A Total of Eight Novel Steroidal Glycosides Based on Spirostan, Furostan, Pseudofurostan, and Cholestane from the Leaves of Cestrum newellii
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Structural Characterization
3.5. Evaluation of Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Begum, A.S.; Goyal, Y. Research and medicinal potential of the genus Cestrum (Solanaceae)—A review. Phcog. Rev. 2007, 1, 320–332. [Google Scholar]
- Ribeiro, P.R.V.; Araújo, A.J.; Costa-Lotufo, L.V.; Braz-Filho, R.; Nobre Junior, H.V.; Silva, C.R.; Neto, J.B.A.; Silveira, E.R.; Lima, M.A.S. Spirostanol glucosides from the leaves of Cestrum laevigatum L. Steroids 2016, 106, 35–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribeiro, P.R.V.; Braz-Filho, R.; Araújo, A.J.; Costa-Lotufo, L.V.; Souza, L.G.S.; Nobre Junior, H.V.; Silva, C.R.; Neto, J.B.A.; Silveira, E.R.; Lima, M.A.S. New epimeric spirostanol and furostanol-type steroidal saponins from Cestrum laevigatum L. J. Braz. Chem. Soc. 2016, 27, 2170–2180. [Google Scholar]
- Ta, C.A.K.; Guerrero-Analco, J.A.; Roberts, E.; Liu, R.; Mogg, C.D.; Saleem, A.; Otárola-Rojas, M.; Poveda, L.; Sanchez-Vindas, P.; Cal, V.; et al. Antifungal saponins from the maya medicinal plant Cestrum schlechtendahlii G. Don (Solanaceae). Phytother. Res. 2016, 30, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, E.M.; Mitaine-Offer, A.C.; Amaro-Luis, J.M.; Miyamoto, T.; Tanaka, C.; Pouységu, L.; Quideau, S.; Rojas, L.B.; Lacaille-Dubois, M.A. Steroidal saponins from the fruits of Cestrum ruizteranianum. Nat. Prod. Commun. 2016, 6, 1825–1826. [Google Scholar]
