Detection Sensitivity Enhancement of Naphthalimide PET Fluorescent Probes by 4-Methoxy-Substitution
Abstract
:1. Introduction
2. Results and Discussion
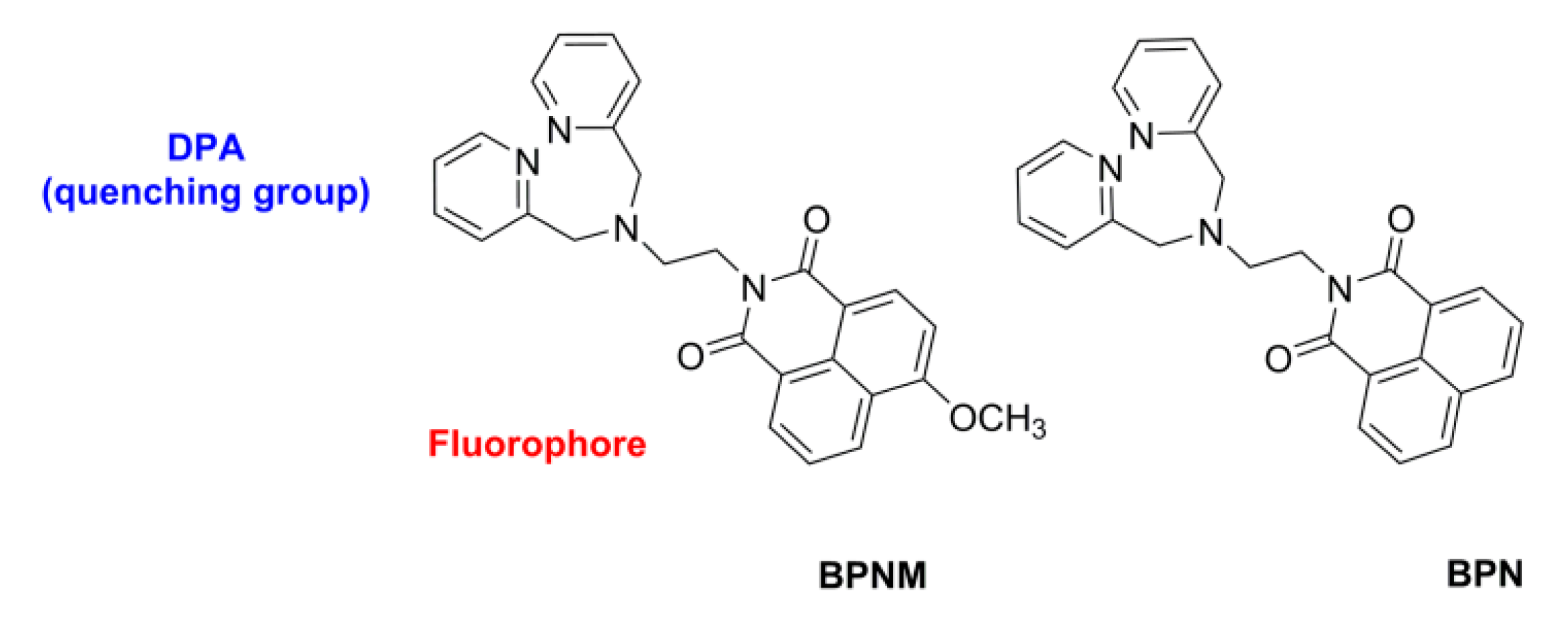
2.1. 4-Methoxy Substitution of Naphthalimide
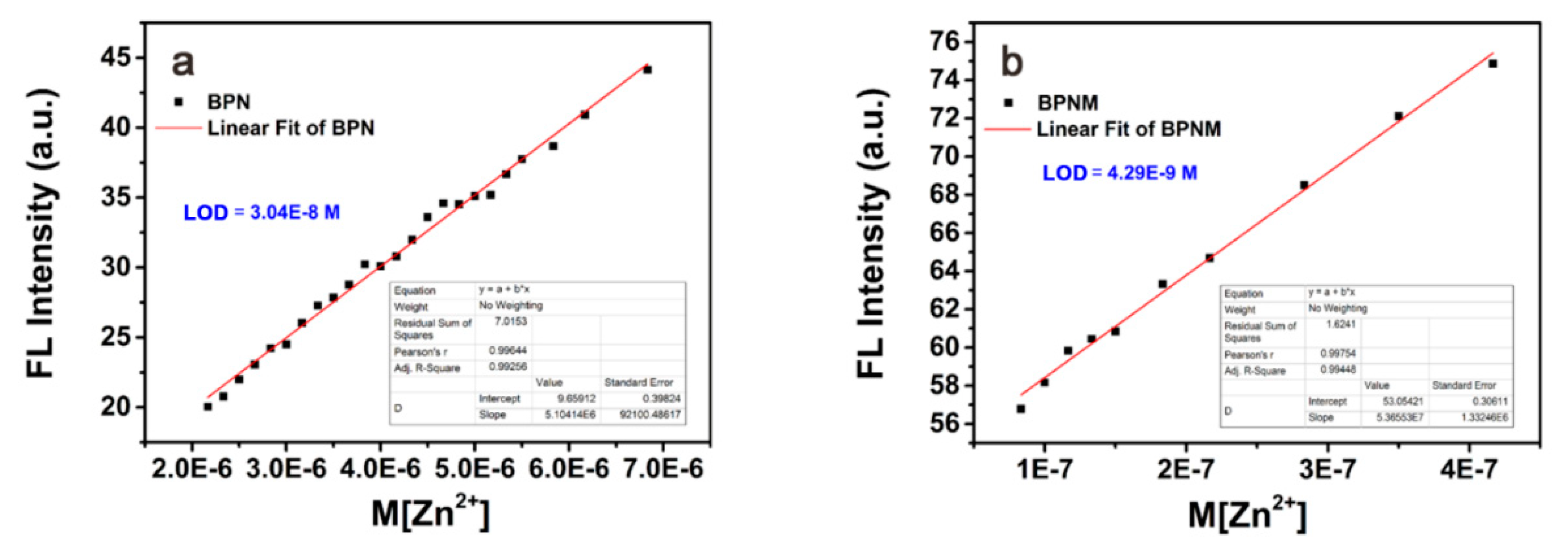
2.2. Zn2+ Titration Experiments of BPNM and BPN
2.3. Determination and Comparison of Limit of Detection (LOD) of BPNM and BPN Probes
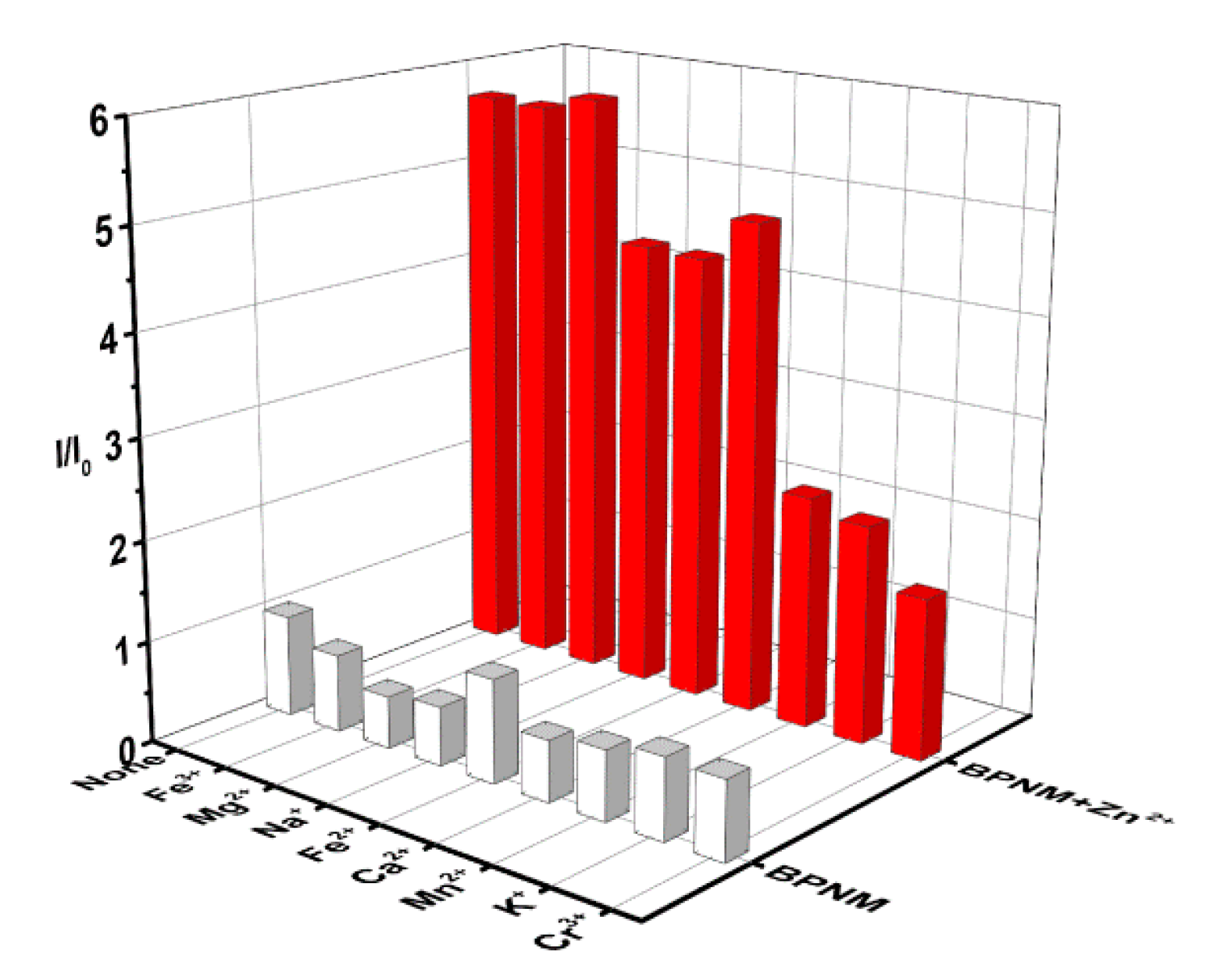
2.4. BPNM and BPN Ion Competitive Experiment
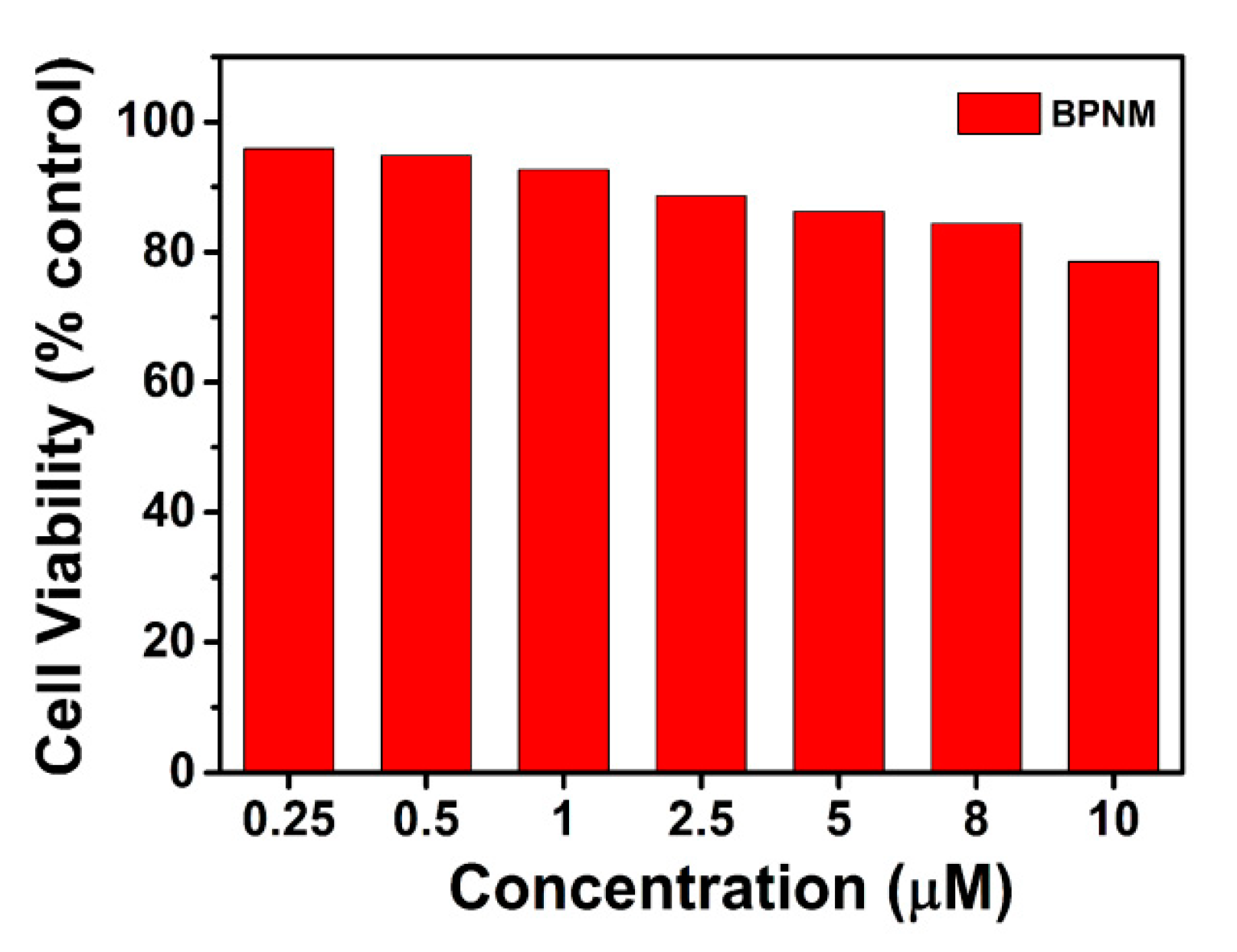
2.5. BPNM and BPN Cytotoxicity Analysis
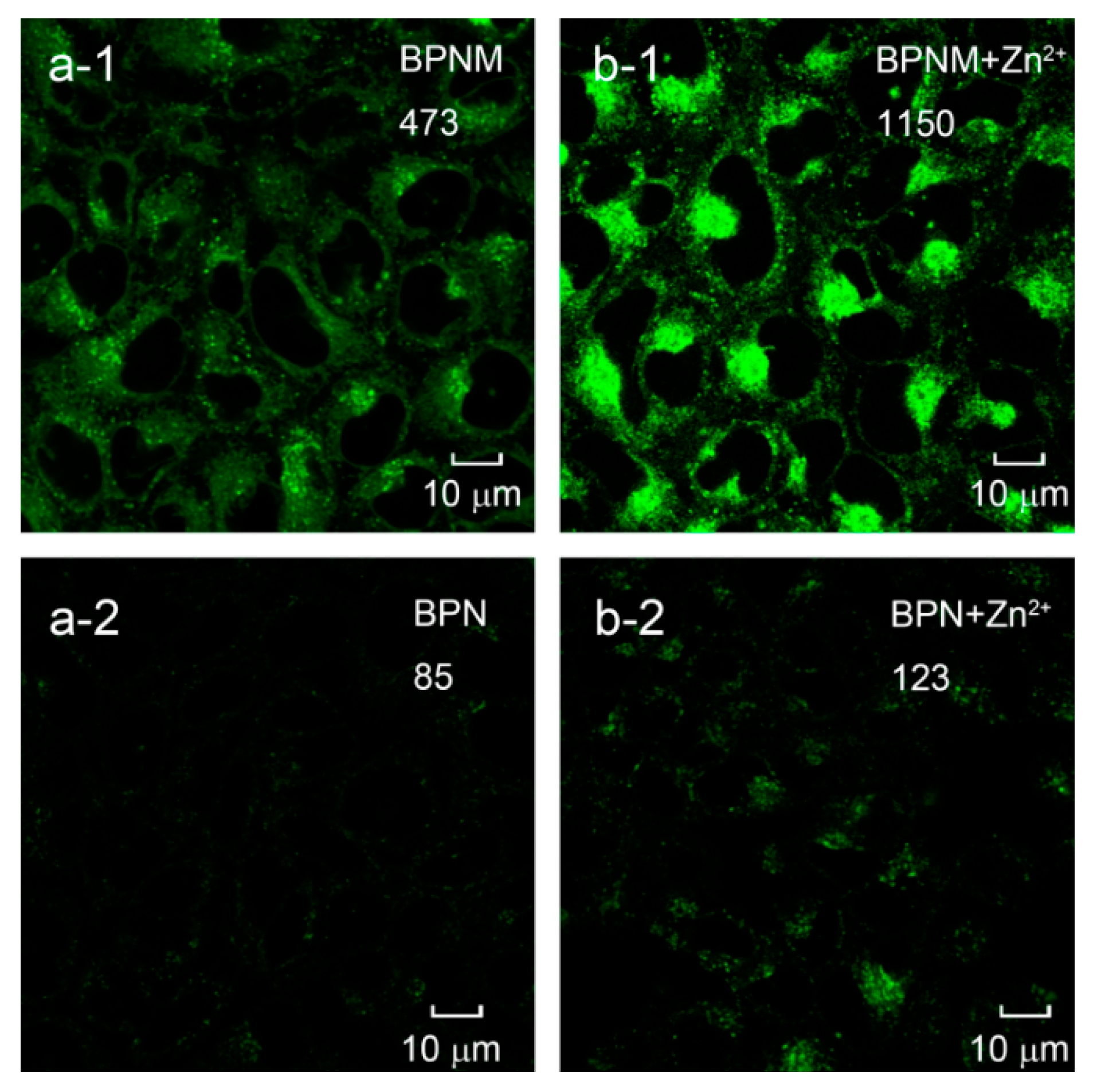
2.6. Confocal Laser Scanning Imaging of BPNM and BPN in Cells
3. Materials and Methods
3.1. Materials and Instrument
3.2. Methods
3.2.1. BPNM and BPN Synthesis Method
3.2.2. Fluorescence Quantum Yield Determination
3.2.3. Cell Culture and MTT Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- de Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Liu, W.M.; Ge, J.C.; Zhang, H.Y.; Wang, P.F. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ju, B.; Zhang, S.; Hou, L. Thermoresponsive cellulose ether and its flocculation behavior for organic dye removal. Carbohydr. Polym. 