Essential Oil Compositions of Three Invasive Conyza Species Collected in Vietnam and Their Larvicidal Activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus
Abstract
1. Introduction
2. Results and Discussion
2.1. Essential Oil Compositions
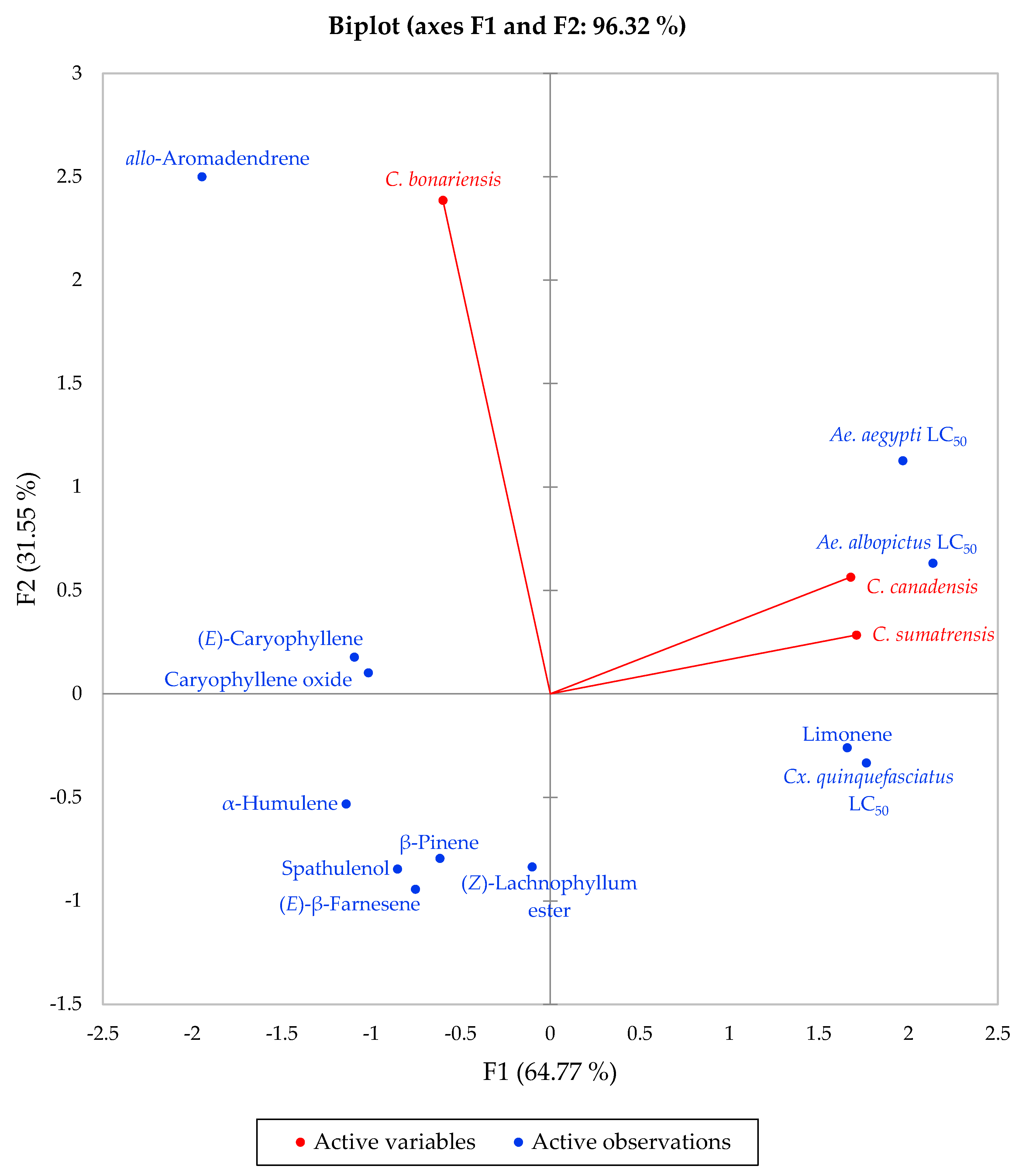
2.2. Mosquito Larvicidal Activity
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Gas Chromatography–Mass Spectrometry
3.4. Mosquito Larvicidal Assay
3.5. Non-Target Insecticidal Assay
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim Lien, P.T.; Briant, L.; Tang, T.B.; Trang, B.M.; Gavotte, L.; Cornillot, E.; Duoc, V.T.; Duong, T.N.; Frutos, R.; Nga, P.T. Surveillance of dengue and chikungunya infection in Dong Thap, Vietnam: A 13-month study. Asian Pac. J. Trop. Med. 2016, 9, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Pham Thi, K.L.; Briant, L.; Gavotte, L.; Labbe, P.; Perriat-Sanguinet, M.; Cornillot, E.; Vu, T.D.; Nguyen, T.Y.; Tran, V.P.; Nguyen, V.S.; et al. Incidence of dengue and chikungunya viruses in mosquitoes and human patients in border provinces of Vietnam. Parasit. Vectors 2017, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R. Mosquito-borne human viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018, 98, 1563–1565. [Google Scholar] [CrossRef] [PubMed]
- Lourenço de Oliveira, R.; Vazeille, M.; de Filippis, A.M.B.; Failloux, A.B. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. Am. J. Trop. Med. Hyg. 2003, 69, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, L.; Scott, T.W.; Gubler, D.J. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010, 4, e646. [Google Scholar] [CrossRef]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, J.S.; Fontenille, D.; Schuffenecker, I.; et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef]
- Wong, P.-S.J.; Li, M.I.; Chong, C.-S.; Ng, L.-C.; Tan, C.-H. Aedes (Stegomyia) albopictus (Skuse): A potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis. 2013, 7, e2348. [Google Scholar] [CrossRef]
- Albuquerque, C.M.R.; Cavalcanti, V.M.S.; Melo, M.A.V.; Verçosa, P.; Regis, L.N.; Hurd, H. Bloodmeal microfilariae density and the uptake and establishment of Wuchereria bancrofti infections in Culex quinquefasciatus and Aedes aegypti. Mem. Inst. Oswaldo Cruz 1999, 94, 591–596. [Google Scholar] [CrossRef]
- Turell, M.J. Members of the Culex pipiens complex as vectors of viruses. J. Am. Mosq. Control Assoc. 2012, 28, 123–127. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Hall-Mendelin, S.; Jansen, C.C.; Higgs, S. Zika virus and Culex quinquefasciatus mosquitoes: A tenuous link. Lancet Infect. Dis. 2017, 17, 1014–1016. [Google Scholar] [CrossRef]
- Thebaud, C.; Abbott, R.J. Characterization of invasive Conyza species (Asteraceae) in Europe: Quantitative trait and isozyme analysis. Am. J. Bot. 1995, 82, 360–368. [Google Scholar] [CrossRef]
- Prieur-Richard, A.-H.; Lavorel, S.; Grigulis, K.; Dos Santos, A. Plant community diversity and invasibility by exotics: Invasion of Mediterranean old fields by Conyza bonariensis and Conyza canadensis. Ecol. Lett. 2000, 3, 412–422. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Sadia, S.; Ali, H.H.; Jabran, K.; Peerzada, A.M.; Chauhan, B.S. Biology and management of two important Conyza weeds: A global review. Environ. Sci. Pollut. Res. 2016, 23, 24694–24710. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Walker, S.; Rollin, M.J.; Tan, D.K.Y.; Robinson, G.; Werth, J. Germination, persistence, and emergence of flaxleaf fleabane (Conyza bonariensis [L.] Cronquist). Weed Biol. Manag. 2007, 7, 192–199. [Google Scholar] [CrossRef]
- Wu, H. The biology of Australian weeds. Plant Prot. Quart. 2007, 22, 122–131. [Google Scholar]
- Pruski, J.F.; Sancho, G. Conyza sumatrensis var. leiotheca (Compositae: Astereae), a new combination for a common Neotropical weed. Novon 2006, 16, 96–101. [Google Scholar] [CrossRef]
