Volatile Compounds and Physicochemical Quality of Four Jabuticabas (Plinia sp.)
Abstract
:1. Introduction
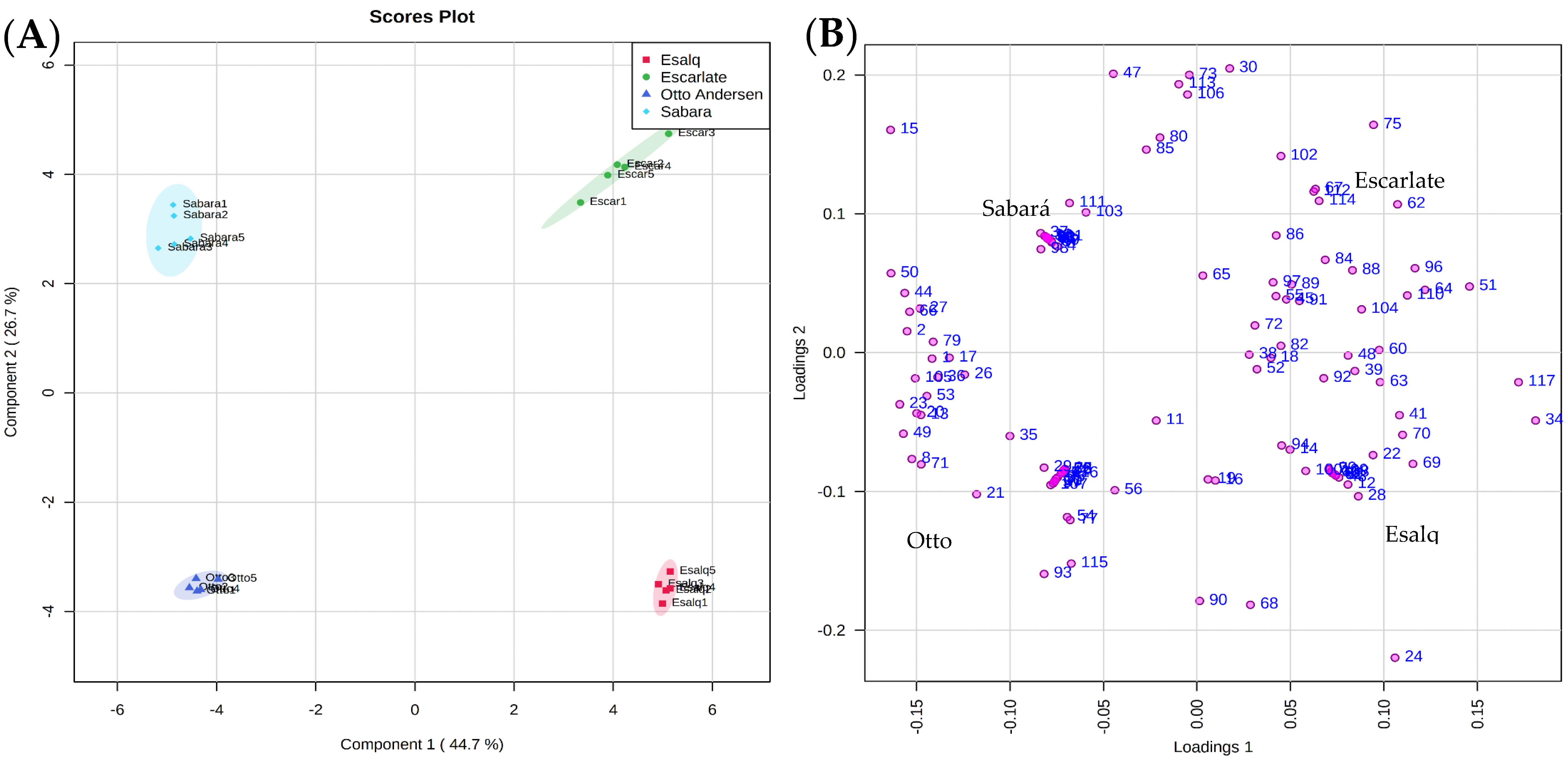
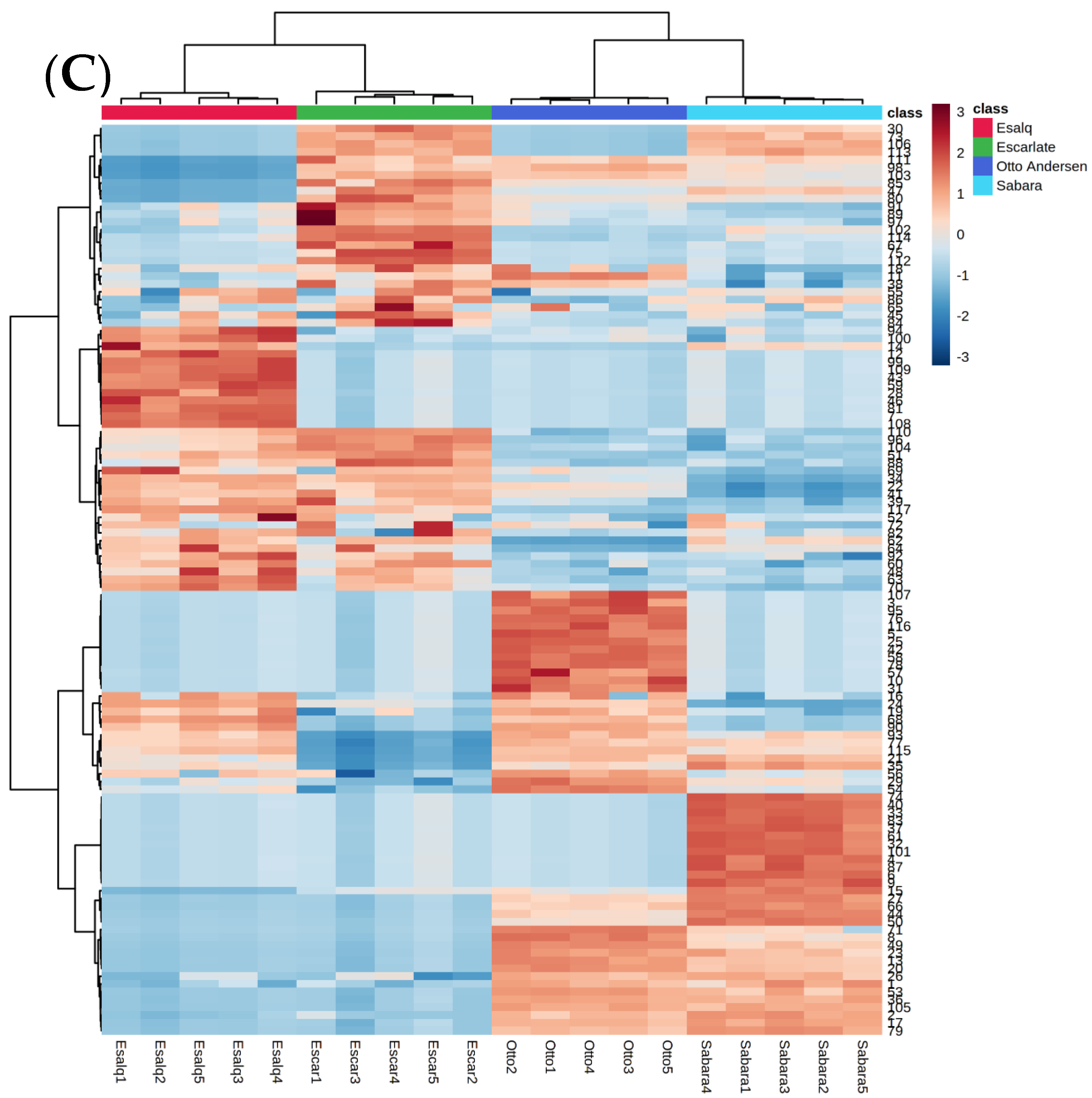
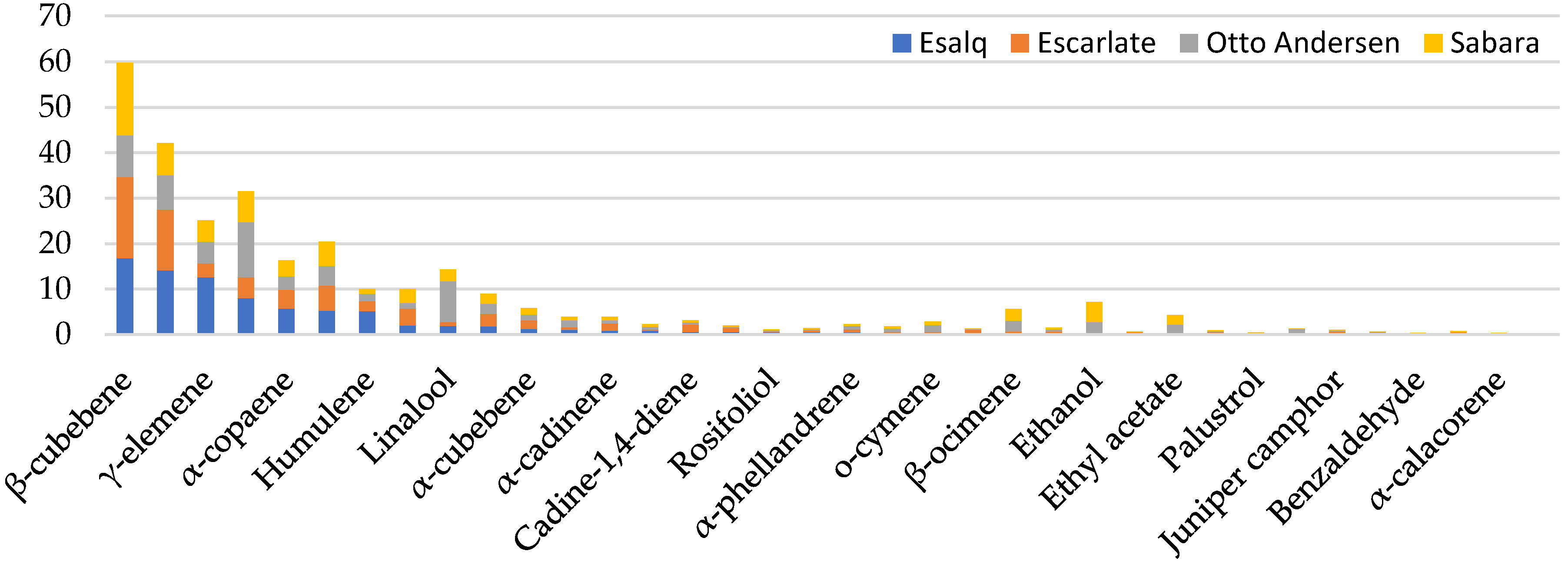
2. Results and Discussion
3. Material and Methods
3.1. Samples
3.2. Quality Parameters
3.3. Volatile Compound Determination
3.3.1. Sample Preparation
3.3.2. GC-MS Analysis
3.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Andrade Neves, N.; Stringheta, P.C.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Flavonols and ellagic acid derivatives in peels of different species of jabuticaba (Plinia spp.) identified by HPLC-DAD-ESI/MSn. Food Chem. 2018, 252, 61–71. [Google Scholar] [CrossRef]
- Suguino, E.; Heiffig, L.S.; Saavedra del Aguila, J.; Minami, K. Mirtáceas com Frutos Comestíveis do Estado de São Paulo: Conhecendo Algumas Plantas, 1st ed.; ESALQ: Piracicaba, Brazil, 2006; ISBN 14144530. [Google Scholar]
- Pereira, M.; de Oliveira, A.L.; Pereira, R.E.D.A.; Sena, J.A.D.; da Costa, J.R.V.; de Almeida, M.; Gonçalves, A.N. Morphologic and molecular characterization of Myrciaria spp species. Rev. Bras. Frutic. 2005, 27, 507–510. [Google Scholar] [CrossRef]
- Salomão, L.C.C.; de Siqueira, D.L.; Aquino, C.F.; de Lins, L.C.R. Jabuticaba—Myrciaria spp. Exot. Fruits 2018, 237–244. [Google Scholar] [CrossRef]
- Wu, S.B.; Long, C.; Kennelly, E.J. Phytochemistry and health benefits of jaboticaba, an emerging fruit crop from Brazil. Food Res. Int. 2013, 54, 148–159. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Potential dietary sources of ellagic acid and other antioxidants among fruits consumed in Brazil: Jabuticaba (Myrciaria jaboticaba (Vell.) Berg). J. Sci. Food Agric. 2012, 92, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.D.C.; Morim, M.P. Subtribos Eugeniinae O. Berg e Myrtinae O. Berg (Myrtaceae) na Restinga da Marambaia, RJ, Brasil. Acta Bot. Bras. 2008, 22, 652–683. [Google Scholar] [CrossRef]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Silva, A.P.G.; Spricigo, P.C.; Purgatto, E.; de Alencar, S.M.; Sartori, S.F.; Jacomino, A.P. Chemical composition, nutritional value and bioactive compounds in six uvaia accessions. Food Chem. 2019, 294, 547–556. [Google Scholar] [CrossRef]
- da Silva, A.P.G.; Spricigo, P.C.; Purgatto, E.; de Alencar, S.M.; Jacomino, A.P. Plinia trunciflora and Plinia cauliflora: Two species rich in bioactive compounds, terpenes, and minerals. J. Food Meas. Charact. 2019, 13, 921–931. [Google Scholar] [CrossRef]
- Rondán, G.; Cabezas, A.; Oliveira, A.; Brousett-Minaya, M.; Narain, N. HS-SPME-GC-MS detection of volatile compounds in Myrciaria jabuticaba Fruit. Sci. Agropecu. 2018, 9, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Zhang, J.; Lin, M.; Su, X.; Li, C.; Wang, H.; Li, B.; Chen, R.; Kang, J. Anti-inflammatory terpenes from Schefflera rubriflora C. J. Tseng & G. Hoo with their TNF-α and IL-6 inhibitory activities. Phytochemistry 2019, 163, 23–32. [Google Scholar] [CrossRef] [PubMed]