2016, 136, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.; Fu, M.; Chen, H.W.; Fang, M.Z. A Water-Soluble Rhodamine-Based Fluorescent Probe for Fe3+ and Its Application in Live Cell Imaging. Chin. J. Org. Chem. 2017, 37, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Lin, W.Y.; Zheng, K.B.; Zhu, S.S. FRET-Based Small-Molecule Fluorescent Probes: Rational Design and Bioimaging Applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef]
- Jia, X.T.; Chen, Q.Q.; Yang, Y.F.; Tang, Y.; Wang, R.; Xu, Y.F.; Zhu, W.P.; Qian, X.H. FRET-Based Mito-Specific Fluorescent Probe for Ratiometric Detection and Imaging of Endogenous Peroxynitrite: Dyad of Cy3 and Cy5. J. Am. Chem. Soc. 2016, 138, 10778–10781. [Google Scholar] [CrossRef]
- Fan, J.; Hu, M.; Zhan, P.; Peng, X. Energy transfer cassettes based on organic fluorophores: Construction and applications in ratiometric sensing. Chem. Soc. Rev. 2013, 42, 29–43. [Google Scholar] [CrossRef]
- Bozdemir, O.A.; Guliyev, R.; Buyukcakir, O.; Selcuk, S.; Kolemen, S.; Gulseren, G.; Nalbantoglu, T.; Boyaci, H.; Akkaya, E.U. Selective Manipulation of ICT and PET Processes in Styryl-Bodipy Derivatives: Applications in Molecular Logic and Fluorescence Sensing of Metal Ions. J. Am. Chem. Soc. 2010, 132, 8029–8036. [Google Scholar] [CrossRef]