- Raghubanshi, A.S.; Rai, L.C.; Gaur, J.P.; Singh, J.S. Invasive alien species and biodiversity in India. Curr. Sci. 2005, 88, 539–540. [Google Scholar]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Kettenring, K.M.; Adams, C.R. Lessons learned from invasive plant control experiments: A systematic review and meta-analysis. J. Appl. Ecol. 2011, 48, 970–979. [Google Scholar] [CrossRef]
- Pasko, S.; Goldberg, J. Review of harvest incentives to control invasive species. Manag. Biol. Invasions 2014, 5, 263–277. [Google Scholar] [CrossRef]
- Stocker, R.K. Mechanical harvesting of Melaleuca quinquenervia in Lake Okeechobee, Florida. Ecol. Eng. 1999, 12, 373–386. [Google Scholar] [CrossRef]
- Carson, B.D.; Lishawa, S.C.; Tuchman, N.C.; Monks, A.M.; Lawrence, B.A.; Albert, D.A. Harvesting invasive plants to reduce nutrient loads and produce bioenergy: An assessment of Great Lakes coastal wetlands. Ecosphere 2018, 9, e02320. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of two invasive plant invaders in Europe (Solidago canadensis and Solidago gigantea) as possible sources of botanical insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Kamrin, M.A. Pesticide Profiles: Toxicity, Environmental Impact, and Fate; CRC Press: Boca Raton, FL, USA, 1997; ISBN 0-56670-190-2. [Google Scholar]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Cuervo-Para, J.A.; Romero Cortés, T.; Ramirez-Lepe, M. Mosquito-borne Diseases, Pesticides Used for Mosquito Control, and Development of Resistance to Insecticides. In Insecticides Resistance; Trdan, S., Ed.; IntechOpen: London, UK, 2016; pp. 111–134. ISBN 978-953-51-2258-6. [Google Scholar]
- Silva, W.J.; Dória, G.A.A.; Maia, R.T.; Nunes, R.S.; Carvalho, G.A.; Blank, A.F.; Alves, P.B.; Marçal, R.M.; Cavalcanti, S.C.H. Effects of essential oils on Aedes aegypti larvae: Alternatives to environmentally safe insecticides. Bioresource Technol. 2008, 99, 3251–3255. [Google Scholar] [CrossRef]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef]
- Masetti, A. The potential use of essential oils against mosquito larvae: A short review. Bull. Insectol. 2016, 69, 307–310. [Google Scholar]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Ntalli, N.; Koliopoulos, G.; Giatropoulos, A.; Menkissoglu-Spiroudi, U. Plant secondary metabolites against arthropods of medical importance. Phytochem. Rev. 2019, 18, 1255–1275. [Google Scholar] [CrossRef]
- Satyal, P.; Hieu, H.V.; Chuong, N.T.H.; Hung, N.H.; Sinh, L.H.; Van The, P.; Tai, T.A.; Hien, V.T.; Setzer, W.N. Chemical composition, Aedes mosquito larvicidal activity, and repellent activity against Triatoma rubrofasciata of Severinia monophylla leaf essential oil. Parasitol. Res. 2019, 118, 733–742. [Google Scholar] [CrossRef]
- Hung, N.H.; Satyal, P.; Hieu, H.V.; Chuong, N.T.H.; Dai, D.N.; Huong, L.T.; Tai, T.A.; Setzer, W.N. Mosquito larvicidal activity of the essential oils of Erechtites species growing wild in Vietnam. Insects 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.H.; Satyal, P.; Do, N.D.; Tai, T.A.; Huong, L.T.; Chuong, N.T.H.; Hieu, H.V.; Tuan, P.A.; Vuong, P.; Van Setzer, W.N. Chemical compositions of Crassocephalum crepidioides essential oils and larvicidal activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, MD, USA, 2016. [Google Scholar]
- NIST17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Thesis, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Maia, J.G.S.; da Silva, M.H.L.; das Gracas, M.B.Z.; Andrade, E. Composition of the essential oils of Conyza bonariensis (L.) Cronquist. J. Essent. Oil Res. 2002, 14, 325–326. [Google Scholar] [CrossRef]
- Souza, M.C.; Siani, A.C.; Ramos, M.F.S.; Menezes-de-lima, O., Jr.; Henriques, M.G.M.O. Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Pharmazie 2003, 58, 582–586. [Google Scholar] [PubMed]
- Barbosa, L.C.A.; Paula, V.F.; Azevedo, A.S.; Silva, E.A.M.; Nascimento, E.A. Essential oil composition from some plant parts of Conyza bonariensis (L.) Cronquist. Flavour Fragr. J. 2005, 20, 39–41. [Google Scholar] [CrossRef]
- Tzakou, O.; Vagias, C.; Gani, A.; Yannitsaros, A. Volatile constituents of essential oils isolated at different growth stages from three Conyza species growing in Greece. Flavour Fragr. J. 2005, 20, 425–428. [Google Scholar] [CrossRef]