- Baqai, F.T.; Ali, A.; Ahmad, V.U. Two new spirostanol glycosides from Cestrum parqui. Helv. Chim. Acta 2001, 84, 3350–3355. [Google Scholar] [CrossRef]
- D’abrosca, B.; Dellagreca, M.; Fiorentino, A.; Monaco, P.; Natale, A.; Oriano, P.; Zarrelli, A. Structural characterization of phytotoxic terpenoids from Cestrum parqui. Phytochemistry 2005, 66, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Mosad, R.R.; Ali, M.H.; Ibrahim, M.T.; Shaaban, H.M.; Emara, M.; Wahba, A.E. New cytotoxic steroidal saponins from Cestrum parqui. Phytochem. Lett. 2017, 22, 167–173. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Baqai, F.T.; Ahmad, R. A tigogenin pentasaccharide from Cestrum diurnum. Phytochemistry 1993, 34, 511–515. [Google Scholar] [CrossRef]
- Fouad, M.A.; Mohamed, K.M.; Kamel, M.S.; Matsunami, K.; Otsuka, H. Cesdiurins I-III, steroidal saponins from Cestrum diurnum L. J. Nat. Med. 2008, 62, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, M.; Motidome, M.; Morita, H.; Takeya, K.; Itokawa, H.; Mimaki, Y.; Sashida, Y. New polyhydroxylated steroidal sapogenin and saponin from the leaves of Cestrum sendtenerianum. Chem. Pharm. Bull. 1999, 47, 582–584. [Google Scholar] [CrossRef][Green Version]
- Haraguchi, M.; Mimaki, Y.; Motidome, M.; Morita, H.; Takeya, K.; Itokawa, H.; Yokosuka, A.; Sashida, Y. Steroidal saponins from the leaves of Cestrum sendtenerianum. Phytochemistry 2000, 55, 715–720. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Baqai, F.T.; Fatima, I.; Ahmad, R. A spirostanol glycoside from Cestrum nocturnum. Phytochemistry 1991, 30, 3057–3061. [Google Scholar] [CrossRef]