- de Lima, D.S.; Lima, J.C.; Calvacanti, R.M.C.B.; dos Santos, B.H.C.; Lima, I.O. Estudo da atividade antibacteriana dos monoterpenos timol e carvacrol contra cepas de Escherichia coli produtoras de β-lactamases de amplo espectro. Rev. Pan Amaz. Saúde 2017, 8, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Apel, M.A.; Sobral, M.; Zuanazzi, J.Å.; Henriques, A.T. Essential oil composition of fourPlinia species (Myrtaceae). Flavour Fragr. J. 2006, 21, 565–567. [Google Scholar] [CrossRef]
- Borges, L.L.; Conceição, E.C.; Silveira, D. Active compounds and medicinal properties of Myrciaria genus. Food Chem. 2014, 153, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Lago, J.H.G.; Souza, E.D.; Mariane, B.; Pascon, R.; Vallim, M.A.; Martins, R.C.C.; Baroli, A.A.; Carvalho, B.A.; Soares, M.G.; dos Santos, R.T.; et al. Chemical and Biological Evaluation of Essential Oils from Two Species of Myrtaceae—Eugenia uniflora L. and Plinia trunciflora (O. Berg) Kausel. Molecules 2011, 16, 9827–9837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasena, V.; Cameron, I. °Brix/Acid ratio as a predictor of consumer acceptability of Crimson seedless table grapes. J. Food Qual. 2008, 31, 736–750. [Google Scholar] [CrossRef]
- Pontes, M.; Marques, J.C.; Câmara, J.S. Headspace solid-phase microextraction-gas chromatography-quadrupole mass spectrometric methodology for the establishment of the volatile composition of Passiflora fruit species. Microchem. J. 2009, 93, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of Selected Aroma-Active Compounds in Strawberries by Headspace Solid-Phase Microextraction Gas Chromatography and Correlation with Sensory Descriptive Analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Bianchi, T.; Weesepoel, Y.; Koot, A.; Iglesias, I.; Eduardo, I.; Gratacós-Cubarsí, M.; Guerrero, L.; Hortós, M.; van Ruth, S. Investigation of the aroma of commercial peach (Prunus persica L. Batsch) types by Proton Transfer Reaction–Mass Spectrometry (PTR-MS) and sensory analysis. Food Res. Int. 2017, 99, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Xie, K.; Duan, W.; Zhu, Y.; Liu, M.; Chen, K.; Klee, H.; Zhang, B. Peach Carboxylesterase PpCXE1 Is Associated with Catabolism of Volatile Esters. J. Agric. Food Chem. 2019, 67, 5189–5196. [Google Scholar] [CrossRef]
- Lee, G.W.; Chung, M.S.; Lee, S.S.; Chung, B.Y.; Lee, S. Transcriptome-guided identification and functional characterization of key terpene synthases involved in constitutive and methyl jasmonate-inducible volatile terpene formation in Eremochloa ophiuroides (Munro)Hack. Plant Physiol. Biochem. 2019, 141, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Lan-Phi, N.T.; Shimamura, T.; Ukeda, H.; Sawamura, M. Chemical and aroma profiles of yuzu (Citrus junos) peel oils of different cultivars. Food Chem. 2009, 115, 1042–1047. [Google Scholar] [CrossRef] [Green Version]
- Rao, B.R.R.; Sastry, K.P.; Saleem, S.M.; Rao, E.V.S.P.; Syamasundar, K.V.; Ramesh, S. Volatile flower oils of three genotypes of rose-scented geranium (Pelargonium sp.). Flavour Fragr. J. 2000, 15, 105–107. [Google Scholar] [CrossRef]
- Jürgens, A.; Webber, A.C.; Gottsberger, G. Floral scent compounds of Amazonian Annonaceae species pollinated by small beetles and thrips. Phytochemistry 2000, 55, 551–558. [Google Scholar] [CrossRef]
- Jürgens, A.; Witt, T.; Gottsberger, G. Flower scent composition in night-flowering Silene species (caryophyllaceae). Biochem. Syst. Ecol. 2002, 30, 383–397. [Google Scholar] [CrossRef]
