Recent Techniques in Nutrient Analysis for Food Composition Database
Abstract
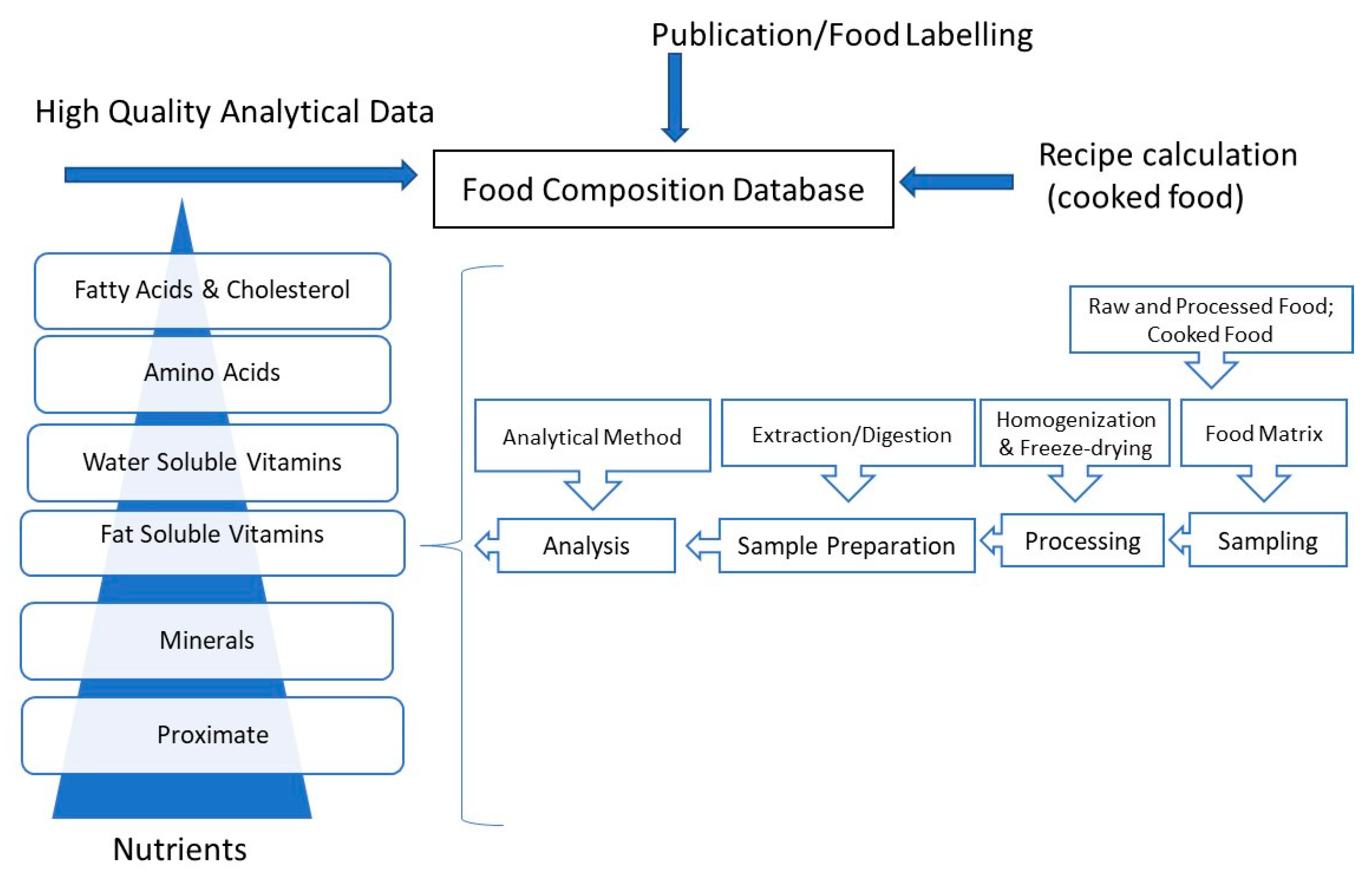
:1. Introduction
2. Proximate
2.1. Moisture
2.2. Protein
2.3. Total Fat
2.4. Total Dietary Fibre (TDF)
2.5. Ash
2.6. Total Sugar
2.7. Carbohydrate
3. Minerals
3.1. Atomic Absorption Spectrometer (AAS)
3.2. Microwave and Inductively Coupled Plasma-Optical Emission Spectrometry/Atomic Emission Spectrometry (ICP-OES/AES))
3.3. Inductively Coupled Plasma-Mass Spectrometer (ICP-MS)
3.4. Energy-Dispersive X-Ray Fluorescence (ED-XRF)
4. Fat Soluble Vitamins and Carotenoids
Sample Preparation and Analytical Technique
5. Water-Soluble Vitamins
Sample Preparation and Analytical Technique
6. Amino Acids
Sample Preparation and Analytical Technique
7. Fatty Acids and Cholesterol
Sample preparation and Analytical Technique
8. Challenges in Generating Data for Food Composition Database
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| AA | Ascorbic acid |
| AAS | Atomic Absorption Spectrometer |
| Ala | Alanine |
| AOAC | Association of Official Analytical Chemists |
| APCI | Atmospheric pressure chemical ionization |
| AQC | 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate |
| Arg | Arginine |
| ASE | Accelerated solvent extraction |
| Asp | Aspartate |
| Asn | Asparagine |
| ATR-FTIR | Attenuated total reflection Fourier-transform infrared spectroscopy |
| BCAA | Branched-chain amino acids |
| β-Ala | β-alanine |
| CE | Capillary electrophoresis |
| CD | Conductometric detector |
| CITP | Capillary isotachophoresis |
| Cit | Citrulline |
| CLA | Conjugated linoleic acid |
| Cys | Cysteine |
| DAD | Diode array detector |
| DEEMM | diethylethoxymethylenemalonate |
| DHAA | Dehydroascorbic acid |
| DLLME | Dispersive liquid-liquid microextraction |
| DMPP | 2,4-dimethoxy-6-piperazin-1-yl pyrimidine |
| DSPE | Dispersive solid-phase extraction |
| 2D-LC | Two-dimensional liquid chromatography |
| EAA | Essential amino acid |
| ECF | Ethylchloroformate |
| ED | Electrochemical detector |
| ED-XRF | Energy-dispersive X-ray fluorescence |
| EDTA | Ethylenediaminetetraacetic acid |
| ELSD | Evaporative light scattering detector |
| ESI | Electrospray ionization |
| FA | Fatty acid |
| FAA | Trifluoroacetylacetone |
| FABE | Fatty acid butyl esters |
| FAME | Fatty acid methyl esters |
| FCD | Food composition database |
| FCT | Food composition tables |
| FFA | Free fatty acid |
| FID | Flame ionization detection |
| FLD | Fluorescence detector |
| FMOC | 9-fluorenylmethyl chloroformate |
| FOS | Fructo-oligosaccharides |
| FSV | Fat-soluble vitamin |
| GABA | γ-aminobutyric acid |
| GC | Gas chromatography |
| GCE | Glassy carbon electrode |
| GCPE | Glassy carbon paste electrode |
| GLC | Gas-liquid chromatography |
| Gln | Glutamine |
| GLP | Good laboratory practice |
| Glu | Glutamic acid |
| Gly | Glycine |
| HCl | Hydrochloric acid |
| His | Histidine |
| HPLC | High performance liquid chromatography |
| HRMS | High resolution mass spectrometry |
| IAA | Indispensable amino acids |
| ICP-MS | Inductively coupled plasma-mass spectrometer |
| ICP-OES/AES | Inductively coupled plasma–optical emission spectrometry/atomic emission spectrometry |
| IEC | Ion-electron chromatography |
| Ile | Isoleucine |
| IR | Infrared radiation |
| KOH | Potassium hydroxide |
| L-AA | L-ascorbic acid |
| LC | Liquid chromatography |
| Leu | Leucine |
| LIF | Laser-induced fluorescence |
| LLE | Liquid-liquid extraction |
| LOQ | Limit of quantification |
| LOD | Limit of detection |
| Lys | Lysine |
| MAE | Microwave-assisted extraction |
| m.c. | Moisture content |
| MCE | Microchip electrophoresis |
| MD-μ-SPE | Dispersive micro-solid phase extraction |
| MEKC | Micellar electro kinetic chromatography |
| Met | Methionine |
| MIC | Microwave induced-combustion |
| MPA | Metaphosphoric acid |
| MRM | Multiple reaction monitoring |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| MSPE | Magnetic solid phase extraction |
| MTBE | Methyl tertiary-butyl ether |
| MTBSTFA | N-methyl-N-(tert-butyldimethylsilyl trifluoroacetamide |
| MUFA | Monounsaturated fatty acids |
| MW | Microwave heating |
| MW-AD | Microwave assisted-digestion |
| NAA | Neutron activation analysis |
| Nano-LC | Nano-liquid chromatography |
| NARP | Non-aqueous reversed-phase |
| NBD-Cl | 4-chloro-7-nitro-2,1,3-benzoxadiazole |
| NEAA | Non-essential amino acid |
| NIR | Near infrared reflectance |
| NMR | Nuclear magnetic resonance |
| NP | Normal phase |
| OPA | O-phthaldialdehyde |
| Orn | Ornithine |
| PAA-T | Tetracycline-grafted polyacrylamide polymer |
| PCA | Perchloric acid |
| PDA | Photo-diode array |
| Phe | Phenylalanine |
| PITC | Phenylisothiocyanate |
| PLE | Pressurized liquid extraction |
| Pro | Proline |
| PUFA | Polyunsaturated fatty acids |
| QC | Quality control |
| QTOF-MS | Quadrupole time-of-flight mass spectrometry |
| RID | Refractive index detector |
| RITDF | Rapid integrated total dietary fiber |
| RP | Reversed-phase |
| SEC | Size-exclusion chromatography |
| Ser | Serine |
| SFA | Saturated fatty acids |
| SFC | Supercritical fluid chromatography |
| SNR | Signal-to-noise ratio |
| SPE | Solid phase extraction |
| SWASV | Square wave anodic stripping voltammetry |
| SWAdSV | Square wave adsorptive stripping voltammetry |
| TCA | Trichloroacetic acid |
| TDF | Total dietary fiber |
| THGA | Transverse heated graphite tube |
| Thr | Threonine |
| TMS-DM | Trimethylsilyl-diazomethane |
| Trp | Tryptophan |
| Tyr | Tyrosine |
| U | Expanded uncertainty |
| UAE | Ultrasound assisted extraction |
| UHPLC | Ultra-high-performance liquid chromatography |
| UPC2 | Ultra-performance convergence chromatography |
| UV/Vis | Ultraviolet/visible |
| Val | Valine |
| VUV | Vacuum ultraviolet |
| WSV | Water soluble vitamin |
References
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Marcus, J.B. Culinary Nutrition: The Science and Practice of Healthy Cooking; Elsevier: Oxford, UK, 2013. [Google Scholar]
- FAO. International Network of Food Data Systems (INFOODS). Available online: http://www.fao.org/infoods/infoods/food-composition-challenges/en/ (accessed on 8 June 2020).