- Mimaki, Y.; Watanabe, K.; Ando, Y.; Sakuma, C.; Sashida, Y.; Furuya, S.; Sakagami, H. Flavonol glycosides and steroidal saponins from the leaves of Cestrum nocturnum and their cytotoxicity. J. Nat. Prod. 2001, 64, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, Y.; Watanabe, K.; Sakagami, H.; Sashida, Y. Steroidal glycosides from the leaves of Cestrum nocturnum. J. Nat. Prod. 2002, 65, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kakiuchi, T.; Ebisawa, H.; Shiono, Y.; Nakamura, T.; Kai, T.; Ikeda, T.; Miyashita, H.; Yoshimitsu, H.; Nohara, T. Steroidal glycosides from the fruits of Solanum viarum. Chem. Pharm. Bull. 2009, 57, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Tsumagari, H.; Honbu, T.; Ikeda, T.; Ono, M.; Nohara, T. Peculiar steroidal saponins with opened E-ring from Solanum genera plants. Tetrahedron Lett. 2001, 42, 8043–8046. [Google Scholar] [CrossRef]
- Honbu, T.; Ikeda, T.; Zhu, X.H.; Yoshihara, O.; Okawa, M.; Nafady, A.M.; Nohara, T. New steroidal glycosides from the fruits of Solanum anguivi. J. Nat. Prod. 2002, 65, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
- Kametani, T.; Katoh, T.; Tsubuki, M.; Honda, T. A facile preparation of ecdysone side chain by utilizing furan derivative. Chem. Lett. 1985, 485–488. [Google Scholar] [CrossRef]
- Iguchi, T.; Kuroda, M.; Naito, R.; Watanabe, T.; Matsuo, Y.; Yokosuka, A.; Mimaki, Y. Cholestane glycosides from Ornithogalum saundersiae bulbs and the induction of apoptosis in HL-60 cells by OSW-1 through a mitochondrial-independent signaling pathway. J. Nat. Med. 2019, 73, 131–145. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
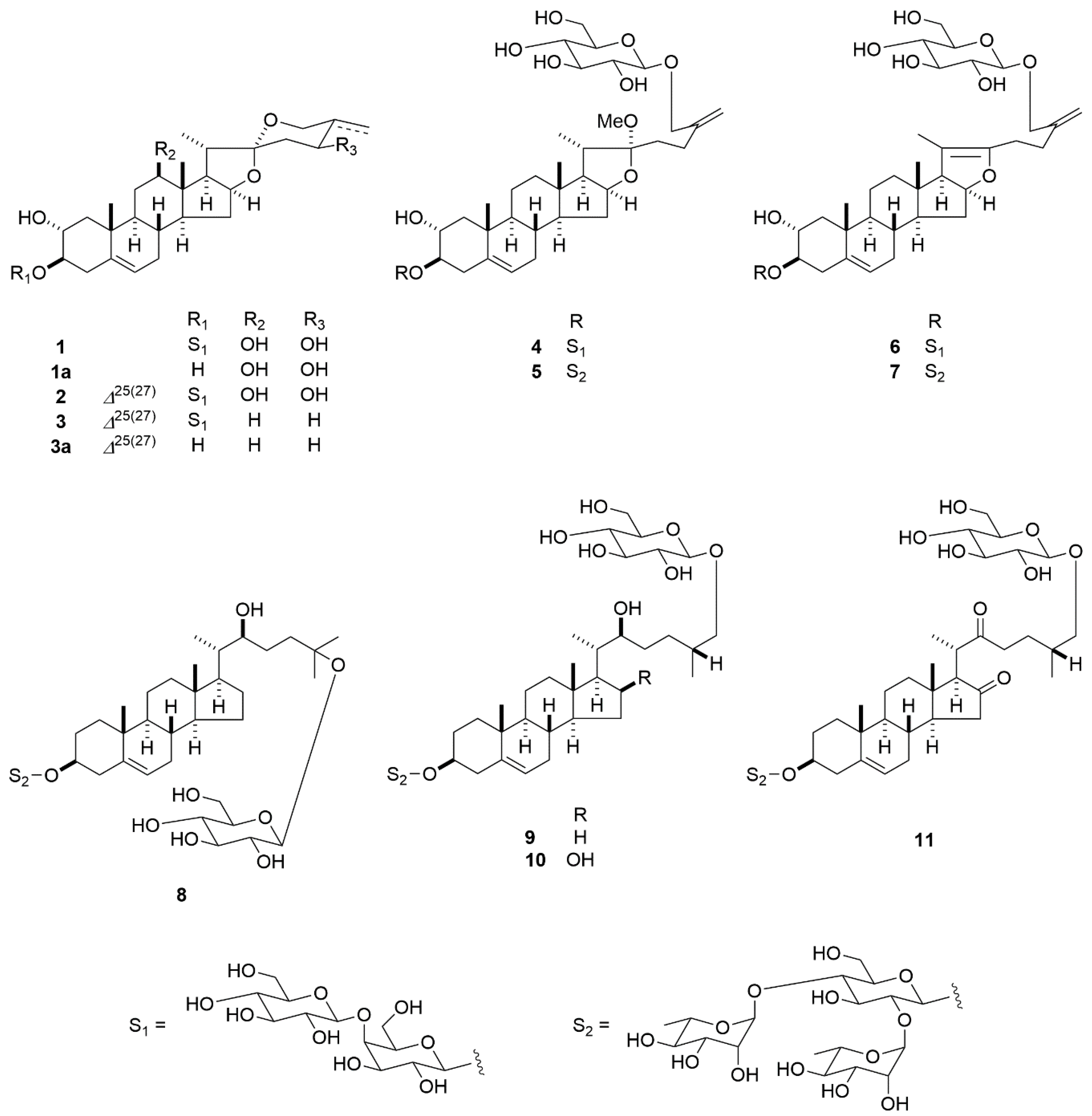
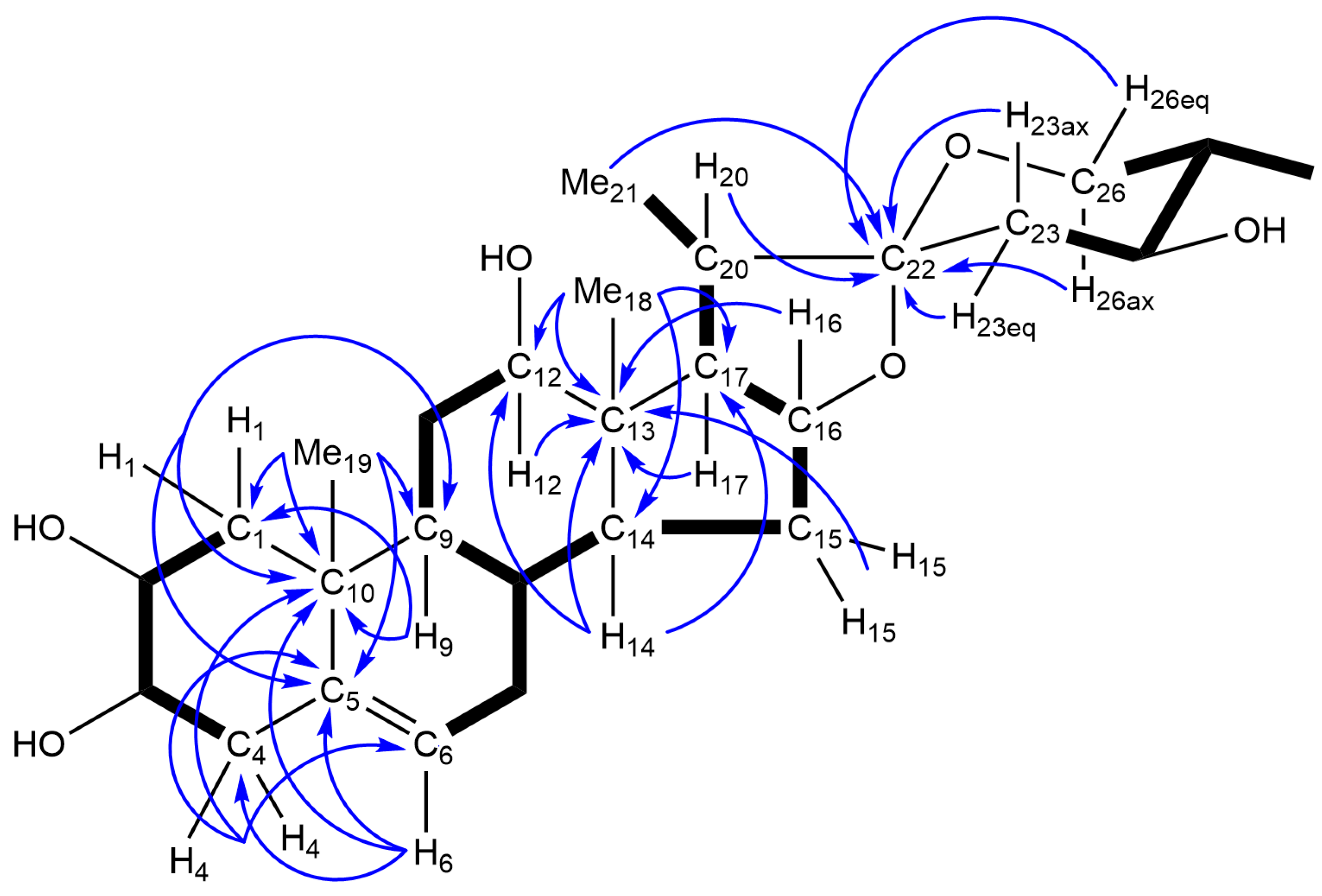