- Borges, R.M.; Bessière, J.M.; Ranganathan, Y. Diel Variation in Fig Volatiles Across Syconium Development: Making Sense of Scents. J. Chem. Ecol. 2013, 39, 630–642. [Google Scholar] [CrossRef]
- Grison-Pigé, L.; Hossaert-McKey, M.; Greeff, J.M.; Bessière, J.M. Fig volatile compounds—A first comparative study. Phytochemistry 2002, 61, 61–71. [Google Scholar] [CrossRef]
- da Silva Barbosa, D.C.; Holanda, V.N.; de Assis, C.R.D.; de Oliveira Farias de Aguiar, J.C.R.; do Nascimento, P.H.; da Silva, W.V.; do Amaral Ferraz Navarro, D.M.; da Silva, M.V.; de Menezes Lima, V.L.; dos Santos Correia, M.T. Chemical composition and acetylcholinesterase inhibitory potential, in silico, of Myrciaria floribunda (H. West ex Willd.) O. Berg fruit peel essential oil. Ind. Crops Prod. 2020, 151, 112372. [Google Scholar] [CrossRef]
- Ramos, M.F.D.S.; Monteiro, S.D.S.; Da Silva, V.P.; Nakamura, M.J.; Siani, A.C. Essential oils from myrtaceae species of the Brazilian Southeastern Maritime forest (Restinga). J. Essent. Oil Res. 2010, 22, 109–113. [Google Scholar] [CrossRef]
- Tucker, A.O.; Maciarello, M.J.; Landrum, L.R. Volatile leaf oils of Caribbean Myrtaceae. II. Pimenta dioica (L.) Merr. of Jamaica. J. Essent. Oil Res. 1991, 3, 195–196. [Google Scholar] [CrossRef]
- Lawal, O.; Oyedeji, A. Chemical Composition of the Essential Oils of Cyperus rotundus L. from South Africa. Molecules 2009, 14, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Vagionas, K.; Ngassapa, O.; Runyoro, D.; Graikou, K.; Gortzi, O.; Chinou, I. Chemical analysis of edible aromatic plants growing in Tanzania. Food Chem. 2007, 105, 1711–1717. [Google Scholar] [CrossRef]
- Costa, T.R.; Fernandes, O.F.L.; Santos, S.C.; Oliveira, C.M.A.; Lião, L.M.; Ferri, P.H.; Paula, J.R.; Ferreira, H.D.; Sales, B.H.N.; Silva, M.D.R.R. Antifungal activity of volatile constituents of Eugenia dysenterica leaf oil. J. Ethnopharmacol. 2000, 72, 111–117. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Ekundayo, O.; Olawore, O.N.; Adeniyi, B.A.; Koenig, W.A. Antimicrobial activity of the essential oils of five Eucalyptus species growing in Nigeria. Fitoterapia 1999, 70, 526–528. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Giordani, C.; Cappellacci, L.; Petrelli, R.; Canale, A. Insecticidal activity of two essential oils used in perfumery (ylang ylang and frankincense). Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Fortes, G.A.C.; Naves, S.S.; Godoi, F.F.F.; Duarte, A.R.; Ferri, P.H.; Santos, S.C. Assessment of a maturity index in jabuticaba fruit by the evaluation of phenolic compounds, essential oil components, sugar content and total acidity. Am. J. Food Technol. 2011, 6, 974–984. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Gutiérrez, A.; Granell, A. Comparative analysis of the volatile fraction of fruit juice from different citrus species. PLoS ONE 2011, 6, e22016. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.; Navaei, M.N.; Dini, M. Chemical composition of the oil ofCleome iberica DC. Flavour Fragr. J. 2005, 20, 434–435. [Google Scholar] [CrossRef]
