Bio-Based Nanoparticles as a Carrier of β-Carotene: Production, Characterisation and In Vitro Gastrointestinal Digestion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimisation of Nanoparticles (NPs) Production
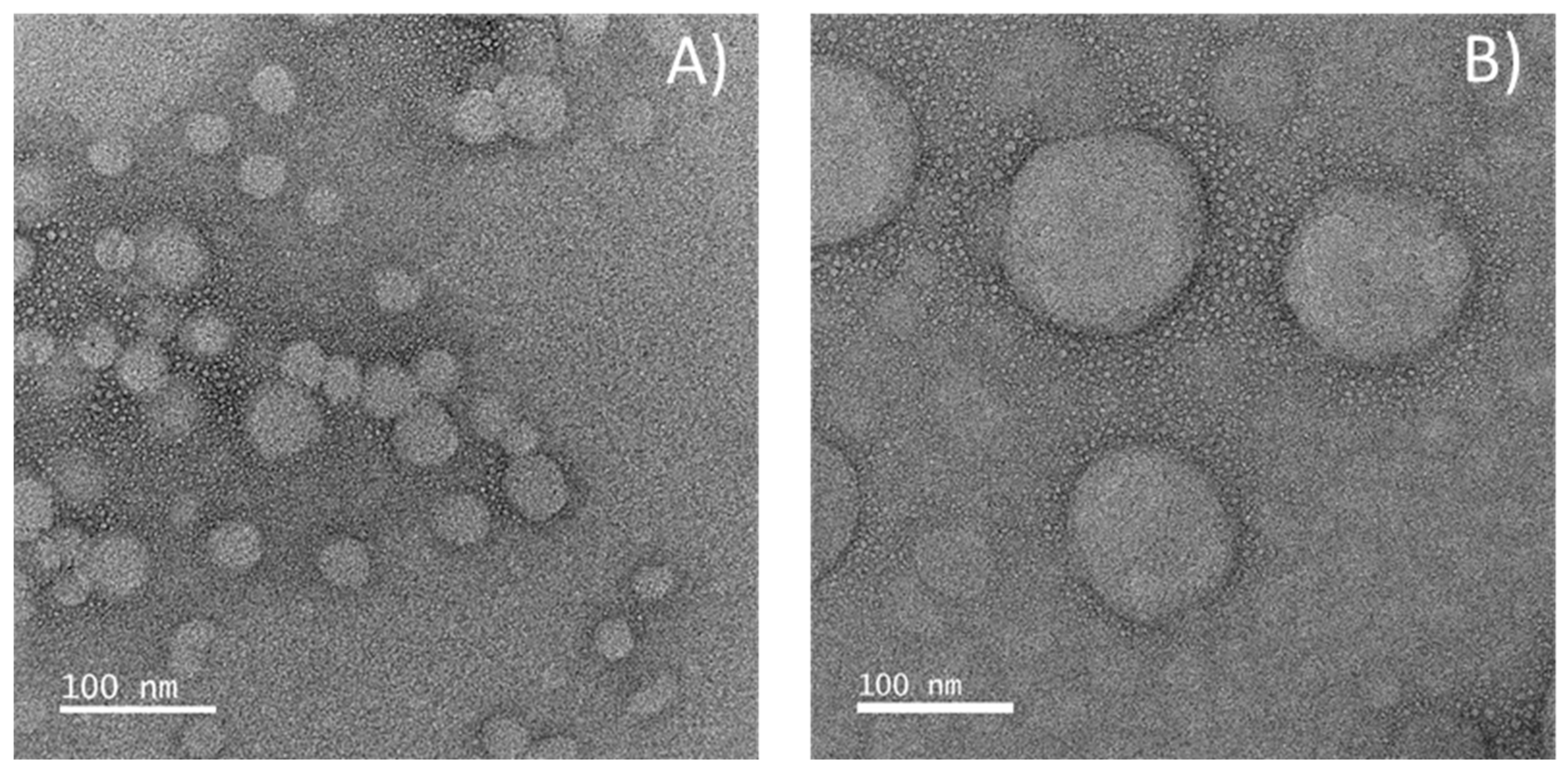
2.2. Encapsulation of β-Carotene
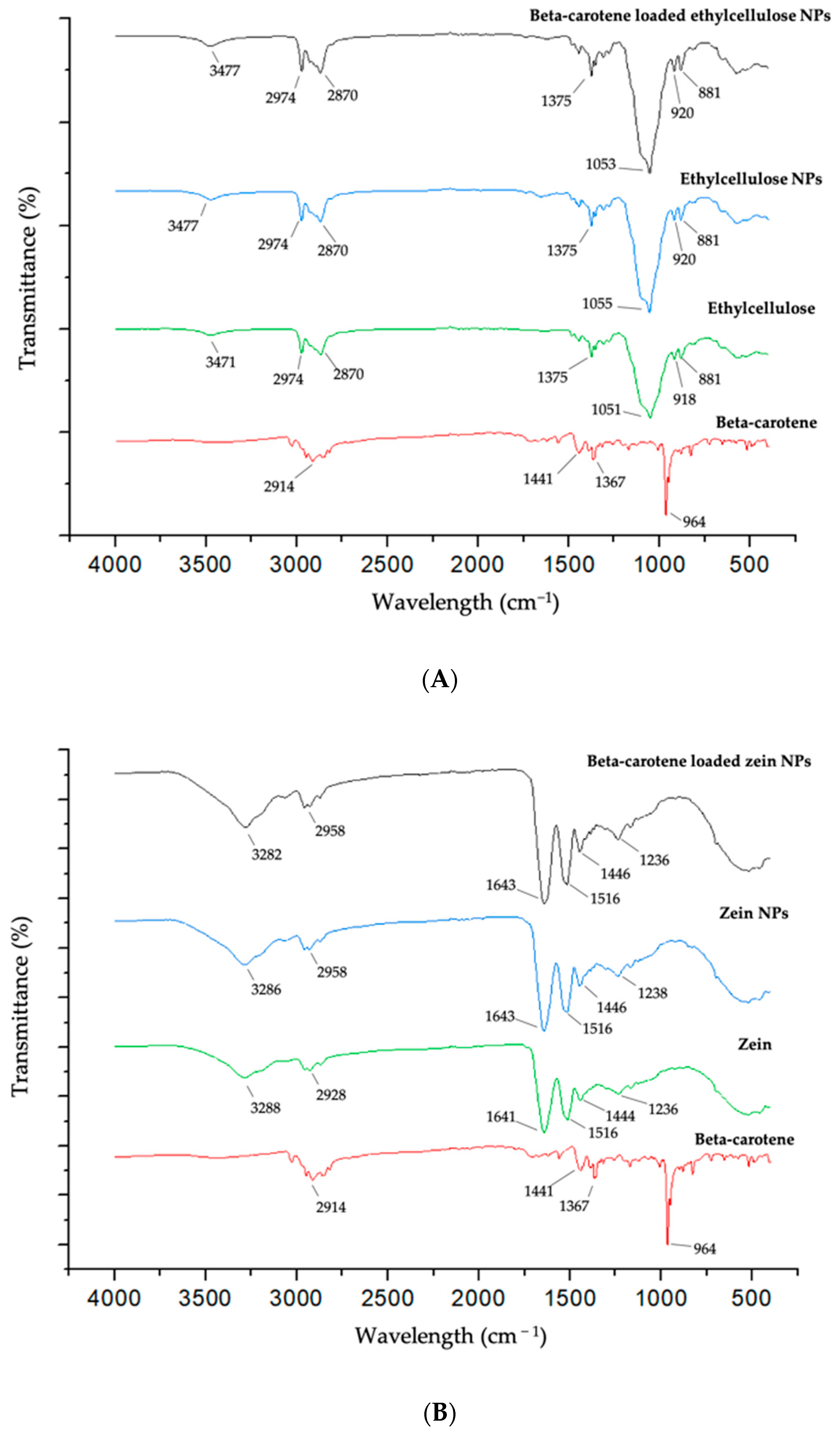
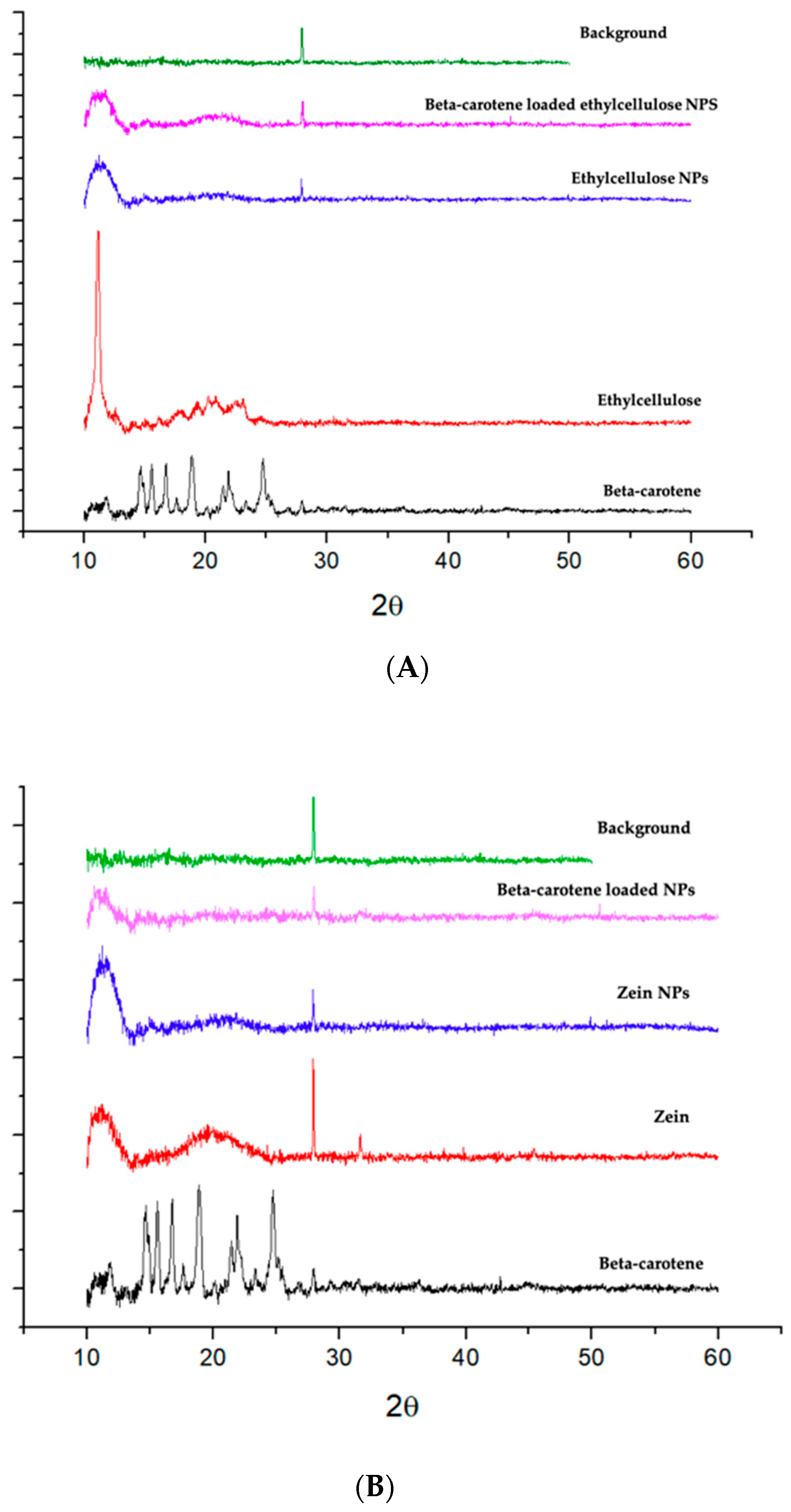
2.3. Fourier Transform Infrared Spectroscopy and X-ray Diffraction
2.4. In Vitro Gastrointestinal Digestion
3. Materials and Methods
3.1. Materials
3.2. Production of Nanoparticles (NPs)
3.3. Nanoparticle Characterisation
3.3.1. Particle Size, Polydispersity Index and Surface Potential
3.3.2. Encapsulation Efficiency
3.3.3. Transmission Electron Microscopy
3.3.4. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.5. X-ray Diffraction
3.4. Bioaccessibility In Vitro Gastrointestinal Digestion
3.4.1. In Vitro Digestion
3.4.2. β-Carotene Extraction
3.4.3. β-Carotene Quantification and Bioaccessibility