- Urdampilleta, J.D.; Amat, A.G.; Bidau, C.J.; Koslobsky, N.K. Biosystematic and chemosystematic studies in five South American species of Conyza (Asteraceae). Bol. Soc. Argent. Bot. 2005, 40, 101–107. [Google Scholar]
- Mabrouk, S.; Elaissi, A.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition of essential oils from leaves, stems, flower heads and roots of Conyza bonariensis L. from Tunisia. Nat. Prod. Res. 2011, 25, 77–84. [Google Scholar] [CrossRef]
- Benzarti, A.; Hammami, S.; Piras, A.; Falconieri, D.; El Mokni, R.; M’Henni, M.F.; Marongiu, B.; Mighri, Z. Effects of different ecological conditions and extraction techniques on the quality of volatile oils from flaxleaf fleabane (Erigeron bonariensis L.). J. Med. Plant Res. 2013, 7, 3059–3065. [Google Scholar]
- Araujo, L.; Moujir, L.M.; Rojas, J.; Carmona, J.; Rondón, M. Chemical composition and biological activity of Conyza bonariensis essential oil collected in Mérida, Venezuela. Nat. Prod. Commun. 2013, 8, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Musembei, R.; Joyce, K.J. Chemical composition and antibacterial activity of essential oil from Kenyan Conyza bonariensis (L.) Cronquist. Sci. Lett. 2017, 5, 180–185. [Google Scholar]
- do Amaral, W.; Deschamps, C.; Biasi, L.A.; Bizzo, H.R.; Machado, M.P.; da Silva, L.E. Yield and chemical composition of the essential oil of species of the Asteraceae family from Atlantic Forest, South of Brazil. J. Essent. Oil Res. 2018, 30, 278–284. [Google Scholar] [CrossRef]
- Stoyanova, A.; Georgiev, E.; Kermedchieva, D.; Lis, A.; Gora, J. Changes in the essential oil of Conyza canadensis (L.) Cronquist. during its vegetation. J. Essent. Oil Res. 2003, 15, 44–45. [Google Scholar] [CrossRef]
- Lis, A.; Piggott, J.R.; Góra, J. Chemical composition variability of the essential oil of Conyza canadensis Cronq. Flavour Fragr. J. 2003, 18, 364–367. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Azar, P.A.; Moradalizadeh, M.; Masoudi, S.; Ameri, N. Volatile constituents of three Compositae herbs: Anthemis altissima L. var. altissima, Conyza canadensis (L.) Cronq. and Grantina aucheri Boiss. growing wild in Iran. J. Essent. Oil Res. 2004, 16, 579–581. [Google Scholar] [CrossRef]
- Choi, H.-J.; Wang, H.-Y.; Kim, Y.-N.; Heo, S.-J.; Kim, N.-K.; Jeong, M.-S.; Park, Y.-H.; Kim, S. Composition and cytotoxicity of essential oil extracted by steam distillation from horseweed (Erigeron canadensis L.) in Korea. J. Korean Soc. Appl. Biol. Chem. 2008, 51, 55–59. [Google Scholar]
- Zeng, D.-Q.; Peng, Y.-H.; Chen, F.-F.; Zhang, Y.; Liu, M. Insecticidal activity of essential oil derived from horseweed Conyza canadensis (L.) Cronq.against two mosquitoes and its volatile components. Acta Entomol. Sin. 2014, 57, 204–211. [Google Scholar]
- Veres, K.; Csupor-Löffler, B.; Lázár, A.; Hohmann, J. Antifungal activity and composition of essential oils of Conyza canadensis herbs and roots. Sci. World J. 2012, 2012, 489646. [Google Scholar] [CrossRef]
- Ayaz, F.; Küçükboyacı, N.; Demirci, B. Chemical composition and antimicrobial activity of the essential oil of Conyza canadensis (L.) Cronquist from Turkey. J. Essent. Oil Res. 2017, 29, 336–343. [Google Scholar] [CrossRef]
- Machado, S.M.F.; Militão, J.S.L.T.; Facundo, V.A.; Ribeiro, A.; de Morais, S.M.; de Alencar, J.W.; Braz Filho, R. Essential oil of Conyza sumatrensis (Retz) Walk. J. Essent. Oil Res. 1995, 7, 83–84. [Google Scholar] [CrossRef]
- Boti, J.B.; Koukoua, G.; N’Guessan, T.Y.; Casanova, J. Chemical variability of Conyza sumatrensis and Microglossa pyrifolia from Côte d’Ivoire. Flavour Fragr. J. 2007, 22, 27–31. [Google Scholar] [CrossRef]
- Mabrouk, S.; Salah, K.B.H.; Elaissi, A.; Jlaiel, L.; Ben Jannet, H.; Aouni, M.; Harzallah-Skhiri, F. Chemical composition and antimicrobial and allelopathic activity of Tunisian Conyza sumatrensis (Retz.) E. Walker essential oils. Chem. Biodivers. 2013, 10, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Dictionary of Natural Products on DVD; CRC Press: Boca Raton, FL, USA, 2019; ISBN 0-412-49150-8.