- Greenfield, H.; Southgate, D.A. Food Composition Data: Production, Management, and Use, 2nd ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Thirumdas, R.; Janve, M.; Siliveru, K.; Kothakota, A. Determination of food quality using atomic emission spectroscopy. In Evaluation Technologies for Food Quality; Elsevier: London, UK, 2019; pp. 175–192. [Google Scholar]
- Egan, H. Report of the Government Chemist; Her Majesty’s Stationery Office: London, UK, 1974. [Google Scholar]
- Büttner, J.; Borth, R.; Boutwell, J.; Broughton, P.; Bowyer, R. International federation of clinical chemistry. Provisional recommendation on quality control in clinical chemistry. Part 1. General principles and terminology. J. Clin. Chem. Clin. Biochem. 1975, 13, 523–531. [Google Scholar]
- Thangaraj, P. Proximate composition analysis. In Pharmacological Assays of Plant-Based Natural Products; Springer: Berlin, Germany, 2016; pp. 21–31. [Google Scholar]
- Riadh, M.H.; Ahmad, S.A.B.; Marhaban, M.H.; Soh, A.C. Infrared heating in food drying: An overview. Dry Technol. 2015, 33, 322–335. [Google Scholar] [CrossRef]
- Nirmaan, A.; Prasantha, B.R.; Peiris, B. Comparison of microwave drying and oven-drying techniques for moisture determination of three paddy (Oryza sativa L.) varieties. Chem. Biol. Technol. Agric. 2020, 7, 1. [Google Scholar] [CrossRef]
- Gao, H.; Wang, G.; Men, J.; Wang, Z. Near-infrared reflectance spectroscopy predicts protein, moisture and ash in beans. Wei Sheng Yan Jiu = J. Hyg. Res. 2017, 46, 461–471. [Google Scholar]
- Duan, W.-J.; Li, Y.; Cui, L.; Liu, F.; Yang, G.-H.; Guo, L.-P.; Wang, X. Analyze moisture transformation and transport rules during processing of paeoniae radix alba by using low-field NMR. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Med. 2017, 42, 2092–2096. [Google Scholar]
- Jung, S.; Rickert, D.; Deak, N.; Aldin, E.; Recknor, J.; Johnson, L.; Murphy, P. Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. J. Am. Oil Chem. Soc. 2003, 80, 1169. [Google Scholar] [CrossRef]
- Pombal, S.; Lourenço, J.; Rocha, P.; Ettlin, D.; Rodilla, J. Comparison of a new total fat quantification method in cheese using microwave assisted extraction (MAE) and soxhlet. Braz. J. Anal. Chem. 2018, 4, 10–15. [Google Scholar]
- AOAC. AOAC Official Method 2002.02 Resistant starch in starch and plant materials. In AOAC Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- AOAC. AOAC Official Method 985.29 Total dietary fibre in foods. In AOAC Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- AOAC. AOAC Official Method 991.43 Total, soluble, and insoluble dietary fiber in foods. In AOAC Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- AOAC. AOAC Official Method 2001.03 Dietary fiber containing supplemented resistant maltodextrin (RMD). In AOAC Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- AOAC. AOAC Official Method 2009.01 Total dietary fiber in foods. In AOAC Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- McCleary, B.V.; Ames, N.C.J.; Iilians, S.; Jin, Y.; Johnson, M.; McCarthy, S.; McKie, V.; Nishibata, T.; Pastell, H.; Plank, D.; et al. Total dietary fiber (CODEX definition) in foods and food ingredients by a rapid enzymatic-gravimetric method and liquid chromatography: Collaborative study, first action 2017.16. J. AOAC Int. 2019, 102, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Weiler, F.H.; dos Santos-Grasel, F.; Lourega, R.V.; da Silva-Ramos, A.; Ferrão, M.F. Simultaneous determination of sulfur, nitrogen and ash for vegetable tannins using ATR-FTIR spectroscopy and multivariate regression. Microchem. J. 2019, 149, 103994. [Google Scholar] [CrossRef]
- Al-Mhanna, N.M.; Huebner, H.; Buchholz, R. Analysis of the sugar content in food products by using gas chromatography mass spectrometry and enzymatic methods. Foods 2018, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Bradley, R.L. Moisture and total solids analysis. In Food Analysis; Springer: Berlin, Germany, 2010; pp. 85–104. [Google Scholar]
- Isengard, H.-D. Water content, one of the most important properties of food. Food Control 2001, 12, 395–400. [Google Scholar] [CrossRef]
- Lewis, M.A.; Trabelsi, S.; Nelson, S.O.; Tollner, E.W.; Haidekker, M.A. An automated approach to peanut drying with real-time microwave monitoring of in-shell kernel moisture content. Appl. Eng. Agric. 2013, 29, 583–593. [Google Scholar]
- Norhayati, M.K.; Mohd, F.; Mohd, N.; Nazline, M.K.; Aswir, A.R.; Wan, S.; Norliza, A.H.; Janarthini, S.; Rusidah, S. Individual and total sugar contents of 83 Malaysian foods. J. Food Res. 2018, 7, 58–63. [Google Scholar]
- Ganzera, M.; Stuppner, H. Evaporative light scattering detection (ELSD) for the analysis of natural products. Curr. Pharm. Anal. 2005, 1, 135–144. [Google Scholar] [CrossRef]
- Megazyme, K.-S. Sucrose, D-Fructose and D-Glucose Assay Procedure; Megazyme International: Bray, Ireland, 2018. [Google Scholar]
- Al-Mhanna, N.M.M. Optimization of differential pH sensors device operation conditions to be used in quantification of low glucose concentration. Int. J. Chem. 2011, 3, 149. [Google Scholar] [CrossRef]
- Montesano, D.; Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.S.; Blasi, F. A simple HPLC-ELSD method for sugar analysis in goji berry. J. Chem. 2016, 2016, 6271808. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO. Carbohydrates in human nutrition: Report of a joint FAO/WHO expert consultation. FAO Food Nutr. Pap. 1998, 66, 1–140. [Google Scholar]
- Poitevin, E. Official methods for the determination of minerals and trace elements in infant formula and milk products: A review. J. Aoac. Int. 2016, 99, 42–52. [Google Scholar] [CrossRef]
- Elmer, P. Atomic Spectroscopy Sample Preparation: Because Preparation is Everything; Perkin Elmer: Walham, MA, USA, 2013. [Google Scholar]
- Souza, J.P.; Cerveira, C.; Miceli, T.M.; Moraes, D.P.; Mesko, M.F.; Pereira, J.S. Evaluation of sample preparation methods for cereal digestion for subsequent As, Cd, Hg and Pb determination by AAS-based techniques. Food Chem. 2020, 321, 126715. [Google Scholar] [CrossRef]
- Anastácio, M.; dos Santos, A.M.; Aschner, M.; Mateus, L. Determination of trace metals in fruit juices in the Portuguese market. Toxicol. Rep. 2018, 5, 434–439. [Google Scholar] [CrossRef]
- De Andrade, C.K.; dos Anjos, V.E.; Felsner, M.L.; Torres, Y.R.; Quináia, S.P. Direct determination of Cd, Pb and Cr in honey by slurry sampling electrothermal atomic absorption spectrometry. Food Chem. 2014, 146, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Daşbaşı, T.; Saçmacı, Ş.; Ülgen, A.; Kartal, Ş. Determination of some metal ions in various meat and baby food samples by atomic spectrometry. Food Chem. 2016, 197, 107–113. [Google Scholar] [CrossRef]
- Dos Santos, W.N.; da Silva, E.G.; Fernandes, M.S.; Araujo, R.G.; Costa, A.N.C.; Vale, M.; Ferreira, S.L. Determination of copper in powdered chocolate samples by slurry-sampling flame atomic-absorption spectrometry. Anal. Bioanal. Chem. 2005, 382, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Pozzatti, M.; Nakadi, F.V.; Vale, M.G.R.; Welz, B. Simultaneous determination of nickel and iron in vegetables of Solanaceae family using high-resolution continuum source graphite furnace atomic absorption spectrometry and direct solid sample analysis. Microchem. J. 2017, 133, 162–167. [Google Scholar] [CrossRef]
- Gamela, R.R.; Duarte, Á.T.; Barrera, E.G.; Welz, B.; Dessuy, M.B.; da Silva, M.M.; Vale, M.G.R. Development of analytical methods for the determination of copper and manganese in infant formula using high resolution continuum source graphite furnace atomic absorption spectrometry and direct solid sample analysis. Anal. Methods 2017, 9, 2321–2327. [Google Scholar] [CrossRef]
- Dos Santos Silva, D.; dos Santos, C.S.; Pando, L.A.; Gomes, M.S.R.; Novaes, C.G.; dos Santos, W.N.L.; Bezerra, M.A. Doehlert design in the optimization of ultrasound assisted dissolution of fish fillet samples with tetramethyl ammonium hydroxide for metals determination using FAAS. Food Chem. 2019, 273, 71–76. [Google Scholar] [CrossRef]
- Almeida, J.S.; Santos, G.L.; Brandão, G.C.; Korn, M.G.; Teixeira, L.S. Multivariate optimization of ultrasound-assisted extraction using Doehlert matrix for simultaneous determination of Fe and Ni in vegetable oils by high-resolution continuum source graphite furnace atomic absorption spectrometry. Food Chem. 2019, 273, 130–135. [Google Scholar] [CrossRef]
- Souza, S.O.; Costa, S.S.L.; Brum, B.C.T.; Santos, S.H.; Garcia, C.A.B.; Araujo, R.G.O. Determination of nutrients in sugarcane juice using slurry sampling and detection by ICP OES. Food Chem. 2019, 273, 57–63. [Google Scholar] [CrossRef]
- Dos Santos Silva, E.; da Silva, E.G.P.; dos Santos Silva, D.; Novaes, C.G.; Amorim, F.A.C.; dos Santos, M.J.S.; Bezerra, M.A. Evaluation of macro and micronutrient elements content from soft drinks using principal component analysis and Kohonen self-organizing maps. Food Chem. 2019, 273, 9–14. [Google Scholar] [CrossRef]
- Hondrogiannis, E.; Peterson, K.; Zapf, C.; Roy, W.; Blackney, B.; Dailey, K. The use of wavelength dispersive X-ray fluorescence and discriminant analysis in the identification of the elemental composition of cumin samples and the determination of the country of origin. Food Chem. 2012, 135, 2825–2831. [Google Scholar] [CrossRef]
- Martın, M.J.; Pablos, F.; González, A. Characterization of green coffee varieties according to their metal content. Anal. Chim. Acta 1998, 358, 177–183. [Google Scholar] [CrossRef]
- Miller-Ihli, N. Trace element determinations in foods and biological samples using inductively coupled plasma atomic emission spectrometry and flame atomic absorption spectrometry. J. Agric. Food Chem. 1996, 44, 2675–2679. [Google Scholar] [CrossRef]
- Lachman, J.; Kolihova, D.; Miholova, D.; Košata, J.; Titěra, D.; Kult, K. Analysis of minority honey components: Possible use for the evaluation of honey quality. Food Chem. 2007, 101, 973–979. [Google Scholar] [CrossRef]
- Pustjens, A.; Muilwijk, M.; Weesepoel, Y.; van Ruth, S. Advances in authenticity testing of geographical origin of food products. In Advances in Food Authenticity Testing; Elsevier: London, UK, 2016; pp. 339–367. [Google Scholar]
- Zheng, Y.; Ruan, G.; Li, B.; Xiong, C.; Chen, S.; Luo, M.; Li, Y.; Du, F. Multicomposition analysis and pattern recognition of Chinese geographical indication product: vinegar. Eur. Food Res. Technol. 2014, 238, 337–344. [Google Scholar] [CrossRef]
- De Higuera, J.M.; da Silva, A.B.S.; de Oliveira, A.F.; de Araujo Nogueira, A.R. Multi-elemental determination in meat samples using multi-isotope calibration strategy by ICP-MS. Food Chem. 2020, 303, 125395. [Google Scholar] [CrossRef]
- Vasić, V.; Đurđić, S.; Tosti, T.; Radoičić, A.; Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Two aspects of honeydew honey authenticity: Application of advance analytical methods and chemometrics. Food Chem. 2020, 305, 125457. [Google Scholar] [CrossRef]
- Dell’Aquila, C.; Neal, A.L.; Shewry, P.R. Development of a reproducible method of analysis of iron, zinc and phosphorus in vegetables digests by SEC-ICP-MS. Food Chem. 2020, 308, 125652. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Marconi, E.; Protano, C.; Canepari, S. Comparative elemental analysis of dairy milk and plant-based milk alternatives. Food Control 2020, 107327. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Li, Z.; Deng, L. Analysis of 17 elements in cow, goat, buffalo, yak, and camel milk by inductively coupled plasma mass spectrometry (ICP-MS). RSC Adv. 2020, 10, 6736–6742. [Google Scholar] [CrossRef]
- Voica, C.; Iordache, A.M.; Ionete, R.E. Multielemental Characterisation of Honey Using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Fused with Chemometrics. J. Mass Spectrom. 2020, 55, e4512. [Google Scholar] [CrossRef]
- Spanu, A.; Langasco, I.; Valente, M.; Deroma, M.A.; Spano, N.; Barracu, F.; Pilo, M.I.; Sanna, G. Tuning of the amount of se in rice (oryza sativa) grain by varying the nature of the irrigation method: Development of an ICP-MS analytical protocol, validation and application to 26 different rice genotypes. Molecules 2020, 25, 1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potortì, A.G.; Bua, G.D.; Turco, V.L.; Tekaya, A.B.; Beltifa, A.; Mansour, H.B.; Dugo, G.; Di Bella, G. Major, minor and trace element concentrations in spices and aromatic herbs from Sicily (Italy) and Mahdia (Tunisia) by ICP-MS and multivariate analysis. Food Chem. 2020, 313, 126094. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, H.; Yu, C.; Tang, J.; Wu, W.; Yang, Q. Determination of the geographical origin of maize (Zea mays L.) using mineral element fingerprints. J. Sci. Food Agric. 2020, 100, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Syahfitri, W.Y.N.; Kurniawati, S.; Adventini, N.; Damastuti, E.; Lestiani, D.D. Macro elemental analysis of food samples by nuclear analytical technique. J. Phys. Conf. Ser. IOP Publ. 2017, 860, 012023. [Google Scholar] [CrossRef] [Green Version]
- Beaty, R.D.; Kerber, J.D. Concepts, Instrumentation and Techniques in Atomic Absorption Spectroscopy, 2nd ed.; The Perkin-Elmer Corporation: Norwalk CT, USA, 1993. [Google Scholar]
- Elmer, P. PinAAcle 900 Series Atomic Absorption Spectrometers. Available online: https://www.perkinelmer.com/lab-solutions/resources/docs/bro_pinaacle900family.pdf (accessed on 8 June 2020).