- Alves, S.F.; Borges, L.L.; de Paula, J.A.M.; Vieira, R.F.; Ferri, P.H.; do Couto, R.O.; de Paula, J.R.; Bara, M.T.F. Chemical variability of the essential oils from fruits of Pterodon emarginatus in the Brazilian Cerrado. Braz. J. Pharmacogn. 2013, 23, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.H.; Yeh, S.Y.; Lin, M.Y.; Shih, M.C.; Yang, K.T.U.; Hwang, S.Y. Major chemotypes and antioxidative activity of the leaf essential oils of Cinnamomum osmophloeum Kaneh. From a clonal orchard. Food Chem. 2007, 105, 133–139. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Ding, X.; Jia, Y.C.; Huang, C.X.; Wang, Y.Z.; Xu, Y.H. Anti-tumor effect of β-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008, 264, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Z.; Yun-Ting, S.; Zeng, Z.; Zhan, X.; Li, C.; Xie, T. A review of medicinal plant species with elemene in China. Afr. J. Pharm. Pharmacol. 2012, 6, 3032–3040. [Google Scholar] [CrossRef] [Green Version]
- Sieniawska, E.; Sawicki, R.; Golus, J.; Swatko-Ossor, M.; Ginalska, G.; Skalicka-Wozniak, K. Nigella damascena L. essential oil-a valuable source of β-elemene for antimicrobial testing. Molecules 2018, 23, 256. [Google Scholar] [CrossRef] [Green Version]
- Sun, J. D-Limonene: Safety and Clinical Applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Mizrahi, B.; Shapira, L.; Domb, A.J.; Houri-Haddad, Y. Citrus Oil and MgCl 2 as Antibacterial and Anti-Inflammatory Agents. J. Periodontol. 2006, 77, 963–968. [Google Scholar] [CrossRef]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Machado Rocha, L.R.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem. Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Manuele, M.G.; Arcos Barreiro, M.L.; Davicino, R.; Ferraro, G.; Cremaschi, G.; Anesini, C. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Investig. 2010, 28, 135–145. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco. Targets. Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.K.; de Sousa Galvão, M.; Soares, A.C.; Nogueira, J.P.; Narain, N. Volatile constituents of jambolan (Syzygium cumini L.) fruits at three maturation stages and optimization of HS-SPME GC-MS method using a central composite design. Food Anal. Methods 2018, 11, 733–749. [Google Scholar] [CrossRef]
- Freitas, T.P.; Spricigo, P.C.; Purgatto, E.; Jacomino, A.P. Aroma and soluble solid contents of the uvaia—A native Atlantic rainforest fruit—are negatively affected by early harvest. J. Food Biochem. 2019, e12881. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and Antioxidant Evaluation of Essential Oil from Eugenia uniflora L., Curzerene-Rich, Thermally Produced in Situ. Biomolecules 2020, 10, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Schie, C.C.N.; Haring, M.A.; Schuurink, R.C. Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol. Biol. 2007, 64, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.M.; Gershenzon, J.; Bohlmann, J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 2003, 132, 1586–1599. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, S.; Hosokawa-Shinonaga, Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant Cell Environ. 2014, 37, 451–461. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Crafts-Brandner, S.J.; Paré, P.W.; Henneberry, T.J. Exogenous methyl jasmonate induces volatile emissions in cotton plants. J. Chem. Ecol. 2001, 27, 679–695. [Google Scholar] [CrossRef]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef] [Green Version]