- Giatropoulos, A.; Papachristos, D.P.; Kimbaris, A.; Koliopoulos, G.; Polissiou, M.G.; Emmanouel, N.; Michaelakis, A. Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their components enantiomeric distribution. Parasitol. Res. 2012, 111, 2253–2263. [Google Scholar] [CrossRef]
- Manimaran, A.; Cruz, M.M.J.J.; Muthu, C.; Vincent, S.; Ignacimuthu, S. Larvicidal and knockdown effects of some essential oils against Culex quinquefasciatus Say, Aedes aegypti (L.) and Anopheles stephensi (Liston). Adv. Biosci. Biotechnol. 2012, 3, 855–862. [Google Scholar] [CrossRef][Green Version]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef]
- Tak, J.H.; Isman, M.B. Penetration-enhancement underlies synergy of plant essential oil terpenoids as insecticides in the cabbage looper, Trichoplusia ni. Sci. Rep. 2017, 7, 42432. [Google Scholar] [CrossRef]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding synergistic toxicity of terpenes as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef]
- Saha, N.; Aditya, G.; Bal, A.; Saha, G.K. A comparative study of predation of three aquatic heteropteran bugs on Culex quinquefasciatus larvae. Limnology 2007, 8, 73–80. [Google Scholar] [CrossRef]
- Huong, L.T.; Hung, N.H.; Dai, D.N.; Tai, T.A.; Hien, V.T.; Satyal, P.; Setzer, W.N. Chemical compositions and mosquito larvicidal activities of essential oils from Piper species. Molecules 2019, 24, 3871. [Google Scholar] [CrossRef]
- Finney, D. Probit Analysis; Reissue, Ed.; Cambridge University Press: Cambridge, UK, 2009; ISBN 978-0521135900. [Google Scholar]
- Travlos, I.S.; Chachalis, D. Glyphosate-resistant hairy fleabane (Conyza bonariensis) is reported in Greece. Weed Technol. 2010, 24, 569–573. [Google Scholar] [CrossRef]
- Koger, C.H.; Poston, D.H.; Hayes, R.M.; Montgomery, R.F. Glyphosate-resistant horseweed (Conyza canadensis) in Mississippi. Weed Technol. 2004, 18, 820–825. [Google Scholar] [CrossRef]
- Santos, G.; Oliveira, R.S., Jr.; Constantin, J.; Francischini, A.C.; Machado, M.F.P.S.; Mangolin, C.A.; Nakajima, J.N. Conyza sumatrensis: A new weed species resistant to glyphosate in the Americas. Weed Biol. Manag. 2014, 14, 106–114. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the Conyza essential oils are no longer available. |
| RIcalc a | RIdb b | Compound | Relative Content % | ||
|---|---|---|---|---|---|
| C. bonariensis | C. canadensis | C. sumatrensis | |||
| 931 | 932 | α-Pinene | 0.5 | 0.5 | 0.2 |
| 948 | 950 | Camphene | tr c | --- | --- |
| 967 | 972 | (3Z)-Octen-2-ol | --- | --- | tr |
| 971 | 972 | Sabinene | tr | 0.1 | 0.1 |
| 976 | 978 | β-Pinene | 0.8 | 8.8 | 3.0 |
| 982 | 984 | 6-Methylhept-5-en-2-one | --- | --- | tr |
| 987 | 989 | Myrcene | tr | 1.2 | 1.0 |
| 1023 | 1025 | p-Cymene | tr | 0.3 | 0.1 |
| 1028 | 1030 | Limonene | 0.2 | 41.5 | 25.5 |
| 1030 | 1031 | β-Phellandrene | --- | tr | --- |
| 1034 | 1034 | (Z)-β-Ocimene | --- | --- | tr |
| 1044 | 1045 | (E)-β-Ocimene | --- | tr | 1.9 |
| 1049 | 1051 | 2,3,6-Trimethylhepta-1,5-diene | --- | tr | --- |
| 1056 | 1057 | γ-Terpinene | --- | tr | --- |
| 1088 | 1091 | p-Cymenene | --- | 0.1 | --- |
| 1090 | 1091 | Rosefuran | --- | --- | 0.1 |
| 1093 | 1097 | α-Pinene oxide | --- | --- | 0.2 |
| 1097 | 1098 | Perillene | --- | 0.1 | --- |
| 1098 | 1101 | Linalool | 0.2 | --- | --- |
| 1101 | 1101 | 6-Methyl-3,5-heptadien-2-one | --- | --- | 0.1 |
| 1103 | 1104 | Nonanal | tr | --- | --- |
| 1112 | 1113 | 4,8-Dimethylnona-1,3,7-triene | --- | --- | 0.2 |
| 1118 | 1119 | endo-Fenchol | tr | --- | --- |
| 1120 | 1121 | trans-p-Mentha-2,8-dien-1-ol | --- | 0.9 | 0.2 |
| 1124 | 1131 | Cyclooctanone | --- | 0.8 | --- |
| 1129 | 1130 | 4-Acetyl-1-methylcyclohexene | --- | 0.1 | --- |
| 1131 | 1132 | cis-Limonene oxide | --- | 0.6 | 0.2 |
| 1134 | 1137 | cis-p-Mentha-2,8-dien-1-ol | --- | 1.2 | 0.3 |
| 1135 | 1137 | trans-Limonene oxide | --- | 0.6 | --- |
| 1137 | 1137 | Nopinone | --- | 0.4 | --- |
| 1137 | 1139 | (E)-Myroxide | --- | --- | 0.1 |
| 1139 | 1141 | trans-Pinocarveol | tr | 1.6 | 0.1 |
| 1150 | 1152 | Citronellal | --- | 0.1 | --- |
| 1160 | 1164 | Pinocarvone | --- | 0.8 | tr |
| 1170 | 1170 | Borneol | tr | --- | --- |
| 1177 | 1179 | 2-Isopropenyl-5-methylhex-4-enal | --- | 0.3 | --- |
| 1182 | 1184 | p-Methylacetophenone | --- | 0.3 | --- |