- Mohebbi, M.; Heydari, R.; Ramezani, M. Determination of Cu, Cd, Ni, Pb and Zn in edible oils using reversed-phase ultrasonic assisted liquid–liquid microextraction and flame atomic absorption spectrometry. J. Anal. Chem. 2018, 73, 30–35. [Google Scholar] [CrossRef]
- Zeiner, M.; Cindrić, I.J.; Kandler, W.; Stingeder, G. Trace determination of skin-irritating metals in tea tree oil by GFAAS. Microchem. J. 2018, 136, 101–105. [Google Scholar] [CrossRef]
- Sarojam, P. Analysis of Fish and Seafoods with AAnalyst 800 Atomic Absorption Spectrophotometer for Trace Metal Contamination, in Accordance with AOAC Methods 999.10 and 999.11; Perkin Elmer, Inc.: Shelton CT, USA, 2009. [Google Scholar]
- Sarojam, P. Analysis of Pb, Cd and As in Tea Leaves Using Graphite Furnace Atomic Absorption Spectrophotometry; Perkin Elmer, Inc.: Shelton CT, USA, 2011. [Google Scholar]
- Alves, M.M.; Medina, A.L.; Pinto, A.M.T.; Antunes, A.C.N.; Sanches Filho, P.J.; Ribeiro, A.S.; Vieira, M.A. Evaluation of the concentration of Cu, Zn, Pb and Cr in different fish species from the São Gonçalo Channel in Pelotas-RS, Brazil. J. Braz. Chem. Soc. 2018, 29, 285–296. [Google Scholar] [CrossRef]
- De Andrade, R.M.; de Gois, J.S.; Toaldo, I.M.; Batista, D.B.; Luna, A.S.; Borges, D.L. Direct determination of trace elements in meat samples via high-resolution graphite furnace atomic absorption spectrometry. Food Anal. Methods 2017, 10, 1209–1215. [Google Scholar] [CrossRef]
- Dos Santos, J.M.; Quináia, S.P.; Felsner, M.L. Fast and direct analysis of Cr, Cd and Pb in brown sugar by GF AAS. Food Chem. 2018, 260, 19–26. [Google Scholar] [CrossRef]
- Boss, C.B.; Fredeen, K.J. Concepts, Instrumentation and Techniques in Inductively Coupled Plasma Optical Emission Spectrometry, 3rd ed.; Perkin Elmer: Norwalk, CO, USA, 2004. [Google Scholar]
- Ozbek, N.; Akman, S. Microwave plasma atomic emission spectrometric determination of Ca, K and Mg in various cheese varieties. Food Chem. 2016, 192, 295–298. [Google Scholar] [CrossRef]
- Baker, S.A.; Miller-Ihli, N.J.; Fodor, P.; Woller, Á. Atomic spectroscopy in food analysis. Enc. Anal. Chem.: Appl. Theory Instrum. 2006. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Rathi, D.-N.; Liew, C.Y.; Fairulnizal, M.M.; Isameyah, D.; Barknowitz, G. Fat-soluble vitamin and carotenoid analysis in cooking oils by ultra-performance convergence chromatography. Food Anal. Methods 2017, 10, 1087–1096. [Google Scholar] [CrossRef]
- Fanali, C.; D’Orazio, G.; Fanali, S.; Gentili, A. Advanced analytical techniques for fat-soluble vitamin analysis. Trac. Trends Anal. Chem. 2017, 87, 82–97. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 992.06 Vitamin A (retinol) in milk-based infant formula. In AOAC Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- AOAC. AOAC Official Method 992.26 Vitamin D3 (cholecalciferol) in ready-to-feed milk-based infant formula. In AOAC Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- AOAC. AOAC Official Method 992.03 Vitamin E activity (all-rac-alpha-tocopherol) in milk-based infant formula. In AOAC Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- AOAC. AOAC Official Method 992.27 Trans-vitamin K1 (phylloquinone) in ready-to-feed milk-based infant formulas. In AOAC Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Gentili, A.; Miccheli, A.; Tomai, P.; Baldassarre, M.E.; Curini, R.; Pérez-Fernández, V. Liquid chromatography–tandem mass spectrometry method for the determination of vitamin K homologues in human milk after overnight cold saponification. J. Food Compos. Anal. 2016, 47, 21–30. [Google Scholar] [CrossRef]
- Jäpelt, R.B.; Jakobsen, J. Analysis of vitamin K1 in fruits and vegetables using accelerated solvent extraction and liquid chromatography tandem mass spectrometry with atmospheric pressure chemical ionization. Food Chem. 2016, 192, 402–408. [Google Scholar] [CrossRef]
- Köseoğlu, K.; Ulusoy, H.İ.; Yilmaz, E.; Soylak, M. Simple and sensitive determination of vitamin A and E in the milk and egg yolk samples by using dispersive solid phase extraction with newly synthesized polymeric material. J. Food Compos. Anal. 2020, 103482. [Google Scholar] [CrossRef]
- Rocchi, S.; Caretti, F.; Gentili, A.; Curini, R.; Perret, D.; Pérez-Fernández, V. Quantitative profiling of retinyl esters in milk from different ruminant species by using high performance liquid chromatography-diode array detection-tandem mass spectrometry. Food Chem. 2016, 211, 455–464. [Google Scholar] [CrossRef]
- Jiao, Z.; Jiao, S.; Guo, Z.; Chen, H.; Zhang, N.; Huang, W. Determination of trace vitamin D in milk samples by graphene-based magnetic solid-phase extraction method coupled with HPLC. Food Anal. Methods 2017, 10, 820–826. [Google Scholar] [CrossRef]
- Sereshti, H.; Toloutehrani, A.; Nodeh, H.R. Determination of cholecalciferol (vitamin D3) in bovine milk by dispersive micro-solid phase extraction based on the magnetic three-dimensional graphene-sporopollenin sorbent. J. Chromatogr. B 2020, 1136, 121907. [Google Scholar] [CrossRef]
- Kamankesh, M.; Mohammadi, A.; Mollahosseini, A.; Jazaeri, S.; Shahdoostkhany, M. Vitamin D3: Preconcentration and determination in cereal samples using ultrasonic-assisted extraction and microextraction method. Cereal Chem. 2017, 94, 532–538. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; Spagnoli, M.; Rocco, A.; Aturki, Z.; Sciubba, F.; De Salvador, F.R.; Engel, P.; Curini, R.; Gentili, A. Non-aqueous reversed-phase liquid-chromatography of tocopherols and tocotrienols and their mass spectrometric quantification in pecan nuts. J. Food Compos. Anal. 2017, 64, 171–180. [Google Scholar] [CrossRef]
- Sýs, M.; Švecová, B.; Švancara, I.; Metelka, R. Determination of vitamin E in margarines and edible oils using square wave anodic stripping voltammetry with a glassy carbon paste electrode. Food Chem. 2017, 229, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.; Fabritius, M.; Yang, B. Determination of vitamin K composition of fermented food. Food Chem. 2019, 275, 515–522. [Google Scholar] [CrossRef]
- Sýs, M.; Jashari, G.; Švecová, B.; Arbneshi, T.; Metelka, R. Determination of vitamin K1 using square wave adsorptive stripping voltammetry at solid glassy carbon electrode. J. Electroanal. Chem. 2018, 821, 10–15. [Google Scholar] [CrossRef]
- Woollard, D.C.; Bensch, A.; Indyk, H.; McMahon, A. Determination of vitamin A and vitamin E esters in infant formulae and fortified milk powders by HPLC: Use of internal standardisation. Food Chem. 2016, 197, 457–465. [Google Scholar] [CrossRef]
- Oberson, J.-M.; Campos-Giménez, E.; Rivière, J.; Martin, F. Application of supercritical fluid chromatography coupled to mass spectrometry to the determination of fat-soluble vitamins in selected food products. J. Chromatogr. B 2018, 1086, 118–129. [Google Scholar] [CrossRef]
- Rathi, D.-N.; Noh, M.F.M.; Rashed, A.A.; Dasuki, I. Simultaneous analysis of vitamin D and K in processed food products via ultra high-performance liquid chromatography (UHPLC). J. Food Meas. Charact. 2019, 13, 1947–1957. [Google Scholar] [CrossRef]
- Cellar, N.A.; McClure, S.C.; Salvati, L.M.; Reddy, T.M. A new sample preparation and separation combination for precise, accurate, rapid, and simultaneous determination of vitamins B1, B2, B3, B5, B6, B7, and B9 in infant formula and related nutritionals by LC-MS/MS. Anal. Chim. Acta 2016, 934, 180–185. [Google Scholar] [CrossRef]
- Melfi, M.T.; Nardiello, D.; Cicco, N.; Candido, V.; Centonze, D. Simultaneous determination of water-and fat-soluble vitamins, lycopene and beta-carotene in tomato samples and pharmaceutical formulations: Double injection single run by reverse-phase liquid chromatography with UV detection. J. Food Compos. Anal. 2018, 70, 9–17. [Google Scholar] [CrossRef]
- Cimpoiu, C.; Hosu, A. Thin layer chromatography for the analysis of vitamins and their derivatives. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 701–728. [Google Scholar] [CrossRef]
- Fatima, Z.; Jin, X.; Zou, Y.; Kaw, H.Y.; Quinto, M.; Li, D. Recent trends in analytical methods for water-soluble vitamins. J. Chromatogr. A 2019, 1606, 360245. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.; Jamroz, J. Precision of dehydroascorbic acid quantitation with the use of the subtraction method–Validation of HPLC–DAD method for determination of total vitamin C in food. Food Chem. 2015, 173, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef]
- Cunha-Santos, E.C.E.; Viganó, J.; Neves, D.A.; Martínez, J.; Godoy, H.T. Vitamin C in camu-camu [Myrciaria dubia (HBK) McVaugh]: evaluation of extraction and analytical methods. Food Res. Int. 2019, 115, 160–166. [Google Scholar] [CrossRef]
- Taujenis, L.; Olšauskaitė, V.; Padarauskas, A. Comparison of RPLC and HILIC coupled with tandem mass spectrometry for the determination of ascorbic and dehydroascorbic acids in fruits. Chemija 2016, 27, 52–59. [Google Scholar]
- Mazurek, A.; Włodarczyk-Stasiak, M.; Pankiewicz, U.; Kowalski, R.; Jamroz, J. Development and validation of a differential pulse polarography method for determination of total vitamin C and dehydroascorbic acid contents in foods. LWT 2020, 118, 108828. [Google Scholar] [CrossRef]
- Elgailani, I.E.H.; Elkareem, M.; Noh, E.; Adam, O.; Alghamdi, A. Comparison of two methods for the determination of vitamin C (ascorbic acid) in some fruits. Am. J. Chem. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Liddicoat, C.; Hucker, B.; Liang, H.; Vriesekoop, F. Thiamin analysis in red wine by fluorescence reverse phase-HPLC. Food Chem. 2015, 177, 325–329. [Google Scholar] [CrossRef]
- Schmidt, A.; Pratsch, H.; Schreiner, M.G.; Mayer, H.K. Determination of the native forms of vitamin B1 in bovine milk using a fast and simplified UHPLC method. Food Chem. 2017, 229, 452–457. [Google Scholar] [CrossRef]
- Fracassetti, D.; Limbo, S.; D’Incecco, P.; Tirelli, A.; Pellegrino, L. Development of a HPLC method for the simultaneous analysis of riboflavin and other flavin compounds in liquid milk and milk products. Eur. Food Res. Technol. 2018, 244, 1545–1554. [Google Scholar] [CrossRef]
- Wu, M.; Gao, F.; Zhang, Y.; Wang, Q.; Li, H. Sensitive analysis of amino acids and vitamin B3 in functional drinks via field-amplified stacking with reversed-field stacking in microchip electrophoresis. Talanta 2015, 131, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Çatak, J. Determination of niacin profiles in some animal and plant based foods by high performance liquid chromatography: association with healthy nutrition. J. Anim. Sci. Technol. 2019, 61, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebiedzińska, A.; Marszałł, M.L.; Grembecka, M.; Czaja, J.; Szefer, P.; Kuta, J.; Garabato, B.D.; Kozlowski, P.M. Detection of B 6 Vitamers in Grain Products: Experimental and Computational Studies. Food Anal. Methods 2018, 11, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Uzuriaga-Sánchez, R.J.; Khan, S.; Wong, A.; Picasso, G.; Pividori, M.I.; Sotomayor, M.D.P.T. Magnetically separable polymer (Mag-MIP) for selective analysis of biotin in food samples. Food Chem. 2016, 190, 460–467. [Google Scholar] [CrossRef]