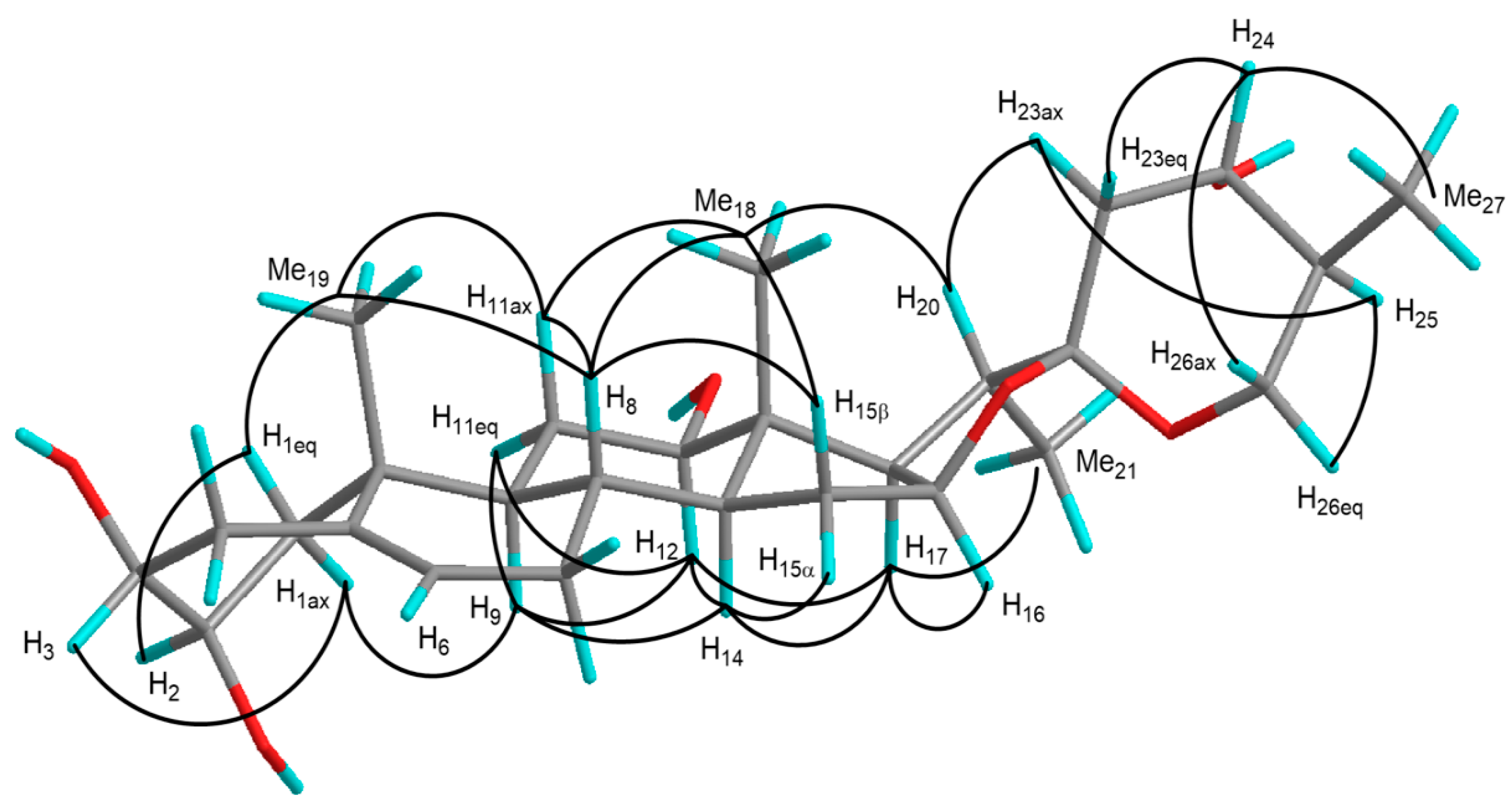

| Positions | 1 | 1a | 2 | 3 | 3a | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45.7 | 46.5 | 45.7 | 45.8 | 46.5 | 45.8 | 45.9 | 45.8 | 46.0 | 37.5 |
| 2 | 69.9 | 72.5 | 69.9 | 70.0 | 72.6 | 70.0 | 70.1 | 70.0 | 70.1 | 30.2 |
| 3 | 84.5 | 76.7 | 84.5 | 84.5 | 76.7 | 84.5 | 84.9 | 84.6 | 84.9 | 78.1 |
| 4 | 37.4 | 40.7 | 37.5 | 37.5 | 40.8 | 37.5 | 37.1 | 37.6 | 37.1 | 39.0 |
| 5 | 140.0 | 141.2 | 140.0 | 140.0 | 141.2 | 140.0 | 139.8 | 140.0 | 139.8 | 140.8 |
| 6 | 121.9 | 121.3 | 121.9 | 121.8 | 121.2 | 121.8 | 121.9 | 121.8 | 121.9 | 122.0 |
| 7 | 31.9 | 32.0 | 31.9 | 32.1 | 32.2 | 32.0 | 32.0 | 32.2 | 32.3 | 32.2 |
| 8 | 30.2 | 30.3 | 30.2 | 31.0 | 31.1 | 30.9 | 31.0 | 30.8 | 30.8 | 32.1 |
| 9 | 49.8 | 50.1 | 49.8 | 50.1 | 50.3 | 50.1 | 50.1 | 50.1 | 50.1 | 50.4 |
| 10 | 37.9 | 38.6 | 38.0 | 37.8 | 38.4 | 37.8 | 37.8 | 37.8 | 37.8 | 36.9 |
| 11 | 31.4 | 31.5 | 31.4 | 21.1 | 21.2 | 21.0 | 21.0 | 21.3 | 21.2 | 21.3 |
| 12 | 78.7 | 78.9 | 78.7 | 39.6 | 39.7 | 39.5 | 39.5 | 39.5 | 39.5 | 40.1 |
| 13 | 46.1 | 46.2 | 46.2 | 40.4 | 40.4 | 40.3 | 40.3 | 43.2 | 43.2 | 42.3 |
| 14 | 55.2 | 55.4 | 55.2 | 56.4 | 56.5 | 56.3 | 56.3 | 54.7 | 54.7 | 57.0 |
| 15 | 31.7 | 31.8 | 31.7 | 32.0 | 32.1 | 32.1 | 32.1 | 34.4 | 34.4 | 24.5 |
| 16 | 81.4 | 81.5 | 81.8 | 81.3 | 81.4 | 81.3 | 81.3 | 84.4 | 84.4 | 28.2 |
| 17 | 62.2 | 62.3 | 62.2 | 62.7 | 62.8 | 63.9 | 63.9 | 64.4 | 64.4 | 53.1 |
| 18 | 10.9 | 11.0 | 11.0 | 16.2 | 16.3 | 16.0 | 16.1 | 14.1 | 14.0 | 12.0 |
| 19 | 20.3 | 20.6 | 20.3 | 20.3 | 20.6 | 20.3 | 20.3 | 20.4 | 20.3 | 19.4 |
| 20 | 43.2 | 43.2 | 43.0 | 41.7 | 41.8 | 40.7 | 40.7 | 103.9 | 103.9 | 41.9 |