- Raguso, R.A. More lessons from linalool: Insights gained from a ubiquitous floral volatile. Curr. Opin. Plant Biol. 2016, 32, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Bernreuther, A.; Schreierc, P. Multidimensional gas chromatography/mass spectrometry: A powerful tool for the direct chiral evaluation of aroma compounds in plant tissues. II. Linalool in essential oils and fruits. Phytochem. Anal. 1991, 2, 167–170. [Google Scholar] [CrossRef]
- Borg-Karlson, A.K.; Englund, F.O.; Unelius, C.R. Dimethyl oligosulphides, major volatiles released from Sauromatum guttatum and Phallus impudicus. Phytochemistry 1994, 35, 321–323. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and Sensory Studies on Tomato Paste Volatiles. J. Agric. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Shimada, T.; Endo, T.; Fujii, H.; Rodríguez, A.; Peña, L.; Omura, M. Characterization of three linalool synthase genes from Citrus unshiu Marc. and analysis of linalool-mediated resistance against Xanthomonas citri subsp. citri and Penicilium italicum in citrus leaves and fruits. Plant Sci. 2014, 229, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ban, Z.; Lu, H.; Li, D.; Poverenov, E.; Luo, Z.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on 4-carvomenthenol. Food Chem. Toxicol. 2008, 46, S91–S94. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.C.B.V.; Baseggio, A.M.; Schlegel, V. Jaboticaba: Chemistry and Bioactivity. Bioactive Mol. Food 2019, 1225–1251. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Manríquez, D.; Luengwilai, K.; González-Agüero, M. Aroma Volatiles: Biosynthesis and Mechanisms of Modulation During Fruit Ripening. Adv. Bot. Res. 2009, 50, 228. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists—International. Official Methods of Analysis, 19th ed.; AOAC: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Carvalho, C.R.L.; Mantovani, D.M.B.; Carvalho, P.R.N.; de Moraes, R.M. Análises Químicas de Alimentos; Manual Téc.; ITAL: Campinas, Brazil, 1990. [Google Scholar]
- Pesis, E.; Ibáñez, A.M.; Phu, M.L.; Mitcham, E.J.; Ebeler, S.E.; Dandekar, A.M. Superficial scald and bitter pit development in cold-stored transgenic apples suppressed for ethylene biosynthesis. J. Agric. Food Chem. 2009, 57, 2786–2792. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Code | Compound | CAS * | Odor Description ** | RI calc a | RI lit b | Class | |
|---|---|---|---|---|---|---|---|
| 1 | Ethyl acetate | 141-78-6 | pineapple | 909 | 907 | ester | |
| 2 | Ethanol | 4-17-5 | alcohol | 929 | 929 | alcohol | |
| 3 | Ethyl propionate | 105-37-3 | fruity, grape, pineapple | 977 | 977 | ester | |
| 4 | Propyl acetate | 109-60-4 | solvent, celery | 989 | 980 | ester | |
| 5 | d-α-pinene | 7785-70-8 | pine, turpentine | 1001 | 1011 | monoterpene | |
| 6 | Cyclofenchene | 488-97-1 | n.d. | 1010 | n.d. | other | |
| 7 | α-thujene | 353313 | wood, green, herb | 1014 | 1021 | monoterpene | |
| 8 | Ethyl butyrate | 105-54-4 | fruity, juicy, pineapple | 1045 | 1047 | ester | |
| 9 | Ethyl isovalerate | 108-64-5 | fruity | 1054 | 1060 | ester | |
