no. 18: 4325. https://doi.org/10.3390/molecules25184325
APA StyleDrobek, M., Frąc, M., Zdunek, A., & Cybulska, J. (2020). The Effect of Cultivation Method of Strawberry (Fragaria x ananassa Duch.) cv. Honeoye on Structure and Degradation Dynamics of Pectin during Cold Storage. Molecules, 25(18), 4325. https://doi.org/10.3390/molecules25184325