- Yang, Z.G.; Cao, J.F.; He, Y.X.; Yang, J.H.; Kim, T.; Peng, X.J.; Kim, J.S. Macro-/micro-environment-sensitive chemosensing and biological imaging. Chem. Soc. Rev. 2014, 43, 4563–4601. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Z.; Lian, P.; Qian, J.; Li, X.; Wang, L.; Fu, W.; Chen, L.; Wei, X.; Li, C. Selective imaging and cancer cell death via pH switchable near-infrared fluorescence and photothermal effects. Chem. Sci. 2016, 7, 5995–6005. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.; Fan, J.L.; Xu, F.; Peng, X.J.; Mu, H.Y.; Wang, J.Y.; Xiong, X.Q. Ratiometric Fluorescence Imaging of Cellular Polarity: Decrease in Mitochondrial Polarity in Cancer Cells. Angew. Chem. Int. Ed. 2015, 54, 2510–2514. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Li, C.W.; Li, J.; Chen, B.; Guo, Y. Novel Coumarin-Based Fluorescent Probes for Detecting Fluoride Ions in Living Cells. Acta Chim. Sinica 2017, 75, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T. Design and synthesis of a library of BODIPY-based environmental polarity sensors utilizing photoinduced electron-transfer-controlled fluorescence ON/OFF switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Gabe, Y.; Urano, Y.; Kikuchi, K.; Kojima, H.; Nagano, T. Highly sensitive fluorescence probes for nitric oxide based on boron dipyrromethene chromophore-rational design of potentially useful bioimaging fluorescence probe. J. Am. Chem. Soc. 2004, 126, 3357–3367. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.L.; Wang, J.Y.; Zhang, S.Z.; Dou, B.R.; Peng, X.J. An Off-On COX-2-Specific Fluorescent Probe: Targeting the Golgi Apparatus of Cancer Cells. J. Am. Chem. Soc. 2013, 135, 11663–11669. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.L.; Wang, J.Y.; Dou, B.R.; Zhou, F.; Cao, J.F.; Qu, J.L.; Cao, Z.; Zhao, W.J.; Peng, X.J. Fluorescence Discrimination of Cancer from Inflammation by Molecular Response to COX-2 Enzymes. J. Am. Chem. Soc. 2013, 135, 17469–17475. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.L.; Wang, J.Y.; Mu, H.Y.; Peng, X.J. An “Enhanced PET”-Based Fluorescent Probe with Ultrasensitivity for Imaging Basal and Elesclomol-Induced HClO in Cancer Cells. J. Am. Chem. Soc. 2014, 136, 12820–12823. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Fan, J.; Pengg, X. Activity-Based Sensing and Theranostic Probes Based on Photoinduced Electron Transfer. Acc. Chem. Res. 2019, 52, 2818–2831. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, Y.; Jin, L. A Lysosome-Targetable and Two-Photon Fluorescent Probe for Monitoring Endogenous and Exogenous Nitric Oxide in Living Cells. J. Am. Chem. Soc. 2012, 134, 17486–17489. [Google Scholar] [CrossRef]