| 10 | Ethyl-2-methyl | 7452-79-1 | sharp, sweet, fruity | 1073 | 1073 | ester | |
| 11 | Hexanal | 66-25-1 | grass, tallow, fat | 1080 | 1084 | aldehyde | |
| 12 | Undecane | 1120-21-4 | alkane, wax | 1109 | 1100 | alkane | |
| 13 | β-pinene | 127-91-3 | pine, resin, turpentine | 1117 | 1116 | monoterpene | |
| 14 | β-thujene | 28634-89-1 | n.d. | 1125 | n.d. | monoterpene | |
| 15 | Isoamyl acetate | 123-92-2 | fresh, banana, sweet | 1132 | 1132 | ester | |
| 16 | β-myrcene | 123-35-3 | balsamic, must, spice | 1144 | 1145 | monoterpene | |
| 17 | Ethyl (Z)-crotonate | 6776-19-8 | n.d. | 1156 | n.d. | ester | |
| 18 | α-Phellandrene | 99-83-2 | turpentine, mint, spice | 1165 | 1166 | monoterpene | |
| 19 | d-limonene | 5989-27-5 | lemon, orange | 1174 | 1178 | monoterpene | |
| 20 | β-phellandrene | 555-10-2 | mint, terpentine | 1209 | 1209 | monoterpene | |
| 21 | 1,8 cineole | 470-82-6 | eucalyptus | 1220 | 1214 | monoterpenoid alcohol | |
| 22 | 2-hexenal | 505-57-7 | apple, green | 1221 | 1220 | aldehyde | |
| 23 | Ethyl hexanoate | 123-66-0 | apple peel, fruity | 1224 | 1223 | ester | |
| 24 | γ-terpinene | 99-85-4 | gasoline, turpentine | 1237 | 1238 | monoterpene | |
| 25 | (E)-ethyl tiglate | 5837-78-5 | sweet, berry, floral | 1239 | n.d. | ester | |
| 26 | β-ocimene | 13877-91-3 | sweet, herb | 1240 | 1242 | monoterpene | |
| 27 | Propyl methacrylate | 2210-28-8 | n.d. | 1241 | n.d. | ester | |
| 28 | 3-octanone | 106-68-3 | fresh, herbal, lavender | 1244 | 1241 | ketone | |
| 29 | o-cymene | 527-84-4 | n.d. | 1260 | 1260 | monoterpene | |
| 30 | Hexyl acetate | 142-92-7 | fruity, apple | 1264 | 1264 | ester | |
| 31 | cis-1,3,3-trimethylbicyclo[3.1.0]hexane-1-carboxaldehyde | 1000365-94-2 | n.d. | 1338 | n.d. | aldehyde | |
| 32 | Ethyl 3-hexenoate | 2396-83-0 | sweet, fruity, pineapple | 1339 | n.d. | ester | |
| 33 | (Z)-3-hexen-1-ol acetate | 3681-71-8 | green, banana | 1340 | 1327 | ester | |
| 34 | 4-hexen-1-ol acetate | 72237-36-6 | n.d. | 1343 | n.d. | ester | |
| 35 | Methyl heptenone | 110-93-0 | citrus, green, lemongrass | 1344 | 1342 | ketone | |
| 36 | Ethyl 2-hexenoate | 1552-67-6 | fruity, green, sweet | 1347 | 1345 | ester | |
| 37 | Butyl 2-butenoate | 7299-91-4 | n.d. | 1352 | n.d. | ester | |
| 38 | Hexanol | 111-27-3 | resin, flower, green | 1362 | 1360 | alcohol | |
| 39 | (Z)-3-hexen-1-ol | 928-96-1 | grass | 1392 | 1391 | alcohol | |
| 40 | Cyclopropanecarboxylic acid,3-methylbutyl ester | 1000245-65-3 | n.d. | 1396 | n.d. | ester | |
| 41 | (E)-2-hexen-1-ol | 928-95-0 | leaf, green, fruity | 1398 | 1401 | alcohol | |
| 42 | Cyclopentane, 1-ethyl-1-methyl- | 16747-50-5 | n.d. | 1400 | n.d. | alkane | |
| 43 | (E)-2-octenal | 2548-87-0 | green, nut, fat, leaf | 1407 | 1408 | aldehyde | |
| 44 | Ethyl octanoate | 106-32-1 | fruity, fat | 1432 | 1436 | ester | |
| 45 | α-cubebene | 17699-14-8 | herb, wax | 1462 | 1463 | sesquiterpene | |
| 46 | 2,2-dimethylhexanal | 996-12-3 | n.d. | 1478 | n.d. | aldehyde | |
| 47 | δ-elemene | 20307-84-0 | wood | 1480 | 1468 | sesquiterpene | |
| 48 | α-copaene | 1000360-33-0 | wood, spice | 1485 | 1488 | sesquiterpene | |
| 49 | Ethyl sorbate | 110318-09-7 | fruity | 1487 | n.d. | ester | |