| 1185 | 1185 | Cryptone | --- | 0.4 | --- |
| 1185 | 1187 | trans-p-Mentha-1(7),8-dien-2-ol | --- | 0.2 | --- |
| 1189 | 1190 | Methyl salicylate | tr | --- | --- |
| 1193 | 1195 | α-Terpineol | 0.1 | --- | 0.1 |
| 1193 | 1196 | Myrtenal | --- | 1.4 | --- |
| 1194 | 1195 | Myrtenol | --- | 1.2 | --- |
| 1196 | 1197 | Methyl chavicol (=Estragol) | --- | 0.2 | --- |
| 1198 | 1201 | cis-Piperitol | --- | 0.8 | 0.1 |
| 1206 | 1207 | Oct-3E-enyl acetate | --- | --- | 0.1 |
| 1217 | 1218 | trans-Carveol | --- | 3.8 | 0.2 |
| 1227 | 1228 | cis-p-Mentha-1(7),8-dien-2-ol | --- | 0.1 | --- |
| 1230 | 1232 | cis-Carveol | --- | 1.1 | 0.1 |
| 1242 | 1242 | Carvone | --- | 3.8 | 0.2 |
| 1247 | 1249 | Linalyl acetate | tr | --- | --- |
| 1266 | 1270 | iso-Piperitenone | --- | 0.6 | --- |
| 1273 | 1277 | Perilla aldehyde | --- | 0.5 | --- |
| 1287 | 1287 | Limonene dioxide | --- | 0.7 | --- |
| 1296 | 1299 | Perilla alcohol | --- | 0.4 | --- |
| 1303 | --- | Unidentified d | --- | 1.1 | --- |
| 1316 | 1324 | Limonene hydroperoxide | --- | 1.1 | --- |
| 1343 | 1346 | Limonene-1,2-diol | --- | 2.6 | --- |
| 1344 | 1349 | 7-epi-Silphiperfol-5-ene | --- | --- | 0.3 |
| 1345 | 1349 | α-Cubebene | 0.2 | --- | --- |
| 1355 | 1340 | p-Mentha-6,8-diene-2-hydroperoxide | --- | 1.2 | --- |
| 1367 | 1371 | α-Ylangene | tr | --- | --- |
| 1374 | 1375 | α-Copaene | 4.5 | --- | 0.1 |
| 1376 | 1380 | Daucene | --- | --- | 0.4 |
| 1377 | 1374 | Isoledene | --- | --- | 0.3 |
| 1379 | 1382 | Modheph-2-ene | --- | --- | 0.4 |
| 1381 | 1382 | β-Bourbonene | tr | --- | --- |
| 1385 | 1387 | β-Cubebene | 0.4 | --- | 0.1 |
| 1386 | 1385 | α-Isocomene | --- | --- | 0.1 |
| 1387 | 1390 | β-Elemene | 0.3 | --- | 0.4 |
| 1392 | 1394 | Sativene | --- | --- | 0.1 |
| 1398 | 1405 | (Z)-Caryophyllene | 0.2 | --- | --- |
| 1404 | 1406 | α-Gurjunene | 0.1 | --- | --- |
| 1408 | 1411 | β-Isocomene | --- | --- | 0.1 |
| 1418 | 1417 | (E)-Caryophyllene | 13.3 | --- | 5.5 |
| 1427 | 1430 | β-Copaene | 0.2 | --- | 0.2 |
| 1430 | 1433 | trans-α-Bergamotene | --- | --- | 1.1 |
| 1432 | 1440 | 6,9-Guaiadiene | --- | --- | 0.2 |
| 1433 | 1436 | α-Guaiene | 1.8 | --- | --- |
| 1436 | 1438 | Aromadendrene | 0.2 | --- | 0.1 |
| 1445 | 1449 | (E)-Lachnophyllum acid | --- | --- | 0.2 |
| 1451 | 1452 | (E)-β-Farnesene | --- | --- | 6.7 |
| 1453 | 1454 | α-Humulene | 5.4 | 0.3 | 0.7 |
| 1457 | 1463 | cis-Cadina-1(6),4-diene | --- | --- | 0.4 |
| 1460 | 1458 | allo-Aromadendrene | 41.2 | --- | --- |
| 1469 | --- | Unidentified e | --- | --- | 1.3 |
| 1472 | 1472 | trans-Cadina-1(6),4-diene | 0.5 | --- | 0.2 |
| 1476 | 1479 | α-Amorphene | 0.1 | --- | --- |
| 1478 | 1483 | Germacrene D | 0.3 | --- | 2.1 |
| 1481 | 1483 | trans-β-Bergamotene | --- | --- | 0.2 |
| 1486 | 1489 | β-Selinene | 0.5 | --- | --- |
| 1488 | 1491 | Viridiflorene | 0.2 | --- | --- |
| 1492 | 1497 | Bicyclogermacrene | --- | --- | 0.3 |
| 1493 | 1497 | α-Selinene | 0.3 | --- | --- |
| 1495 | 1497 | α-Muurolene | 0.4 | --- | 0.1 |
| 1498 | 1505 | α-Bulnesene | 1.8 | --- | --- |
| 1501 | 1505 | (E,E)-α-Farnesene | --- | --- | 0.1 |
| 1504 | 1514 | (Z)-Lachnophyllum acid | --- | 0.2 | 0.8 |
| 1507 | 1510 | (E)-Lachnophyllum ester | --- | --- | 0.4 |
| 1510 | 1512 | γ-Cadinene | 0.4 | --- | 0.1 |
| 1515 | 1515 | (Z)-Lachnophyllum ester | --- | 5.5 | 20.7 |
| 1515 | 1518 | δ-Cadinene | 0.6 | --- | --- |
| 1518 | 1519 | trans-Calamenene | 0.3 | --- | --- |
| 1521 | 1523 | β-Sesquiphellandrene | --- | --- | 0.3 |
| 1531 | 1532 | Tridec-11-yn-1-ol | --- | --- | 0.3 |
| 1533 | 1538 | α-Cadinene | 0.1 | --- | --- |
| 1538 | 1541 | α-Calacorene | 0.1 | --- | --- |
| 1556 | 1557 | Germacrene B | --- | --- | 0.1 |
| 1558 | 1560 | (E)-Nerolidol | --- | 0.2 | 1.8 |
| 1559 | 1564 | β-Calacorene | 0.1 | --- | --- |
| 1565 | 1566 | 1,5-Epoxysalvial-4(14)-ene | --- | --- | 0.2 |
| 1566 | 1568 | Dendrolasin | --- | --- | 0.1 |
| 1567 | 1567 | Palustrol | 0.1 | --- | --- |
| 1574 | 1576 | Spathulenol | 1.3 | --- | 5.2 |
| 1580 | 1577 | Caryophyllene oxide | 12.2 | 1.1 | 5.8 |
| 1582 | 1590 | Globulol | 0.4 | --- | 0.5 |
| 1589 | 1593 | Salvial-4(14)-en-1-one | --- | 0.1 | 0.2 |
| 1590 | 1594 | Viridiflorol | 0.8 | --- | 0.3 |
| 1593 | 1599 | Cubeban-11-ol | 0.2 | --- | --- |
| 1599 | 1601 | Carotol | --- | --- | 1.1 |
| 1601 | 1605 | Ledol | 0.6 | --- | --- |
