Ethyne Functionalized Meso-Phenothiazinyl-Phenyl-Porphyrins: Synthesis and Optical Properties of Free Base Versus Protonated Species
Abstract
:1. Introduction
2. Results and Discussions
2.1. Chemical Synthesis
2.2. UV-Vis Absorption and Emission Properties of Ethynyl Functionalized MPP Derivatives 2A–L
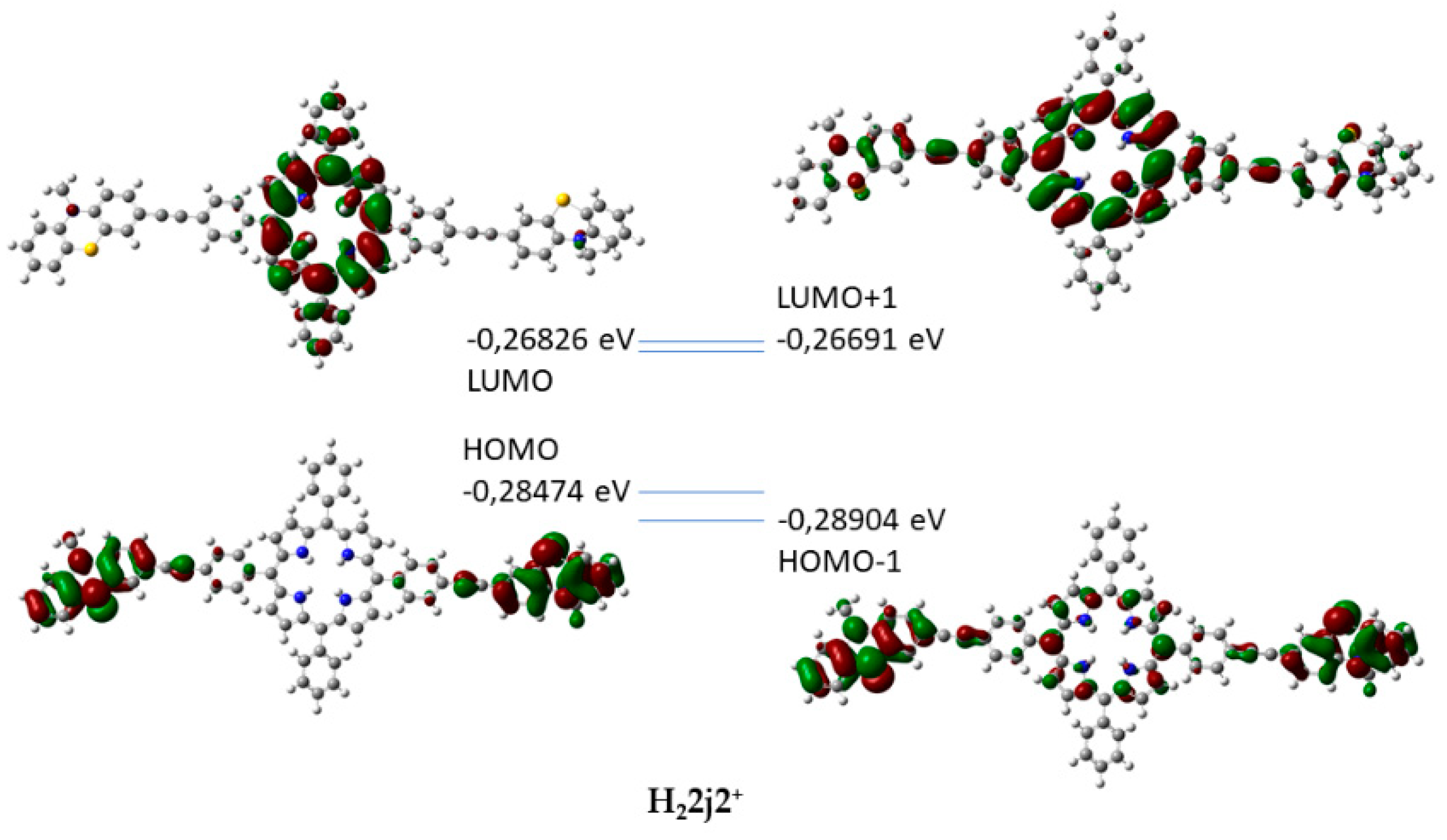
2.3. Optical Properties of the Protonated Ethynyl-MPP Derivatives
3. Materials and Methods
General Procedure for the Synthesis of 2A–L
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Louda, J.W. Porphyrins. Encycl. Earth Sci. Ser. 2018, 1247–1253. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, R.; Zhou, H.; Zhang, X.; Xu, T.; Liu, X.; Zhao, Y. Synthesis of Phenothiazine-Functionalized Porphyrins with High Fluorescent Quantum Yields. Tetrahedron Lett. 2008, 49, 7446–7449. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Lim, J.M.; Osuka, A.; Kim, D. Various Strategies for Highly-Efficient Two-Photon Absorption in Porphyrin Arrays. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 13–28. [Google Scholar] [CrossRef]
- Mahmood, A.; Hu, J.Y.; Xiao, B.; Tang, A.; Wang, X.; Zhou, E. Recent Progress in Porphyrin-Based Materials for Organic Solar Cells. J. Mater. Chem. A 2018, 6, 16769–16797. [Google Scholar] [CrossRef]
- Jagadeeswari, S.; Paramaguru, G.; Renganathan, R. Synthesis and Characterization of Free Base and Metal Porphyrins and Their Interaction with CdTe QDs. J. Photochem. Photobiol. A Chem. 2014, 276, 104–112. [Google Scholar] [CrossRef]
- Verykios, A.; Papadakis, M.; Soultati, A.; Skoulikidou, M.C.; Papaioannou, G.; Gardelis, S.; Petsalakis, I.D.; Theodorakopoulos, G.; Petropoulos, V.; Palilis, L.C.; et al. Functionalized Zinc Porphyrins with Various Peripheral Groups for Interfacial Electron Injection Barrier Control in Organic Light Emitting Diodes. ACS Omega 2018, 3, 10008–10018. [Google Scholar] [CrossRef]
- De Amorim Lima, N.M.; Camargo Avila, H.J.; do Nascimento Marchiori, C.F.; Gondim Sampaio, S.; Ferreira Mota, J.P.; Gomes Pereira Ribeiro, V.; da Silva Clemente, C.; Mele, G.; Cremona, M.; Mazzetto, S.E. Light-Emitting Porphyrin Derivative Obtained from a Subproduct of the Cashew Nut Shell Liquid: A Promising Material for OLED Applications. Materials 2019, 12, 1063. [Google Scholar] [CrossRef] [Green Version]
- Janghouri, M.; Adineh, M. Color Optimization of Red Organic Light Emitting Diodes (OLEDs) through Dihydroxyphenyl-Substituted Zinc Porphyrins Emitters. J. Photochem. Photobiol. A Chem. 2017, 341, 31–38. [Google Scholar] [CrossRef]
- Ptaszek, M. Rational Design of Fluorophores for in Vivo Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 113. [Google Scholar]