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martins, J.T.; Ramos, Ó.L.; Pinheiro, A.C.; Bourbon, A.I.; Silva, H.D.; Rivera, M.C.; Cerqueira, M.A.; Pastrana, L.; Malcata, F.X.; González-Fernández, Á.; et al. Edible Bio-Based Nanostructures: Delivery, Absorption and Potential Toxicity. Food Eng. Rev. 2015, 7, 491–513. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Pinheiro, A.C.; Silva, H.D.; Ramos, P.E.; Azevedo, M.A.; Flores-López, M.L.; Rivera, M.C.; Bourbon, A.I.; Ramos, Ó.L.; Vicente, A.A. Design of Bio-nanosystems for Oral Delivery of Functional Compounds. Food Eng. Rev. 2014, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Calderó, G.; Leitner, S.; García-Celma, M.J.; Solans, C. Modulating size and surface charge of ethylcellulose nanoparticles through the use of cationic nano-emulsion templates. Carbohydr. Polym. 2019, 225, 115201. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Rieger, J. Organic Nanoparticles in the Aqueous Phase—Theory, Experiment, and Use. Angew. Chem. Int. Ed. 2001, 40, 4330–4361. [Google Scholar] [CrossRef]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Particle Preparation to In Vivo Applications. In Polymer Nanoparticles for Nanomedicines; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–53. [Google Scholar] [CrossRef]
- Lepeltier, E.; Bourgaux, C.; Couvreur, P. Nanoprecipitation and the “Ouzo effect”: Application to drug delivery devices. Adv. Drug Deliv. Rev. 2014, 71, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Urbán-Morlán, Z.; Mendoza-Elvira, S.E.; Hernández-Cerón, R.S.; Alcalá-Alcalá, S.; Ramírez-Mendoza, H.; Ciprián-Carrasco, A.; Piñón-Segundo, E.; Quintanar-Guerrero, D. Preparation of ethyl cellulose nanoparticles by Solvent-Displacement using the conventional method and a recirculation system. J. Mex. Chem. Soc. 2015, 59, 173–180. [Google Scholar] [CrossRef]
- Avanço, G.B.; Braschi, M.L. Preparation and characterisation of ethylcellulose microparticles containing propolis. Rev. Ciências Farm. Básica Apl. 2008, 29, 129–135. [Google Scholar]
- Lai, H.L.; Pitt, K.; Craig, D.Q.M. Characterisation of the thermal properties of ethylcellulose using differential scanning and quasi-isothermal calorimetric approaches. Int. J. Pharm. 2010, 386, 178–184. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; ISBN 978-1-58212-135-2. [Google Scholar]
- Rekhi, G.S.; Jambhekar, S.S. Ethylcellulose-A Polymer Review. Drug Dev. Ind. Pharm. 1995, 21, 61–77. [Google Scholar] [CrossRef]
- Ahmadi, P.; Jahanban-Esfahlan, A.; Ahmadi, A.; Tabibiazar, M.; Mohammadifar, M. Development of Ethyl Cellulose-based Formulations: A Perspective on the Novel Technical Methods. Food Rev. Int. 2020, 1–48. [Google Scholar] [CrossRef]
- Murtaza, G. Ethylcellulose microparticles: A review. Acta Pol. Pharm. Drug Res. 2012, 69, 11–22. [Google Scholar]
- European Comission. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Commun. 2008, 50, 18. [Google Scholar]
- U.S. Food & Drug Administration. CFR-Code of Federal Regulations Title 21: Ethylcellulose. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.868 (accessed on 15 July 2020).