| 1606 | 1611 | Humulene epoxide II | 2.2 | 2.9 | 0.4 |
| 1624 | 1628 | 1-epi-Cubenol | 0.2 | --- | --- |
| 1629 | 1629 | iso-Spathulenol | --- | --- | 0.6 |
| 1633 | 1635 | Caryophylla-4(12),8(13)-dien-5β-ol | 0.2 | --- | --- |
| 1635 | 1632 | Muurola-4,10(14)-dien-1β-ol | --- | --- | 0.7 |
| 1638 | 1643 | τ-Cadinol | 0.2 | --- | 0.4 |
| 1640 | 1644 | τ-Muurolol | 0.1 | --- | 0.3 |
| 1643 | 1643 | α-Muurolol | 0.2 | --- | --- |
| 1643 | 1644 | allo-Aromadendrene epoxide | --- | 0.3 | --- |
| 1652 | 1655 | α-Cadinol | 0.6 | 0.3 | 0.4 |
| 1655 | 1655 | Eudesma-4(15),7-dien-1α-ol | --- | --- | 0.1 |
| 1661 | 1664 | cis-Calamenen-10-ol | 0.1 | --- | --- |
| 1666 | 1666 | 14-Hydroxy-9-epi-(E)-caryophyllene | 0.1 | --- | --- |
| 1669 | 1677 | Cadalene | 0.1 | --- | --- |
| 1686 | 1685 | Eudesma-4(15),7-dien-1β-ol | --- | 0.4 | 0.1 |
| 1698 | 1704 | cis-Thujopsenol | 0.1 | --- | --- |
| 1717 | --- | Unidentified f | --- | 1.0 | --- |
| 1738 | 1740 | 8α,11-Elemodiol | 0.1 | --- | --- |
| 1751 | 1748 | Khusimol | 1.5 | --- | --- |
| 1790 | 1792 | 14-Hydroxy-δ-cadinene | --- | --- | 0.2 |
| 1800 | --- | Unidentified g | 1.1 | --- | --- |
| 1833 | 1836 | Neophytadiene | --- | --- | 0.2 |
| 1857 | 1860 | Platambin | 0.1 | 0.5 | 0.1 |
| 1882 | 1884 | Corymbolone | 0.2 | --- | --- |
| 2103 | 2102 | Phytol | tr | --- | 0.1 |
| Monoterpene hydrocarbons | 1.5 | 52.7 | 31.8 | ||
| Oxygenated monoterpenoids | 0.3 | 26.4 | 1.9 | ||
| Sesquiterpene hydrocarbons | 73.7 | 0.3 | 20.7 | ||
| Oxygenated sesquiterpenoids | 21.3 | 5.7 | 18.5 | ||
| Diterpenoids | trace | --- | 0.4 | ||
| Others | trace | 7.2 | 22.9 | ||
| Total Identified | 96.8 | 92.3 | 96.1 | ||
| Conyza Species (Collection Site) | Major Components (>5%) | Ref. |
|---|---|---|
| C. bonariensis aerial parts EO (Chapada dos Guimarães, Mato Grosso, Brazil) | limonene (6.9%), (E)-caryophyllene (14.4%), (E)-β-farnesene (23.3%), germacrene D (15.3%), bicyclogermacrene (8.3%), spathulenol (7.6%) | [40] |
| C. bonariensis aerial parts EO (Melgaço, Pará, Brazil) | limonene (22.9%), (E)-caryophyllene (13.3%), trans-α-bergamotene (5.3%), (E)-β-farnesene (20.1%), bicyclogermacrene (6.6%), spathulenol (6.3%) | [40] |
| C. bonariensis aerial parts EO (Peixe-Boi, Pará, Brazil) | (E)-caryophyllene (13.3%), trans-α-bergamotene (8.1%), (E)-β-farnesene (30.9%) | [40] |
| C. bonariensis aerial parts EO (alta Floresta, Mato Grosso, Brazil) | limonene (12.6%), (E)-caryophyllene (13.0%), (E)-β-farnesene (19.1%), germacrene D (13.2%), bicyclogermacrene (6.3%), spathulenol (5.7%) | [40] |
| C. bonariensis aerial parts EO (Macapá, Amapá, Brazil) | limonene (58.4%), (E)-β-farnesene (7.0%) | [40] |
| C. bonariensis aerial parts EO (Rio de Janeiro, Brazil) | limonene (45.0%), (E)-β-ocimene (13.0%), (E)-β-farnesene (6.6%), germacrene D (6.4%) | [41] |
| C. bonariensis leaf EO (Minas Gerais State, Brazil) | limonene (29.6%), trans-α-bergamotene (10.3%), matricaria methyl ester (8.3%), β-copaen-4α-ol (7.4%) | [42] |
| C. bonariensis aerial parts EO (Athens, Greece) | limonene (8.3%), (E)-β-ocimene (11.5%), (E)-β-farnesene (8.1%), (Z)-lachnophyllum ester (21.2%), matricaria ester (17.5%) | [43] |
| C. bonariensis aerial parts EO (Southwestern Misiones Province, Argentina) | limonene (13.5%), (E)-β-ocimene (13.3%), p-mentha-1,3,8-triene (5.2%), germacrene D (14.6%), bicyclogermacrene (6.6%) | [44] |
| C. bonariensis leaf EO (Monastir, Tunisia) | limonene (5.8%), terpinolene (5.3%), (E)-β-farnesene (7.5%), matricaria ester (17.8%), caryophyllene oxide (7.8%) | [45] |
| C. bonariensis aerial parts EO (Cagliari, Sardinia, Italy) | limonene (5.1%), carvacrol (9.8%), α-curcumene (10.2%), spathulenol (18.6%), caryophyllene oxide (18.7%), neophytadiene (6.1%) | [46] |
| C. bonariensis leaf EO (Mérida State, Venezuela) | limonene (5.1%), (Z)-β-ocimene (5.1%), (E)-β-ocimene (20.7%), (E)-β-farnesene (37.8%), α-farnesene (5.6%), β-sesquiphellandrene (9.8%) | [47] |
| C. bonariensis leaf EO (Kabianga, Kericho, Kenya) | β-pinene (5.4%), limonene (8.3%), 2,6,7,7a-tetrahydro-1,5-dimethyl-1H-indene-3-carboxaldehyde (49.1%) a | [48] |
| C. bonariensis aerial parts EO (Parana State, Brazil) | limonene (66.3%), 2-heptyl acetate (6.9%) | [49] |
| C. bonariensis aerial parts EO | (E)-caryophyllene (13.3%), α-humulene (5.4%), allo-aromadendrene (41.2%), caryophyllene oxide (12.2%) | this work |