| 21 | 14.2 | 14.2 | 14.1 | 14.9 | 14.9 | 16.1 | 16.1 | 11.7 | 11.7 | 12.5 |
| 22 | 112.0 | 112.1 | 112.0 | 109.4 | 109.4 | 112.3 | 112.3 | 151.6 | 151.6 | 73.2 |
| 23 | 41.8 | 41.9 | 43.5 | 33.1 | 33.1 | 31.5 | 31.5 | 24.6 | 24.6 | 30.5 |
| 24 | 70.6 | 70.6 | 67.0 | 28.8 | 28.9 | 28.0 | 28.0 | 31.0 | 31.0 | 39.3 |
| 25 | 39.8 | 39.9 | 149.3 | 144.3 | 144.4 | 146.7 | 146.7 | 146.1 | 146.1 | 77.4 |
| 26 | 65.2 | 65.3 | 64.6 | 64.9 | 64.9 | 71.9 | 71.9 | 71.6 | 71.6 | 27.1 |
| 27 | 13.6 | 13.6 | 106.4 | 108.7 | 108.7 | 111.0 | 111.0 | 111.6 | 111.6 | 27.1 |
| OMe | 47.3 | 47.3 | ||||||||
| Gal | Gal | Gal | Gal | Glc (I) | Gal | Glc (I) | Glc (I) | |||
| 1′ | 103.2 | 103.3 | 103.3 | 103.3 | 100.9 | 103.4 | 100.9 | 100.2 | ||
| 2′ | 73.0 | 73.0 | 73.0 | 73.0 | 77.6 | 73.0 | 77.7 | 77.8 | ||
| 3′ | 75.0 | 75.1 | 75.1 | 75.0 | 77.6 | 75.1 | 77.7 | 77.9 | ||
| 4′ | 79.9 | 80.0 | 80.0 | 80.0 | 78.5 | 80.0 | 78.5 | 78.5 | ||
| 5′ | 75.8 | 75.9 | 75.9 | 75.8 | 76.9 | 75.9 | 77.0 | 76.9 | ||
| 6′ | 60.9 | 60.9 | 60.9 | 60.9 | 61.0 | 60.9 | 61.0 | 61.2 | ||
| Glc | Glc | Glc | Glc (I) | Rha (I) | Glc (I) | Rha (I) | Rha (I) | |||
| 1′′ | 106.9 | 106.9 | 106.9 | 106.9 | 101.9 | 107.0 | 101.9 | 102.0 | ||
| 2′′ | 75.7 | 75.7 | 75.7 | 75.7 | 72.2 | 75.7 | 72.3 | 72.5 | ||
| 3′′ | 78.5 | 78.6 | 78.6 | 78.6 | 72.7 | 78.6 | 72.7 | 72.8 | ||
| 4′′ | 72.0 | 72.1 | 72.0 | 72.0 | 73.9 | 72.1 | 74.0 | 74.1 | ||
| 5′′ | 78.3 | 78.4 | 78.4 | 78.3 | 69.4 | 78.4 | 69.5 | 69.5 | ||
| 6′′ | 62.8 | 62.9 | 62.9 | 62.9 | 18.5 | 63.0 | 18.5 | 18.6 | ||
| Glc (II) | Rha (II) | Glc (II) | Rha (II) | Rha (II) | ||||||
| 1′′′ | 103.7 | 102.7 | 103.7 | 102.7 | 102.8 | |||||