| 50 | 2-ethyl-1-hexanol | 104-76-7 | rose, green | 1493 | 1487 | alcohol | |
| 51 | β-bourbonene | 5208-59-3 | herbal | 1495 | 1495 | sesquiterpene | |
| 52 | Benzaldehyde | 100-52-7 | almond, burnt sugar | 1498 | 1502 | aldehyde | |
| 53 | Grape butyrate | 5405-41-4 | marshmallow | 1522 | 1524 | ester | |
| 54 | Linalool | 78-70-6 | flower, lavender | 1533 | 1537 | sesquiterpenoid alcohol | |
| 55 | β-cubebene | 13744-15-5 | citrus, fruity | 1555 | 1546 | sesquiterpene | |
| 56 | 4-terpineol | 562-74-3 | pepper, woody, earth | 1596 | 1585 | monoterpenoid alcohol | |
| 57 | δ-selinene | 28624-23-9 | n.d. | 1601 | n.d. | sesquiterpene | |
| 58 | l-bornyl acetate | 5655-61-8 | pine | 1603 | 1600 | sesquiterpenoid ester | |
| 59 | (E)-2-octen-1-ol | 18409-17-1 | mushroom (soap, plastic) | 1604 | 1590 | alcohol | |
| 60 | β-elemene | 515-13-9 | herb, wax, fresh | 1610 | 1595 | sesquiterpene | |
| 61 | (Z)-3-hexenyl (E)-2-butenoate | 65405-80-3 | green | 1611 | 1610 | ester | |
| 62 | Isoledene | 1000156-10-8 | n.d. | 1622 | n.d. | sesquiterpene | |
| 63 | γ-elemene | 490377 | green, wood, oil | 1637 | 1636 | sesquiterpene | |
| 64 | Alloaromadendrene | 25246-27-9 | wood | 1639 | 1639 | sesquiterpene | |
| 65 | 1-epi-bicyclosesquiphellandrene | 54274-73-6 | n.d. | 1642 | n.d. | sesquiterpene | |
| 66 | Aristolene | 6831-16-9 | n.d. | 1644 | n.d | sesquiterpene | |
| 67 | α-muurolene | 31983-22-9 | wood | 1647 | n.d. | sesquiterpene | |
| 68 | Methyl benzoate | 93-58-3 | prune, lettuce, herb, sweet | 1651 | 1640 | ester | |
| 69 | cis-muurola-4(14),5-diene | 1000365-95-4 | n.d. | 1661 | n.d. | sesquiterpene | |
| 70 | Humulene | 6753-98-6 | wood | 1662 | 1663 | sesquiterpene | |
| 71 | Ethyl benzoate | 93-89-0 | camomile, flower, fruity | 1665 | 1658 | ester | |
| 72 | γ-muurolene | 30021-74-0 | herb, wood, spice | 1675 | 1681 | sesquiterpene | |
| 73 | γ-gurjunene | 22567-17-5 | musty | 1678 | n.d | sesquiterpene | |
| 74 | Nonanol | 143-08-8 | fat, green | 1678 | 1666 | alcohol | |
| 75 | β-patchoulene | 514-51-2 | n.d. | 1681 | n.d. | sesquiterpene | |
| 76 | Viridiflorene | 21747-46-6 | n.d. | 1699 | n.d. | sesquiterpene | |
| 77 | β-selinene | 17066-67-0 | herb | 1715 | 1711 | sesquiterpene | |
| 78 | α-selinene | 473-13-2 | pepper, orange | 1719 | 1724 | sesquiterpene | |
| 79 | (E)-germacrene D | 23986-74-5 | wood, spice | 1731 | 1724 | sesquiterpene | |
| 80 | α-amorphene | 483-75-0 | n.d. | 1742 | 1752 | sesquiterpene | |
| 81 | Benzyl acetate | 140-11-4 | floral, fruity, jasmin | 1755 | 1747 | ester | |
| 82 | δ-cadinene | 483-76-1 | thyme, medicine, wood | 1759 | 1749 | sesquiterpene | |
| 83 | 2-phenylacetamide, N-(1-phenyl-2-propyl)- | 1000223-70-1 | n.d. | 1762 | n.d. | other | |
| 84 | Cadine-1,4-diene | 16728-99-7 | spice, fruity | 1785 | 1786 | sesquiterpene | |
| 85 | Selina-3,7(11)-diene | 6813-21-4 | n.d. | 1789 | 1789 | sesquiterpene | |
| 86 | γ-cadinene | 39029-41-9 | wood | 1790 | 1776 | sesquiterpene | |
| 87 | Citronellyl butyrate | 141-16-2 | fruity, sweet, rose | 1802 | 1809 | monoterpenoid ester | |
| 88 | α-cadinene | 24406-05-1 | woody, dry | 1817 | 1815 | sesquiterpene | |
| 89 | Calamenene | 483-77-2 | herb, spice | 1824 | 1822 | sesquiterpene | |