- Rodríguez, D.; Torres, L.; Parra, J. Analysis of folic acid in white rice by ultra-fast liquid chromatography. J. Phys. Conf. Ser. IOP Publ. 2019, 1388, 012037. [Google Scholar] [CrossRef]
- Ložnjak, P.; García-Salinas, C.; de la Garza, R.I.D.; Bysted, A.; Jakobsen, J. The use of a plant enzyme for rapid and sensitive analysis of naturally-occurring folates in food by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1594, 34–44. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; Gentili, A.; Martinelli, A.; Caretti, F.; Curini, R. Evaluation of oxidized buckypaper as material for the solid phase extraction of cobalamins from milk: Its efficacy as individual and support sorbent of a hydrophilic–lipophilic balance copolymer. J. Chromatogr. A 2016, 1428, 255–266. [Google Scholar] [CrossRef]
- Nakos, M.; Pepelanova, I.; Beutel, S.; Krings, U.; Berger, R.; Scheper, T. Isolation and analysis of vitamin B12 from plant samples. Food Chem. 2017, 216, 301–308. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Li, J.; Li, L.; Yuan, X.; Xu, L.; Shi, Z.-g. Fast separation of water-soluble vitamins by hydrophilic interaction liquid chromatography based on submicrometer flow-through silica microspheres. Food Chem. 2020, 307, 125531. [Google Scholar] [CrossRef]
- Ostovan, A.; Ghaedi, M.; Arabi, M.; Yang, Q.; Li, J.; Chen, L. Hydrophilic multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B-family vitamins. ACS Appl. Mater. Interfaces 2018, 10, 4140–4150. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.A.; Young, M.F.; Taneja, S.; Rangiah, K. Quantification of B-vitamins from different fresh milk samples using ultra-high performance liquid chromatography mass spectrometry/selected reaction monitoring methods. J. Chromatogr. A 2020, 1609, 460452. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Sagratini, G.; Vittori, S.; Torregiani, E. Optimization of an extraction procedure for the simultaneous quantification of riboflavin, nicotinamide and nicotinic acid in anchovies (Engraulis enrasicolus) by high-performance liquid chromatography–tandem mass spectrometry. J. Food Compos. Anal. 2018, 66, 23–29. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Karimi-Maleh, H.; Khoshnama, Z.; Hassankhani, A.; Abbasghorbani, M. A voltammetric sensor for simultaneous determination of vitamin C and vitamin B 6 in food samples using ZrO2 nanoparticle/ionic liquids carbon paste electrode. Food Anal. Methods 2015, 8, 549–557. [Google Scholar] [CrossRef]
- Navarro-Pascual-Ahuir, M.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M. Determination of water-soluble vitamins in energy and sport drinks by micellar electrokinetic capillary chromatography. Food Control 2016, 63, 110–116. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 967.21.Vitamin C in juices and vitamin preparations. In AOAC Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005; p. 45.41.14. [Google Scholar]
- De Quirós, A.R.-B.; Fernández-Arias, M.; López-Hernández, J. A screening method for the determination of ascorbic acid in fruit juices and soft drinks. Food Chem. 2009, 116, 509–512. [Google Scholar] [CrossRef]
- Schmidt, A.; Schreiner, M.; Mayer, H. Rapid determination of the various native forms of vitamin B6 and B2 in cow’s milk using ultra-high performance liquid chromatography. J. Chromatogr. A 2017, 1500, 89–95. [Google Scholar] [CrossRef]
- Blake, C.J. Analytical procedures for water-soluble vitamins in foods and dietary supplements: A review. Anal. Bioanal. Chem. 2007, 389, 63–76. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Laskowski, W.; Kulykovets, O.; Kudlińska-Chylak, A.; Czeczotko, M.; Rejman, K. Food products as sources of protein and amino acids—The case of Poland. Nutrients 2018, 10, 1977. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: a review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sharma, A.; Kohli, S.K.; Yadav, P.; Bali, S.; Bakshi, P.; Parihar, R.D.; Yuan, H.; Yan, D.; He, Y. Amino acids distribution in economical important plants: a review. Biotechnol. Res. Innov. 2019, 3, 197–207. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, G. Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv. Nutr. 2017, 8, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ueno, H.; Kikuzaki, H. Construction of a free-form amino acid database for vegetables and mushrooms. Integr. Food Nutr. Metab. 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Ueno, H.; Kikuzaki, H. Free amino acid compositions for fruits. J. Nutr. Diet. Pr. 2017, 1, 1–5. [Google Scholar]
- Rutherfurd, S.M.; Dunn, B.M. Quantitative amino acid analysis. Curr. Protoc. Protein Sci. 2011, 63, 2–3. [Google Scholar] [CrossRef]
- Otter, D.E. Standardised methods for amino acid analysis of food. Br. J. Nutr. 2012, 108, S230–S237. [Google Scholar] [CrossRef] [Green Version]
- AOAC. AOAC Official Method 985.28. Sulfur amino acids in food and feed ingredients. In AOAC Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 1988. [Google Scholar]
- AOAC. AOAC Official Method 2018.06. Total Amino Acids in Infant Formulas and Adult Nutritionals. In AOAC Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 1988. [Google Scholar]
- AOAC. AOAC Official Method 2019. Total proteinogenic amino acids and taurine. In AOAC Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Corleto, K.A.; Singh, J.; Jayaprakasha, G.; Patil, B.S. A sensitive HPLC-FLD method combined with multivariate analysis for the determination of amino acids in l-citrulline rich vegetables. J. Food Drug Anal. 2019, 27, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, Z.; Liu, Z.; Feng, Z.; Zhang, L.; Wan, X.; Yang, X. Identification of d-amino acids in tea leaves. Food Chem. 2020, 317, 126428. [Google Scholar] [CrossRef]
- Dong, M.; Qin, L.; Xue, J.; Du, M.; Lin, S.-Y.; Xu, X.-B.; Zhu, B.-W. Simultaneous quantification of free amino acids and 5′-nucleotides in shiitake mushrooms by stable isotope labeling-LC-MS/MS analysis. Food Chem. 2018, 268, 57–65. [Google Scholar] [CrossRef]
- Redruello, B.; Ladero, V.; Del Rio, B.; Fernández, M.; Martín, M.C.; Alvarez, M.A. A UHPLC method for the simultaneous analysis of biogenic amines, amino acids and ammonium ions in beer. Food Chem. 2017, 217, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rojas, J.M.; Moreno-Ortega, A.; Ordóñez, J.L.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Pereira-Caro, G. Development and validation of UHPLC-HRMS methodology for the determination of flavonoids, amino acids and organosulfur compounds in black onion, a novel derived product from fresh shallot onions (Allium cepa var. aggregatum). LWT 2018, 97, 376–383. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Papageorgiou, M.; Kalogiannis, S. Validation of a HILIC UHPLC-MS/MS method for amino acid profiling in triticum species wheat flours. Foods 2019, 8, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Zhao, F.; Chen, M.; Ye, N.; Lin, Q.; Ouyang, L.; Cai, X.; Meng, P.; Gong, X.; Wang, Y. Determination of 21 free amino acids in 5 types of tea by ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC–MS/MS) using a modified 6-aminoquinolyl-I-hydroxysuccinimidyl carbamate (AQC) method. J. Food Compos. Anal. 2019, 81, 46–54. [Google Scholar] [CrossRef]
- Majidano, S.; Khuhawar, M.; Zounr, R.; Channar, A.; Jahangir, T.; Mughal, M. Determination of amino acids in jams, fruits and pharmaceutical preparations by gas chromatography using trifluoroacetylacetone and ethylchloroformate as derivatizing reagents. Anal. Methods 2015, 7, 3148–3156. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Barroso, M.A.; Ruiz, J.; Antequera, T. A rapid and accurate extraction procedure for analysing free amino acids in meat samples by GC-MS. Int. J. Anal. Chem. 2015, 2015, 209214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menestrina, F.; Grisales, J.O.; Castells, C.B. Chiral analysis of derivatized amino acids from kefir by gas chromatography. Microchem. J. 2016, 128, 267–273. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Seraglio, S.K.T.; Rocha, G.; Balderas, C.B.; Piovezan, M.; Gonzaga, L.V.; de Barcellos Falkenberg, D.; Fett, R.; de Oliveira, M.A.L.; Costa, A.C.O. Free amino acid determination by GC-MS combined with a chemometric approach for geographical classification of bracatinga honeydew honey (Mimosa scabrella Bentham). Food Control 2017, 78, 383–392. [Google Scholar] [CrossRef]
- Jastrzębska, A.; Piasta, A.M.; Szłyk, E. Optimization of cheese sample preparation methodology for free amino acid analysis by capillary isotachophoresis. J. Food Compos. Anal. 2015, 40, 136–142. [Google Scholar] [CrossRef]
- Omar, M.M.A.; Elbashir, A.A.; Schmitz, O.J. Capillary electrophoresis method with UV-detection for analysis of free amino acids concentrations in food. Food Chem. 2017, 214, 300–307. [Google Scholar] [CrossRef]
- Luo, T.; Ke, J.; Xie, Y.; Dong, Y. Determination of underivatized amino acids to evaluate quality of beer by capillary electrophoresis with online sweeping technique. J. Food Drug Anal. 2017, 25, 789–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, T.; Xie, Y.; Dong, Y.; Liu, A.; Dong, Y. Quality assessment of soy sauce using underivatized amino acids by capillary electrophoresis. Int. J. Food Prop. 2017, 20, S3052–S3061. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 982.3.00. In AOAC Official Methods of Analysis, 17th ed.; Gaithersburg, MD, USA, 2000; p. 45.43.05. [Google Scholar]
- Jaudzems, G.; Guthrie, J.; Lahrichi, S.; Fuerer, C. Total amino acids by UHPLC–UV in infant formulas and adult nutritionals, first action 2018.06. J. Aoac. Int. 2019, 102, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Mustățea, G.; Ungureanu, E.; Iorga, E. Protein acidic hydrolysis for amino acids analysis in food-progress over time: a short review. J. Hyg. Eng. Des. 2019, 26, 81–87. [Google Scholar]
- Rutherfurd, S.M.; Gilani, G.S. Amino acid analysis. Curr. Protoc. Protein Sci. 2009, 58, 9–11. [Google Scholar] [CrossRef]
- Themelis, T.; Gotti, R.; Orlandini, S.; Gatti, R. Quantitative amino acids profile of monofloral bee pollens by microwave hydrolysis and fluorimetric high performance liquid chromatography. J. Pharm. Biomed. Anal. 2019, 173, 144–153. [Google Scholar] [CrossRef]
- Bedin, S.; Zanella, K.; Bragagnolo, N.; Taranto, O.P. Implication of Microwaves on the Extraction Process of Rice Bran Protein. Braz. J. Chem. Eng. 2019, 36, 1653–1665. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Wu, Z.; Jia, S.; Wu, G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J. Chromatogr. B 2014, 964, 116–127. [Google Scholar] [CrossRef]
- Zhao, D.; Li, S.; Han, X.; Li, C.; Ni, Y.; Hao, J. Physico-chemical properties and free amino acids profiles of six wolfberry cultivars in Zhongning. J. Food Compos. Anal. 2020, 88, 103460. [Google Scholar] [CrossRef]