| C. canadensis aerial parts EO (Plovdiv, Bulgaria) | limonene (77.7–89.4%) | [50] |
| C. canadensis aerial parts EO (Łódź, Poland) | limonene (76.3%) | [51] |
| C. canadensis aerial parts EO (Alps, France) | limonene (83.2%) | [51] |
| C. canadensis aerial parts EO (Rome, Italy) | limonene (70.3%), (E)-β-ocimene (5.5%) | [51] |
| C. canadensis aerial parts EO (Seville, Spain) | limonene (51.4%), (E)-β-ocimene (13.4%), trans-α-bergamotene (11.9%) | [51] |
| C. canadensis aerial parts EO (Belgium) | limonene (68.0%), (E)-β-ocimene (5.1%), trans-α-bergamotene (5.4%), germacrene D (7.3%) (Z,Z)-matricaria ester (6.1%) | [51] |
| C. canadensis aerial parts EO (Plovdiv, Bulgaria) | limonene (87.9%) | [51] |
| C. canadensis aerial parts EO (Vilnius, Lithuania) | limonene (77.7%), trans-α-bergamotene (5.5%) | [51] |
| C. canadensis aerial parts EO (Israel) | limonene (54.9%), (Z)-β-farnesene (6.3%) (Z,Z)-matricaria ester (7.7%) | [51] |
| C. canadensis aerial parts EO (Kerman, Iran) | myrcene (8.9%), limonene (12.3%), (E)-β-farnesene (14.6%), ar-curcumene (7.8%), zingiberene (5.5%), spathulenol (14.1%), isospathulenol (7.7%), phytol (7.3%) | [52] |
| C. canadensis aerial parts EO (Athens, Greece) | β-pinene (9.5%), limonene (57.3%), matricaria ester (14.4%) | [43] |
| C. canadensis aerial parts EO (Korea) | limonene (68.3%), (E)-β-ocimene (15.9%) b | [53] |
| C. canadensis EO (China) | limonene (14.8%), epi-bicyclosesquiphellandrene (11.0%), C7H30B4Si (25.1%) c, 1-phenyl-1-nonyne (7.3%) | [54] |
| C. canadensis aerial parts EO (Szeged, Hungary) | limonene (79.2%) | [55] |
| C. canadensis aerial parts EO (Manavgat, Antalya, Turkey) | β-pinene (9.7%), limonene (28.1%), spathulenol (16.3%) | [56] |
| C. canadensis aerial parts EO | β-pinene (8.8%), limonene (41.5%), (Z)-lachnophyllum ester (5.5%) | this work |
| C. sumatrensis aerial parts EO (Rondôndia state, Brazil) | sabinene (5.3%), limonene (22.9%), (E)-β-ocimene (5.0%), (E)-β-farnesene (5.3%), (Z)-lachnophyllum ester (43.7%) | [57] |
| C. sumatrensis leaf EO (N’gorato village, Côte d’Ivoire) | limonene (13.0%), (E)-β-ocimene (6.5%), (E)-caryophyllene (10.5%), (E)-β-farnesene (17.0%), (Z)-lachnophyllum ester (5.9%), germacrene D (13.6%), bicyclogermacrene (5.2%) | [58] |
| C. sumatrensis leaf EO (Monastir, Tunisia) | matricaria ester (7.5%), spathulenol (13.8%), caryophyllene oxide (20.5%) | [59] |
| C. sumatrensis aerial parts EO | limonene (25.5%), (E)-caryophyllene (5.5%), (E)-β-farnesene (6.7%), (Z)-lachnophyllum ester (20.7%), spathulenol (5.2%), caryophyllene oxide (5.8%) | this work |
| 24 h | |||||
| Essential Oil or Major Compound | LC50 (95% Limits), μg/mL | LC90 (95% Limits), μg/mL | χ2 | p | Slope |
| Aedes aegypti | |||||
| C. bonariensis | 69.71 (64.82–75.36) | 88.61 (82.13–97.54) | 9.39 | 0.009 | 9.45 |
| C. canadensis | 9.801 (8.730–10.986) | 23.27 (19.93–28.36) | 8.70 | 0.069 | 12.18 |
| C. sumatrensis | 21.74 (20.16–23.36) | 31.02 (28.29–35.50) | 0.131 | 0.988 | 7.98 |
| β-Pinene | 23.63 (22.16-25.33) | 32.12 (29.47-36.00) | 0.225 | 0.994 | 7.69 |
| Limonene | 17.66 (16.45–18.97) | 23.62 (22.03–25.73) | 0.784 | 0.941 | 10.68 |
| (E)-Caryophyllene | 70.80 (65.49–76.69 | 107.2 (98.4–118.6) | 4.08 | 0.395 | 12.75 |
| α-Humulene | 53.05 (48.69–58.08) | 82.78 (75.81–91.87) | 15.9 | 0.003 | 12.79 |
| Caryophyllene oxide | 136.6 (129.2–143.9) | 180.2 (171.4–191.2) | 30.1 | 0.000 | 12.37 |
| Permethrin control | 0.000643 (0.000551–0.00753) | 0.00246 (0.00192–0.00344) | 12.5 | 0.006 | 11.57 |
| Aedes albopictusa | |||||
| C. bonariensis | 81.13 (74.61–87.97) | 127.1 (117.5–139.9) | 0.395 | 0.821 | 11.44 |
| C. canadensis | 18.04 (16.71–19.52) | 26.20 (24.22–28.82) | 1.46 | 0.834 | 11.30 |
| C. sumatrensis | 19.13 (17.73–20.66) | 27.49 (25.41–30.38) | 3.19 | 0.364 | 9.97 |
| Permethrin control | 0.0024 (0.0021–0.0026) | 0.0042 (0.0038–0.0049) | 4.64 | 0.031 | 8.45 |
| Culex quinquefasciatus | |||||
| C. bonariensis | 130.0 (122.5–138.8) | 178.4 (165.6–197.2) | 0.675 | 0.713 | 8.97 |
| C. canadensis | 39.37 (36.83–42.00) | 52.29 (49.04–56.56) | 0.493 | 0.974 | 10.49 |
| C. sumatrensis | 26.74 (24.80–29.20) | 36.83 (33.56–41.92) | 8.97 | 0.030 | 7.96 |
| β-Pinene | 30.46 (28.21–33.21) | 41.58 (38.10–46.58) | 0.399 | 0.983 | 9.38 |