- Purrello, R.; Gurrieri, S.; Lauceri, R. Porphyrin Assemblies as Chemical Sensors. Coord. Chem. Rev. 1999, 190, 683–706. [Google Scholar] [CrossRef]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Azhar Khan, M.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakow, D.; Böker, B.; Brandhorst, K.; Burghaus, O.; Bröring, M. 10-Heterocorroles: Ring-contracted porphyrinoids with fine-tuned aromatic and metal-binding properties. Angew. Chem. Int. Ed. 2013, 52, 4912–4915. [Google Scholar] [CrossRef] [PubMed]
- Neda, I.; Farkens, M.; Fischer, A.; Jones, P.G.; Schmutzler, R. Chemistry of the l,3,5-Triaza-2-phosphinane-4,6-diones, Part V Synthesis of Phosphoryl(III)(λ4P) and Thiophosphoryl(III)(λ4P) Derivatives of 1,3,5-Triaza-2-phosphinane-4,6- diones, Reactions with Ketones. Z. Naturforsch. 1993, B48, 860–866. [Google Scholar] [CrossRef]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-Based Sensor Nanoarchitectonics in Diverse Physical Detection Modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef] [PubMed]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lembo, A.; Tagliatesta, P.; Guldi, D.M. Synthesis and Photophysical Investigation of New Porphyrin Derivatives with β-Pyrrole Ethynyl Linkage and Corresponding Dyad with [60] Fullerene. J. Phys. Chem. A 2006, 110, 11424–11434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temelli, B.; Gündüz, M.; Yüksel, D. Ethynyl-bridged porphyrin-corrole dyads and triads: Synthesis, properties and DFT calculations. Tetrahedron 2018, 74, 4476–4488. [Google Scholar] [CrossRef]
- Zhao, H.; Long, J.; Luo, X.; Zhao, B.; Tan, S. 2-Ethynyl-6-methylthieno [3,2-b] thiophene as an efficient p spacer for porphyrin-based dyes. Dyes Pigm. 2015, 122, 168–176. [Google Scholar] [CrossRef]
- Shia, K.; Yaoa, H.; Zoub, Y.; Weia, Y.; Songa, N.; Zhanga, S.; Tiana, Y.; Zhua, S.; Zhanga, B.; Guan, S. Crosslinked porphyrin-based polyimides: Tunable porosity parameters and carbon dioxide adsorption. Microporous Mesoporous Mater. 2019, 287, 246–253. [Google Scholar] [CrossRef]
- Wang, W.-C.; Lin, Y.-W.; Peng, S.-H.; Chuang, C.-T.; Chang, C.-C.; Hsu, C.-S. A strategy of designing near-infrared porphyrin-based non-fullerene acceptors for panchromatic organic solar cells. Org. Electron. 2020, 86, 105899. [Google Scholar] [CrossRef]