- Ferré, S.; González-Ruiz, V.; Guillarme, D.; Rudaz, S. Analytical strategies for the determination of amino acids: past, present and future trends. J. Chromatogr. B 2019, 121819. [Google Scholar] [CrossRef]
- Lu, W.; Bennett, B.D.; Rabinowitz, J.D. Analytical strategies for LC–MS-based targeted metabolomics. J. Chromatogr. B 2008, 871, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazier, R.A.; Papadopoulou, A. Recent advances in the application of capillary electrophoresis for food analysis. Electrophoresis 2003, 24, 4095–4105. [Google Scholar] [CrossRef] [PubMed]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Free fatty acids quantification in dairy products. Int. J. Dairy Technol. 2016, 69, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Odegaard, A.O. Dietary fatty acids in the etiology of type 2 diabetes. In Nutrition and Type 2 Diabetes: Etiology and Prevention; Taylor and Francis Group: Abingdon, UK, 2013; p. 55. [Google Scholar]
- Liu, Z.; Ezernieks, V.; Rochfort, S.; Cocks, B. Comparison of methylation methods for fatty acid analysis of milk fat. Food Chem. 2018, 261, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L. Dietary cholesterol and cardiovascular risk: A science advisory from the american heart association. Circulation 2019, 141, e39–e53. [Google Scholar] [CrossRef] [Green Version]
- Mihai, A.L.; Negoita, M.; Belc, N. Evaluation of fatty acid profile of oils/fats by GC-MS through two quantification approaches. Rom. Biotech. Lett. 2019, 24, 973–985. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 989.05. Fat in milk. Modified mojonnier ether extraction method. In AOAC Official Method of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- AOAC. AOAC official method 969.33 Fatty acids in oils and fats. Preparation of methyl esters. Boron trifluoride method. In AOAC Official Methods of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Mannion, D.T.; Furey, A.; Kilcawley, K.N. Development and validation of a novel free fatty acid butyl ester gas chromatography method for the determination of free fatty acids in dairy products. J. Agric. Food Chem. 2018, 67, 499–506. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Mantzourani, C.; Babaiti, R.; Kokotos, G. Study of the royal jelly free fatty acids by liquid chromatography-high resolution mass spectrometry (LC-HRMS). Metabolites 2020, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Agnew, M.P.; Craigie, C.R.; Weralupitiya, G.; Reis, M.M.; Johnson, P.L.; Reis, M.G. Comprehensive evaluation of parameters affecting one-step method for quantitative analysis of fatty acids in meat. Metabolites 2019, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Salimon, J.; Omar, T.A.; Salih, N. An accurate and reliable method for identification and quantification of fatty acids and trans fatty acids in food fats samples using gas chromatography. Arab. J. Chem. 2017, 10, S1875–S1882. [Google Scholar] [CrossRef] [Green Version]
- Naviglio, D.; Dellagreca, M.; Ruffo, F.; Andolfi, A.; Gallo, M. Rapid analysis procedures for triglycerides and fatty acids as pentyl and phenethyl esters for the detection of butter adulteration using chromatographic techniques. J. Food Qual. 2017, 2017, 9698107. [Google Scholar] [CrossRef]
- Parks, P.; Goins, R. In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in food. J. Food Sci. 1994, 59, 1262–1266. [Google Scholar] [CrossRef]
- Trigueros, L.; Sendra, E. Fatty acid and conjugated linoleic acid (CLA) content in fermented milks as assessed by direct methylation. LWT-Food Sci. Technol. 2015, 60, 315–319. [Google Scholar] [CrossRef]
- Masson, L.; Alfaro, T.; Camilo Manríquez, C.; Carvalho, A.; Illesca, P.; Torres, R.; Tavares do Carmo, M.; Mancini Filho, J.; Bernal, C. Fatty acid composition of soybean/sunflower mix oil, fish oil and butterfat applying the AOCS Ce 1j-07 method with a modified temperature program. Grasas Y Aceites 2015, 66, 1–16. [Google Scholar]
- Fan, H.; Smuts, J.; Bai, L.; Walsh, P.; Armstrong, D.W.; Schug, K.A. Gas chromatography–vacuum ultraviolet spectroscopy for analysis of fatty acid methyl esters. Food Chem. 2016, 194, 265–271. [Google Scholar] [CrossRef]
- Talebi, M.; Patil, R.A.; Sidisky, L.M.; Berthod, A.; Armstrong, D.W. Branched-chain dicationic ionic liquids for fatty acid methyl ester assessment by gas chromatography. Anal. Bioanal. Chem. 2018, 410, 4633–4643. [Google Scholar] [CrossRef]
- Zhou, T.; Leng, J.; Peng, Y.; Zhang, L.; Guo, Y. Mass spectrometric analysis of free fatty acids in infant milk powders by frozen pretreatment coupled with isotope-labeling derivatization. J. Sep. Sci. 2016, 39, 873–879. [Google Scholar] [CrossRef]
- Qiu, J.; Hou, H.-Y.; Yang, I.-S.; Chen, X.-B. Raman spectroscopy analysis of free fatty acid in olive oil. Appl. Sci. 2019, 9, 4510. [Google Scholar] [CrossRef] [Green Version]
- Martha, R.; Toharmat, T.; Rofiah, N.; Anggraeni, D. Comparison of extraction methods for fatty acid and conjugated linoleic acid quantification in milk. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 546, p. 042022. [Google Scholar]
- Kokotou, M.G.; Mantzourani, C.; Kokotos, G. Development of a liquid chromatography–high resolution mass spectrometry method for the determination of free fatty acids in milk. Molecules 2020, 25, 1548. [Google Scholar] [CrossRef] [Green Version]
- Grasso, S.; Harrison, S.M.; Monahan, F.J.; Brunton, N.P. A validated method for cholesterol determination in turkey meat products using relative response factors. Foods 2019, 8, 684. [Google Scholar] [CrossRef] [Green Version]
- Puertas, G.; Vázquez, M. Cholesterol determination in egg yolk by UV-VIS-NIR spectroscopy. Food Control 2019, 100, 262–268. [Google Scholar] [CrossRef]
- Albuquerque, T.G.; Oliveira, M.B.P.; Sanches-Silva, A.; Costa, H.S. Cholesterol determination in foods: Comparison between high performance and ultra-high performance liquid chromatography. Food Chem. 2016, 193, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Adu, J.K.; Amengor, C.D.; Kabiri, N.; Orman, E.; Patamia, S.A.G.; Okrah, B.K. Validation of a simple and robust liebermann–burchard colorimetric method for the assay of cholesterol in selected milk products in ghana. Int. J. Food Sci. 2019, 2019, 9045938. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Hassan, M.F.; Rauf, A. Determination of trans fat in selected fast food products and hydrogenated fats of India using attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy. J. Oleo Sci. 2017, 66, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Tarhan, İ.; Ismail, A.A.; Kara, H. Quantitative determination of free fatty acids in extra virgin olive oils by multivariate methods and Fourier transform infrared spectroscopy considering different absorption modes. Int. J. Food Prop. 2017, 20, S790–S797. [Google Scholar] [CrossRef]
- Jiang, R.; Jiao, Y.; Zhang, P.; Liu, Y.; Wang, X.; Huang, Y.; Zhang, Z.; Xu, F. Twin derivatization strategy for high-coverage quantification of free fatty acids by liquid chromatography–tandem mass spectrometry. Anal. Chem. 2017, 89, 12223–12230. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J. Pharm. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-H.; Dutkiewicz, E.P.; Huang, Y.-C.; Zhou, H.-B.; Hsu, C.-C. Analytical methods for cholesterol quantification. J. Food Drug Anal. 2019, 27, 375–386. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Method 994.10. Cholesterol in foods-direct saponification-gas chromatographic methods. In AOAC Official Methods of Analysis; AOAC International: Arlington, VA, USA, 1996. [Google Scholar]
- Kamelska, A.M.; Jarmołowska, B.; Bryl, K. A simplified enzymatic method for total cholesterol determination in milk. Int. Dairy J. 2015, 50, 50–57. [Google Scholar] [CrossRef]
- Charrondiere, U.R.; Stadlmayr, B.; Wijesinha-Bettoni, R.; Rittenschober, D.; Nowak, V.; Burlingame, B. INFOODS contributions to fulfilling needs and meeting challenges concerning food composition databases. Procedia Food Sci. 2013, 2, 35–45. [Google Scholar] [CrossRef] [Green Version]
| Sample Preparation | Instrument | Food Matrix | Advantages of Current Improved Technique | Ref. |
|---|---|---|---|---|
| Moisture | ||||
| Heating of sample through absorption of IR radiation from a halogen radiator. Continual determination of mass during drying process. The moisture content percentage is determined from the difference in weight before and after drying. | Halogen moisture analysers | All type of food matrix | Highly energy-efficient, less water-consuming, and environmentally friendly compared to conventional heating. Further, it is also characterized by homogeneity of heating, high heat transfer rate, low heating time, low energy consumption, improved product quality, and food safety | [9] |
| Weighed and spread the sample as thin layer in a Petri dish before placing on the circular asbestos sheet nearby the center. Heated at different watts of absorbed microwave heating (MW) power output settings. Prior to obtaining the weight, MW dried sample was stored in a desiccator containing silica gel to decrease the surface moisture and high temperature development in the sample. The weight loss after each MW drying was expressed as the apparent moisture content (m.c.) of the samples. | Microwave oven | Paddy varieties | An extremely rapid method of drying up a sample but the temperatures achieved is very high, making it suitable only for very thermostable materials. It is also not useful if the moisture content is below 2%. | [10] |
| Samples were evenly spread in a glass Petri dish. A circular black paper was placed at the bottom of the Petri dish under the samples to avoid specular reflections from the bottom of the dish. The Petri dish was placed on the turntable, the turn table was set in motion and the reflectance spectrum was recorded while scanning the sample along a wide periphery within the Petri dish. Reflectance spectra were collected in the wave-length range between 1000 nm and 1800 nm, at 1 nm intervals. An integration time of 10 ms was used throughout the measurements | NIR | Cereal grains | Demonstrated reliable prediction of wheat composition directly on the whole kernels, which represented a great advance with benefits in terms of sample preparation, cost, and applicability | [11] |
| The experiments were carried out with two unilateral magnets. One of the magnets (denoted as magnet A). The other magnet (magnet B) has a non-linear magnetic field. Magnet B features a high signal-to-noise ratio (SNR) due to a large sensitive spot. Both magnets were integrated with a Bruker Minispec console. The samples were packed into 1 × 1 × 4.5 cm plastic prism containers, chosen to fit the linear gradient region of magnet A for testing the diffusion-weighted methodology. | NMR | Beverages, oils and lipids, vegetables, meat, and dairy products | A robust method, which can rapidly analyse mixtures at the molecular level without requiring separation and/or purification steps | [12] |
| Total Protein | ||||