- Zhang, Y.; Cui, L.; Che, X.; Zhang, H.; Shi, N.; Li, C.; Chen, Y.; Kong, W. Zein-based films and their usage for controlled delivery: Origin, classes and current landscape. J. Control. Release 2015, 206, 206–219. [Google Scholar] [CrossRef]
- Li, F.; Chen, Y.; Liu, S.; Qi, J.; Wang, W.; Wang, C.; Zhong, R.; Chen, Z.; Li, X.; Guan, Y.; et al. Size-controlled fabrication of zein nano/microparticles by modified anti-solvent precipitation with/without sodium caseinate. Int. J. Nanomed. 2017, 12, 8197–8209. [Google Scholar] [CrossRef] [Green Version]
- Corradini, E.; Curti, P.; Meniqueti, A.; Martins, A.; Rubira, A.; Muniz, E. Recent Advances in Food-Packing, Pharmaceutical and Biomedical Applications of Zein and Zein-Based Materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [Green Version]
- Holding, D.R. Recent advances in the study of prolamin storage protein organization and function. Front. Plant Sci. 2014, 5, 276. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Sharma, G.; Kushwah, V.; Ghoshal, G.; Jain, A.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Βeta carotene-loaded zein nanoparticles to improve the biopharmaceutical attributes and to abolish the toxicity of methotrexate: A preclinical study for breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Sessa, D.J.; Eller, F.J.; Palmquist, D.E.; Lawton, J.W. Improved methods for decolorizing corn zein. Ind. Crop. Prod. 2003, 18, 55–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Li, F.; Shi, N.; Li, C.; Yu, X.; Chen, Y.; Kong, W. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int. J. Pharm. 2016, 513, 191–210. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. CFR-Code of Federal Regulations Title 21: Zein. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1984 (accessed on 16 September 2019).
- de Freitas Zômpero, R.H.; López-Rubio, A.; de Pinho, S.C.; Lagaron, J.M.; de la Torre, L.G. Hybrid encapsulation structures based on β-carotene-loaded nanoliposomes within electrospun fibers. Colloids Surf. B Biointerfaces 2015, 134, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.M.; Azevedo, M.A.; Teixeira, J.A.; Pastrana, L.M.; Gonçalves, C.; Cerqueira, M.A. Food Applications of Nanotechnology, 1st ed.; Engineered Nanostructures for Enrichment and Fortification of Foods; CRC Press: Boca Raton, FL, USA, 2019; pp. 61–86. [Google Scholar]
- Albanes, D. β-Carotene and lung cancer: A case study. Am. J. Clin. Nutr. 1999, 69, 1345S–1350S. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, Y.; Chen, L. Intestinal uptake of barley protein-based nanoparticles for β-carotene delivery. Acta Pharm. Sin. B 2019, 9, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Perez-Masiá, R.; González-Barrio, R.; Periago, M.J.; López-Rubio, A. Potential of microencapsulation through emulsion-electrospraying to improve the bioaccesibility of β-carotene. Food Hydrocoll. 2017, 73, 1–12. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; McClements, D.J.; Gao, Y. Development of polyphenol-protein-polysaccharide ternary complexes as emulsifiers for nutraceutical emulsions: Impact on formation, stability, and bioaccessibility of β-carotene emulsions. Food Hydrocoll. 2016, 61, 578–588. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Micro- and nano-encapsulation of β-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Chow, S.F.; Wan, K.Y.; Cheng, K.K.; Wong, K.W.; Sun, C.C.; Baum, L.; Chow, A.H. Development of highly stabilized curcumin nanoparticles by flash nanoprecipitation and lyophilization. Eur. J. Pharm. Biopharm. 2015, 94, 436–449. [Google Scholar] [CrossRef]
- Thioune, O.; Briançon, S.; Devissaguet, J.P.; Fessi, H. Development of a new ethylcellulose pseudolatex for coating. Drug Dev. Res. 2000, 50, 157–162. [Google Scholar] [CrossRef]