- Goldberg, P.K.; Pundsack, T.J.; Splan, K.E. Photophysical Investigation of Neutral and Diprotonated Free-Base Bis(Arylethynyl)porphyrins. J. Phys. Chem. A 2011, 115, 10452–10460. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wamser, C.C. Hyperporphyrin Effects in the Spectroscopy of Protonated Porphyrins with 4-Aminophenyl and 4-Pyridyl Meso Substituents. J. Phys. Chem. A 2014, 118, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Rudine, A.B.; Delfatti, B.D.; Wamser, C.C. Spectroscopy of Protonated Tetraphenylporphyrins with Amino/Carbomethoxy Substituents: Hyperporphyrin Effects and Evidence for a Monoprotonated Porphyrin. J. Org. Chem. 2013, 78, 6040–6049. [Google Scholar] [CrossRef] [PubMed]
- Dyrda, G.; Słota, R.; Broda, M.A.; Mele, G. Meso-Aryl-Substituted Free-Base Porphyrins: Formation, Structure and Photostability of Diprotonated Species. Res. Chem. Intermed. 2016, 42, 3789–3804. [Google Scholar] [CrossRef] [Green Version]
- Webb, M.J.; Bampos, N. Noncovalent Interactions in Acid-Porphyrin Complexes. Chem. Sci. 2012, 3, 2351–2366. [Google Scholar] [CrossRef]
- Meot-Ner, M.; Adler, A.D. Substituent Effects in Noncoplanar π Systems. Ms-Porphins. J. Am. Chem. Soc. 1975, 97, 5107–5111. [Google Scholar] [CrossRef]
- Presselt, M.; Dehaen, W.; Maes, W.; Klamt, A.; Martínez, T.; Beenken, W.J.D.; Kruk, M. Quantum chemical insights into the dependence of porphyrin basicity on the meso-aryl substituents: Thermodynamics, buckling, reaction sites and molecular flexibility. Phys. Chem. Chem. Phys. 2015, 17, 14096–14106. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.M.; Liu, X.; He, T.J.; Liu, F.C. Density functional theory investigation of porphyrin diacid:electronic absorption spectrum and conformational inversion. Chem. Phys. 2003, 289, 397–407. [Google Scholar] [CrossRef]
- Gal, E.; Brem, B.; Pereţeanu, I.; Gǎinǎ, L.; Lovasz, T.; Perde-Schrepler, M.; Silaghi-Dumitrescu, L.; Cristea, C.; Silaghi-Dumitrescu, L. Novel Meso-Phenothiazinylporphyrin Dyes: Synthesis, Optical, Electrochemical Properties and PDT Assay. Dyes Pigm. 2013, 99, 144–153. [Google Scholar] [CrossRef]
- Brem, B.; Gal, E.; Gəinə, L.; Cristea, C.; Gəbudean, A.M.; Aştilean, S.; Silaghi-Dumitrescu, L. Metallo Complexes of Meso-Phenothiazinylporphyrins: Synthesis, Linear and Nonlinear Optical Properties. Dyes Pigm. 2015, 123, 386–395. [Google Scholar] [CrossRef]
- Kramer, C.S.; Muller, T.J.J. Synthesis and Electronic Properties of Alkynylated Phenothiazines. Eur. J. Org. Chem. 2003, 18, 3534–3548. [Google Scholar] [CrossRef]
- Molnar, E.; Gal, E.; Gaina, L.; Cristea, C.; Fischer-Fodor, E.; Perde-Schrepler, M.; Achimas-Cadariu, P.; Focsan, M.; Silaghi-Dumitrescu, L. Novel Phenothiazine-Bridged Porphyrin-(Hetero)aryl dyads: Synthesis, Optical Properties, In Vitro Cytotoxicity and Staining of Human Ovarian Tumor Cell Lines. Int. J. Mol. Sci. 2020, 21, 3178. [Google Scholar] [CrossRef] [PubMed]