| A sample of known mass is combusted in a high temperature (about 900 °C) chamber in the presence of oxygen. This leads to the release of carbon dioxide, water, and nitrogen. The nitrogen content is then measured by passing the remaining gasses through a column that has a thermal conductivity detector at the end. Thus, the signal from the thermal conductivity detector can be converted into nitrogen content. | Enhanced Dumas method | All food matrix | It is much faster than the Kjeldahl method (under 4 min per measurement, compared to 1–2 h for Kjeldahl). It doesn’t need toxic chemicals or catalysts. Many samples can be measured automatically. It is easy to use. | [13] |
| Total Fat | ||||
| Liquid-phase MAE process is based upon the ability of a matrix to absorb microwave energy. | MAE | Cheese | MAE offers a range of benefits over other solvent extraction methods, since MAE is faster and more effective, has lower consumption of energy and solvents, and, above all, uses less toxic solvents. It performs two steps in only a single step, i.e., hydrolysis and extraction simultaneously | [14] |
| Total Dietary Fibre (TDF) | ||||
| RITDF method combines the key attributes of AOAC Official Methods 2002.02 [15], 985.29 [16], 991.43 [17], 2001.03 [18] and 2009.01 [19]. | Integrated Total Dietary Fiber Assay Kit | All food matrices | RITDF method is more accurate because it is specifically designed to overcome both potential inaccuracies: the double measurement of some fibres and the lack of measurement of other fibres. Since RITDF method improves the accuracy of fibre analysis, the determination of available carbohydrate will also be more accurate. This test may replace the need for multiple tests, highlights possibility for potential savings. | [20] |
| Ash | ||||
| Depends on sample preparation and measurement method. First—the samples were covered with a slide window and clamped to the ATR diamond crystal using pressure gauges; second—the samples were placed on the ATR diamond crystal and clamped using pressure gauges. The tight fit of the ATR clamp head shape to the gap above the crystal allowed an accurate and even coverage of the crystal with a thin layer of the sample. | ATR-FTIR | Vegetable/Plant | In general, the proposed method requires a small drop/amount of sample on the ATR base-plate reagent consumption, being much faster than traditional techniques, allowing potential applications for simultaneous determination of sulphur, nitrogen and ash contents for routine analysis of plant/vegetable tannins by FTIR data. | [21] |
| Total Sugar | ||||
| Date syrup was dried before dissolving in pyridine and placed in an ultrasonic bath. The sample was mixed well by vortex and centrifuged to remove any insoluble materials. Part of the supernatant was taken for the oximation-silylation step. | GC-MS | Date juice (possibility of other food matrices) | Rapid sugar identification GCMS results determined the appropriate enzymatic assays for quantifying the sugars in date juice. These results were similar to those of the two enzymatic methods (standard enzymatic assay and measuring the change in pH by CL10 analyser). | [22] |
| Sample Preparation | Instrument | Application/Food Matrix | Advantages of Current Improved Technique | Ref. |
|---|---|---|---|---|
| Open Digestion Open vessel hot block digestion uses atmospheric pressure digestion for extraction of high throughput samples [33]. Microwave 1. Microwave assisted-digestion (MW-AD) 2. Microwave induced-combustion (MIC) Slurry Sampling A technique of direct sample preparation applicable only for Graphite Furnace AAS. | Flame & Graphite Furnace AAS | Brown sugar, wine, fruit juice, honey, meat and baby foods, chocolate, vegetables, infant formula, fish fillet, vegetable oil | Open digestion offers high throughput, AAS provides high sensitivity, good precision, low cost, relative simplicity | [35,36,37,38,39,40,41,42] |
| ICP-OES/AES | Almonds kernel, tea leaves, coffee, cereals, mussel tissues, mushrooms, seafood, cow’s milk, legumes, wine, nuts, cheese, onion, garlic, honey, barley, bread, fish, sugarcane juice, soft drinks | MW-AD able to digest difficult food samples matrix quickly, completely, minimum loss of volatile compounds and reduces risk of contamination. MIC uses diluted solutions and lower reagent comsumption accordance with green chemistry recommendations. Slurry Sampling capable minimising drawbacks of manual and automated sample digestion. ICP-OES/AES provides rapid elemental determination techniques, Multiple elements can be analysed from small volume of samples, Refractory samples that are lower concentration can also be determined, By using plasma source, non-metals can be determined | [43,44,45,46,47,48,49,50] | |
| ICP-MS | Meat, honeydew honey, vegetables, milk, rice, spices and aromatic herbs. maize | High sensitivity for trace element detection, multi-elemental and isotopic analysis, and high sample throughput. | [51,52,53,54,55,56,57,58,59] | |
| Dried samples powder was pressed until the surface was homogenous and ready for analysis. | ED-XRF | Fruits and vegetables, cumin spice | Simple sample preparation, direct measurement, Multi-element analysis, fast analysis. | [45,60] |
| Sample Preparation | Instrument | Food Matrix | Advantages of Current Improved Technique | Ref. |
|---|---|---|---|---|
| Vitamin A | ||||
| Deproteinization with ethanol followed by direct hexane extraction | HPLC-DAD-MS/MS-APCI (+)-NARP | Cow, buffalo, goat and ewe’s milk | Novel analytical method with increased selectivity, sensitivity for characterization of retinoic acid, retinal, retinol and fourteen retinyl esters. | [83] |
| Vitamin D | ||||
| Graphene-coated magnetic particle (Fe3O4@Graphene based Magnetic Solid-Phase Extraction (MSPE) | HPLC-UV | Milk | Reduced time, lower consumption of organic solvent, improved sensitivity and accuracy, eliminates the need for protein removal prior to extraction of vitamin D (D2, D3). | [84] |
| Magnetic three-dimensional graphene-sporopollenin sorbent (3DG-Fe3O4@Sp) based dispersive micro-solid phase extraction (MD-μ-SPE) | HPLC-UV | Bovine milk | New sorbent material (3DG-Fe3O4@Sp) is synthesised and applied for the extraction of vitamin D3. Proposed technique is advantageous for its low solvent consumption, low sorbent dose, as well as rapid extraction and analysis. | [85] |
| Ultrasound assisted extraction (UAE) followed by DLLME | HPLC-UV | Wheat flour, bread | Accurate, precise, reliable sample pre-treatment method with reduced sample-matrix interference and good detection limit for trace levels of vitamin D3. | [86] |
| Vitamin E | ||||
| Overnight cold saponification followed by LLE with hexane | HPLC-APCI (+)-MS/MS-isocractic NARP | Pecan nuts | Simultaneous quantification of four tocopherols and tocotrienols, each with a highly sensitive method that explains detection of minor homologues (δ-tocopherols and tocotrienols) for the first time. | [87] |
| Extraction into silicone oil, acting as lipophilic binder of GCPE | ED by SWASV | Margarines and edible oils | Results obtained are comparable to HPLC, with respect to total tocopherol content. Electrochemical approach provides an option for easy sample preparation, rapid and cheaper analysis. | [88] |
| Vitamin K | ||||
| Overnight cold saponification followed by LLE with hexane | HPLC-APCI (+)-MS/MS-NARP | Human milk | Simultaneous detection of phylloquinone (vitamin K1), menaquinone-4 (MK-4) and menaquinone-7 (MK-7) in human milk with high accuracy and precision. Utilizes fewer samples with a simplified and inexpensive extraction procedure. | [80] |
| ASE system followed by extract clean-up via SPE | LC-APCI-MS/MS | Fruits and vegetables | Combination of ASE and LC-APCI-MS/MS technique provides a sensitive, selective and rapid approach for vitamin K1 analysis in fruits and vegetables. | [81] |
| Ultrasonic assisted solvent extraction and SPE | UHPLC-APCI (+)-MS/MS | Fermented foods | Minimal use of chlorinated solvents and columns with smaller core shell particles dimension enable lower flow rate with good resolution. Post-column derivatization is eliminated with the use of tandem-MS and results in better detection limits. Proposed technique offers excellent selectivity, sensitivity and rapid analysis of phylloquinone and menaquinones. | [89] |
| Adsorptive accumulation onto solid GCE surface | ED by (SWAdSV) | Extra virgin olive oil | Electrochemical approach offers benefits of lower solvent consumption, easier sample preparation as well as lower cost. Better analytical performance is also seen in comparison to other electroanalytical methods. Results obtained are comparable with HPLC technique. | [90] |
| Simultaneous Analysis of Selected Vitamins | ||||
| Protease digestion | HPLC-dual wavelength FLD and DAD | Infant formulae and fortified milk powders | Simultaneous detection of vitamin A, E esters and β-carotene. Method eliminates saponification that allows other esters to be used as an internal standard. Faster extraction with adequate precision and recovery using protease digestion. | [91] |
| Direct solvent extraction using enzyme-assisted matrix disintegration and methanolic protein precipitation | SFC-APCI (+)-MS/MS | Milk-based infant formula, infant cereals, adult nutritionals, frozen mixed meals | Simultaneous analysis of vitamin A (retinyl acetate, palmitate, retinol), vitamin E (α-tocopherol, α-tocopheryl acetate), vitamin K (phylloquinone, menaquinone-4) via direct injection and vitamin D (D2, D3) upon derivatization. Fast, easy, robust, safe, lower cost and reliable technique for all four FSV analyses. | [92] |
| Simplified saponification and solvent extraction | UHPLC-DAD with on-line SPE | Cereal and flour products | Simultaneous analysis of vitamin D, K (D2, D3, K1, K2) with on-line SPE application for further sample purification and better detection at trace levels. Simple and reliable UHPLC method of high accuracy, repeatability and recovery. | [93] |
| DSPE with newly synthesized polymeric material consisting of PAA-T | HPLC-DAD | Milk and egg yolk | Simple, cost-effective, sensitive technique for simultaneous determination of vitamin A and E. First reported application of polyacyril amide and tetracycline as solid phase sorbent; where the use of tetracycline is useful as it is of low cost and provides good zones for the interaction of vitamin molecules. | [82] |
| Dilution with MTBE, sonication, filtration | UPC2-PDA | Canola, sunflower, vegetable, mixed, and coconut oil | Rapid detection of seven FSV (retinol, retinyl acetate, D2, D3, α-tocopherol, K1, K2) and carotenoids (lutein, lycopene, β-carotene) within 8 and 3 min, respectively. UPC2 technique environmental-friendly, cost-effective, with improved repeatability and faster analysis compared to HPLC. | [74] |
| Sample Preparation | Instrument | Food Matrix | Advantages of Current Improved Technique | Ref. |
|---|---|---|---|---|
| Vitamin C | ||||