- Lee, M.H.; Han, J.H.; Lee, J.H.; Choi, H.G.; Kang, C.; Kim, J.S. Mitochondrial Thioredoxin-Responding Off-On Fluorescent Probe. J. Am. Chem. Soc. 2012, 134, 17314–17319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Duan, D.Z.; Liu, Y.P.; Ge, C.P.; Cui, X.M.; Sun, J.Y.; Fang, J.G. Highly Selective Off-On Fluorescent Probe for Imaging Thioredoxin Reductase in Living Cells. J. Am. Chem. Soc. 2014, 136, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.L.; Han, Z.C.; Kang, Y.; Peng, X.J. A Two-Photon Fluorescent Probe for Lysosomal Thiols in Live Cells and Tissues. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Thivierge, C.; Bandichhor, R.; Burgess, K. Spectral dispersion and water solubilization of BODIPY dyes via palladium-catalyzed C-H functionalization. Org. Lett. 2007, 9, 2135–2138. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Peng, X.J.; Guo, B.C.; Fan, J.L.; Zhang, Z.C.; Wang, J.Y.; Cui, A.J.; Gao, Y.L. Boron dipyrromethene fluorophore based fluorescence sensor for the selective imaging of Zn(II) in living cells. Org. Biomol. Chem. 2005, 3, 1387–1392. [Google Scholar] [CrossRef]