| Limonene | 31.63 (29.37–34.50) | 41.51 (38.03–46.78) | 0.874 | 0.928 | 8.23 |
| (E)-Caryophyllene | 165.4 (157.5–174.0) | 220.6 (207.8–238.5) | 10.0 | 0.040 | 9.91 |
| α-Humulene | 108.3 (101.4–115.5) | 158.2 (148.5–170.5) | 1.0 | 0.910 | 13.32 |
| Caryophyllene oxide | 98.52 (90.70–108.68) | 144.5 (129.6–165.7) | 1.60 | 0.809 | 9.20 |
| Permethrin control | 0.0165 (0.0149–0.0181) | 0.0305 (0.0266–0.0367) | 5.24 | 0.073 | 10.12 |
| Diplonychus rusticusa | |||||
| C. canadensis | 135.7 (129.3–142.8) | 182.5 (172.6–195.5) | 7.78 | 0.051 | 12.35 |
| C. sumatrensis | 111.0 (106.1–116.7) | 137.0 (129.5–147.6) | 16.1 | 0.001 | 9.85 |
| 48 h | |||||
| Essential Oil or Major Compound | LC50 (95% Limits), μg/mL | LC90 (95% Limits), μg/mL | χ2 | p | Slope |
| Aedes aegypti | |||||
| C. bonariensis | 63.85 (59.07–70.75) | 81.84 (74.16–94.79) | 3.43 | 0.180 | 6.89 |
| C. canadensis | 7.091 (6.099–8.141) | 22.46 (18.63–28.59) | 5.98 | 0.201 | 11.63 |
| C. sumatrensis | 22.52 (21.18–23.87) | 29.00 (27.23–31.68) | 0.0488 | 0.997 | 10.12 |
| β-Pinene | 22.91 (21.29–24.85) | 31.37 (29.03–35.03) | 0.323 | 0.988 | 9.08 |
| Limonene | 17.43 (16.24–18.74) | 23.17 (21.58–25.28) | 0.664 | 0.956 | 10.48 |
| (E)-Caryophyllene | 65.92 (60.45–72.08) | 106.4 (98.4–116.7) | 14.2 | 0.007 | 13.10 |
| α-Humulene | 46.25 (42.27–50.94) | 74.14 (67.47–82.99) | 19.2 | 0.001 | 12.21 |
| Caryophyllene oxide | 120.2 (112.7–127.5) | 165.4 (156.4–176.6) | 19.8 | 0.001 | 12.34 |
| Permethrin control | 0.000575 (0.000483–0.00688) | 0.00281 (0.00208–0.00423) | 5.29 | 0.152 | 10.93 |
| Aedes albopictusa | |||||
| C. bonariensis | 69.42 (63.20–75.93) | 113.2 (103.8–125.8) | 3.10 | 0.212 | 10.72 |
| C. canadensis | 15.12 (13.93–16.47) | 22.67 (20.84–25.09) | 7.23 | 0.124 | 12.22 |
| C. sumatrensis | 18.43 (17.05–19.93) | 26.76 (24.71–29.58) | 4.25 | 0.236 | 8.44 |
| Culex quinquefasciatus | |||||
| C. bonariensis | 108.1 (101.4–115.1) | 152.1 (142.4–165.1) | 2.32 | 0.313 | 10.84 |
| C. canadensis | 29.81 (27.33–32.68) | 47.06 (43.03–52.39) | 14.5 | 0.006 | 12.17 |
| C. sumatrensis | 22.95 (21.22-25.08) | 33.06 (30.07-37.60) | 2.38 | 0.498 | 9.37 |
| β-Pinene | 28.36 (26.20–31.19) | 39.01 (35.41–44.50) | 2.41 | 0.661 | 8.39 |
| Limonene | 29.15 (26.89–31.98) | 40.83 (37.19–46.07) | 7.05 | 0.133 | 9.50 |
| (E)-Caryophyllene | 138.5 (129.3–148.5) | 215.3 (200.1–234.9) | 13.5 | 0.009 | 13.11 |
| α-Humulene | 87.81 (81.14–94.89) | 140.0 (130.0–152.7) | 9.80 | 0.044 | 13.50 |
| Caryophyllene oxide | 95.19 (86.69–106.26) | 141.0 (127.6–160.8) | 4.01 | 0.405 | 10.12 |
| Diplonychus rusticusa | |||||
| C. canadensis | 124.0 (118.0–130.4) | 165.0 (156.1–176.6) | 1.17 | 0.760 | 12.17 |
| C. sumatrensis | 107.8 (103.1–113.4) | 133.6 (126.1–144.4) | 8.07 | 0.045 | 9.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoi, T.M.; Huong, L.T.; Chinh, H.V.; Hau, D.V.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Hien, V.T.; Setzer, W.N. Essential Oil Compositions of Three Invasive Conyza Species Collected in Vietnam and Their Larvicidal Activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Molecules 2020, 25, 4576. https://doi.org/10.3390/molecules25194576
Hoi TM, Huong LT, Chinh HV, Hau DV, Satyal P, Tai TA, Dai DN, Hung NH, Hien VT, Setzer WN. Essential Oil Compositions of Three Invasive Conyza Species Collected in Vietnam and Their Larvicidal Activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Molecules. 2020; 25(19):4576. https://doi.org/10.3390/molecules25194576
Chicago/Turabian StyleHoi, Tran Minh, Le Thi Huong, Hoang Van Chinh, Dang Viet Hau, Prabodh Satyal, Thieu Anh Tai, Do Ngoc Dai, Nguyen Huy Hung, Vu Thi Hien, and William N Setzer. 2020. "Essential Oil Compositions of Three Invasive Conyza Species Collected in Vietnam and Their Larvicidal Activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus" Molecules 25, no. 19: 4576. https://doi.org/10.3390/molecules25194576
APA StyleHoi, T. M., Huong, L. T., Chinh, H. V., Hau, D. V., Satyal, P., Tai, T. A., Dai, D. N., Hung, N. H., Hien, V. T., & Setzer, W. N. (2020). Essential Oil Compositions of Three Invasive Conyza Species Collected in Vietnam and Their Larvicidal Activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Molecules, 25(19), 4576. https://doi.org/10.3390/molecules25194576