| Addition of MPA, centrifugation, reduction to DHAA | HPLC-DAD | Juices, fruits, vegetables, fruit cream powder and infant milk formula | Selective and precise method for determination of vitamin C in foods. | [98] |
| Addition of MPA, centrifugation, dilution | UPLC-PDA and HPLC-PDA | Fruit beverages | UPLC method is faster, more sensitive, consumes less eluent, cheaper and more eco-friendly than the conventional HPLC method. | [99] |
| PLE, acid extraction and maceration | UHPLC-DAD | Camu-came fruit | PLE technique give extracts rich in vitamin C and using nontoxic solvents. Fast, higher resolution, greater sensitivity and specificity for determination of L-AA and DHAA. | [100] |
| Homogenized, Addition of EDTA, centrifugation, dilution | LC-MS | Fruits (apple, kiwi and orange) | Higher sensitivity and selectivity for determination of the L-AA and DHAA. | [101] |
| Addition of MPA, centrifugation, filtration, derivatisation | Voltammetric trace analyser 746 VA | Juices, fruits and vegetables, fruit cream powder and infant milk formula | High selectivity, lower costs, shorter time and simple method for determination of total vitamin C and DHAA contents in food. | [102] |
| Liquid extraction | UV-Visible Spectrophotometer | Fruits | Simple and fast method for determination of AA. | [103] |
| Thiamine (Vitamin B1) | ||||
| PVPP pre-treatment and derivatization | HPLC-FLD | Red wines | Higher recoveries and accurate method for determination of thiamine vitamers (thiamine diphosphate, thiamine monophosphate and thiamine) in wines. | [104] |
| Protein precipitation, enzymatic treatment | UPLC-FLD | Milk | Simple, fast, cost effective UHPLC method for the determination of the three most relevant vitamin B1 active compounds, namely thiamine, thiamine monophosphate and thiamine diphosphate. | [105] |
| Riboflavin (Vitamin B2) | ||||
| Centrifugal skimming, ultrafiltration | HPLC-FLD | Milk and milk products | Reliable and accurate method without strong acidic conditions for determination of riboflavin and the related flavins (flavin mononucleotide and flavin adenine dinucleotide). | [106] |
| Niacin (Vitamin B3) | ||||
| Dilution and derivatization | MCE-LIF | Functional Drink | Rapid, low sample consumption, miniaturization and high sensitivity. | [107] |
| Acid treatment, protein precipitation, filtration | HPLC-FLD | Meat, cereal and legume | Accurate method for determination of vitamin B3 (nicotinic acid and nicotinamide) profiles in animal and plant-based foods. | [108] |
| Pyridoxine (Vitamin B6) | ||||
| Acid digestion, enzyme treatment | HPLC-ED | Cereals products | Simple, fast sample preparation, sensitivity and selective method for simultaneous analysis of three vitamin B6 vitamers (pyridoxamine, pyridoxal and pyridoxine). | [109] |
| Biotin (Vitamin B7) | ||||
| Acid treatment | HPLC-UV | Milk | Rapid, selective, reproducible and high adsorption capacity for determination of biotin in milk food samples. | [110] |
| Folates (Vitamin B9) | ||||
| Buffer extraction, enzymatic treatment, filtration | UFLC-DAD | White rice | Fast and good recovery method for analysis of folic acid in white rice. | [111] |
| Enzyme treatment, SPE | LC-MS/MS | Dairy products, cereals, legumes, fruit, vegetables, offal and meat | Rapid, sensitive and reproducible method for analysis of six folates in food. | [112] |
| Cobalamins (Vitamin B12) | ||||
| Protein precipitation, SPE | LC-MS/MS | Cow’s milk | Fast and better selectivity for determination of vitamin B12 homologues. | [113] |
| Enzymatic treatment, centrifugation, filtration, purification | HPLC-DAD | Vegetables and fruits | Good selectivity, recovery and repeatability for the accurate determination of vitamin B12 in complex matrices. | [114] |
| Simultaneous Method of Water-Soluble Vitamins | ||||
| Filtration, Degassing | HPLC-DAD | Functional beverages | Fast, high accuracy and good reproducibility for determination of seven WSVs (vitamin C (AA), vitamins B6, B2, B3 (nicotinamide and nicotinic acid), B9 and B12) in two functional beverages. | [115] |
| Filtration, d-SPE | HPLC-UV | Orange Juice | Less consumption of organic solvents. High selectivity and satisfactory recovery for determination of vitamins B2, B3 and B6 in juice. | [116] |
| Sonication, protein precipitation, extraction with diethyl ether | LC-MS | Fresh Milk | Low volume of samples and simple sample preparation. Highly sensitive methods to quantify vitamins B1, B2, B3, B5, B6, B7 and B9 from milk samples. | [117] |
| Acid hydrolysis, acidic hydrolysis plus peptide precipitation, acidic plus enzymatic hydrolysis and enzymatic hydrolysis | LC-MS/MS | Anchovies | Fast and high specificity for simultaneous quantification of riboflavin, nicotinamide and nicotinic acid in anchovies. | [118] |
| Centrifugation | Autolab with PGSTAT 302N | Fruit Juices and energy drinks | Fast, simple, selective and sensitive method for determination of AA and vitamin B6 in fruit juices and energy drinks. | [119] |
| Degassing, centrifugation, addition of MPA, filtration | MEKC-UV | Energy drink, sport drink and fruit nectars | Minimal sample preparation and reagent consumption. Simultaneous determination of eight WSVs (vitamins B1, B2, B3 (nicotinamide and nicotinic acid), B5, B6, B12 and C). | [120] |
| Sample Preparation | Instrument | Food Matrix | Advantages of Current Improved Technique | Ref. |
|---|---|---|---|---|
| Sonicated samples and pre-column derivatization using O-phthalaldehyde (OPA). | HPLC-FLD | Vegetables and commercial juices | First reported OPA derivatives to analyse amino acids using C8 column. A rapid, sensitive, accurate and reproducible method for simultaneous determination of twenty-one amino acids (Asp, Glu, Asn, His, Ser, Gln, Cit, Arg, Gly, Thr, Ala, β-ala, Tyr, Met, Val, Trp, Phe, Ile, Leu, Lys), including non-proteinogenic amino acid, Orn. | [137] |
| SPE-concentrated samples without derivatization except for the analysis of DL-theanine, samples were pre-column derivatized using AccQ-Tag reagents. | HPLC-DAD-QTOF-MS (Chiral) | Tea | Rapid sample preparation (underivatized) and sensitive method for the detection of eleven types of D-amino acids (Thea, Thr, Leu/Ile, Phe, and Tyr) including L-form of theanine in tea infusion. | [138] |
| Deproteinization of samples with ice-cold methanol at 4 °C for 10 min, underivatized. | HPLC-MS/MS | Shitake mushroom | The use of LC-MS/MS eliminates derivatization step and allows for overlapping amino acid retention times, shortening the analysis time of determining simultaneously twenty amino acids (Pro, Thr, Cys, Asn, Lys, Met, Phe, Arg, Asp, His, Gly, Glu, Ala, Ile, Leu, Ser, Trp, Tyr, Val, Gln) and six 5′-nucleotides using a C18 column. Ion-pairing reagent, acetonitrile and water with 0.1% formic acid were shown to improve the separation of amino acids and 5′-nucleotides, achieving good resolution and symmetric peak shapes for all analytes. | [139] |
| Derivatization using diethylethoxymethylenemalonate (DEEMM). | UHPLC-PDA | Beverage (Beer) | Rapid analysis, high sensitivity and reproducibility. Used less solvent and can be a potential routine analysis for safety and quality of beers or other similar beverages. A novel UHPC method using a C18 column for a simultaneous determination of twenty-one amino acids (Asp, Glu, Asn, Ser, Gln, His, Gly, Thr, Arg, Ala, Pro, Tyr, Val, Met, Trp, Ile, Leu, Lys and Phe) including Orn and GABA, 9 biogenic amines and ammonium ions in beer. | [140] |
| Hydrolysed samples with a mixture of deionized water and methanol (20:80, v/v) acidified with 1% formic acid, underivatized. | UHPLC-PDA-HRMS | Vegetables (Fresh shallot and black onions) | Better separation of Leu and Ile isomers. Ammonium salts increased the MS chromatogram signal and peak. Simultaneous detection of twenty-one amino acids (Leu, Ile, Phe, Trp, Met, Val, Pro, Tyr, Ala, Thr, Gly, Glu, Gln, Ser, Asn, Lys, His, Asp, Arg, Orn and GABA), using BEH amide column (HILIC). Potential applicability to other similar vegetables. | [141] |
| Hydrolysed samples with 6M HCL with reducing agent, 4% (v/v) thioglycolic acid, underivatized. | UHPLC-HILIC-MS/MS | Cereal (Wheat flour) | The use of HILIC column enhanced the sensitivity of electrospray ionization-mass spectrometry (ESI-MS) detection. Tandem MS increases resolution and decreases run time, shorter separation time, high resolution and sensitive for a simultaneous determination of seventeen amino acids (Gly, Ala, Ser, Pro, Val, Thr, Asp, Glu, Ile, Leu, Asn, Lys, Met, His, Phe, Arg, Tyr and Cys). | [142] |
| Samples extraction using water (30 min), pre-column derivatization using 6-Aminoquinolyl-N hydroxysuccinimidyl carbamate (AQC) (AccQ-Tag reagent). | UHPLC-TQ-MS/MS | Tea | Simple extraction method. AQC reduced derivatization time, stabile at room temperature for several days, low toxicity, simple derivatization process and fewer side reactions. TQ-MS/MS improved detection sensitivity and resolution, increasing the separation of co-eluting compounds and shorten the chromatographic run time for simultaneous detection of twenty-one free amino acids (Asp, Glu, Hy-pro, Ser, Gly, His, Thr, Ala, Arg, Pro, Thea, Cys, Tyr, Val, Met, Ile, Lys, Leu, Phe, Trp and GABA) using a C18 column. | [143] |
| Derivatization using Trifluoroacetylacetone (FAA) and Ethylchloroformate (ECF). | GC-MS | Jams, fruits and pharmaceutical preparations | Two-stage derivatization with FAA and ECF in an aqueous phase showed better sensitivity and selectivity. This method simultaneously analysed nineteen amino acids (Gly, Ala, Val, Leu, Ile, His, Ser, Thr, Cys, Met, Asp, Asn, Pro, Glu, Gln, Lys, Tyr, Trp and Phe) using HP-5 column. | [144] |
| Hydrolysed samples with 0.1M HCL, deproteinised with acetonitrile and derivatization with N-methyl-N-(tert-butyldimethylsilyl trifluoroacetamide (MTBSTFA). | GC-MS | Meat (Dry-cured ham and Fresh pork loin) | Lesser time, a lower amount of sample and solvent required, cost and time-effective. Good recovery and excellent linearity except for Trp. Simultaneous detection of twenty-one amino acids (Ala, Gly, Val, Leu, Ile, Pro, Met, Ser, Thr, Phe, Asp, Cys, Glu, Asp, Lys, Gln, Arg, His, Tyr and Trp) including Hydroxyproline. | [145] |
| Derivatization with ethyl chloroformate/ethanol mixture. | GC-MS (Chiral) | Kefir (Fermented milk) | Combination of ethyl chloroformate and ethanol was found to be the best derivatization reagent to separate and quantify a higher number of enantiomer amino acid derivatives. This method successfully detected d- and l-ala, d- and l-val, d-pro, l-thr, Asp and Glu, Met and Cys. | [146] |
| Derivatization consisted of solid-phase extraction clean up and using reagent alkyl chloroformate. | GC-MS | Honeydew honey | Alkyl chloroformate produced stable derivatives at room temperature. Fast analysis (7 min) and no matrix effects were detected in the studied range. The standard mix of thirty-two amino acids was successfully separated. | [147] |