- Jaunet-Lahary, T.; Jacquemin, D.; Legouin, B.; Le Questel, J.Y.; Cupif, J.F.; Toupet, L.; Uriac, P.; Graton, J. Dissymmetric Molecular Tweezers in Host-Guest Complexes: Internal or External Complexation? J. Phys. Chem. C 2015, 119, 3771–3779. [Google Scholar] [CrossRef]
- Li, M.; Fan, J.L.; Du, J.J.; Cao, J.F.; Peng, X.J. Inhibiting proton interference in PET chemosensors by tuning the HOMO energy of fluorophores. Sens. Actuators B Chem. 2018, 259, 626–632. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| λabs (nm) 1 | λem (nm) 2 | Φf3 | Φf4 | |
|---|---|---|---|---|
| BPNM | 379 | 457 | 0.058 | 0.628 |
| BPN | 345 | 396 | 0.003 | 0.014 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, M.; Liu, Y. Detection Sensitivity Enhancement of Naphthalimide PET Fluorescent Probes by 4-Methoxy-Substitution. Molecules 2020, 25, 4465. https://doi.org/10.3390/molecules25194465
Tian Y, Li M, Liu Y. Detection Sensitivity Enhancement of Naphthalimide PET Fluorescent Probes by 4-Methoxy-Substitution. Molecules. 2020; 25(19):4465. https://doi.org/10.3390/molecules25194465
Chicago/Turabian StyleTian, Ye, Miao Li, and Ying Liu. 2020. "Detection Sensitivity Enhancement of Naphthalimide PET Fluorescent Probes by 4-Methoxy-Substitution" Molecules 25, no. 19: 4465. https://doi.org/10.3390/molecules25194465