| 90 | Geraniol | 106-24-1 | rose, geranium | 1845 | 1847 | monoterpenoid alcohol | |
| 91 | α-calacorene | 21391-99-1 | wood | 1890 | 1901 | sesquiterpene | |
| 92 | Palustrol | 95975-84-1 | n.d. | 1910 | n.d. | sesquiterpenoid alcohol | |
| 93 | β-caryophyllene oxide | 1139-30-6 | herb, sweet, spice | 2020 | 2014 | sesquiterpene | |
| 94 | Ledol | 577-27-5 | sweet, green | 2040 | 2043 | sesquiterpenoid alcohol | |
| 95 | Methyl cinnamate | 103-26-4 | strawberry, cherry | 2065 | 2056 | ester | |
| 96 | Humulane-1,6-dien-3-ol | 1000140-23-1 | n.d. | 2081 | n.d. | sesquiterpenoid alcohol | |
| 97 | Mansonone | 5574-34-5 | n.d. | 2095 | n.d. | other | |
| 98 | Cubenol | 21284-22-0 | spice, herb, green tea | 2097 | 2097 | sesquiterpenoid alcohol | |
| 99 | p-vinylbenzohydrazide | 1000244-74-9 | n.d. | 2105 | n.d. | other | |
| 100 | Rosifoliol | 63891-61-2 | n.d. | 2115 | n.d. | sesquiterpenoid alcohol | |
| 101 | Ethyl cinnamate | 103-36-6 | sweet, fruity, spicy, berry plum | 2136 | 2139 | ester | |
| 102 | Selina-6-en-4-ol | 1000140-23-2 | n.d. | 2141 | n.d. | sesquiterpenoid alcohol | |
| 103 | Carotol | 465-28-1 | pleasent mild | 2149 | n.d. | sesquiterpenoid alcohol | |
| 104 | T-cadinol | 1474790 | wood, balsamic | 2150 | 2155 | sesquiterpenoid alcohol | |
| 105 | T-muurolol | 19912-62-0 | herb, weak spice, honey | 2157 | 2148 | sesquiterpenoid alcohol | |
| 106 | Spathulenol | 6750-60-3 | earthy, herbal, fruity | 2170 | 2153 | sesquiterpenoid alcohol | |
| 107 | Methyl isoeugenol | 93-16-3 | spicy, clove, blossom | 2180 | 2196 | other | |
| 108 | 2,9-bornanediol | 54831-21-9 | n.d. | 2185 | n.d. | monoterpenoid alcohol | |
| 109 | α-eudesmol | 473-16-5 | sweet, wood | 2190 | 2208 | sesquiterpenoid alcohol | |
| 110 | α-cadinol | 481-34-5 | herb, wood | 2192 | 2191 | sesquiterpenoid alcohol | |
| 111 | Cadalene | 483-78-3 | n.d. | 2196 | 2203 | sesquiterpene | |
| 112 | 3,6-Dimethyl-4H-furo[3,2-c]pyran-4-one | 36745-38-7 | n.d. | 2201 | n.d. | ketone | |
| 113 | Occidentalol | 29484-47-7 | n.d. | 2204 | n.d. | sesquiterpenoid alcohol | |
| 114 | Juniper camphor | 473-04-1 | camphor | 2207 | n.d. | sesquiterpenoid alcohol | |
| 115 | Tetracyclo[6.3.2.0(2,5).0(1,8)]tridecan-9-ol, 4,4-dimethyl | 1000157-75-1 | n.d. | 2211 | n.d. | alcohol | |
| 116 | β-eudesmol | 473-15-4 | wood, green | 2225 | 2214 | sesquiterpenoid alcohol | |
| 117 | Galaxolide 2 | 1000285-26-7 | musk | 2230 | n.d. | other |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, T.P.; Taver, I.B.; Spricigo, P.C.; do Amaral, L.B.; Purgatto, E.; Jacomino, A.P. Volatile Compounds and Physicochemical Quality of Four Jabuticabas (Plinia sp.). Molecules 2020, 25, 4543. https://doi.org/10.3390/molecules25194543
Freitas TP, Taver IB, Spricigo PC, do Amaral LB, Purgatto E, Jacomino AP. Volatile Compounds and Physicochemical Quality of Four Jabuticabas (Plinia sp.). Molecules. 2020; 25(19):4543. https://doi.org/10.3390/molecules25194543
Chicago/Turabian StyleFreitas, Thais Pádua, Isabela Barroso Taver, Poliana Cristina Spricigo, Lucas Bueno do Amaral, Eduardo Purgatto, and Angelo Pedro Jacomino. 2020. "Volatile Compounds and Physicochemical Quality of Four Jabuticabas (Plinia sp.)" Molecules 25, no. 19: 4543. https://doi.org/10.3390/molecules25194543