| Hydrolysed samples with 0.1M HCl, triple extractions (30 min each process), underivatized. | CITP-CD | Cheese | Much simpler compared to LC due to the direct injection of samples without derivatization, high sensitivity and precision. CD and PTFE pre-separation capillary analysed six amino acids (His, Phe, Lys, Arg, Tyr and Orn) with short running time. | [148] |
| Hydrolysed samples using 80% (v/v) ethanol and pre-column derivatization with 4-chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl). | CE-UV | Potato, eggplant, chickpeas, soft wheat flour and Sorghum Durra flour | NBD-CI is low cost and produces a low number of by-products for fluorescent labelling and UV detection using fused silica capillary column. Superior resolution and sensitivity compared to HPLC technique for the determination of six amino acids (Ala, Asp, Glu, Pro, Ser and Val) in various foods matrices. | [149] |
| Samples extraction using carbon tetrachloride, underivatized. | CE-UV (with online sweeping technique) | Beverage (Beer) | A novel method for the analysis of amino acids in beer without derivatization. The use of copper ions helps to enhance the UV absorption by forming complexes [Cu (AA)n]+2 that has a stronger absorption rate. An online sweeping technique enhanced the sensitivity of the amino acids, improved 25 ~ 35-fold. Fourteen amino acids (Lys, Gly, His, Ala, Ser, Val, Met, Phe, Leu, Ile, Try, Pro and Glu) were separated and determined. | [150] |
| Sample extraction using carbon tetrachloride, underivatized. | CE-UV (with online sweeping technique) | Fermented soy sauce | Higher resolution, shorter analysis time and sensitive compared to the LC technique. Fourteen amino acids (Lys, Gly, His, Ala, Ser, Thr, Val, Phe, Leu, Ile, Trp, Pro, Glu and Asp) were separated and determined using uncoated fused silica capillary column. | [151] |
| Sample Preparation | Instrument | Food Matrix | Advantages of Current Improved Technique | Ref. |
|---|---|---|---|---|
| Fatty acids | ||||
| Combination of lipid extraction and derivatization with the base-catalyzed method followed by trimethylsilyl-diazomethane (TMS-DM) | GC | Margarines | This method was found to be effective tools for analyzing dietary fats and oils in complex mixtures of food products for monitoring of low levels of FA and TFA, and the control of labelling authenticity. | [173] |
| The first method for triglycerides analysis requires dissolution of the sample in n-hexane and GC analysis using a capillary column. The second method is based on the transesterification of triglycerides as pentyl esters in a single- step reaction using sodium pentanoate in pentanol. The third method involves the transesterification of triglycerides in fat through reaction with 2-phenylethanol in a single step | GC | Butter | The first method does not require the stabilization phase of the GC system that is required in the official method. It is a simple method based on the chromatogram overlap. The advantage of second method; using pentyl esters reduces the volatility of short-chain FAs, and substantial recoveries were obtained compared with methyl ester analysis. The third method allows LC analysis at room temperature without degradation | [174] |
| Total lipid was extracted by the Folch method. Three methods of transesterification were compared. Method 1: 0.2 M KOH in methanol at 50 °C for 20 min; Method 2: 6% H2SO4 in methanol at 60 °C for 2 h; Method 3:6% H2SO4 in methanol at 80 °C for 60 min. | GC-MS and LC-MS | Milk | This study proposed simple one-step protocol based on 0.2 M methanolic KOH, a short reaction time (20 min) and a mild reaction temperature (50 °C) for milk FAME preparation | [165] |
| Two solid-liquid extraction; Protocol A using methanol and Protocol B using diethyl ether/isopropanol | LC-HRMS | Royal Jelly | Using a new platform, LC/HRMS to analyze free fatty acids in royal jelly. Solid liquid extraction protocol gives good recoveries and the method allows fast and direct quantification of FFA besides offering an advantage to simultaneously screen for additional potential fatty acids in royal jelly without the need of reference compound. | [171] |
| FAMEs were prepared using in situ methylation according to [175] with extraction process | GC | Fermented milk | Direct methylation reduces time, solvent consumption, being cost efficient and environmentally friendly, whereas free of interferences due to solvent affinity. This method allows quantification of CLA with good reproducibility | [176] |
| FAME derivatization procedure using BF3 14% in methanol according to International Standards—ISO 5509 (2000), indicated by the AOCS Method Ce 1j-07. The FAMEs in the fat samples were identified by the GLC procedure using the modified temperature program, by comparison of their relative retention times calculated to 18:0 with the respective relative retention times of the 52 FAMEs in the GLC-463 standard | Gas-Liquid Chromatography (GLC) | Mixture of soybean and sunflower oil, fish oil, butterfat | The proposed method allows to completely separate butyric acid from the solvent, trans-18:1 from cis-18:1, 20:1 isomers from 18:3n-3, 22:1n-9 from 20:4n-6, 20:5n-3 from 24:0 and the main CLA isomers | [177] |
| Oil samples were trans-esterified using 3 N methanolic HCl | GC-VUV | Olive oil, canola, vegetable, corn, sunflower and peanut oil | GC-VUV has niche selectivity and able to distinguish unsaturated FAMEs easily and differentiate cis/trans-isomeric FAMEs with enhanced chromatographic separation supported by deconvolution capabilities of the VUV detector and software | [178] |
| Fatty acid methyl ester (FAME) sample separation performed isothermally at 180 °C. | GC | Rapeseed oil mix | Ionic liquid (IL)-based column exhibited good selectivity in the analysis of the cis/trans C18:1 isomers of a partially hydrogenated vegetable oil sample on 30-m columns | [179] |
| Solid phase extraction and converted to fatty acid butyl esters (FABE) | GC-FID | Dairy products | FABE method overcomes limitations associated with direct on-column injection, such as either column-phase absorption or deterioration, accurate quantification of short-chain free fatty acids, and underestimation of polyunsaturated free fatty acid. This method is applicable for the quantification of FFAs in a wide range of dairy products | [170] |
| Frost pre-treatment of samples and coupled with an n-hexane extraction. The samples were further derivatized using 2,4-dimethoxy-6-piperazin-1-yl pyrimidine (DMPP) | UHPLC-ESI-MS/MS | Milk powder | Frozen pre-treatment of samples improved the extraction efficiency of FFAs in infant milk powder due to the increased polarity from water to ice while decreases impurities in the extracts. High sensitivity and specificity of DMPP labelling coupled with MS reduced sample amount. | [180] |
| Samples were directly used without any treatment | Raman Spectroscopy | Olive oil | Relative Raman intensity analysis useful for quick quality evaluation of extra virgin olive oils | [181] |
| Samples were extracted using acid and base catalyst method. Acid catalyst were carried out in H2SO4/methanol solvents. Methylation was performed for 2 h at 80 °C, and FA methyl esters were recovered for chromatographic analysis by the addition of isooctane. The extraction using base catalysts was conducted by AOAC 989.05 [168] official method, followed by AOAC 969.33 [169] for methylation. | GC | Milk products | Acid catalysts method is able to extract CLA and some fatty acids with higher yield compared to base catalyst. This method minimizes sample loss and contamination through reduced sample manipulation. | [182] |
| All meat samples were freeze dried and ground to fine powder. FA derivatized using bi-methylation procedure | GC-FID | Meat | This method has fewer steps and can be performed under non-anhydrous conditions. This method is also applicable to meat samples from different species, covering a broad range of fat content and offers a simplified and reliable method for analysis of fatty acids from meat samples. | [172] |
| Liquid/liquid extraction protocol involving the addition of methanol for the protein precipitation. | LC-HRMS | Milk | The current method involves mild sample preparation conditions, excluding the hydrolysis of esterified fatty acids of triacylglycerols or other lipid classes and avoids time-consuming extraction pre-separation, or derivatization procedures. It is rapid and robust, permitting the quantification of twenty-two FFAs in a 10-min single run | [183] |
| Cholesterol | ||||
| Hot saponification with subsequent derivatization to trimethylsilyl ether | GC | Turkey meat | An easy, quick and sensitive GC method for the determination of cholesterol in turkey meat products in the range of 0.4–8 mg cholesterol/g using relative response factors | [184] |
| Saponification at 80 °C for 3 h for a complete cholesterol extraction | UV-VIS-NIR spectroscopy and enzymatic method | Egg yolk | The UV-VIS-NIR spectroscopy combined with chemometric tools demonstrated to be a useful, rapid, clean and cheap technique for determination of egg yolk cholesterol | [185] |
| Saponification process with different amount of samples (0.1, 0.25, 0.5, 0.75 and 1.0 g, wide range of ethanolic KOH concentrations (0.1, 0.2, 0.3 and 0.4 M) and different saponification reaction time (30, 60, 90 and 120 min) | HPLC and UHPLC | Sour cream, egg, egg yolk and chicken nugget | UHPLC method allowed reduction in the consumption of organic solvents (8 times lower) and decreased analysis time (4 min), being more eco-friendly, than conventional HPLC methods | [186] |
| Saponification with 10% methanolic KOH and separated in a solvent-solvent extraction with diethyl ether:water (5:2) | Spectrophotometer | Dairy products | Liebermann-Burchard reaction via colorimetric method is a robust and reliable alternative method for analysis of cholesterol in dairy products | [187] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Noh, M.F.; Gunasegavan, R.D.-N.; Mustafa Khalid, N.; Balasubramaniam, V.; Mustar, S.; Abd Rashed, A. Recent Techniques in Nutrient Analysis for Food Composition Database. Molecules 2020, 25, 4567. https://doi.org/10.3390/molecules25194567
Md Noh MF, Gunasegavan RD-N, Mustafa Khalid N, Balasubramaniam V, Mustar S, Abd Rashed A. Recent Techniques in Nutrient Analysis for Food Composition Database. Molecules. 2020; 25(19):4567. https://doi.org/10.3390/molecules25194567
Chicago/Turabian StyleMd Noh, Mohd Fairulnizal, Rathi Devi-Nair Gunasegavan, Norhayati Mustafa Khalid, Vimala Balasubramaniam, Suraiami Mustar, and Aswir Abd Rashed. 2020. "Recent Techniques in Nutrient Analysis for Food Composition Database" Molecules 25, no. 19: 4567. https://doi.org/10.3390/molecules25194567
APA StyleMd Noh, M. F., Gunasegavan, R. D.-N., Mustafa Khalid, N., Balasubramaniam, V., Mustar, S., & Abd Rashed, A. (2020). Recent Techniques in Nutrient Analysis for Food Composition Database. Molecules, 25(19), 4567. https://doi.org/10.3390/molecules25194567