- Kaur, K.; Kumar, P.; Kush, P. Amphotericin B loaded ethyl cellulose nanoparticles with magnified oral bioavailability for safe and effective treatment of fungal infection. Biomed. Pharmacother. 2020, 128, 110297. [Google Scholar] [CrossRef]
- Eltayeb, M.; Stride, E.; Edirisinghe, M. Preparation, characterization and release kinetics of ethylcellulose nanoparticles encapsulating ethylvanillin as a model functional component. J. Funct. Foods 2015, 14, 726–735. [Google Scholar] [CrossRef]
- Hayden, D.R.; Kibbelaar, H.V.M.; Imhof, A.; Velikov, K.P. Fully-biobased UV-absorbing nanoparticles from ethyl cellulose and zein for environmentally friendly photoprotection. RSC Adv. 2018, 8, 25104–25111. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-P.; Zhang, Y.-Y.; Yu, D.-G.; Wu, D.; Li, H.-L. Fabrication of sustained-release zein nanoparticles via modified coaxial electrospraying. Chem. Eng. J. 2018, 334, 807–816. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid–liquid dispersion method. LWT Food Sci. Technol. 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Cheng, C.J.; Ferruzzi, M.; Jones, O.G. Fate of lutein-containing zein nanoparticles following simulated gastric and intestinal digestion. Food Hydrocoll. 2019, 87, 229–236. [Google Scholar] [CrossRef]
- Ahmed, B.; Yusuf, M.; Khan, M.; Khan, R. Plausible antioxidant biomechanics and anticonvulsant pharmacological activity of brain-targeted β-carotene nanoparticles. Int. J. Nanomed. 2012, 7, 4311. [Google Scholar] [CrossRef] [Green Version]
- Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: Curcumin and caffeine as model compounds. J. Food Eng. 2016, 180, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.P.; Vaz, A.F.M.; Correia, M.T.S.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Quercetin-Loaded Lecithin/Chitosan Nanoparticles for Functional Food Applications. Food Bioprocess Technol. 2014, 7, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Fu, Y.; Chen, G.; Shi, Y.; Li, X.; Zhang, H.; Shen, Y. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Neha, T.; Shishir, T.; Ashutosh, D. Fourier transform infrared spectroscopy (FTIR) profiling of red pigment produced by Bacillus subtilis PD5. Afr. J. Biotechnol. 2017, 16, 1507–1512. [Google Scholar] [CrossRef] [Green Version]
- Hayden, D.R.; Kibbelaar, H.V.M.; Imhof, A.; Velikov, K.P. Size and Optically Tunable Ethyl Cellulose Nanoparticles as Carriers for Organic UV Filters. ChemNanoMat 2018, 4, 301–308. [Google Scholar] [CrossRef]
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. β-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40. [Google Scholar] [CrossRef]
- Guo, J.-X.; Gray, D.G. Preparation, characterization, and mesophase formation of esters of ethylcellulose and methylcellulose. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 889–896. [Google Scholar] [CrossRef]
- Khan, S.A.; Ahmad, M.; Aamir, M.N.; Murtaza, G.; Rasool, F.; Akhtar, M. Study of Nimesulide Release From Ethylcellulose Microparticles and Drug-Study of Nimesulide Release From Ethylcellulose Microparticles and Drug-Polymer Compatibility Analysis. Lat. Am. J. Pharm. 2010, 29, 554–561. [Google Scholar]