- Gǎinǎ, L.; Gal, E.; Mataranga-Popa, L.; Porumb, D.; Nicolescu, A.; Cristea, C.; Silaghi-Dumitrescu, L. Synthesis, structural investigations, and DFT calculations on novel 3-(1,3-dioxan2-yl)-10-methyl-10H-phenothiazine derivatives with fluorescence properties. Tetrahedron 2012, 68, 2465–2470. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2a–l are available from the authors. |








| Cpd | λabs [nm] | λem [nm] | Stokes Shift [cm−1] | ΦFb | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ptz | Soret a ε [mol−1 cm−1] | Q4 | Q3 | Q2 | Q1 | ||||
| 2a | 251 | 422 (239167) | 519 | 556 | 593 | 650 | 658 | 8499 | 0.23 |
| 2b | 252 | 419 (172178) | 518 | 555 | 591 | 649 | 655 | 8485 | 0.21 |
| 2b | 252 | 424 (165464) | 520 | 558 | 594 | 651 | 659 | 8410 | 0.21 |
| 3b | 253 | 421 (79531) | 520 | 557 | 591 | 651 | 658 | 8443 | 0.20 |
| 2c | 252 | 421 (283687) | 518 | 554 | 591 | 651 | 659 | 8691 | 0.22 |
| 2d | 252 | 420 (248390) | 519 | 553 | 589 | 648 | 655 | 8542 | 0.21 |
| 3c | 252 | 423 (189598) | 520 | 559 | 590 | 650 | 658 | 8555 | 0.21 |
| 3d | 252 | 421 (111910) | 519 | 556 | 591 | 650 | 657 | 8532 | 0.20 |
| 4a | 252 | 420 (95878) | 514 | 553 | 591 | 648 | 651 | 8448 | 0.23 |
| 5a | 252 | 422 (173379) | 518 | 554 | 591 | 649 | 656 | 8452 | 0.25 |
| 4b | 253 | 419 (184003) | 517 | 555 | 592 | 649 | 655 | 8599 | 0.21 |
| 5b | 251 | 421 (173811) | 519 | 557 | 593 | 651 | 654 | 8462 | 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar, E.; Gál, E.; Găină, L.; Cristea, C.; Silaghi-Dumitrescu, L. Ethyne Functionalized Meso-Phenothiazinyl-Phenyl-Porphyrins: Synthesis and Optical Properties of Free Base Versus Protonated Species. Molecules 2020, 25, 4546. https://doi.org/10.3390/molecules25194546
Molnar E, Gál E, Găină L, Cristea C, Silaghi-Dumitrescu L. Ethyne Functionalized Meso-Phenothiazinyl-Phenyl-Porphyrins: Synthesis and Optical Properties of Free Base Versus Protonated Species. Molecules. 2020; 25(19):4546. https://doi.org/10.3390/molecules25194546
Chicago/Turabian StyleMolnar, Eva, Emese Gál, Luiza Găină, Castelia Cristea, and Luminița Silaghi-Dumitrescu. 2020. "Ethyne Functionalized Meso-Phenothiazinyl-Phenyl-Porphyrins: Synthesis and Optical Properties of Free Base Versus Protonated Species" Molecules 25, no. 19: 4546. https://doi.org/10.3390/molecules25194546
APA StyleMolnar, E., Gál, E., Găină, L., Cristea, C., & Silaghi-Dumitrescu, L. (2020). Ethyne Functionalized Meso-Phenothiazinyl-Phenyl-Porphyrins: Synthesis and Optical Properties of Free Base Versus Protonated Species. Molecules, 25(19), 4546. https://doi.org/10.3390/molecules25194546