| 2′′′ | 75.0 | 72.3 | 75.1 | 72.4 | 72.4 | |||||
| 3′′′ | 78.5 | 72.6 | 78.5 | 72.6 | 72.7 | |||||
| 4′′′ | 71.6 | 73.8 | 71.6 | 73.8 | 73.9 | |||||
| 5′′′ | 78.4 | 70.3 | 78.4 | 70.3 | 70.4 | |||||
| 6′′′ | 62.7 | 18.4 | 62.7 | 18.4 | 18.5 | |||||
| Glc (II) | Glc (II) | Glc (II) | ||||||||
| 1′′′′ | 103.7 | 103.7 | 98.6 | |||||||
| 2′′′′ | 75.0 | 75.1 | 75.4 | |||||||
| 3′′′′ | 78.5 | 78.5 | 78.8 | |||||||
| 4′′′′ | 71.6 | 71.6 | 71.8 | |||||||
| 5′′′′ | 78.4 | 78.4 | 78.0 | |||||||
| 6′′′′ | 62.7 | 62.6 | 62.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iguchi, T.; Takahashi, N.; Mimaki, Y. A Total of Eight Novel Steroidal Glycosides Based on Spirostan, Furostan, Pseudofurostan, and Cholestane from the Leaves of Cestrum newellii. Molecules 2020, 25, 4462. https://doi.org/10.3390/molecules25194462
Iguchi T, Takahashi N, Mimaki Y. A Total of Eight Novel Steroidal Glycosides Based on Spirostan, Furostan, Pseudofurostan, and Cholestane from the Leaves of Cestrum newellii. Molecules. 2020; 25(19):4462. https://doi.org/10.3390/molecules25194462
Chicago/Turabian StyleIguchi, Tomoki, Naoki Takahashi, and Yoshihiro Mimaki. 2020. "A Total of Eight Novel Steroidal Glycosides Based on Spirostan, Furostan, Pseudofurostan, and Cholestane from the Leaves of Cestrum newellii" Molecules 25, no. 19: 4462. https://doi.org/10.3390/molecules25194462
APA StyleIguchi, T., Takahashi, N., & Mimaki, Y. (2020). A Total of Eight Novel Steroidal Glycosides Based on Spirostan, Furostan, Pseudofurostan, and Cholestane from the Leaves of Cestrum newellii. Molecules, 25(19), 4462. https://doi.org/10.3390/molecules25194462