- Tao, J.; Chow, S.F.; Zheng, Y. Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Pharm. Sin. B 2019, 9, 4–18. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Wright, A.; Pietrangelo, C.; Macnaughton, A. Influence of simulated upper intestinal parameters on the efficiency of β carotene micellarisation using an in vitro model of digestion. Food Chem. 2007, 107, 1253–1260. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose–A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Yeom, D.W.; Kim, S.R.; Yoon, H.Y.; Kim, C.H.; Son, H.Y.; Kim, J.H.; Lee, S.; Choi, Y.W. Development of a chitosan based double layer-coated tablet as a platform for colon-specific drug delivery. Drug Des. Dev. Ther. 2016, 11, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, F.; Garekani, H.A.; Sadeghi, R. Comparison of ethylcellulose matrix characteristics prepared by solid dispersion technique or physical mixing. DARU J. Pharm. Sci. 2003, 11, 7–13. [Google Scholar]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef] [PubMed]
- Duodu, K.; Taylor, J.R.; Belton, P.; Hamaker, B. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Amado, I.R.; Vázquez, J.A. Mussel processing wastewater: A low-cost substrate for the production of astaxanthin by Xanthophyllomyces dendrorhous. Microb. Cell Fact. 2015, 14, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Liao, W.; Qi, X.; Yu, W.; Wu, J. Identification of immunomodulatory peptides from zein hydrolysates. Eur. Food Res. Technol. 2020, 246, 931–937. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of Carotenoids and Vitamin E from Their Main Dietary Sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Huo, T.; Ferruzzi, M.G.; Schwartz, S.J.; Failla, M.L. Impact of Fatty Acyl Composition and Quantity of Triglycerides on Bioaccessibility of Dietary Carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [Google Scholar] [CrossRef] [Green Version]
- Craft, N.E.; Soares, J.H. Relative solubility, stability, and absorptivity of lutein and β-carotene in organic solvents. J. Agric. Food Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Simões, L.S.; Abrunhosa, L.; Vicente, A.A.; Ramos, O.L. Suitability of β-lactoglobulin micro- and nanostructures for loading and release of bioactive compounds. Food Hydrocoll. 2020, 101, 105492. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |

| Sample | Average Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Ethylcellulose NPs | 69 ± 2ab | 0.18 ± 0.04a | −61 ± 2b |
| Loaded Ethylcellulose NPs * | 60 ± 9a | 0.27 ± 0.02b | −93 ± 3a |
| Zein NPs | 82 ± 7bc | 0.34 ± 0.05b | 64 ± 2c |
| Loaded Zein NPs * | 83 ± 8c | 0.29 ± 0.06b | 70.5 ± 0.7d |
| Sample | Bioaccessibility (%) | |
|---|---|---|
| Gastric Phase | Intestinal Phase | |
| Loaded ethylcellulose NPs | 2.7 ± 0.1 | 8.3 ± 0.1a |
| Loaded zein NPs | - * | 37 ± 1b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, B.S.; Azevedo, A.G.; Gonçalves, C.; Amado, I.R.; Ferreira, E.C.; Pastrana, L.M.; Cerqueira, M.A. Bio-Based Nanoparticles as a Carrier of β-Carotene: Production, Characterisation and In Vitro Gastrointestinal Digestion. Molecules 2020, 25, 4497. https://doi.org/10.3390/molecules25194497
Afonso BS, Azevedo AG, Gonçalves C, Amado IR, Ferreira EC, Pastrana LM, Cerqueira MA. Bio-Based Nanoparticles as a Carrier of β-Carotene: Production, Characterisation and In Vitro Gastrointestinal Digestion. Molecules. 2020; 25(19):4497. https://doi.org/10.3390/molecules25194497
Chicago/Turabian StyleAfonso, Beatriz S., Ana G. Azevedo, Catarina Gonçalves, Isabel R. Amado, Eugénio C. Ferreira, Lorenzo M. Pastrana, and Miguel A. Cerqueira. 2020. "Bio-Based Nanoparticles as a Carrier of β-Carotene: Production, Characterisation and In Vitro Gastrointestinal Digestion" Molecules 25, no. 19: 4497. https://doi.org/10.3390/molecules25194497
APA StyleAfonso, B. S., Azevedo, A. G., Gonçalves, C., Amado, I. R., Ferreira, E. C., Pastrana, L. M., & Cerqueira, M. A. (2020). Bio-Based Nanoparticles as a Carrier of β-Carotene: Production, Characterisation and In Vitro Gastrointestinal Digestion. Molecules, 25(19), 4497. https://doi.org/10.3390/molecules25194497










